In Silico Insights into the Mechanism of Action of Epoxy-α-Lapachone and Epoxymethyl-Lawsone in Leishmania spp.
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Chemical Structures
3.2. Protein Structures
3.3. Prediction of the Interaction of Compounds on the Enzymes
4. Conclusions and Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- World Health Organization (WHO). Control of Leishmaniases: Report of the Meeting of the WHO Expert Committee on the Control of Leishmaniases; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Ready, P.D. Epidemiology of visceral Leishmaniasis. Clin. Epidemiol. 2014, 6, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Pace, D. Leishmaniasis. J. Infect. 2014, 69 (Suppl. 1), S10–S18. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.F.; Schubach, A. Systematic review of the adverse effects of cutaneous leishmaniasis treatment in the New World. Acta Trop. 2011, 18, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Berman, J.D.; Waddell, D. Biochemical Mechanisms of the Antileishmanial Activity of Sodium Stibogluconate. Antimicrob. Agents Chemother. 1985, 27, 916–920. [Google Scholar] [CrossRef]
- Wyllie, S.; Cunningham, M.L. Dual action of antimonial drugs on thiol redox metabolism in the human pathogen Leishmania donovani. J. Biol. Chem. 2004, 279, 39925–39932. [Google Scholar] [CrossRef]
- Baiocco, P.; Colotti, G. Molecular Basis of Antimony Treatment in Leishmaniasis. J. Med. Chem. 2009, 52, 2603–2612. [Google Scholar] [CrossRef]
- Yardley, V.; Croft, S.L. A comparison of the activities of three amphotericin B lipid formulations against experimental visceral and cutaneous leishmaniasis. Int. J. Antimicrob. Agents 2000, 13, 243–248. [Google Scholar] [CrossRef]
- Sievers, T.M.; Kubak, B.M. Safety and efficacy of Intralipid emulsions of Amphotericin B. J. Antimicrob. Chemother 1996, 38, 333–347. [Google Scholar] [CrossRef]
- Sundar, S. Treatment of visceral leishmaniasis. Med. Microbiol. Immunol 19. [CrossRef]
- Wolday, D.; Berhe, N. Emerging Leishmania/HIV Co-infection in Africa. Med. Microbiol. Immunol. 2001, 19, 65–67. [Google Scholar] [CrossRef]
- Brynceton, A. Current issues in the treatment of visceral leishmaniasis. Med. Microbiol. Immunol. 2001, 190, 81–84. [Google Scholar] [CrossRef]
- Croft, S.L.; Engel, J. Miltefosine discovery of the antileishmanial activity of phospholipid derivatives. Trans R Soc Trop Med Hyg. 2006, 100 (Suppl. 1), S4–S8. [Google Scholar] [CrossRef]
- Fisher, C.; Voss, A. Development status of miltefosine as first oral drug in visceral and cutaneous leishmaniasis. Med. Microb. Immunol. 2001, 190, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Ben Salah, A.; Zakraoui, H. A randomized, placebocontrolled trial in Tunisia treating cutaneous leishmaniasis with paromomycin ointment. Am. J. Trop. Med. Hyg. 1995, 5, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Croft, S.L.; Yardley, V. Drug sensitivity of Leishmania species: Some unresolved problems. Trans. R. Soc. Trop. Med. Hyg. 2002, 96, S127–S129. [Google Scholar] [CrossRef]
- Sundar, S.; Jha, T.K. Injectable paromomycin for visceral leishmaniasis in India. N. Engl. J. Med. 2007, 356, 2571–2581. [Google Scholar] [CrossRef] [PubMed]
- Souza-Silva, F.; do Nascimento, S.B. Evidences for leishmanicidal activity of the naphthoquinone derivative epoxy—lapachone. Exp. Parasitol. 2014, 147, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Souza-Silva, F.; Bourguignon, S.C. Epoxy-a-lapachone has in vitro and in vivo anti-leishmania (Leishmania) amazonensis effects and inhibits serine proteinase activity in this parasite. Antimicrob. Agents Chemother. 2015, 59, 1910–1918. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.F.G.; Souza-Silva, F. Evidence for tissue toxicity in BALB/c exposed to a long-term treatment with oxiranes compared to meglumine antimoniate. Biomed. Res. Int. 2017, 9840210. [Google Scholar] [CrossRef]
- Oliveira, L.F.G.; Souza-Silva, F. Antileishmanial activity of 2-methoxy-4H-spiro-[naphthalene-1,2′-oxiran]-4-one (Epoxymethoxy-lawsone): A promising new drug candidate for leishmaniasis treatment. Molecules 2018, 23, 864. [Google Scholar] [CrossRef]
- Gonçalves-Oliveira, L.F.; Souza-Silva, F. The combination therapy of meglumine antimoniate and oxiranes (epoxy-α-lapachone and epoxymethyl-lawsone) enhance the leishmanicidal effect in mice infected by Leishmania (Leishmania) amazonensis. Int. J. Parasitol. Drugs Drug Resist. 2019, 10, 101–108. [Google Scholar] [CrossRef]
- Paterno, E. Ricerche Sull Acido Lapacico. Gazz. Chim. Ital. 1882, 12, 337–339. [Google Scholar]
- Hooker, S.C. The Constitution of Lapachic Acid (Lapachol) and Derivatives. J. Chem. Soc. 1892, 61, 611–650. [Google Scholar] [CrossRef][Green Version]
- Hooker, S.C. The Constitution of Lapachol and its Derivatives. The Struture of the Amylene Chain. J. Chem. Soc. 1896, 69, 1355–1381. [Google Scholar] [CrossRef]
- Ferreira, V.F.; Pinto, A.V. 2,3-Dihydro-3,3-dimethylspiro- [1H-4-oxanthracene-5,2′-oxiran]-10(5H)-one. Acta Crystallogr. 2002, 58, 560. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, P.F.; do Nascimento, S.B. New oxirane derivatives of 1, 4-naphthoquinones and their evaluation against T. cruzi epimastigote forms. Bioorganic Med. Chem. Lett. 2012, 20, 4995–5000. [Google Scholar] [CrossRef]
- Bourguignon, S.C.; Cavalcanti, D.F. Trypanosoma cruzi: Insights into naphthoquinone effects on growth and proteinase activity. Exp. Parasitol. 2010, 127, 160–166. [Google Scholar] [CrossRef]
- O’Brien, P.J. Molecular mechanisms of quinone cytotoxicity. Chem.-Biol. Interact. 1991, 80, 1–41. [Google Scholar] [CrossRef]
- Monks, T.J.; Hanzlik, R.P. Quinone chemistry and toxicity. Toxicol. Appl. Pharmacol. 1992, 112, 2–16. [Google Scholar] [CrossRef]
- Monks, T.J.; Jones, D.C. The metabolism and toxicity of quinones, quinonimines, quinone methides, and quinonethioethers. Curr. Drug Metab. 2002, 3, 425–438. [Google Scholar] [CrossRef]
- Klopčič, I.; Dolenc, M.S. Chemicals and drugs forming reactive quinone and quinone imine metabolites. Chem. Res. Toxicol. 2018, 32, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, P.; Bastow, K.F. Novel mechanisms of DNA topoisomerase II inhibition by pyranonaphthoquinone derivatives-eleutherin, alpha lapachone, and beta lapachone. Biochem. Pharmacol. 2000, 60, 1367–1379. [Google Scholar] [CrossRef]
- Jorqueira, A.; Gouvêa, R.M. Oxyrane derivative of α-lapachone is potent growth inhibitor of Trypanosoma cruzi epimastigote forms. Parasitol. Res. 2006, 99, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.F.; Jorqueira, A. Trypanocidal agents with low cytotoxicity to mammalian cell line: A comparison of the theoretical and biological features of lapachone derivatives. Bioorganic Med. Chem. 2006, 14, 5459–5466. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, P.F.; Pinto, M.C.R.F. Synthesis and antimalarial activity of quinones and structurally-related oxirane derivatives. Eur. J. Med. Chem. 2016, 108, 134–140. [Google Scholar] [CrossRef] [PubMed]
- López, L.I.L.; Flores, S.D.N. Naphthoquinones: Biological properties and synthesis of lawsone and derivates-a structured review. Vitae 2014, 21, 248–258. [Google Scholar]
- Verlinde, C.L.; Hannaert, V. Glycolysis as a target for the design of new anti-trypanosome drugs. Drug. Resist. Updates 2001, 4, 50–65. [Google Scholar] [CrossRef] [PubMed]
- McCall, L.I.; El Aroussi, A. Targeting Ergosterol biosynthesis in Leishmania donovani: Essentiality of sterol 14 alpha-demethylase. PLoS Negl. Trop. Dis 2015, 9, e0003588. [Google Scholar] [CrossRef]
- Lepesheva, G.I.; Hargrove, T.Y. VFV as a New Effective CYP51 Structure-Derived Drug Candidate for Chagas Disease and Visceral Leishmaniasis. J. Infect. Dis. 2015, 212, 1439–1448. [Google Scholar] [CrossRef]
- Brilhante, R.S.; Caetano, É.P. Terpinen-4-ol, tyrosol, and β-lapachone as potential antifungals against dimorphic fungi. Braz. J. Microbiol. 2016, 47, 917–924. [Google Scholar] [CrossRef]
- Choi, J.Y.; Podust, L.M. Drug strategies targeting CYP51 in neglected tropical diseases. Chem. Rev. 2014, 114, 11242–11271. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Hsu, F.F. Sterol biosynthesis is required for heat resistance but not extracellular survival in Leishmania. PLoS Pathog. 2014, 10, e1004427. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Moitra, S. Sterol 14-α-demethylase is vital for mitochondrial functions and stress tolerance in Leishmania major. PLoS Pathog. 2020, 16, e1008810. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.W.; McLeod, R. Fatty acid and sterol metabolism: Potential antimicrobial targets in apicomplexan and trypanosomatid parasitic protozoa. Mol. Biochem. Parasitol. 2003, 126, 129–142. [Google Scholar] [CrossRef]
- Kim, I.S.; Kim, Y. Effects of β-lapachone, a new anticancer candidate, on cytochrome P450-mediated drug metabolism. Cancer Chemother. Pharmacol. 2013, 72, 699–702. [Google Scholar] [CrossRef]
- Niwa, T.; Imagawa, Y. Drug interactions between nine antifungal agents and drugs metabolized by human cytochromes P450. Curr. Drug Metab. 2014, 15, 651–679. [Google Scholar] [CrossRef]
- Pisani, D.; Elliot, A.J. Relationship between inhibition of mitochondrial respiration by naphthoquinones, their antitumor activity, and their redox potential. Biochem. Pharmacol. 1986, 35, 3791–3798. [Google Scholar] [CrossRef]
- Menna-Barreto, R.F.S.; Goncalves, R.L.S. The effects on Trypanosoma cruzi of novel synthetic naphthoquinones are mediated by mitochondrial dysfunction. Free Radic. Biol. Med. 2009, 47, 644–653. [Google Scholar] [CrossRef]
- Mendonça, D.V.C.; Lage, D.P. Antileishmanial activity of a naphthoquinone derivate against promastigote and amastigote stages of Leishmania infantum and Leishmania amazonensis and its mechanism of action against L. amazonensis species. Parasitol. Res. 2018, 117, 391–403. [Google Scholar] [CrossRef]
- McHardy, N.; Wekesa, L.S. Anti-theilerial activity of BW720C (buparvaquone): A comparison with parvaquone. Res. Vet. Sci. 1985, 39, 29–33. [Google Scholar] [CrossRef]
- Croft, S.L.; Hogg, J. The activity of hydroxynaphthoquinones against Leishmania donovani. J. Antimicrob. Chemother. 1992, 30, 827–832. [Google Scholar] [CrossRef]
- Ortiz, D.; Forquer, I. Targeting the Cytochrome bc1 Complex of Leishmania Parasites for Discovery of Novel Drugs. Antimicrob. Agents Chemother. 2016, 60, 4972–4982. [Google Scholar] [CrossRef]
- Siregar, J.E.; Kurisub, G. Direct evidence for the atovaquone action on the Plasmodium cytochrome bc1 complex. Parasitol. Int. 2015, 64, 295–300. [Google Scholar] [CrossRef]
- Beal, M.F. Mitochondria take center stage in aging and neurodegeneration. Ann. Neurol. 2005, 58, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D.; Nicholls, D.G. Assessing mitochondrial dysfunction in cells. Biochem J. 2011, 435, 297–312. [Google Scholar] [CrossRef]
- de Sena Pereira, V.S.; da Silva Emery, F. In vitro antiplasmodial activity, pharmacokinetic profiles and interference in isoprenoid pathway of 2-aniline-3-hydroxy-1.4-naphthoquinone derivatives. Malar. J. 2018, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, M.D.; Curtis, D.E. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 1–17. [Google Scholar] [CrossRef] [PubMed]
- de Magalhães, C.S.; Almeida, D.M. A dynamic niching genetic algorithm strategy for docking highly flexible ligands. Inf. Sci. 2014, 289, 206–224. [Google Scholar] [CrossRef]
- Burza, S.; Croft, S.L. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- Ponte-Sucre, A.; Gamarro, F. Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLoS Negl. Trop. Dis. 2017, 14, e0006052. [Google Scholar] [CrossRef] [PubMed]
- Braga, S.S. Multi-target drugs active against leishmaniasis: A paradigm of drug repurposing. Eur. J. Med. Chem. 2019, 183, 111660. [Google Scholar] [CrossRef] [PubMed]
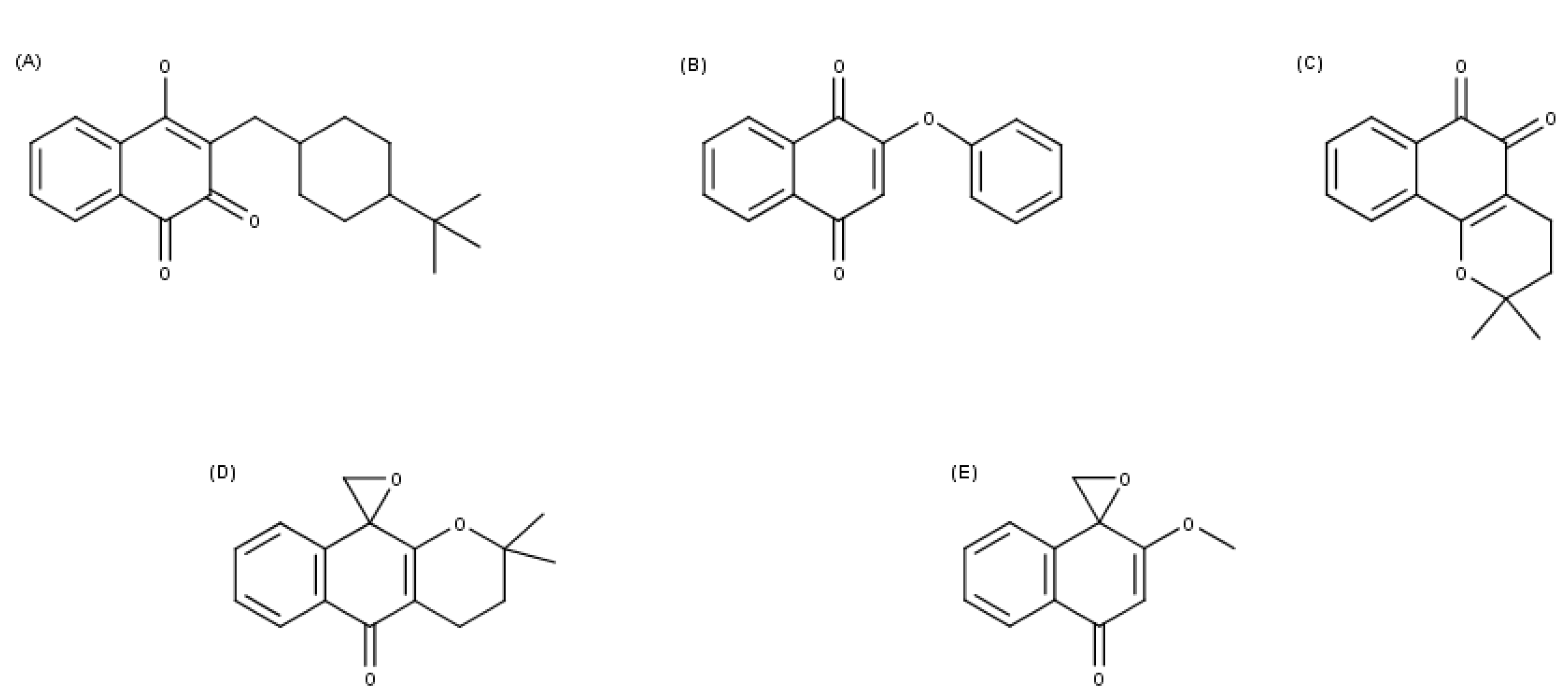
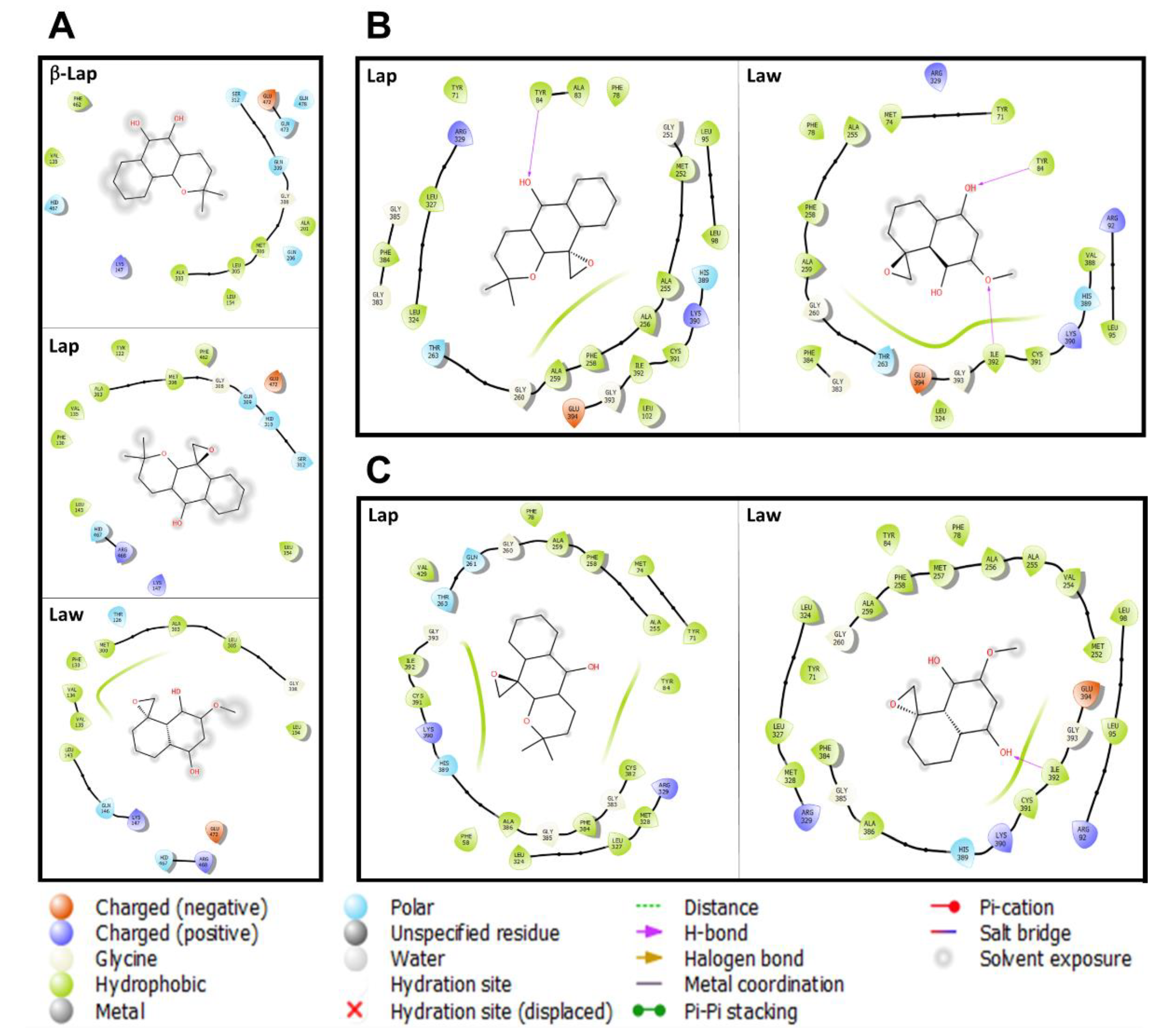
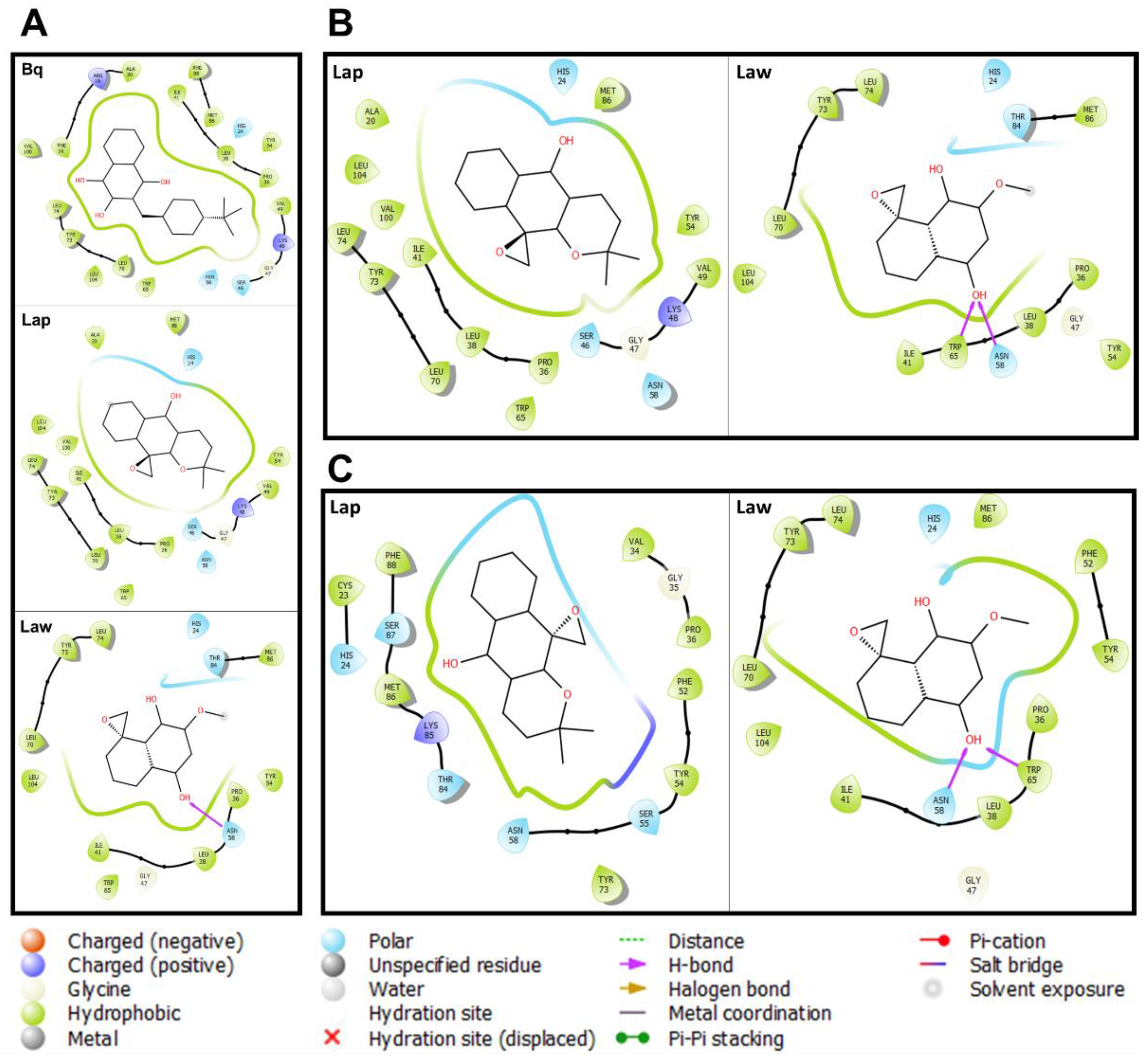

| Parasites | Enzymes | Compounds | ||||
|---|---|---|---|---|---|---|
| Lap | Law | B6 | Buparvaquone | β-lapachone | ||
| Coccidioides posadasii | Lanosterol C-14 demethylase | −8.0 | −8.0 | ND | ND | −8.4 |
| Leishmania (L.) mexicana | Cytochrome C | −10.2 | −8.9 | ND | −10.0 | ND |
| Trypanosoma brucei | Glyceraldehyde-3-phosphate | −8.1 | −7.5 | −8.0 | ND | ND |
| Leishmania (L.) amazonensis | Lanosterol C-14 demethylase | −8.4 | −7.4 | ND | ND | ND |
| Cytochrome C | −10.0 | −8.8 | ND | ND | ND | |
| Glyceraldehyde-3-phosphate | −8.5 | −7.6 | ND | ND | ND | |
| Leishmania (V.) braziliensis | Lanosterol C-14 demethylase | −8.2 | −7.4 | ND | ND | ND |
| Cytochrome C | −9.0 | −8.8 | ND | ND | ND | |
| Glyceraldehyde-3-phosphate | −8.3 | −7.7 | ND | ND | ND | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peixoto, J.F.; Oliveira, A.d.S.; Monteiro, P.Q.; Gonçalves-Oliveira, L.F.; Andrade-Neto, V.V.; Ferreira, V.F.; Souza-Silva, F.; Alves, C.R. In Silico Insights into the Mechanism of Action of Epoxy-α-Lapachone and Epoxymethyl-Lawsone in Leishmania spp. Molecules 2021, 26, 3537. https://doi.org/10.3390/molecules26123537
Peixoto JF, Oliveira AdS, Monteiro PQ, Gonçalves-Oliveira LF, Andrade-Neto VV, Ferreira VF, Souza-Silva F, Alves CR. In Silico Insights into the Mechanism of Action of Epoxy-α-Lapachone and Epoxymethyl-Lawsone in Leishmania spp. Molecules. 2021; 26(12):3537. https://doi.org/10.3390/molecules26123537
Chicago/Turabian StylePeixoto, Juliana Figueiredo, Adriane da Silva Oliveira, Patrícia Queiroz Monteiro, Luiz Filipe Gonçalves-Oliveira, Valter Viana Andrade-Neto, Vitor Francisco Ferreira, Franklin Souza-Silva, and Carlos Roberto Alves. 2021. "In Silico Insights into the Mechanism of Action of Epoxy-α-Lapachone and Epoxymethyl-Lawsone in Leishmania spp." Molecules 26, no. 12: 3537. https://doi.org/10.3390/molecules26123537
APA StylePeixoto, J. F., Oliveira, A. d. S., Monteiro, P. Q., Gonçalves-Oliveira, L. F., Andrade-Neto, V. V., Ferreira, V. F., Souza-Silva, F., & Alves, C. R. (2021). In Silico Insights into the Mechanism of Action of Epoxy-α-Lapachone and Epoxymethyl-Lawsone in Leishmania spp. Molecules, 26(12), 3537. https://doi.org/10.3390/molecules26123537









