Detection of Frozen–Thawed Duck Fatty Liver by MALDI-TOF Mass Spectrometry: A Chemometrics Study
Abstract
1. Introduction
2. Results and Discussion
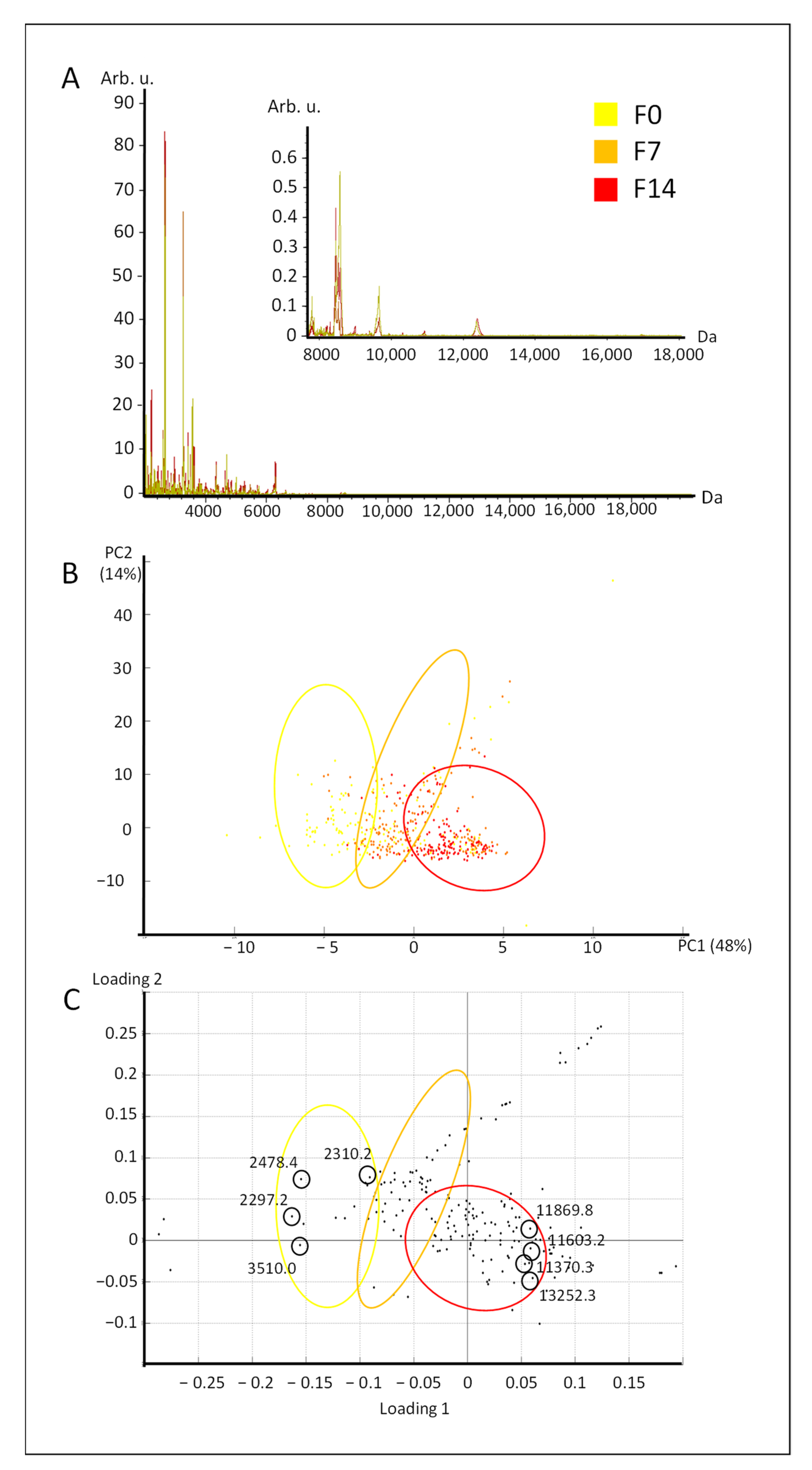
2.1. Study of the Variability under Commercial Conditions
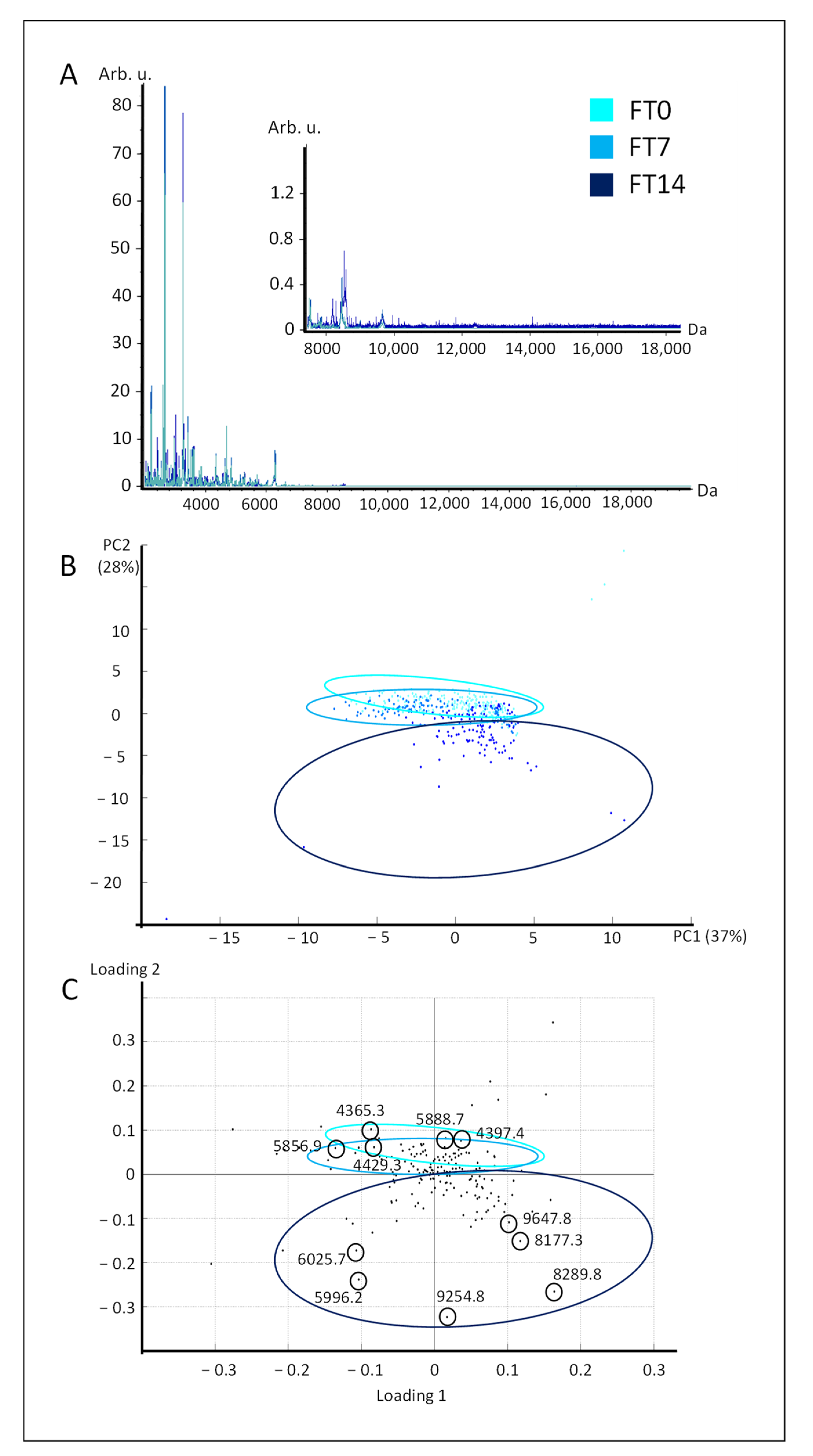
2.2. Discrimination of Fresh and Frozen–Thawed Livers
3. Materials and Methods
3.1. Chemical and Reagents
3.2. Experimental Design and Liver Sampling
3.3. Proteins Extraction
3.4. MALDI-TOF Mass Spectrometry Protein Fingerprinting
3.5. Chemometrics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Regulation EU No. 1308/2013 of the European Parliament and of the Council of 17 December 2013. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32013R1308 (accessed on 17 April 2021).
- Soyer, A.; Ozalp, B.; Dalmis, U.; Bilgin, V. Effect of freezing temperature and duration of frozen storage on lipid and protein oxidation in chicken meat. Food Chem. 2010, 120, 1025–1030. [Google Scholar] [CrossRef]
- Xiong, Y.L. Protein oxidation and implications for muscle food quality. In Antioxidants in Muscle Foods; Decker, E., Faustman, C., Lopez-Bote, C.J., Eds.; John Wiley & Sons: Chicester, UK, 2000; ISBN 0471314544. [Google Scholar]
- Théron, L.; Fernandez, X.; Marty-Gasset, N.; Chambon, C.; Viala, D.; Pichereaux, C.; Rossignol, M.; Astruc, T.; Molette, C. Proteomic analysis of duck fatty liver during post-mortem storage related to the variability of fat loss during cooking of “Foie Gras”. J. Agric. Food Chem. 2013, 61, 920–930. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.; Diaz, M.Y.; Martinez, B.; Garcia-Cachan, M.D. Effect of frozen storage conditions (temperature and length of storage) on microbial and sensory quality of rustic crossbred beef at different stages of aging. Meat Sci. 2009, 83, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Nomura, F. Proteome-based bacterial identification using matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS): A revolutionary shift in clinical diagnostic microbiology. Biochim. Bioph. Acta-Proteins Proteom. 2015, 1854, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, S.; Beindorf, P.M.; Biermaier, M.; Schwaiger, K.; Gareis, M.; Gottschalk, C. A novel approach for the determination of freshness and identity of trouts by MALDI-TOF mass spectrometry. Food Control 2017, 80, 281–289. [Google Scholar] [CrossRef]
- Stahl, A.; Schroder, U. Development of a MALDI−TOF MS-based protein fingerprint database of common food fish allowing fast and reliable identification of fraud and substitution. J. Agric. Food Chem. 2017, 65, 7519–7527. [Google Scholar] [CrossRef] [PubMed]
- Arena, S.; Salzano, A.M.; Scaloni, A. Identification of protein markers for the occurrence of defrosted material in milk through a MALDI-TOF MS profiling approach. J. Proteom. 2016, 147, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Théron, L.; Sayd, T.; Chambon, C.; Vautier, A.; Ferreira, C.; Aubry, L.; Ferraro, V.; Santé-Lhoutellier, V. Toward the prediction of PSE-like muscle defect in hams: Using chemometrics for the spectral fingerprint of plasma. Food Control 2020, 109, 106929. [Google Scholar] [CrossRef]
- Li, C.; Li, Z.; Tuo, Y.; Ma, D.; Shi, Y.; Zhang, Q.; Zuo, X.; Deng, K.; Chen, Y.; Wang, Z.; et al. MALDI-TOF MS as a Novel Tool for the Estimation of Postmortem Interval in Liver Tissue Samples. Sci. Rep. 2017, 7, 4887. [Google Scholar] [CrossRef] [PubMed]
- Simonovia, A.; Rohlik, B.A.; Skorpilova, T.; Petrova, M.; Pipek, P. Differentiation between fresh and thawed chicken meats. Czech J. Food Sci. 2013, 31, 108–115. [Google Scholar] [CrossRef]
- Zhang, S.X.; Farouk, M.M.; Young, O.A.; Wieliczko, K.J.; Podmore, C. Functional stability of frozen normal and high pH beef. Meat Sci. 2005, 69, 765–772. [Google Scholar] [CrossRef]
- Kristensen, L.; Christensen, M.; Ertbjerg, P. Activities of calpastatin, μ-calpain and m-calpain are stable during frozen storage of meat. Meat Sci. 2006, 72, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Ying, X. An overview of overfitting and its solution. J. Phys. Conf. Ser. 2019, 1168, 022022. [Google Scholar] [CrossRef]
- Hammer, B.; Strickert, M.; Villmann, T. Supervised neural gas with general similarity measure. Neural Process. Lett. 2005, 21, 21–44. [Google Scholar] [CrossRef]
- Holland, J.H. Adaptation in Natural and Artificial Systems; University of Michigan Press: Ann Arbor, MI, USA, 1975. [Google Scholar]
- Esener, N.; Green, M.J.; Emes, R.D.; Jowett, B.; Davies, P.L.; Bradley, A.J.; Dottorini, T. Discrimination of contagious and environmental strains of Streptococcus uberis in dairy herds by means of mass spectrometry and machine-learning. Sci. Rep. 2018, 8, 17517. [Google Scholar] [CrossRef] [PubMed]
- Metz, C.E. Basic principles of ROC analysis. Semin. Nucl. Med. 1978, 8, 283–298. [Google Scholar] [CrossRef]
- Pepe, M.S.; Thompson, M.L. Combining diagnostic test results to increase accuracy. Biostatistics 2000, 1, 123–140. [Google Scholar] [CrossRef]
- Sayd, T.; Morzel, M.; Chambon, C.; Franck, M.; Figwer, P.; Larzul, C.; Le Roy, P.; Monin, G.; Chérel, P.; Laville, E. Proteome analysis of the sarcoplasmic fraction of pig semimembranosus muscle: Implications on meat color development. J. Agric. Food Chem. 2006, 54, 2732–2737. [Google Scholar] [CrossRef] [PubMed]
| QC | SNN | GA | ||
|---|---|---|---|---|
| Cross-validation | Fresh livers | 83.1% | 69.7% | 79.6% |
| Frozen–thawed livers | 84.1% | 85.4% | 93.2% | |
| Global | 83.6% | 77.6% | 86.4% | |
| Recognition capability | 88.2% | 49.4% | 93.3% | |
| Number of peaks involved | 21 | 1 | 5 | |
| Weight | AUC | ||||||
|---|---|---|---|---|---|---|---|
| Mass, in Da | Fresh Livers | Frozen–Thawed Livers | p-Value | QC | SNN | GA | |
| 2073.37 | 7.96 ± 1.38 | 5.99 ± 0.91 | <0.000001 | 0.32 | 0.65 | ||
| 2202.14 | 15.62 ± 4.75 | 24.04 ± 4.72 | <0.000001 | 5.3 | 0.96 | ||
| 2596.51 | 20.00 ± 3.37 | 18.28 ± 3.69 | <0.000001 | 0.24 | 0.61 | ||
| 2691.06 | 6.77 ± 0.44 | 9.88 ± 0.97 | <0.000001 | 9.4 | 0.74 | ||
| 2965.47 | 5.34 ± 0.56 | 4.29 ± 0.39 | <0.000001 | 5.3 | 0.79 | ||
| 3541.38 | 3.75 ± 0.31 | 3.91 ± 0.22 | 0 | 1.4 | 0.78 | ||
| 3555.07 | 18.97 ± 5.70 | 11.84 ± 3.62 | <0.000001 | 5.9 | 0.77 | ||
| 3570.96 | 7.02 ± 0.96 | 5.89 ± 1.12 | <0.000001 | 1.09 | 0.76 | ||
| 3606.55 | 10.06 ± 1.52 | 8.57 ± 1.17 | <0.000001 | 3.6 | 0.82 | ||
| 4262.37 | 3.48 ± 0.23 | 3.97 ± 0.35 | <0.000001 | 1.8 | 0.69 | 0.76 | |
| 4316.21 | 9.4 ± 0.98 | 9.5 ± 1.21 | 0.0000378 | 4.5 | 0.79 | ||
| 4347.77 | 4.21 ± 0.36 | 3.8 ± 0.33 | <0.000001 | 1.6 | 0.70 | ||
| 4414.55 | 2.55 ± 0.32 | 3.17 ± 0.63 | <0.000001 | 0.06 | 0.63 | ||
| 4628.01 | 5.13 ± 0.85 | 7.07 ± 0.91 | <0.000001 | 2.2 | 0.82 | ||
| 4743.13 | 3.24 ± 0.64 | 3.89 ± 0.54 | <0.000001 | 7.2 | 1.06 | 0.88 | |
| 4822.69 | 3.29 ± 0.65 | 7.20 ± 1.65 | <0.000001 | 1.7 | 0.84 | ||
| 4833.79 | 1.99 ± 0.23 | 1.78 ± 0.19 | <0.000001 | 2.7 | 0.86 | ||
| 4990.13 | 4.13 ± 1.03 | 2.33 ± 0.31 | <0.000001 | 1.7 | 0.83 | ||
| 5160.81 | 2.19 ± 0.21 | 2.49 ± 2.31 | <0.000001 | 1.4 | 0.77 | ||
| 5225.46 | 2.37 ± 0.17 | 2.65 ± 0.25 | <0.000001 | 1.6 | 0.74 | ||
| 5460.16 | 2.3 ± 0.28 | 2.47 ± 0.31 | 0.00000193 | 3.5 | 0.72 | ||
| 6243.72 | 0.96 ± 0.10 | 0.99 ± 0.10 | <0.000001 | 2.2 | 0.82 | ||
| 6283.88 | 5.84 ± 1.14 | 8.12 ± 1.27 | <0.000001 | 1.7 | 0.82 | ||
| 6640.20 | 1.43 ± 0.16 | 1.41 ± 0.18 | <0.000001 | 9.4 | 0.86 | 0.82 | |
| Storage Time (in Days) | Weight Range (in Grams) | Number of Livers | |
|---|---|---|---|
| Fresh | F0 | <500 | 15 |
| 500–600 | 15 | ||
| >600 | 15 | ||
| F7 | <500 | 10 | |
| 500–600 | 10 | ||
| >600 | 10 | ||
| F14 | <500 | 15 | |
| 500–600 | 15 | ||
| >600 | 15 | ||
| Frozen–thawed | FT0 | <500 | 12 |
| 500–600 | 12 | ||
| >600 | 12 | ||
| FT7 | <500 | 12 | |
| 500–600 | 12 | ||
| >600 | 12 | ||
| FT14 | <500 | 12 | |
| 500–600 | 12 | ||
| >600 | 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aubry, L.; Sayd, T.; Ferreira, C.; Chambon, C.; Vénien, A.; Blinet, S.; Bourin, M.; Travel, A.; Halgrain, M.; Santé-Lhoutellier, V.; et al. Detection of Frozen–Thawed Duck Fatty Liver by MALDI-TOF Mass Spectrometry: A Chemometrics Study. Molecules 2021, 26, 3508. https://doi.org/10.3390/molecules26123508
Aubry L, Sayd T, Ferreira C, Chambon C, Vénien A, Blinet S, Bourin M, Travel A, Halgrain M, Santé-Lhoutellier V, et al. Detection of Frozen–Thawed Duck Fatty Liver by MALDI-TOF Mass Spectrometry: A Chemometrics Study. Molecules. 2021; 26(12):3508. https://doi.org/10.3390/molecules26123508
Chicago/Turabian StyleAubry, Laurent, Thierry Sayd, Claude Ferreira, Christophe Chambon, Annie Vénien, Sylvie Blinet, Marie Bourin, Angélique Travel, Maeva Halgrain, Véronique Santé-Lhoutellier, and et al. 2021. "Detection of Frozen–Thawed Duck Fatty Liver by MALDI-TOF Mass Spectrometry: A Chemometrics Study" Molecules 26, no. 12: 3508. https://doi.org/10.3390/molecules26123508
APA StyleAubry, L., Sayd, T., Ferreira, C., Chambon, C., Vénien, A., Blinet, S., Bourin, M., Travel, A., Halgrain, M., Santé-Lhoutellier, V., & Théron, L. (2021). Detection of Frozen–Thawed Duck Fatty Liver by MALDI-TOF Mass Spectrometry: A Chemometrics Study. Molecules, 26(12), 3508. https://doi.org/10.3390/molecules26123508