Investigation of Antifungal Mechanisms of Thymol in the Human Fungal Pathogen, Cryptococcus neoformans
Abstract
:1. Introduction
2. Results
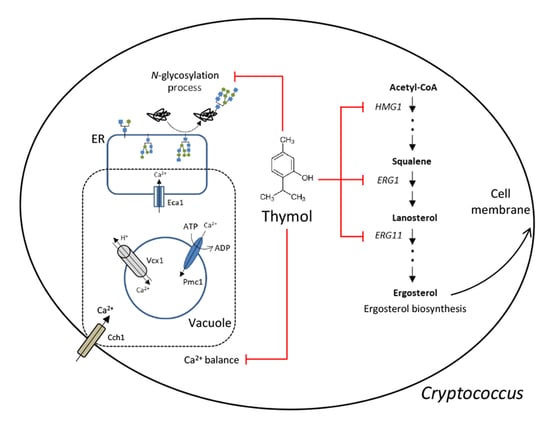
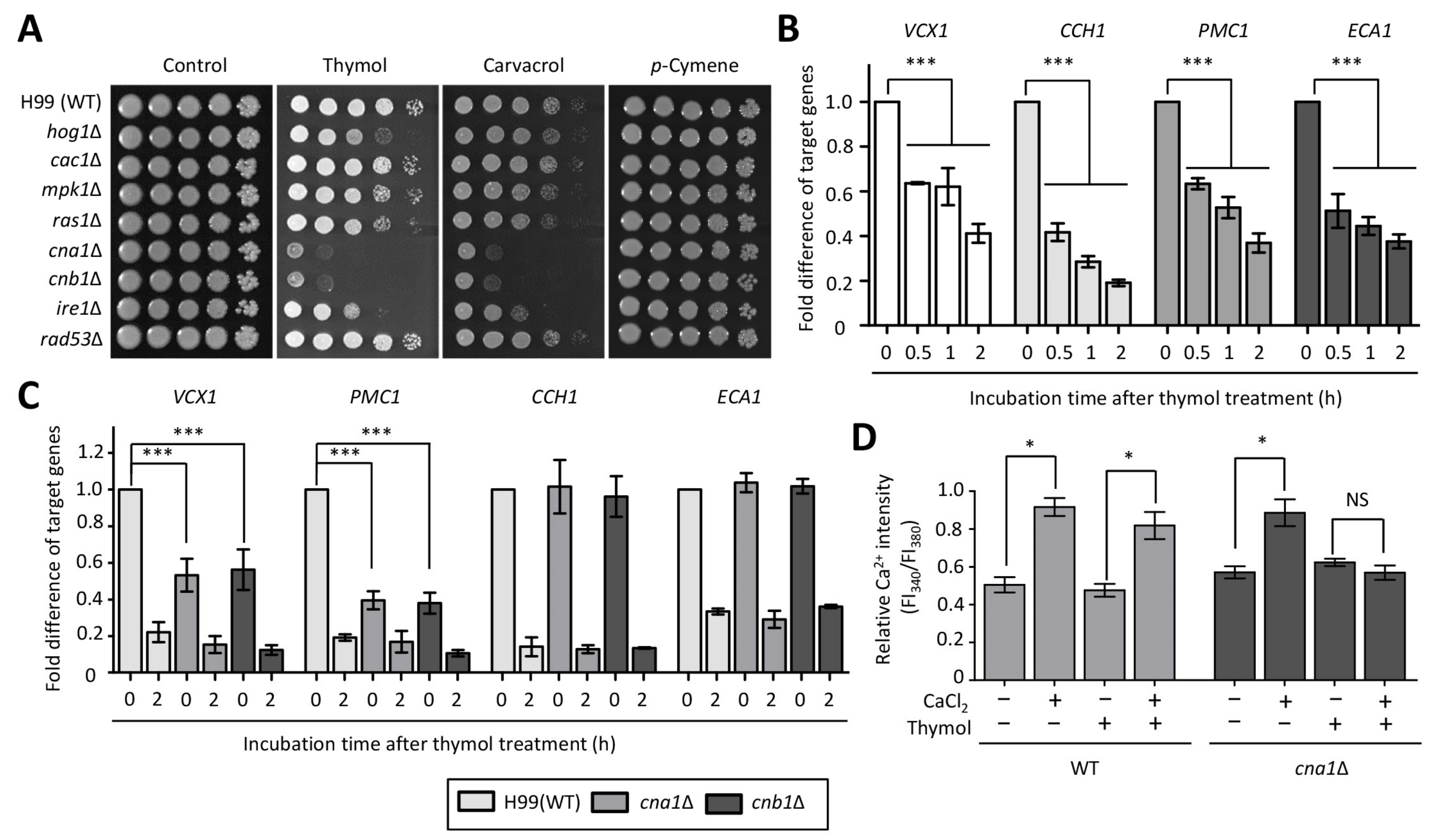
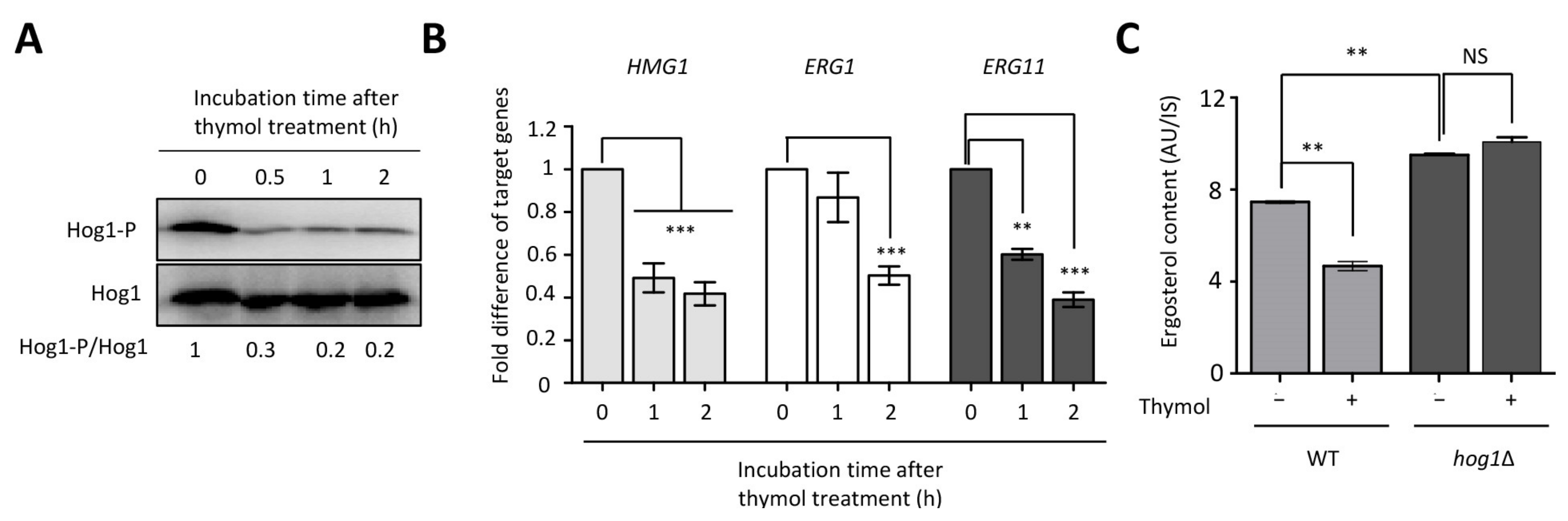
2.1. Identification of Thymol-Responsive Signaling Pathways in C. neoformans
2.2. The Effect of Thymol Treatment on the Intracellular Ca2+ Balance in C. neoformans
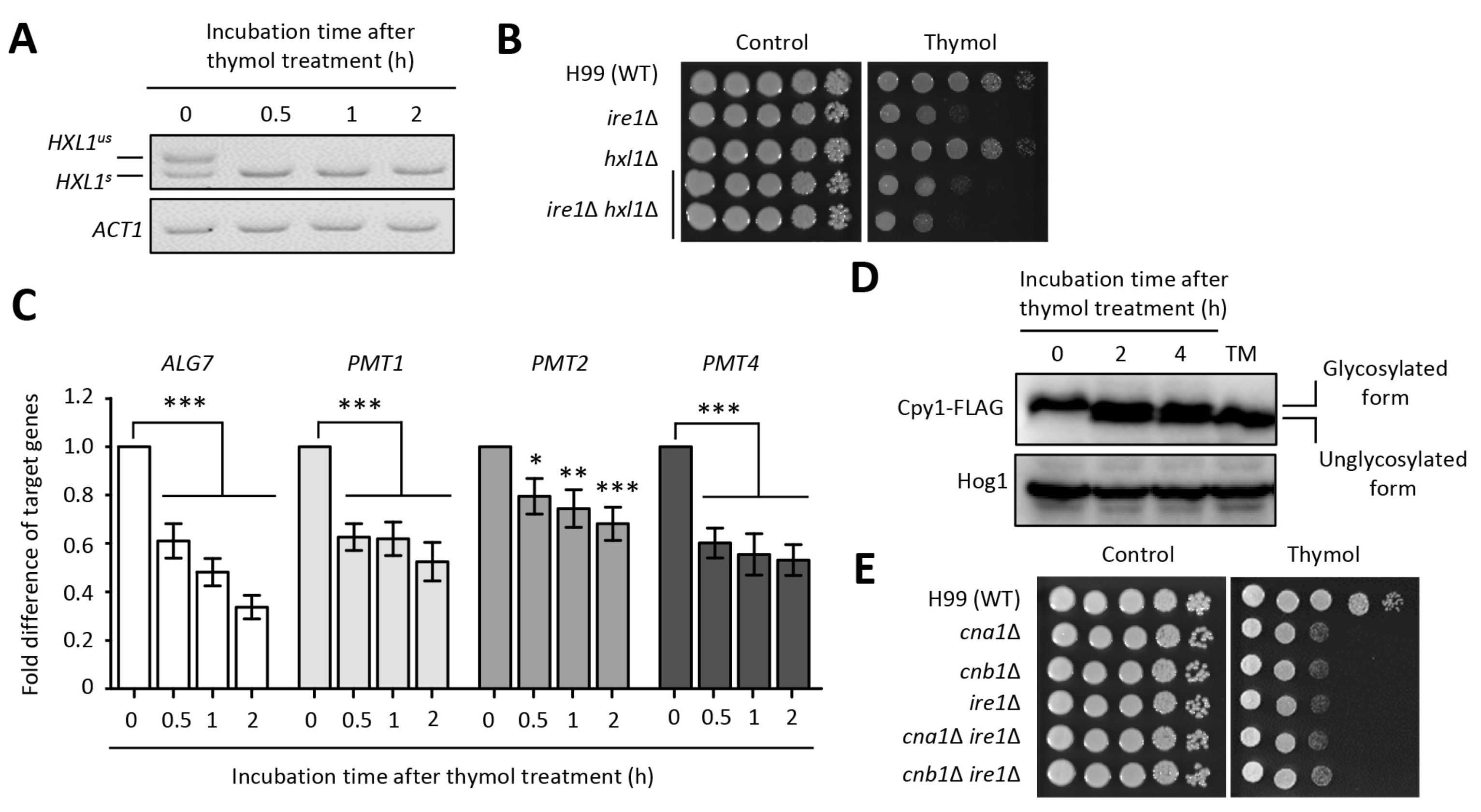
2.3. The Effect of Thymol on the ER Stress in C. neoformans
2.4. The Effect of Thymol on the Ergosterol Biosynthesis in C. neoformans
3. Discussion
4. Materials and Methods
4.1. Strain and Media
4.2. Construction of C. neoformans Mutant Strains
4.3. Total RNA Isolation, cDNA Synthesis, and Quantitative Reverse Transcription PCR
4.4. Protein Extraction and Western Blot Analysis
4.5. Spotting Assay
4.6. Determination of Relative Intracellular Ca2+ Levels
4.7. Construction of Strain Containing CPY-FLAG
4.8. ROS Measurement Assay
4.9. Measurement of Ergosterol Content
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Scorzoni, L.; de Paula, E.S.A.C.; Marcos, C.M.; Assato, P.A.; de Melo, W.C.; de Oliveira, H.C.; Costa-Orlandi, C.B.; Mendes-Giannini, M.J.; Fusco-Almeida, A.M. Antifungal Therapy: New Advances in the Understanding and Treatment of Mycosis. Front. Microbiol. 2017, 8, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostrosky-Zeichner, L.; Casadevall, A.; Galgiani, J.N.; Odds, F.C.; Rex, J.H. An insight into the antifungal pipeline: Selected new molecules and beyond. Nat. Rev. Drug Discov. 2010, 9, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A. Antifungal drug resistance: Mechanisms, epidemiology, and consequences for treatment. Am. J. Med. 2012, 125, S3–S13. [Google Scholar] [CrossRef] [PubMed]
- VanEtten, H.D.; Mansfield, J.W.; Bailey, J.A.; Farmer, E.E. Two Classes of Plant Antibiotics: Phytoalexins versus “Phytoanticipins”. Plant Cell 1994, 6, 1191–1192. [Google Scholar] [CrossRef] [PubMed]
- Grayer, R.J.; Kokubun, T. Plant-fungal interactions: The search for phytoalexins and other antifungal compounds from higher plants. Phytochemistry 2001, 56, 253–263. [Google Scholar] [CrossRef]
- Amiri, H. Essential oils composition and antioxidant properties of three Thymus species. Evid.-Based Complement. Altern. Med. eCAM 2012, 2012, 728065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehi, B.; Mishra, A.P.; Shukla, I.; Sharifi-Rad, M.; Contreras, M.D.M.; Segura-Carretero, A.; Fathi, H.; Nasrabadi, N.N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, thyme, and other plant sources: Health and potential uses. Phytother. Res. PTR 2018, 32, 1688–1706. [Google Scholar] [CrossRef] [PubMed]
- Nostro, A.; Blanco, A.R.; Cannatelli, M.A.; Enea, V.; Flamini, G.; Morelli, I.; Sudano Roccaro, A.; Alonzo, V. Susceptibility of methicillin-resistant staphylococci to oregano essential oil, carvacrol and thymol. FEMS Microbiol. Lett. 2004, 230, 191–195. [Google Scholar] [CrossRef] [Green Version]
- Olasupo, N.A.; Fitzgerald, D.J.; Gasson, M.J.; Narbad, A. Activity of natural antimicrobial compounds against Escherichia coli and Salmonella enterica serovar typhimurium. Lett. Appl. Microbiol. 2003, 37, 448–451. [Google Scholar] [CrossRef] [Green Version]
- Braga, P.C.; Alfieri, M.; Culici, M.; Dal Sasso, M. Inhibitory activity of thymol against the formation and viability of Candida albicans hyphae. Mycoses 2007, 50, 502–506. [Google Scholar] [CrossRef]
- Shen, Q.; Zhou, W.; Li, H.; Hu, L.; Mo, H. ROS Involves the Fungicidal Actions of Thymol against Spores of Aspergillus flavus via the Induction of Nitric Oxide. PLoS ONE 2016, 11, e0155647. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, S.; Du, S.; Chen, S.; Sun, H. Antifungal activity of thymol and carvacrol against postharvest pathogens Botrytis cinerea. J. Food Sci. Technol. 2019, 56, 2611–2620. [Google Scholar] [CrossRef]
- De Lira Mota, K.S.; de Oliveira Pereira, F.; de Oliveira, W.A.; Lima, I.O.; de Oliveira Lima, E. Antifungal activity of Thymus vulgaris L. essential oil and its constituent phytochemicals against Rhizopus oryzae: Interaction with ergosterol. Molecules 2012, 17, 14418–14433. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, A.P.C.; Nobrega, R.O.; Lima, E.O.; Araujo, W.O.; Lima, I.O. Antifungal activity study of the monoterpene thymol against Cryptococcus neoformans. Nat. Prod. Res. 2020, 34, 2630–2633. [Google Scholar] [CrossRef]
- Rajasingham, R.; Smith, R.M.; Park, B.J.; Jarvis, J.N.; Govender, N.P.; Chiller, T.M.; Denning, D.W.; Loyse, A.; Boulware, D.R. Global burden of disease of HIV-associated cryptococcal meningitis: An updated analysis. Lancet Infect. Dis. 2017, 17, 873–881. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Heitman, J. The biology of the Cryptococcus neoformans species complex. Annu. Rev. Microbiol. 2006, 60, 69–105. [Google Scholar] [CrossRef]
- Idnurm, A.; Bahn, Y.S.; Nielsen, K.; Lin, X.; Fraser, J.A.; Heitman, J. Deciphering the model pathogenic fungus Cryptococcus neoformans. Nat. Rev. Microbiol. 2005, 3, 753–764. [Google Scholar] [CrossRef]
- Jung, K.W.; Lee, K.T.; So, Y.S.; Bahn, Y.S. Genetic Manipulation of Cryptococcus neoformans. Curr. Protoc. Microbiol. 2018, 50, e59. [Google Scholar] [CrossRef]
- Bahn, Y.S.; Kojima, K.; Cox, G.M.; Heitman, J. Specialization of the HOG pathway and its impact on differentiation and virulence of Cryptococcus neoformans. Mol. Biol. Cell 2005, 16, 2285–2300. [Google Scholar] [CrossRef] [Green Version]
- Kraus, P.R.; Fox, D.S.; Cox, G.M.; Heitman, J. The Cryptococcus neoformans MAP kinase Mpk1 regulates cell integrity in response to antifungal drugs and loss of calcineurin function. Mol. Microbiol. 2003, 48, 1377–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odom, A.; Muir, S.; Lim, E.; Toffaletti, D.L.; Perfect, J.; Heitman, J. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J. 1997, 16, 2576–2589. [Google Scholar] [CrossRef]
- Cheon, S.A.; Jung, K.W.; Chen, Y.L.; Heitman, J.; Bahn, Y.S.; Kang, H.A. Unique evolution of the UPR pathway with a novel bZIP transcription factor, Hxl1, for controlling pathogenicity of Cryptococcus neoformans. PLoS Pathog. 2011, 7, e1002177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alspaugh, J.A.; Cavallo, L.M.; Perfect, J.R.; Heitman, J. RAS1 regulates filamentation, mating and growth at high temperature of Cryptococcus neoformans. Mol. Microbiol. 2000, 36, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Alspaugh, J.A.; Pukkila-Worley, R.; Harashima, T.; Cavallo, L.M.; Funnell, D.; Cox, G.M.; Perfect, J.R.; Kronstad, J.W.; Heitman, J. Adenylyl cyclase functions downstream of the Gα protein Gpa1 and controls mating and pathogenicity of Cryptococcus neoformans. Eukaryot. Cell 2002, 1, 75–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, K.W.; Yang, D.H.; Kim, M.K.; Seo, H.S.; Lim, S.; Bahn, Y.S. Unraveling Fungal Radiation Resistance Regulatory Networks through the Genome-Wide Transcriptome and Genetic Analyses of Cryptococcus neoformans. mBio 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, K.W.; Lee, Y.; Huh, E.Y.; Lee, S.C.; Lim, S.; Bahn, Y.S. Rad53- and Chk1-Dependent DNA Damage Response Pathways Cooperatively Promote Fungal Pathogenesis and Modulate Antifungal Drug Susceptibility. mBio 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Rao, A.; Zhang, Y.; Muend, S.; Rao, R. Mechanism of antifungal activity of terpenoid phenols resembles calcium stress and inhibition of the TOR pathway. Antimicrob. Agents Chemother. 2010, 54, 5062–5069. [Google Scholar] [CrossRef] [Green Version]
- Kmetzsch, L.; Staats, C.C.; Cupertino, J.B.; Fonseca, F.L.; Rodrigues, M.L.; Schrank, A.; Vainstein, M.H. The calcium transporter Pmc1 provides Ca2+ tolerance and influences the progression of murine cryptococcal infection. FEBS J. 2013, 280, 4853–4864. [Google Scholar] [CrossRef] [Green Version]
- Park, H.S.; Chow, E.W.; Fu, C.; Soderblom, E.J.; Moseley, M.A.; Heitman, J.; Cardenas, M.E. Calcineurin Targets Involved in Stress Survival and Fungal Virulence. PLoS Pathog. 2016, 12, e1005873. [Google Scholar] [CrossRef] [Green Version]
- Winther, J.R.; Stevens, T.H.; Kielland-Brandt, M.C. Yeast carboxypeptidase Y requires glycosylation for efficient intracellular transport, but not for vacuolar sorting, in vivo stability, or activity. Eur. J. Biochem. 1991, 197, 681–689. [Google Scholar] [CrossRef]
- Ko, Y.J.; Yu, Y.M.; Kim, G.B.; Lee, G.W.; Maeng, P.J.; Kim, S.S.; Floyd, A.; Heitman, J.; Bahn, Y.S. Remodeling of global transcription patterns of Cryptococcus neoformans genes mediated by the stress-activated HOG signaling pathways. Eukaryot. Cell 2009, 8, 1197–1217. [Google Scholar] [CrossRef] [Green Version]
- Veen, M.; Stahl, U.; Lang, C. Combined overexpression of genes of the ergosterol biosynthetic pathway leads to accumulation of sterols in Saccharomyces cerevisiae. FEMS Yeast Res. 2003, 4, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Carrasco, H.; Raimondi, M.; Svetaz, L.; Di Liberto, M.; Rodriguez, M.V.; Espinoza, L.; Madrid, A.; Zacchino, S. Antifungal activity of eugenol analogues. Influence of different substituents and studies on mechanism of action. Molecules 2012, 17, 1002–1024. [Google Scholar] [CrossRef] [Green Version]
- Chaillot, J.; Tebbji, F.; Remmal, A.; Boone, C.; Brown, G.W.; Bellaoui, M.; Sellam, A. The Monoterpene Carvacrol Generates Endoplasmic Reticulum Stress in the Pathogenic Fungus Candida albicans. Antimicrob. Agents Chemother. 2015, 59, 4584–4592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansari, M.A.; Fatima, Z.; Hameed, S. Anticandidal Effect and Mechanisms of Monoterpenoid, Perillyl Alcohol against Candida albicans. PLoS ONE 2016, 11, e0162465. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.; Khan, A.; Akhtar, F.; Yousuf, S.; Xess, I.; Khan, L.A.; Manzoor, N. Fungicidal activity of thymol and carvacrol by disrupting ergosterol biosynthesis and membrane integrity against Candida. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 41–50. [Google Scholar] [CrossRef]
- Upadhya, R.; Kim, H.; Jung, K.W.; Park, G.; Lam, W.; Lodge, J.K.; Bahn, Y.S. Sulphiredoxin plays peroxiredoxin-dependent and -independent roles via the HOG signalling pathway in Cryptococcus neoformans and contributes to fungal virulence. Mol. Microbiol. 2013, 90, 630–648. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Shao, Y.L.; Tang, Y.J.; Zhou, W.W. Antifungal Activity of Essential oil Compounds (Geraniol and Citral) and Inhibitory Mechanisms on Grain Pathogens (Aspergillus flavus and Aspergillus ochraceus). Molecules 2018, 23, 2108. [Google Scholar] [CrossRef] [Green Version]
- Darvishi, E.; Omidi, M.; Bushehri, A.A.; Golshani, A.; Smith, M.L. Thymol antifungal mode of action involves telomerase inhibition. Med. Mycol. 2013, 51, 826–834. [Google Scholar] [CrossRef]
- Durr, G.; Strayle, J.; Plemper, R.; Elbs, S.; Klee, S.K.; Catty, P.; Wolf, D.H.; Rudolph, H.K. The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting, and endoplasmic reticulum-associated protein degradation. Mol. Biol. Cell 1998, 9, 1149–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colinet, A.S.; Sengottaiyan, P.; Deschamps, A.; Colsoul, M.L.; Thines, L.; Demaegd, D.; Duchene, M.C.; Foulquier, F.; Hols, P.; Morsomme, P. Yeast Gdt1 is a Golgi-localized calcium transporter required for stress-induced calcium signaling and protein glycosylation. Sci. Rep. 2016, 6, 24282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunes-Bayir, A.; Kocyigit, A.; Guler, E.M.; Dadak, A. In Vitro Hormetic Effect Investigation of Thymol on Human Fibroblast and Gastric Adenocarcinoma Cells. Molecules 2020, 25, 3270. [Google Scholar] [CrossRef] [PubMed]
- So, Y.S.; Yang, D.H.; Jung, K.W.; Huh, W.K.; Bahn, Y.S. Molecular Characterization of Adenylyl Cyclase Complex Proteins Using Versatile Protein-Tagging Plasmid Systems in Cryptococcus neoformans. J. Microbiol. Biotechnol. 2017, 27, 357–364. [Google Scholar] [CrossRef]
- Schneider Rde, O.; Diehl, C.; dos Santos, F.M.; Piffer, A.C.; Garcia, A.W.; Kulmann, M.I.; Schrank, A.; Kmetzsch, L.; Vainstein, M.H.; Staats, C.C. Effects of zinc transporters on Cryptococcus gattii virulence. Sci. Rep. 2015, 5, 10104. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, K.-W.; Chung, M.-S.; Bai, H.-W.; Chung, B.-Y.; Lee, S. Investigation of Antifungal Mechanisms of Thymol in the Human Fungal Pathogen, Cryptococcus neoformans. Molecules 2021, 26, 3476. https://doi.org/10.3390/molecules26113476
Jung K-W, Chung M-S, Bai H-W, Chung B-Y, Lee S. Investigation of Antifungal Mechanisms of Thymol in the Human Fungal Pathogen, Cryptococcus neoformans. Molecules. 2021; 26(11):3476. https://doi.org/10.3390/molecules26113476
Chicago/Turabian StyleJung, Kwang-Woo, Moon-Soo Chung, Hyoung-Woo Bai, Byung-Yeoup Chung, and Sungbeom Lee. 2021. "Investigation of Antifungal Mechanisms of Thymol in the Human Fungal Pathogen, Cryptococcus neoformans" Molecules 26, no. 11: 3476. https://doi.org/10.3390/molecules26113476
APA StyleJung, K.-W., Chung, M.-S., Bai, H.-W., Chung, B.-Y., & Lee, S. (2021). Investigation of Antifungal Mechanisms of Thymol in the Human Fungal Pathogen, Cryptococcus neoformans. Molecules, 26(11), 3476. https://doi.org/10.3390/molecules26113476