Synthetic Routes to Coumarin(Benzopyrone)-Fused Five-Membered Aromatic Heterocycles Built on the α-Pyrone Moiety. Part II: Five-Membered Aromatic Rings with Multi Heteroatoms
Abstract
1. Introduction
2. Synthesis of Benzopyrone-Fused Five-Membered Aromatic Heterocycles
2.1. Five-Membered Aromatic Rings with Two Heteroatoms
2.1.1. Two Identical Heteroatoms (N-N)
Pyrazole
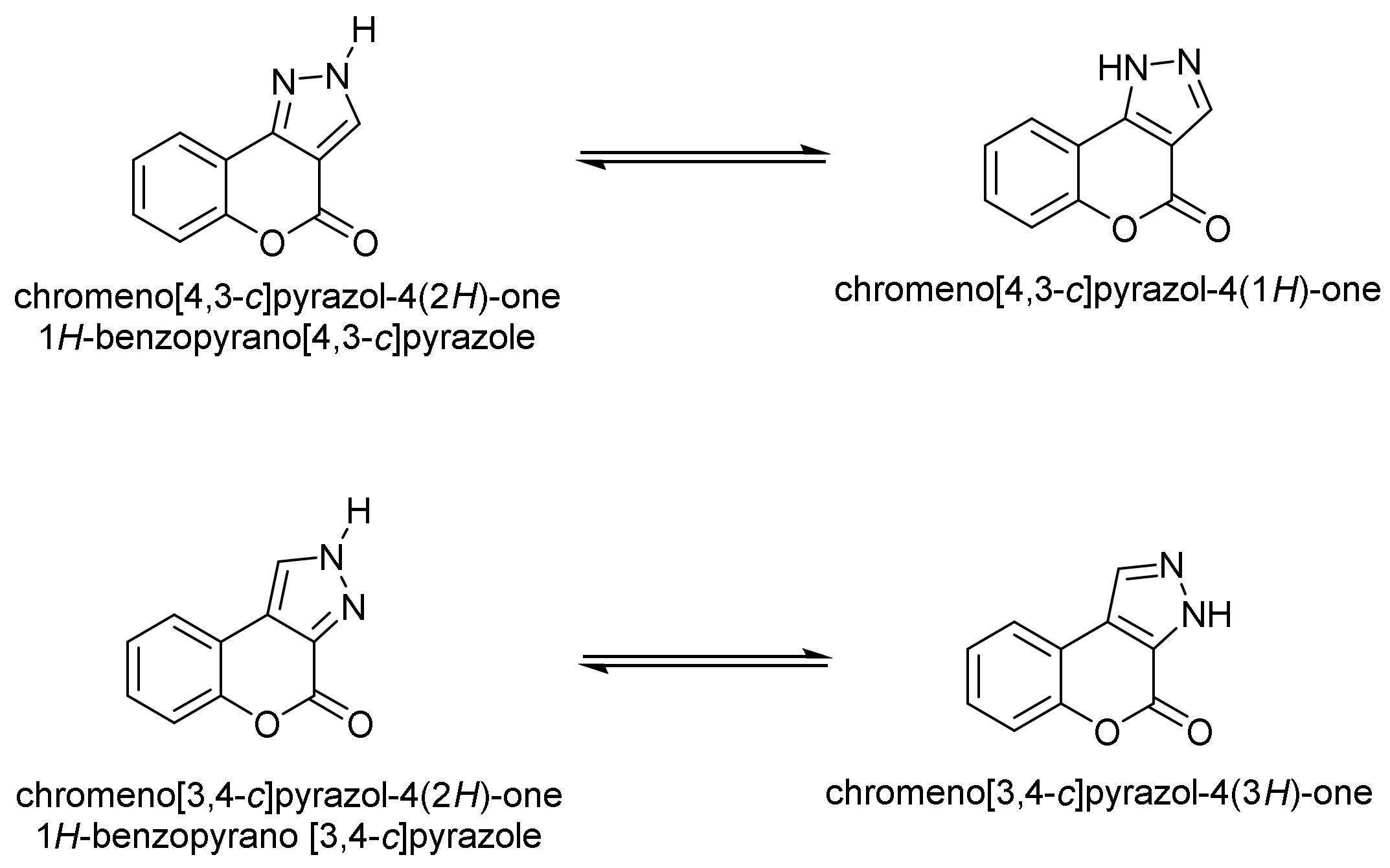
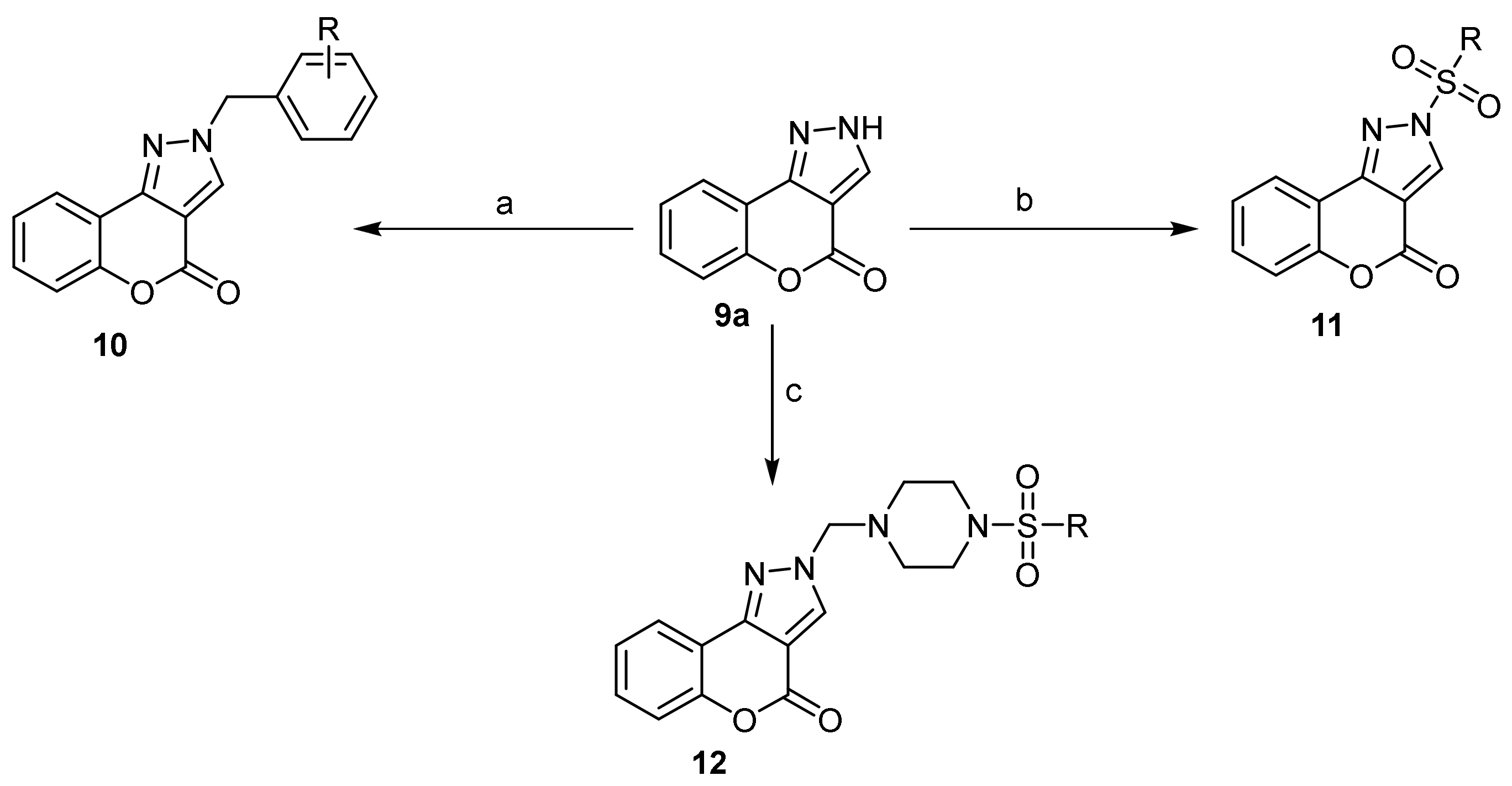
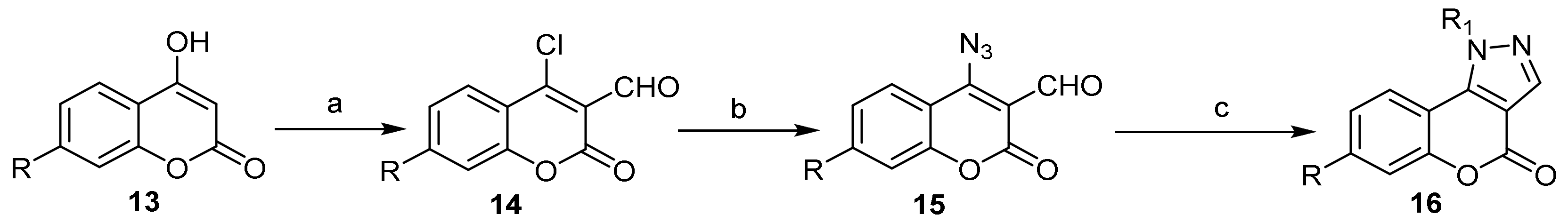
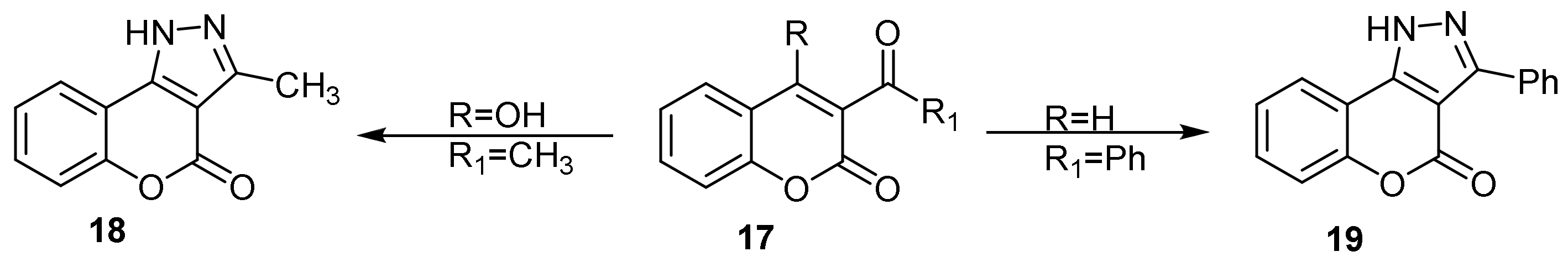
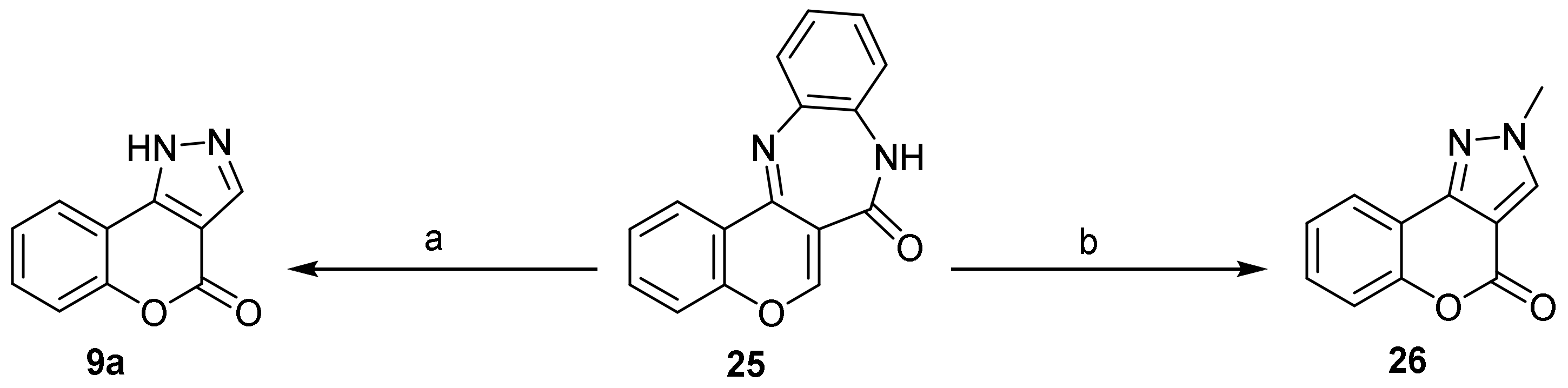
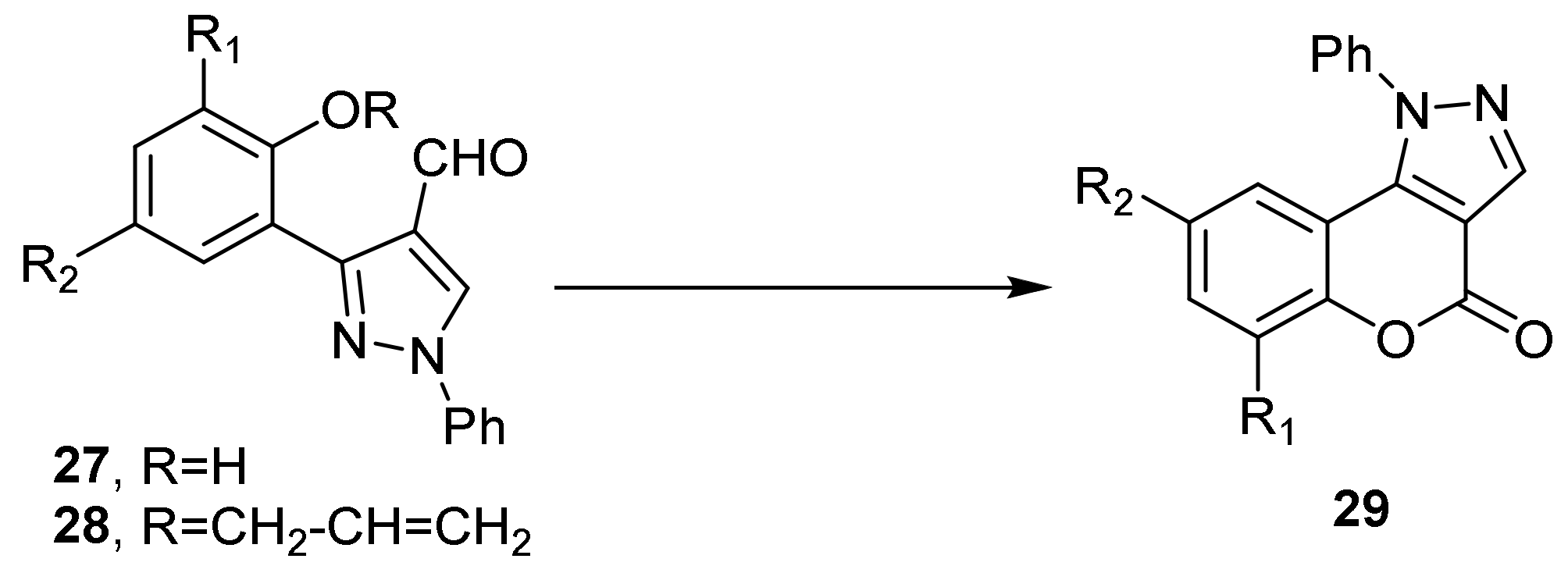
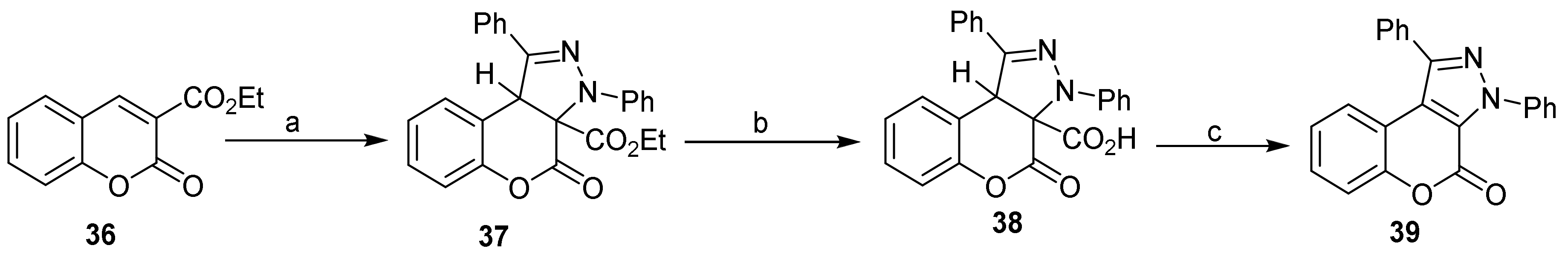
- Chromeno[4,3-c]pyrazol-4(2H)-one; (1H-Benzopyrano[4,3-c]pyrazole)
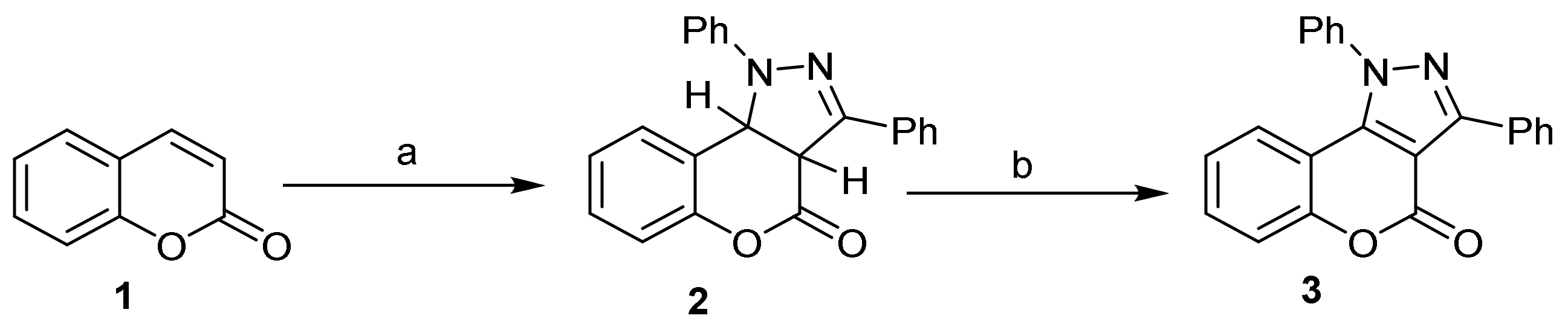
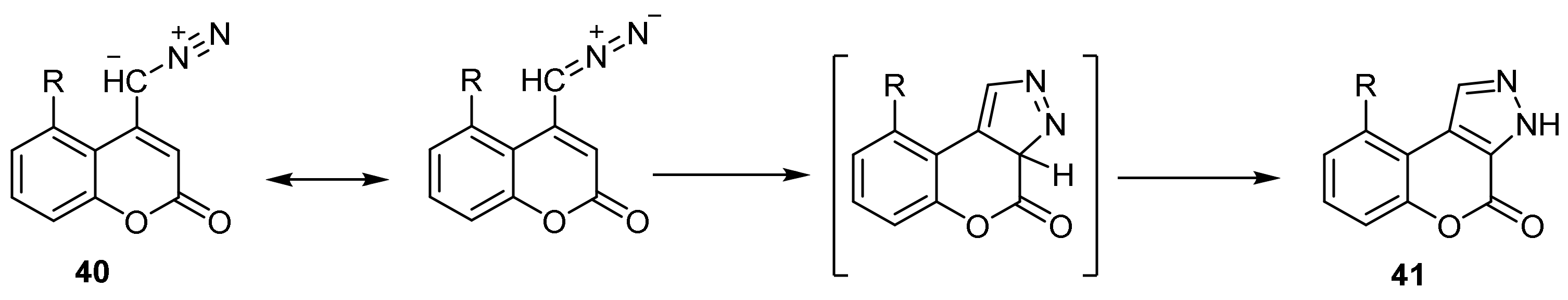
- Pyrazole Construction
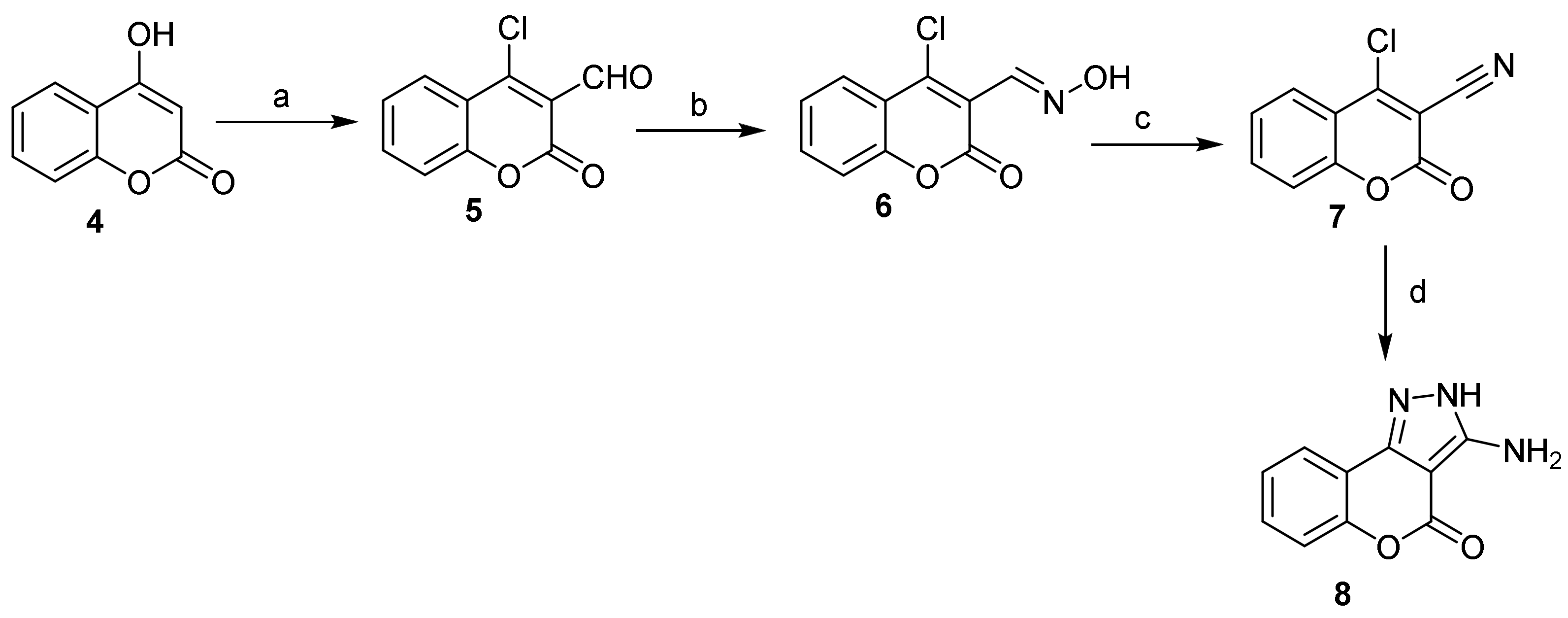
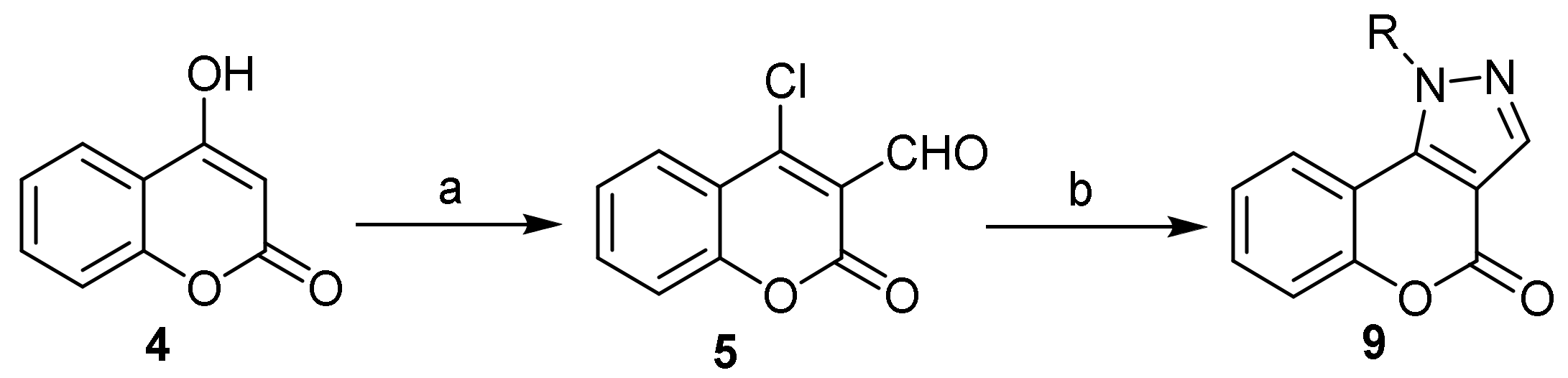
- Pyrone Construction
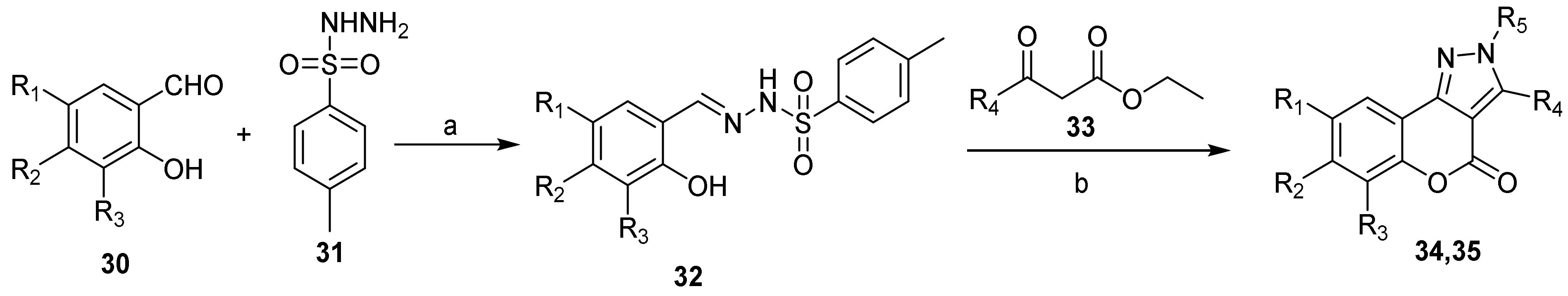
- Chromeno[3,4-c]pyrazol-4(2H)-one; (1H-Benzopyrano[3,4-c]pyrazole)
- Pyrazole Construction
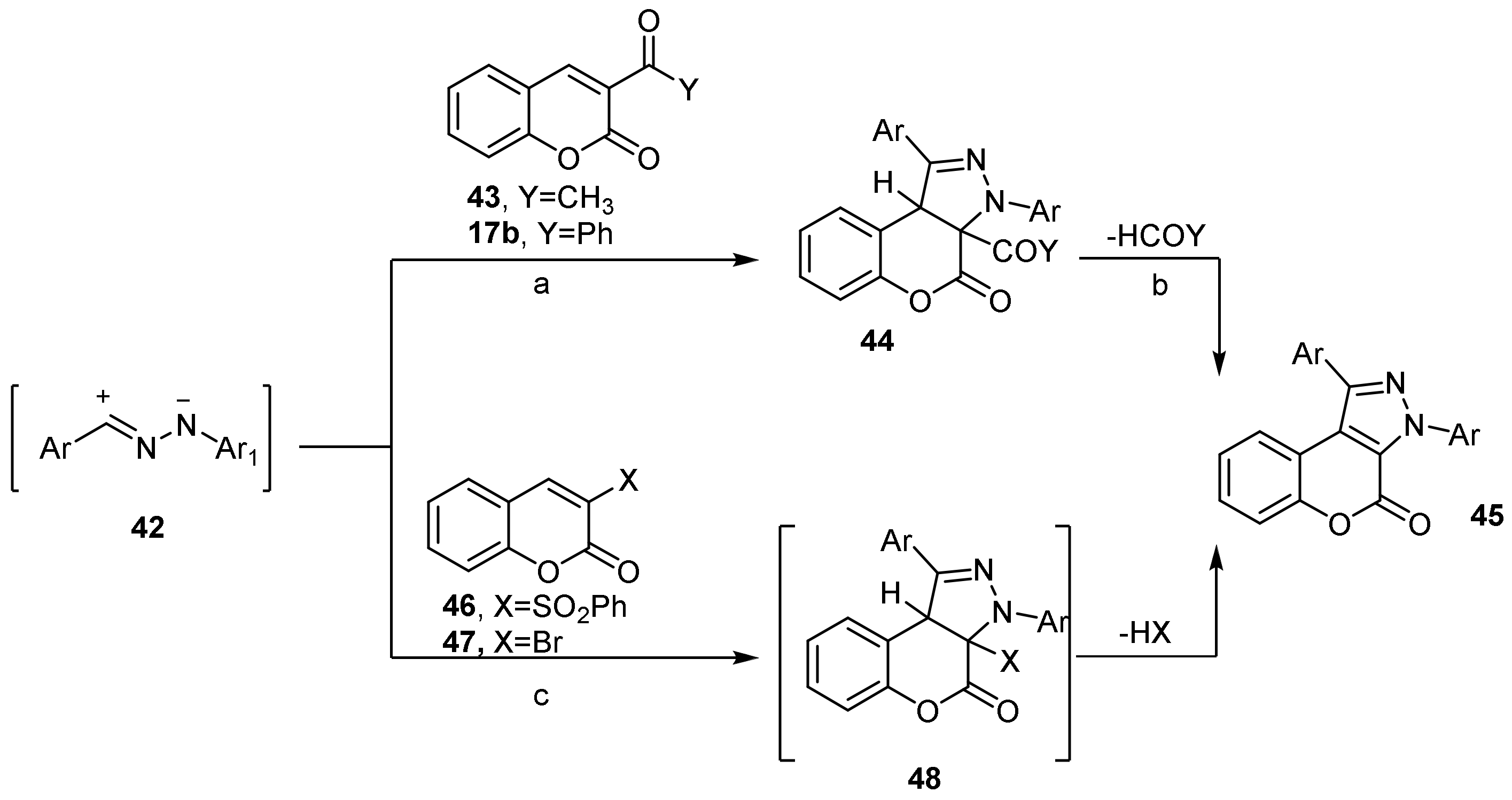
Imidazole
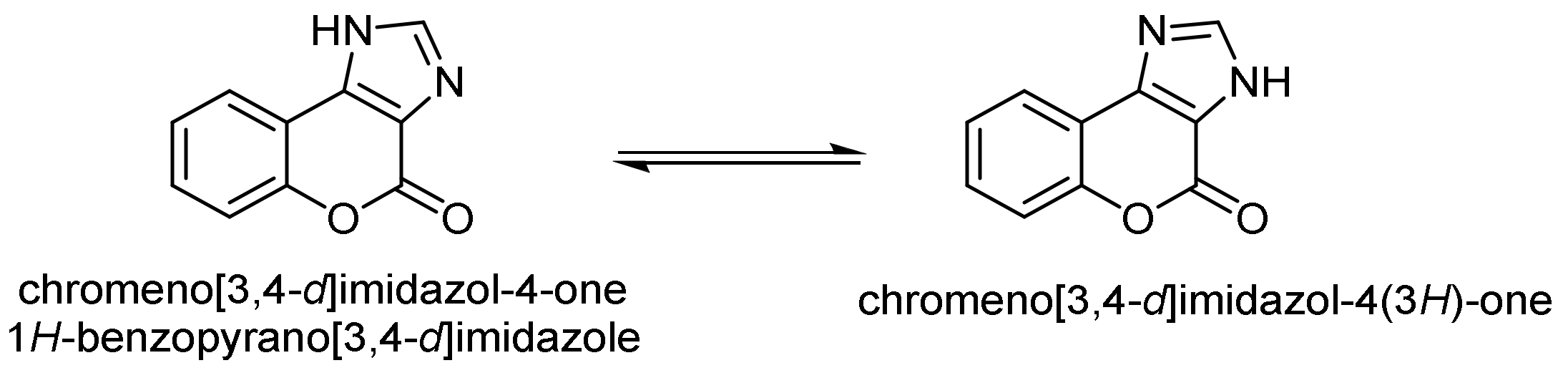
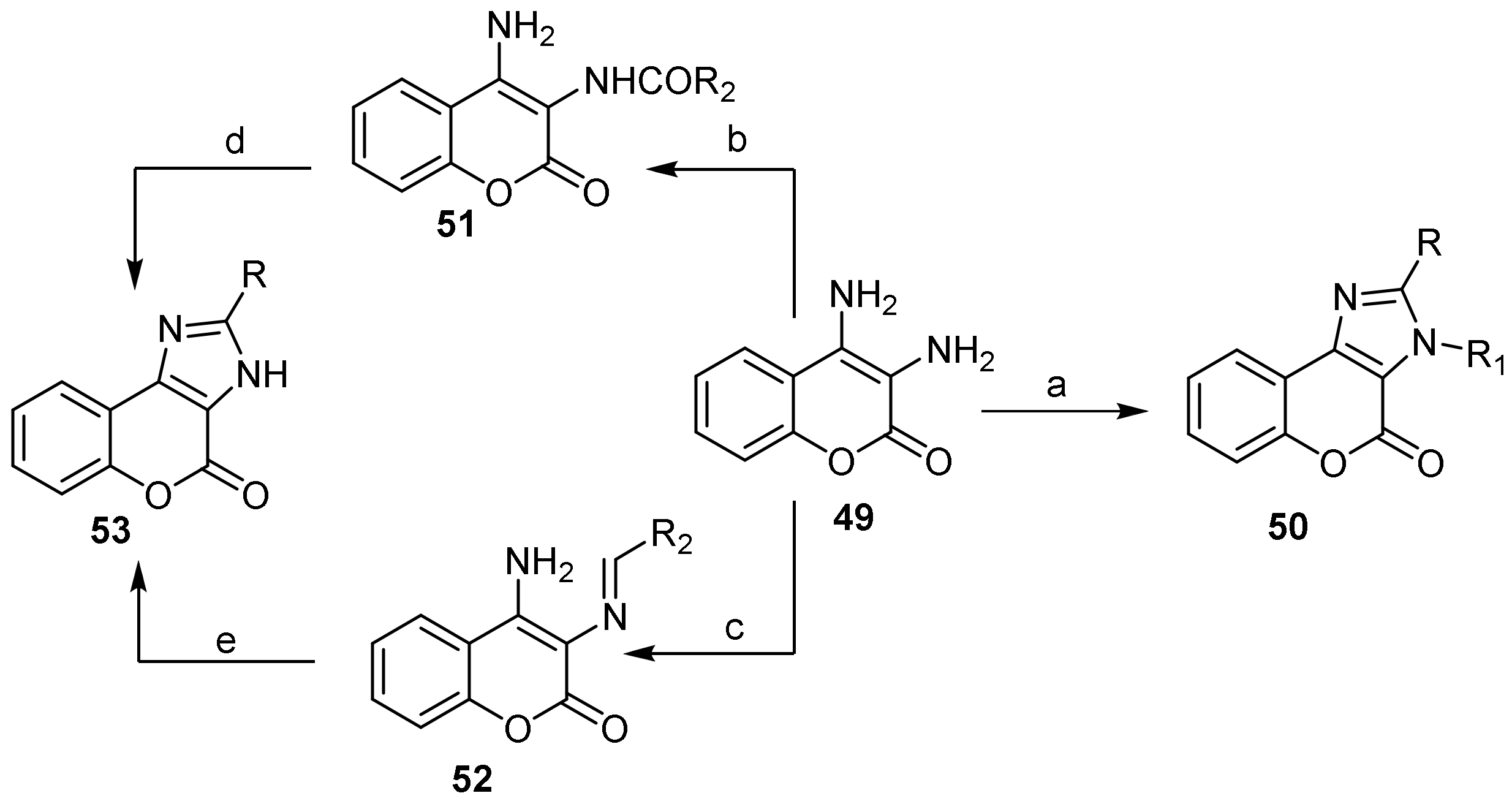
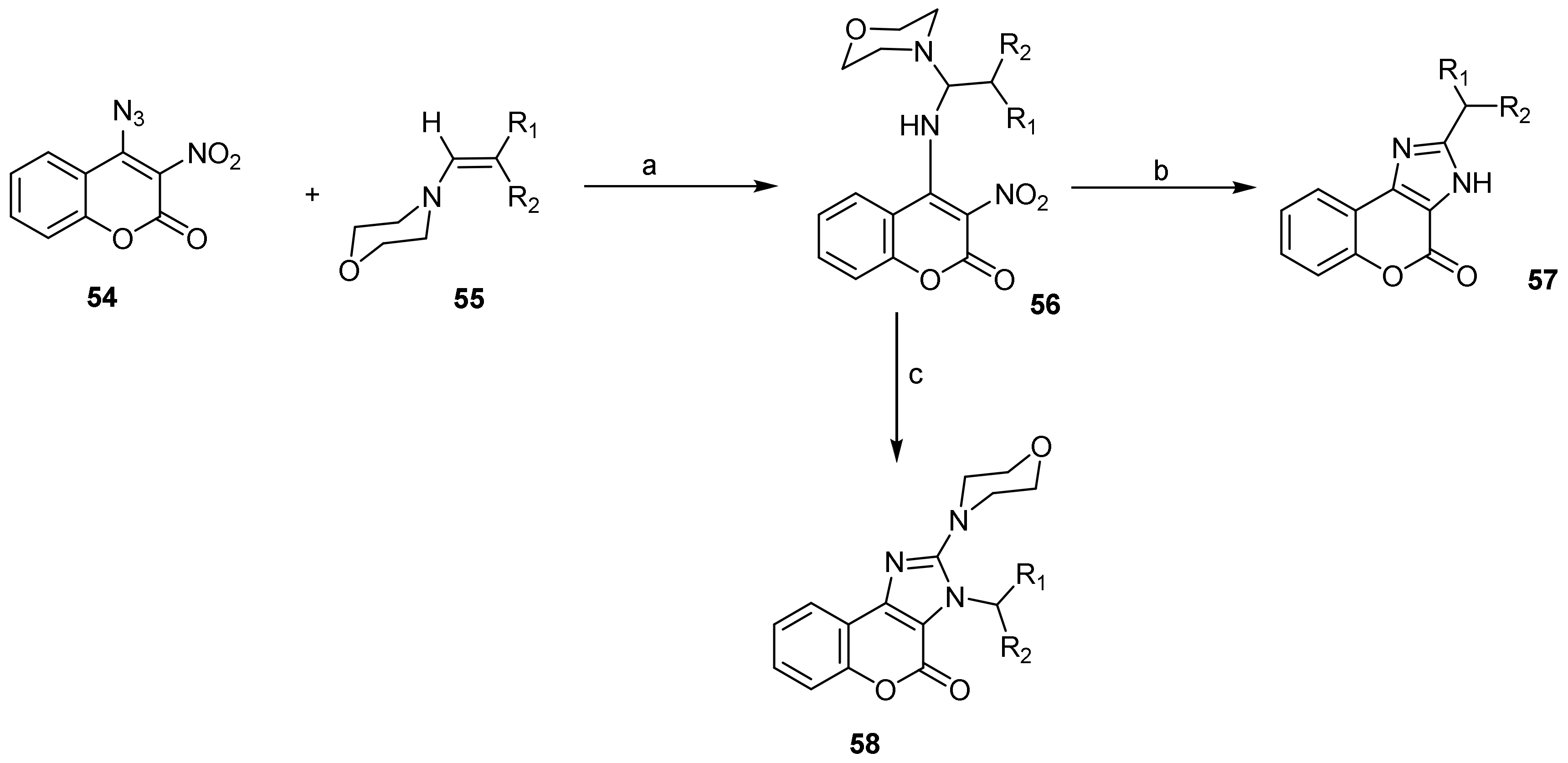
- Chromeno[3,4-d]imidazol-4-one; (1H-Benzopyrano[3,4-d]imidazole)
- Imidazole Construction
2.1.2. Two Different Heteroatoms
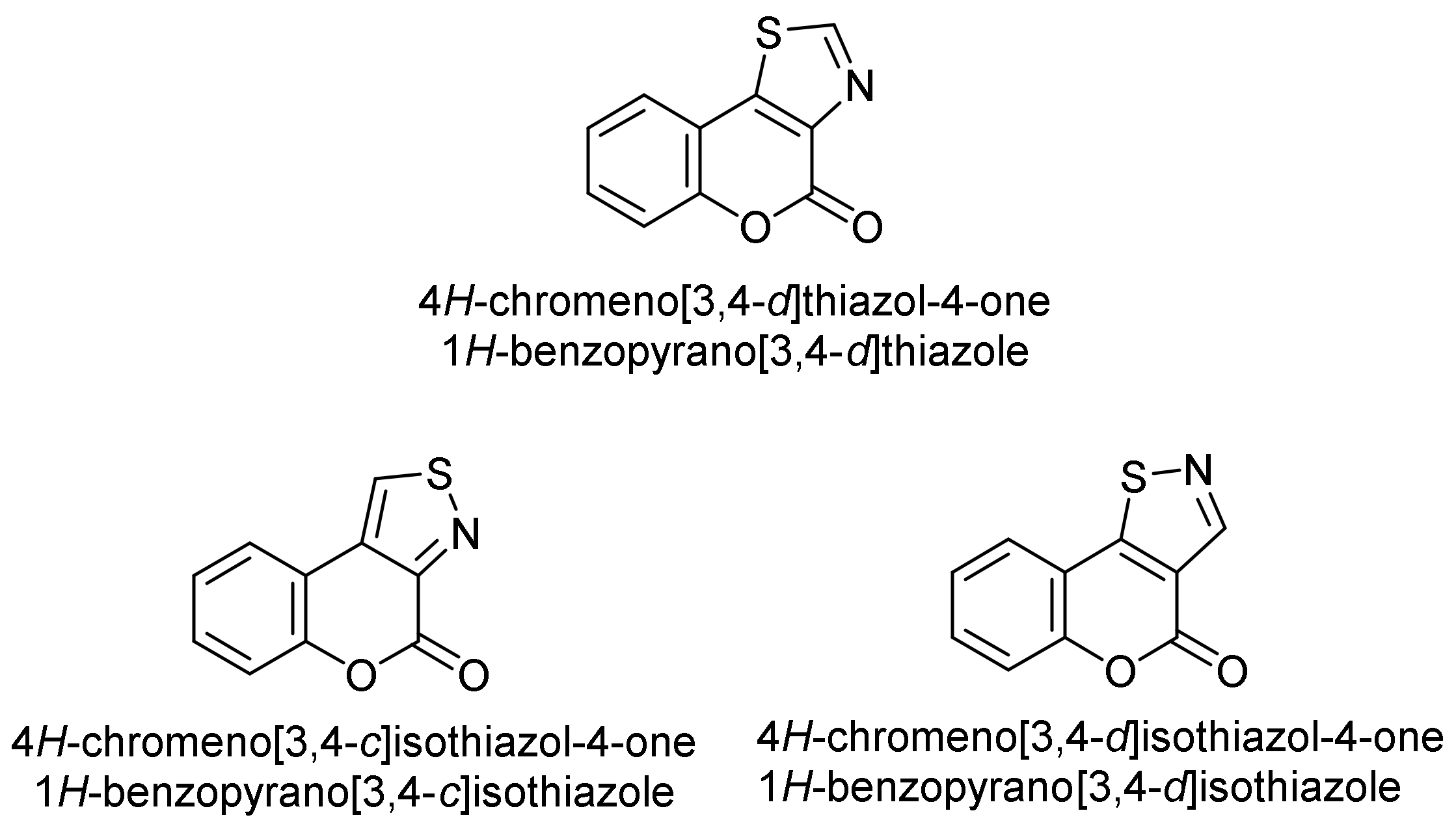
Thiazole and Isothiazole
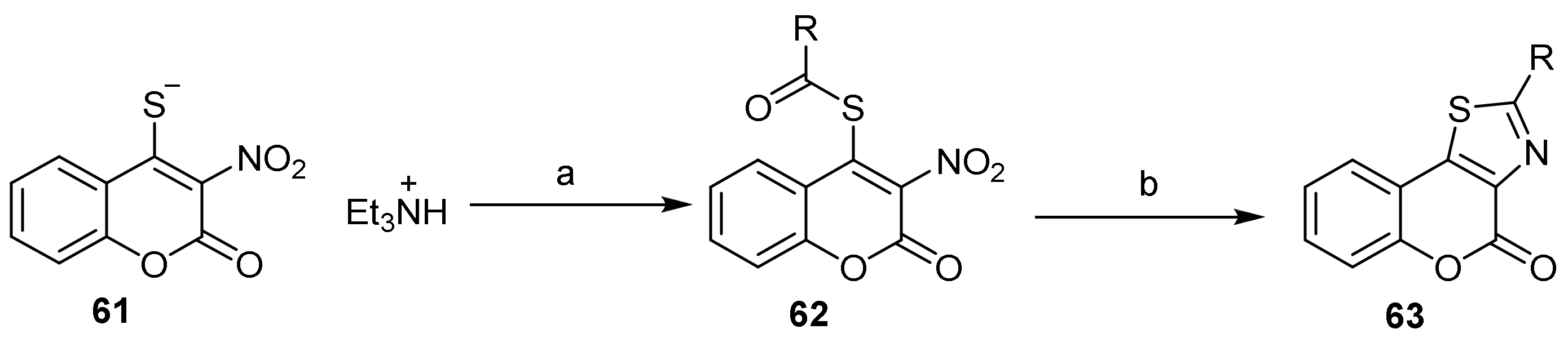
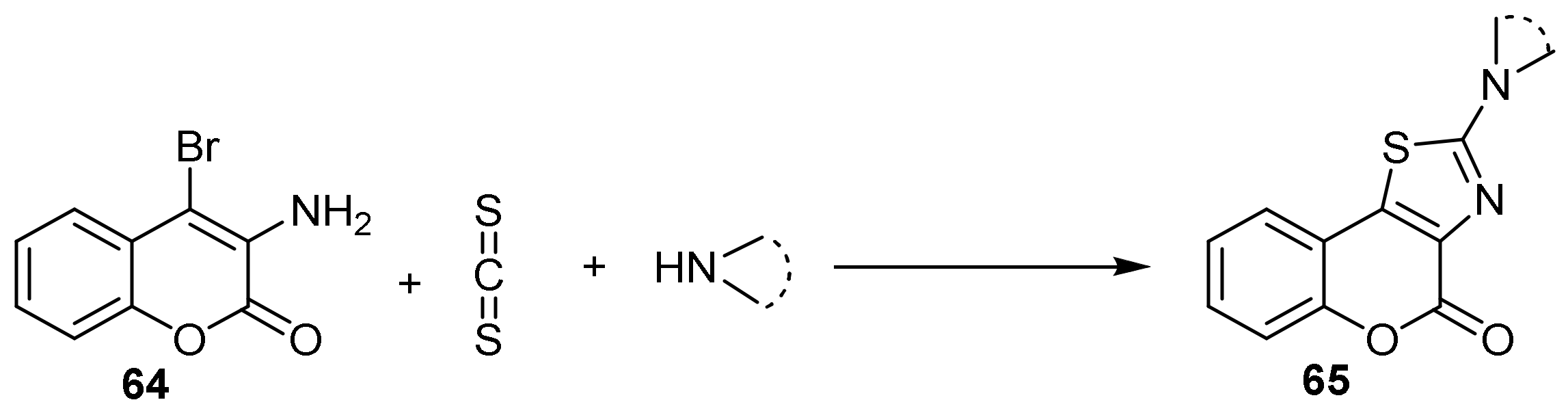
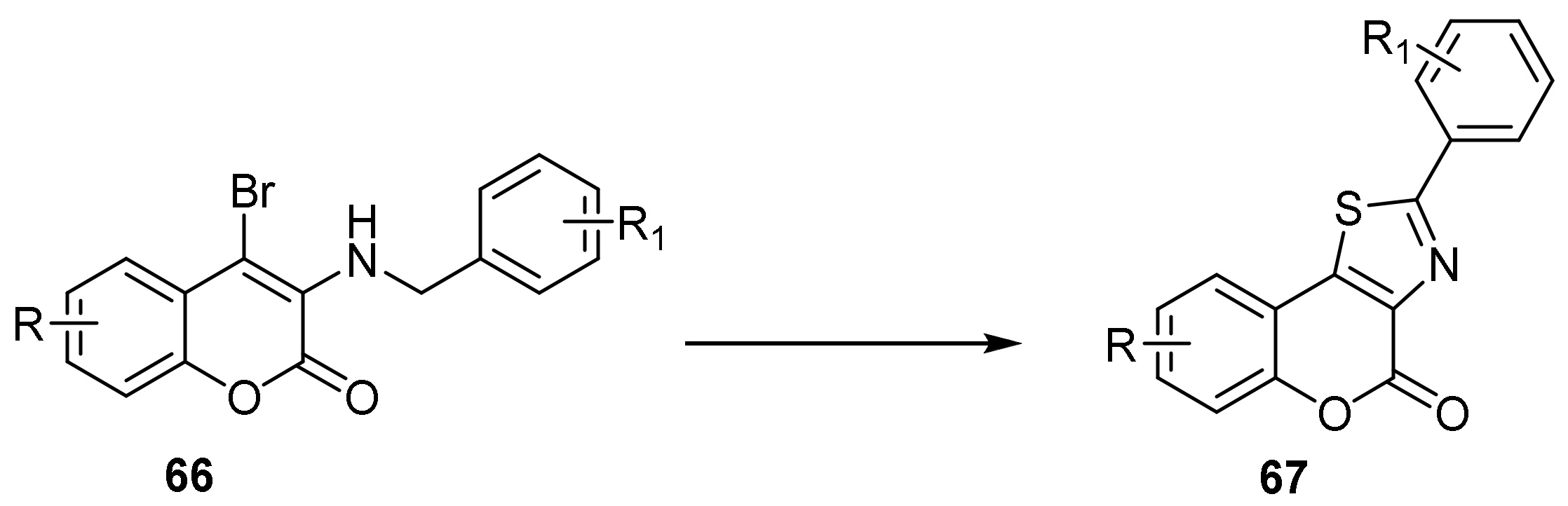
- 4H-Chromeno[3,4-d]thiazol-4-one
- Thiazole Construction
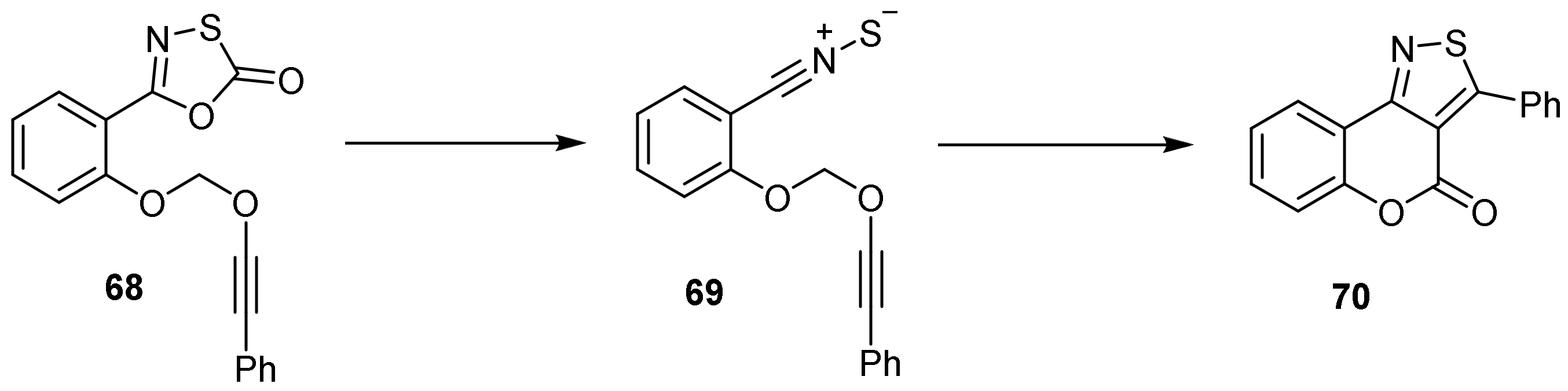
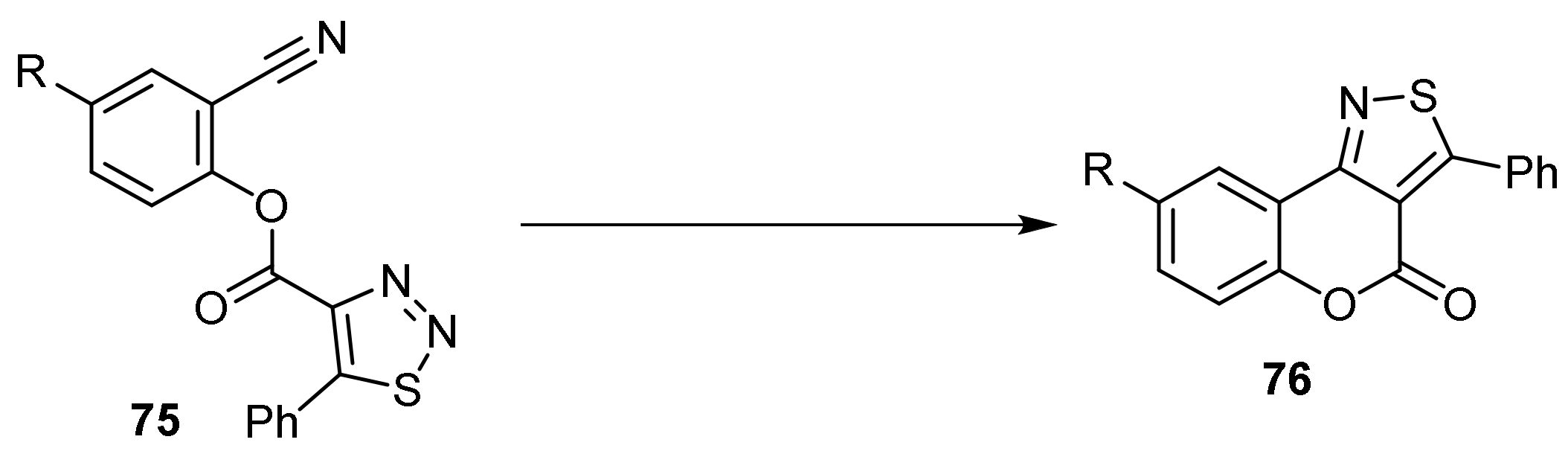
- 4H-Chromeno[3,4-c]isothiazol-4-one
- Pyrone Construction
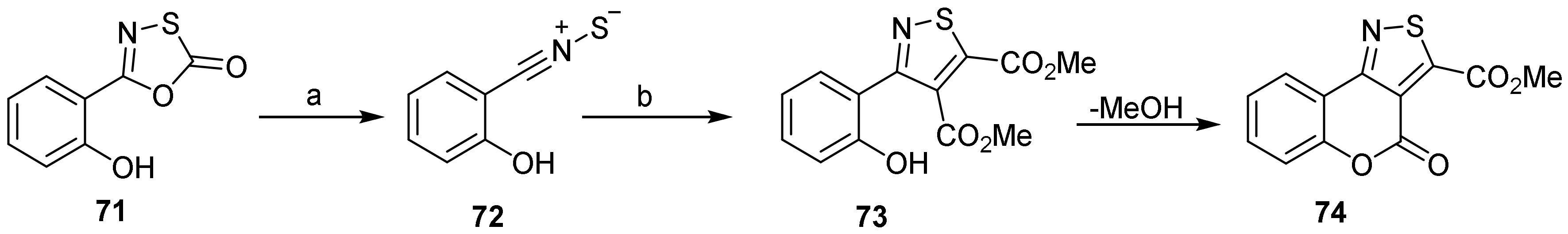
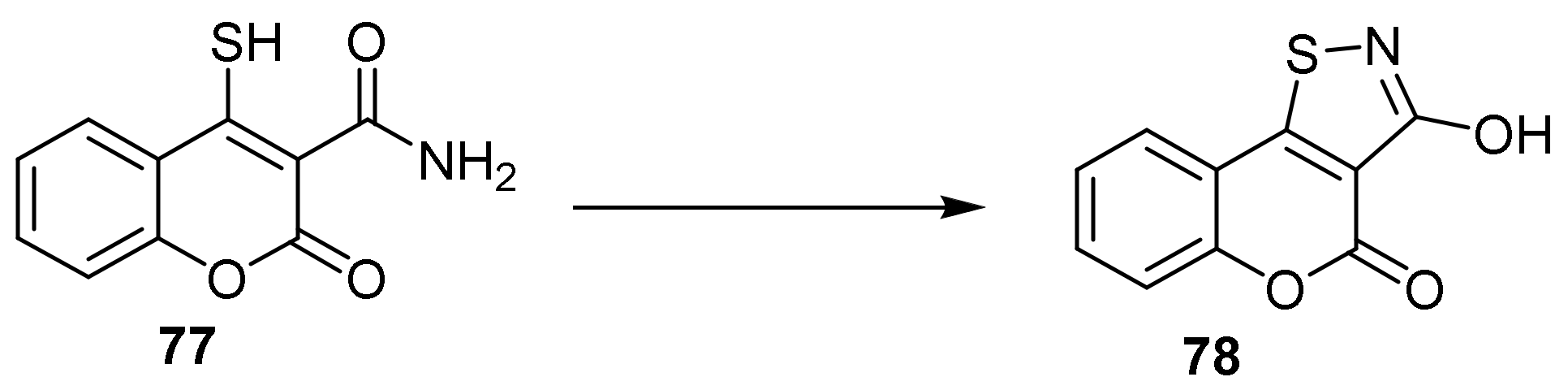
- 4H-Chromeno[3,4-d]isothiazol-4-one
Oxazole and Isoxazole
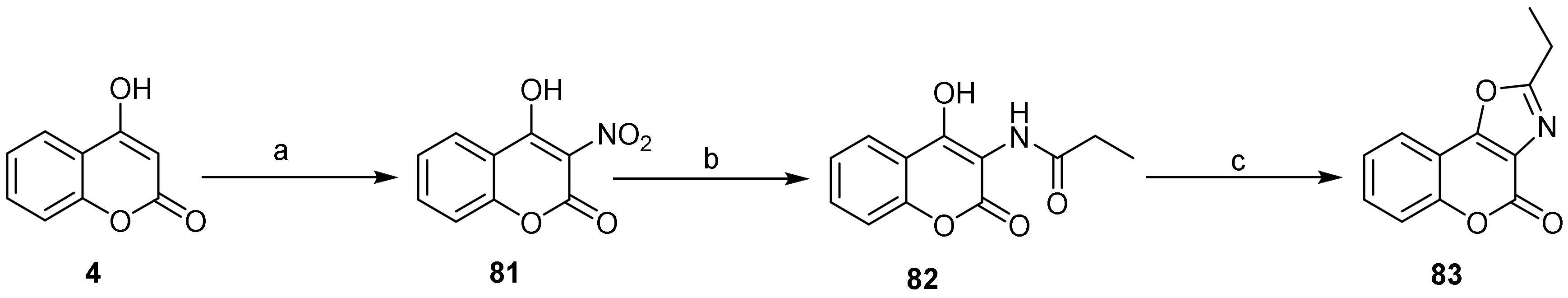
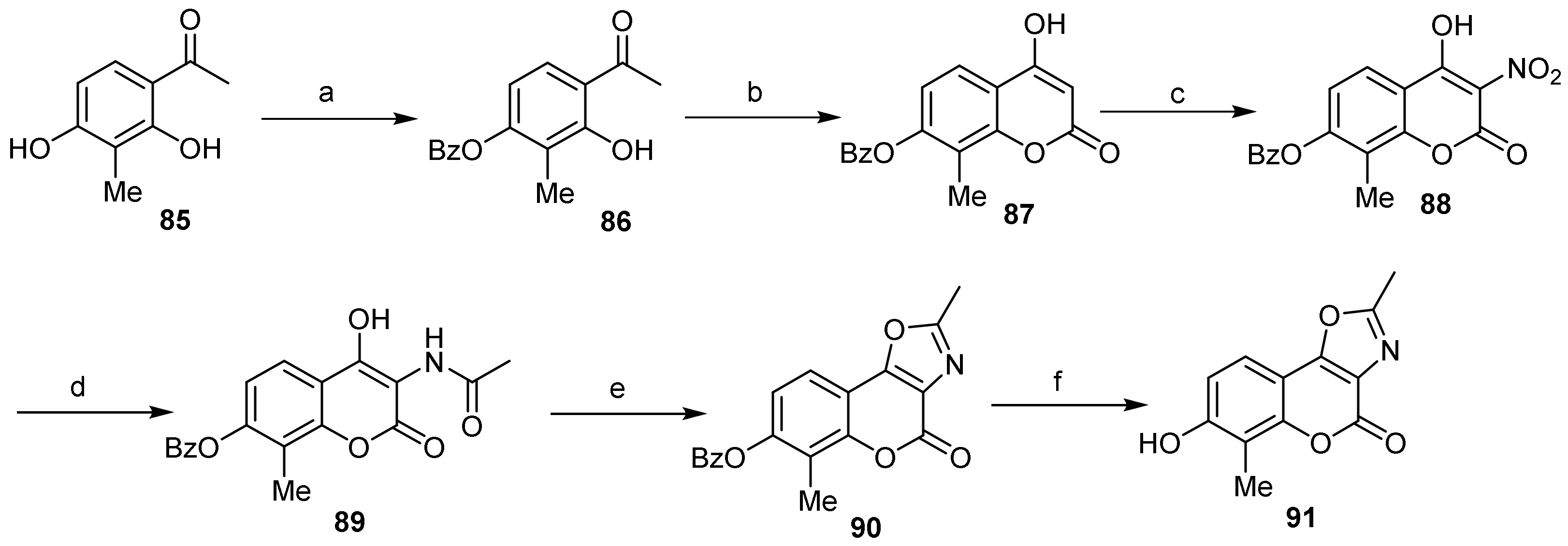
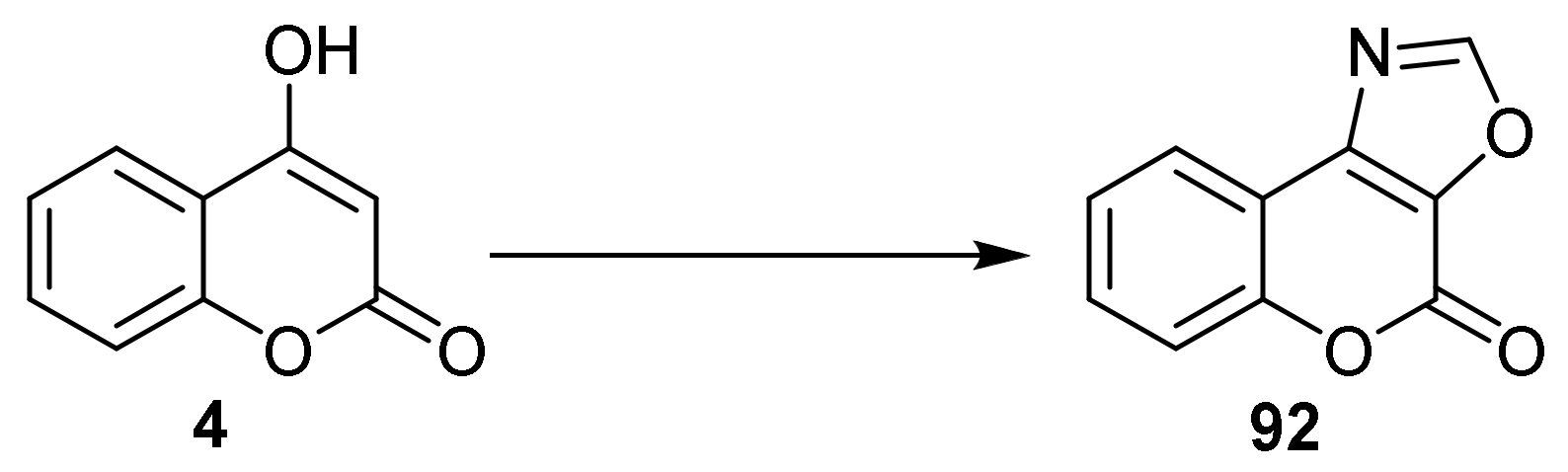
- 4H-Chromeno[3,4-d]oxazol-4-one
- Oxazole Construction
- Pyrone and Oxazole Construction
- 4H-Chromeno[4,3-d]oxazol-4-one
- Oxazole Construction
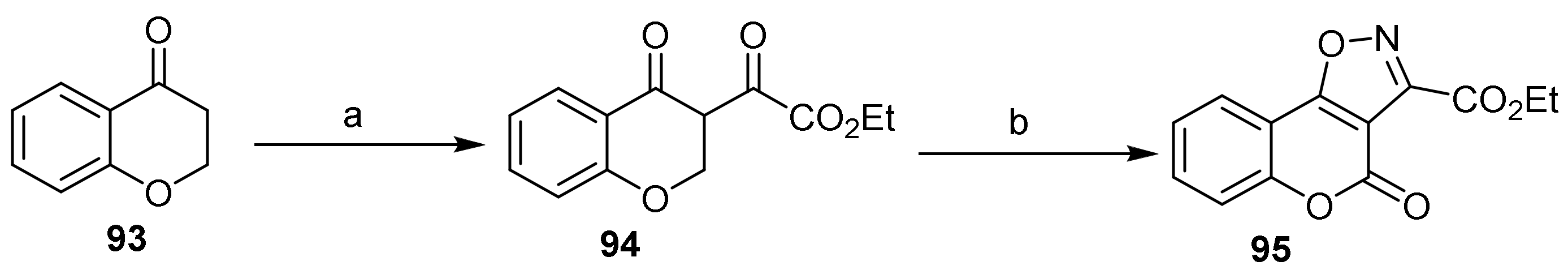
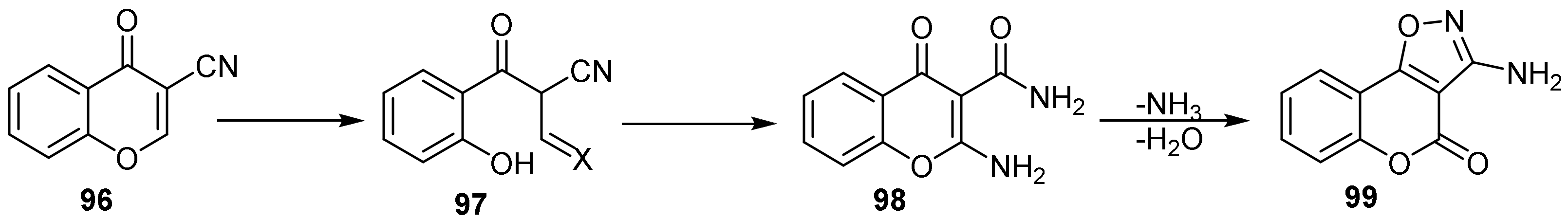
- 4H-Chromeno[3,4-d]isoxazol-4-ones
- Isoxazole Construction
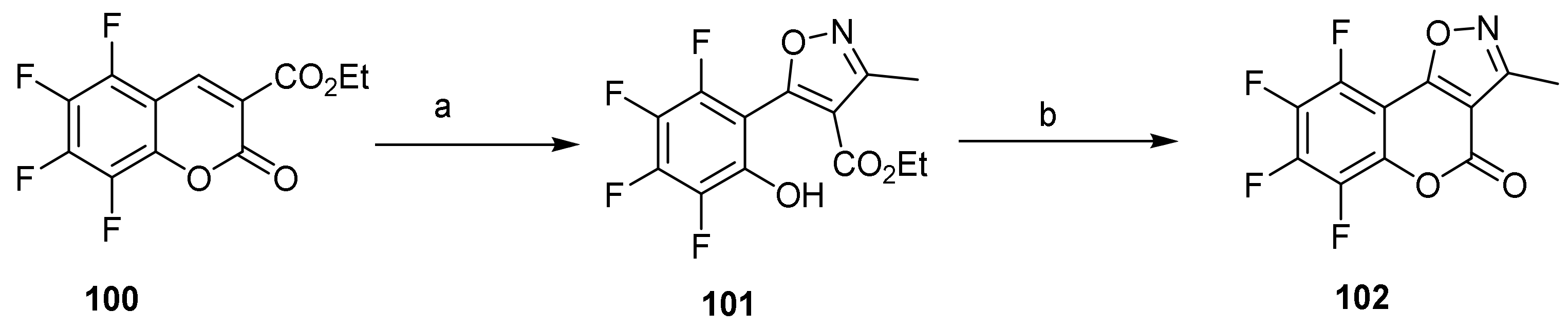
- 4H-Chromeno[4,3-c]isoxazol-4-one
- Isoxazole Construction
2.2. Five-Membered Aromatic Rings with Three Heteroatoms
2.2.1. Three Identical Heteroatoms
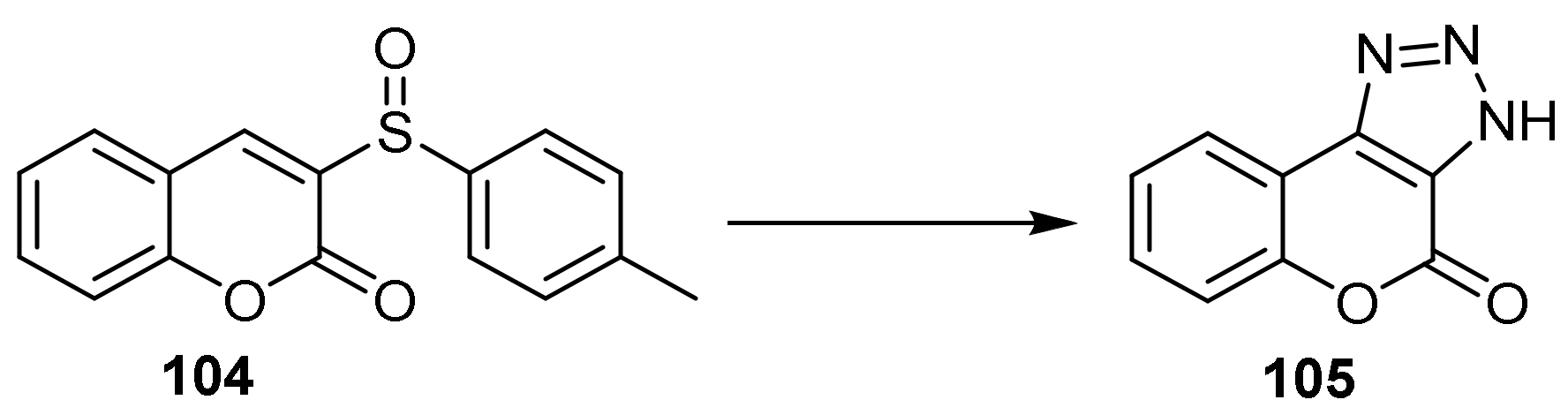
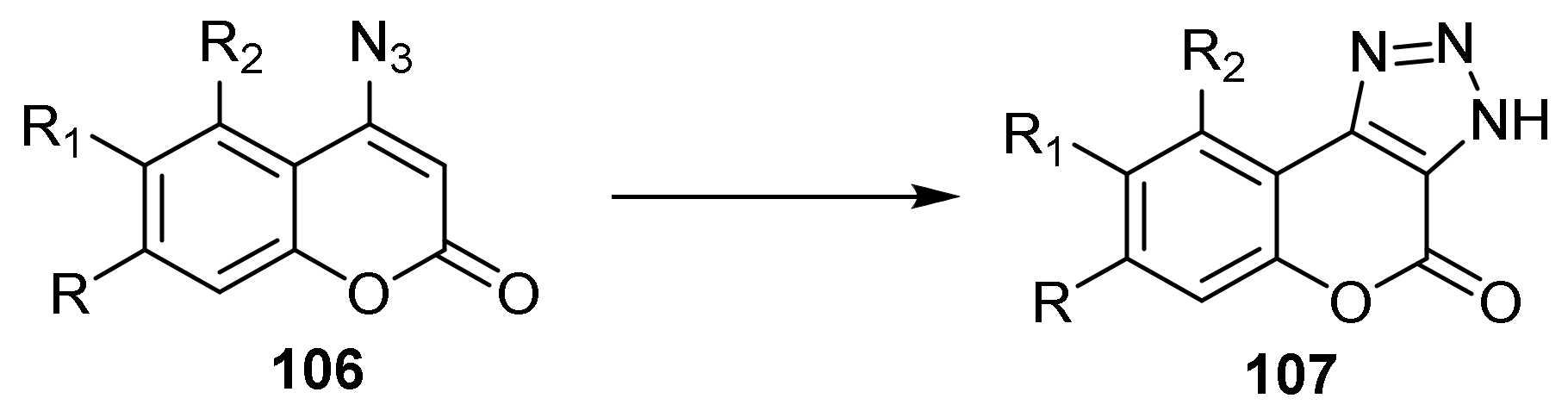
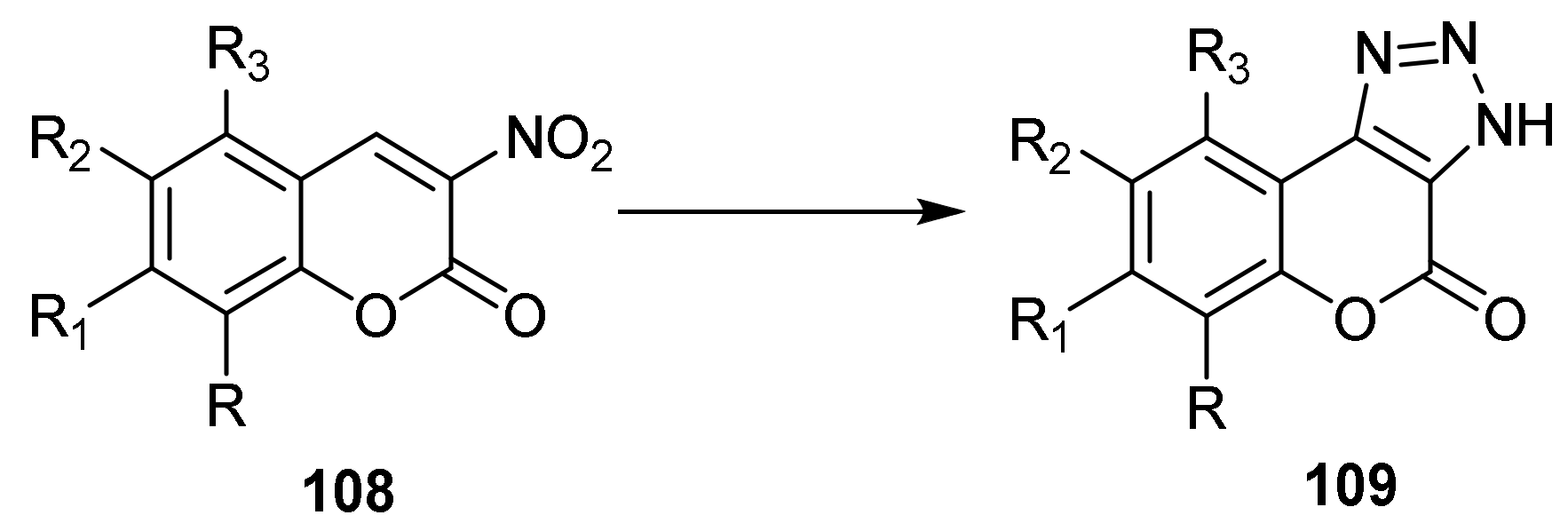
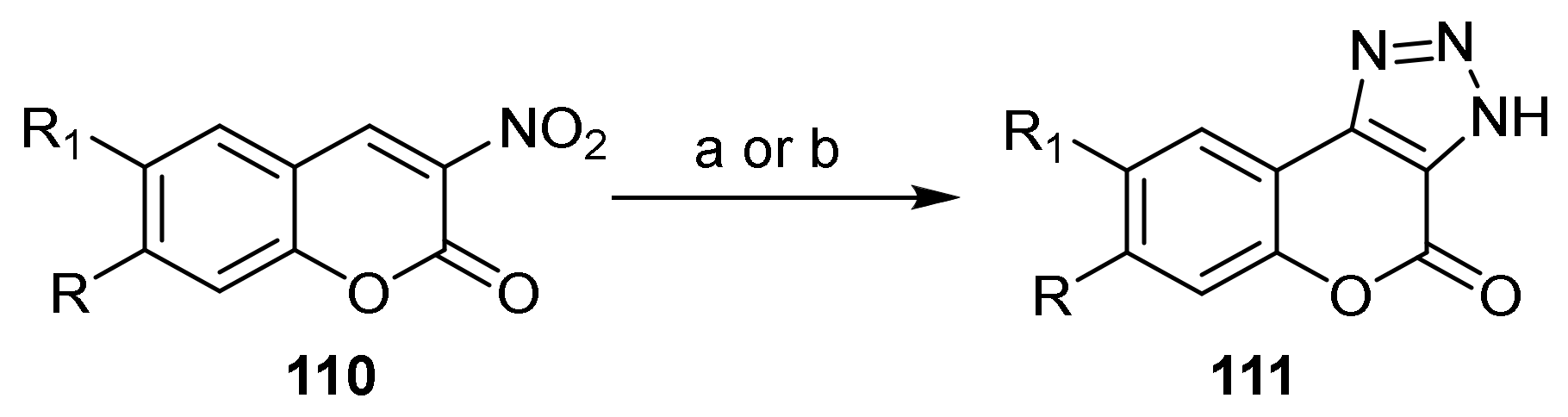
Triazole
- Chromeno[3,4-d][1,2,3]triazol-4(9bH)-one
- Triazole Construction
2.2.2. Three Different Heteroatoms
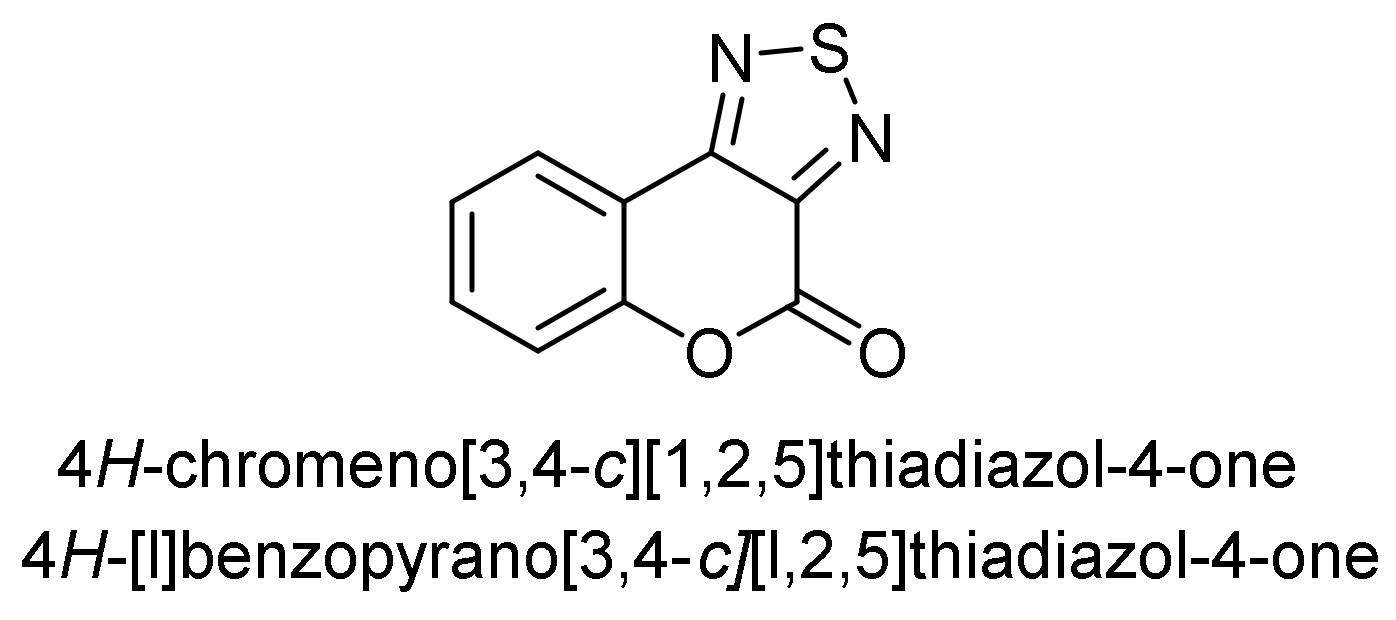
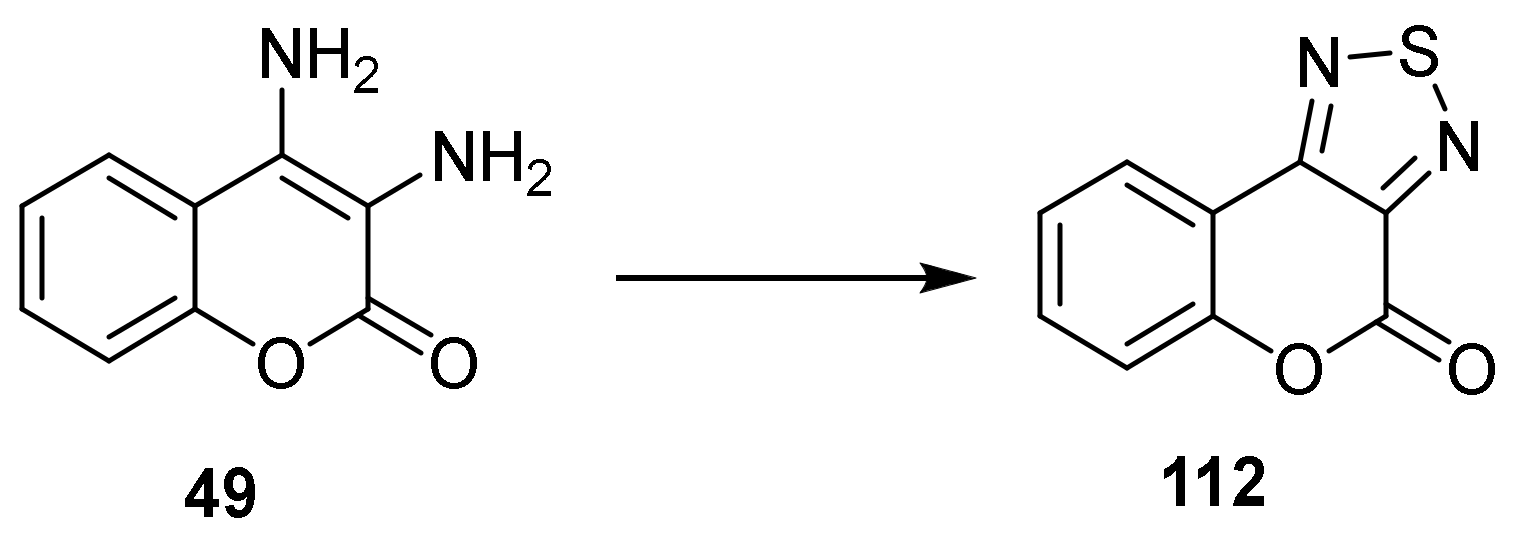
Thiadiazole
- 4H-Chromeno[3,4-c][1,2,5]thiadiazol-4-one
- Thiadiazole Construction
Author Contributions
Funding
Conflicts of Interest
References
- Sethna, S.M.; Shah, N.M. The Chemistry of Coumarins. Chem. Rev. 1945, 36, 1–62. [Google Scholar] [CrossRef]
- Medina, F.G.; Marrero, J.G.; Macías-Alonso, M.; González, M.C.; Córdova-Guerrero, I.; García, A.G.T.; Osegueda-Robles, S. Coumarin heterocyclic derivatives: Chemical synthesis and biological activity. Nat. Prod. Rep. 2015, 32, 1472–1507. [Google Scholar] [CrossRef]
- Molnar, M.; Lončarić, M.; Kovač, M. Green Chemistry Approaches to the Synthesis of Coumarin Derivatives. Curr. Org. Chem. 2020, 24, 4–43. [Google Scholar] [CrossRef]
- Salehian, F.; Nadri, H.; Jalili-Baleh, L.; Youseftabar-Miri, L.; Bukhari, S.N.A.; Foroumadi, A.; Küçükkilinç, T.T.; Sharifzadeh, M.; Khoobi, M. A review: Biologically active 3,4-heterocycle-fused coumarins. Eur. J. Med. Chem. 2021, 212, 113034. [Google Scholar] [CrossRef]
- Geetika, P.; Subhash, B. Review on Synthesis of Bio-Active Coumarin-Fused Heterocyclic Molecules. Curr. Org. Chem. 2020, 24, 2566–2587. [Google Scholar]
- Lončar, M.; Jakovljevi, Ć.M.; Šubarić, D.; Pavlić, M.; Služek, V.B.; Cindrić, I.; Molnar, M. Coumarins in Food and Methods of Their Determination. Foods 2020, 9, 645. [Google Scholar] [CrossRef]
- Trkovnik, M.; Kalaj, V.; Kitan, D. Synthesis of new heterocyclocoumarins from 3,4-diamino- and 4-chloro-3-nitrocoumarins. Org. Prep. Proced. Int. 1987, 19, 450–455. [Google Scholar] [CrossRef]
- Önder, F.C.; Durdaği, S.; Sahin, K.; Özpolat, B.; Ay, M. Design, Synthesis, and Molecular Modeling Studies of Novel Coumarin Carboxamide Derivatives as eEF-2K Inhibitors. J. Chem. Inf. Model. 2020, 60, 1766–1778. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, C.R.; Sahoo, J.; Mahapatra, M.; Lenka, D.; Sahu, P.K.; Dehury, B.; Padhy, R.N.; Paidesetty, S.K. Coumarin derivatives as promising antibacterial agent(s). Arab. J. Chem. 2021, 14, 102922. [Google Scholar] [CrossRef]
- Mulwad, V.; Shirodkar, J. Synthesis of Some of the Antibacterial Compounds from 4-Hydroxycoumarins: Part II. Ind. J. Chem. 2002, 41B, 1263–1267. [Google Scholar]
- Abunada, N.M.; Hassaneen, H.M.; Abu Samaha, A.S.M.; Miqdad, O.A. Synthesis and antimicrobial evaluation of some new pyrazole, pyrazoline and chromeno[3,4-c]pyrazole derivatives. J. Braz. Chem. Soc. 2009, 20, 975–987. [Google Scholar] [CrossRef]
- El-Saghier, A.M.M.; Naili, M.B.; Rammash, B.K.; Saleh, N.A.; Kreddan, K.M. Synthesis and antibacterial activity of some new fused chromenes. Arkivoc 2007, 2007, 83–91. [Google Scholar] [CrossRef]
- Mishra, S.; Pandey, A.; Manvati, S. Coumarin: An emerging antiviral agent. Heliyon 2020, 6, e03217. [Google Scholar] [CrossRef]
- Reddy, M.V.R.; Rao, M.R.; Rhodes, D.; Hansen, M.S.T.; Rubins, K.; Bushman, F.D.; Venkateswarlu, Y.; Faulkner, D.J. Lamellarin α 20-Sulfate, an Inhibitor of HIV-1 Integrase Active against HIV-1 Virus in Cell Culture. J. Med. Chem. 1999, 42, 1901–1907. [Google Scholar] [CrossRef] [PubMed]
- Boger, D.L.; Soenen, D.R.; Boyce, C.W.; Hedrick, M.P.; Jin, Q. Total Synthesis of Ningalin B Utilizing a Heterocyclic Azadiene Diels−Alder Reaction and Discovery of a New Class of Potent Multidrug Resistant (MDR) Reversal Agents. J. Org. Chem. 2000, 65, 2479–2483. [Google Scholar] [CrossRef] [PubMed]
- Neagoie, C.; Vedrenne, E.; Buron, F.; Mérour, J.-Y.; Roşca, S.; Bourg, S.; Lozach, O.; Meijer, L.; Baldeyrou, B.; Lansiaux, A.; et al. Synthesis of chromeno[3,4-b]indoles as Lamellarin D analogues: A novel DYRK1A inhibitor class. Eur. J. Med. Chem. 2012, 49, 379–396. [Google Scholar] [CrossRef]
- Rajabi, M.; Hossaini, Z.; Khalilzadeh, M.A.; Datta, S.; Halder, M.; Mousa, S.A. Synthesis of a new class of furo[3,2-c]coumarins and its anticancer activity. J. Photochem. Photobiol. B Biol. 2015, 148, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Al-Ayed, A.S. Synthesis of New Substituted Chromen[4,3-c]pyrazol-4-ones and Their Antioxidant Activities. Molecules 2011, 16, 10292–10302. [Google Scholar] [CrossRef]
- Hamdi, N.; Fischmeister, C.; Puerta, C.; Valerga, P. A rapid access to new coumarinyl chalcone and substituted chromeno[4,3-c]pyrazol-4(1H)-ones and their antibacterial and DPPH radical scavenging activities. Med. Chem. Res. 2010, 20, 522–530. [Google Scholar] [CrossRef]
- Hosni, H.M.; Abdulla, M. Anti-inflammatory and analgesic activities of some newly synthesized pyridinedicarbonitrile and benzopyranopyridine derivatives. Acta Pharm. 2008, 58, 175–186. [Google Scholar] [CrossRef]
- Khan, I.A.; Kulkarni, M.V.; Gopal, M.; Shahabuddin, M.; Sun, C.-M. Synthesis and biological evaluation of novel angularly fused polycyclic coumarins. Bioorg. Med. Chem. Lett. 2005, 15, 3584–3587. [Google Scholar] [CrossRef] [PubMed]
- El-Sawy, E.; Abdelwahab, A.; Kirsch, G. Synthetic Routes to Coumarin(Benzopyrone)-Fused Five-Membered Aromatic Heterocycles Built on the α-Pyrone Moiety. Part 1: Five-Membered Aromatic Rings with One Heteroatom. Molecules 2021, 26, 483. [Google Scholar] [CrossRef]
- Shawali, A.S.; Elanadouli, B.E.; Albar, H.A. Cycloaddition of diphenylnitrilimine to coumarins. The synthesis of 3a,9b-dihydro-4-oxo-1H-benzopyrano [4,3-c]pyrazole derivatives. Tetrahedron 1985, 41, 1877–1884. [Google Scholar] [CrossRef]
- El-Dean, A.M.K.; Zaki, R.M.; Geies, A.A.; Radwan, S.M.; Tolba, M.S. Synthesis and antimicrobial activity of new heterocyclic compounds containing thieno[3,2-c]coumarin and pyrazolo[4,3-c]coumarin frameworks. Russ. J. Bioorg. Chem. 2013, 39, 553–564. [Google Scholar] [CrossRef]
- Strakova, I.; Petrova, M.; Belyakov, S.; Strakovs, A. Reactions of 4-Chloro-3-formylcoumarin with Arylhydrazines. Chem. Heterocycl. Compd. 2003, 39, 1608–1616. [Google Scholar] [CrossRef]
- Yin, Y.; Wu, X.; Han, H.-W.; Sha, S.; Wang, S.-F.; Qiao, F.; Lü, A.-M.; Lv, P.-C.; Zhu, H.-L. Discovery and synthesis of a novel series of potent, selective inhibitors of the PI3K?: 2-alkyl-chromeno[4,3-c]pyrazol-4(2H)-one derivatives. Org. Biomol. Chem. 2014, 12, 9157–9165. [Google Scholar] [CrossRef] [PubMed]
- Chekir, S.; Debbabi, M.; Regazzetti, A.; Dargère, D.; Laprévote, O.; Ben Jannet, H.; Gharbi, R. Design, synthesis and biological evaluation of novel 1,2,3-triazole linked coumarinopyrazole conjugates as potent anticholinesterase, anti-5-lipoxygenase, anti-tyrosinase and anti-cancer agents. Bioorg. Chem. 2018, 80, 189–194. [Google Scholar] [CrossRef]
- Yin, Y.; Hu, J.-Q.; Wu, X.; Sha, S.; Wang, S.-F.; Qiao, F.; Song, Z.-C.; Zhu, H.-L. Design, synthesis and biological evaluation of novel chromeno[4,3-c]pyrazol-4(2H)-one derivates containing sulfonamido as potential PI3Kα inhibitors. Bioorg. Med. Chem. 2019, 27, 2261–2267. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Sha, S.; Wang, K.; Zhang, Y.-H.; Liu, Y.-D.; Ju, G.-D.; Wang, B.; Zhu, H.-L. Discovery of Chromeno[4,3-c]pyrazol-4(2H)-one Containing Carbonyl or Oxime Derivatives as Potential, Selective Inhibitors PI3Kα. Chem. Pharm. Bull. 2016, 64, 1576–1581. [Google Scholar] [CrossRef]
- Yin, Y.; Sha, S.; Wu, X.; Wang, S.-F.; Qiao, F.; Song, Z.-C.; Zhu, H.-L. Development of novel chromeno[4,3-c]pyrazol-4(2H)-one derivates bearing sulfonylpiperazine as antitumor inhibitors targeting PI3Kα. Eur. J. Med. Chem. 2019, 182, 111630. [Google Scholar] [CrossRef] [PubMed]
- Steinfiihrer, T.; Hantschmann, A.; Pietsch, M.; WeiDenfels, M. Heterocyclisch [c]-Anellierte Cumarine Aus 4-Azido-3-Cumarincarbaldehyden. Liebigs Ann. Chem. 1992, 1992, 23–28. [Google Scholar] [CrossRef]
- Mustafa, A.; Hishmat, O.H.; Nawar, A.A.; Khalil, K.H.M.A. Pyrazolo-cumarine und Pyrazolyl-cumarone. Eur. J. Org. Chem. 1965, 684, 194–200. [Google Scholar] [CrossRef]
- Ibrahim, M.A. Ring transformation of chromone-3-carboxylic acid under nucleophilic conditions. Arkivoc 2008, 2008, 192. [Google Scholar] [CrossRef]
- Ibrahim, M.A. Ring Transformation of Chromone-3-Carboxamide under Nucleophilic Conditions. J. Braz. Chem. Soc. 2013, 24, 1754–1763. [Google Scholar] [CrossRef]
- Ibrahim, M.; Badran, A.; El-Gohary, N.; Hashiem, S. Studies on the Chemical Reactions of Some 3-Substituted-6,8-dimethylchromones with Nucleophilic Reagents. J. Heterocycl. Chem. 2018, 55, 2315–2324. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; El-Gohary, N.M.; Said, S. Reactivity of 6-Methylchromone-3-carbonitrile Towards Some Nitrogen Nucleophilic Reagents. Heterocycles 2018, 96, 690. [Google Scholar] [CrossRef]
- Trimeche, B.; Gharbi, R.; Martin, M.-T.; Nuzillard, J.M.; Mighri, Z.; El Houla, S. Reactivity of [1]benzopyrano[4,3-c][1,5]benzodiazepin-7(8H)-ones towards some N-binucleophiles. J. Chem. Res. 2004, 2004, 170–173. [Google Scholar] [CrossRef]
- Lokhande, P.; Hasanzadeh, K.; Konda, S.G. A novel and efficient approach for the synthesis of new halo substituted 2-arylpyrazolo[4,3-c] coumarin derivatives. Eur. J. Chem. 2011, 2, 223–228. [Google Scholar] [CrossRef]
- Stadlbauers, W.; Hojas, G. Ring closure reactions of 3-arylhydrazonoalkyl-quinolin-2-ones to 1-aryl-pyrazolo[4,3-c]quinolin-2-ones. J. Heterocycl. Chem. 2004, 41, 681–690. [Google Scholar] [CrossRef]
- Hariprasad, K.S.; Anand, A.; Rathod, B.B.; Zehra, A.; Tiwari, A.K.; Prakasham, R.S.; Raju, B.C. Neoteric Synthesis and Biological Activities of Chromenopyrazolones, Tosylchromenopyrazolones, Benzoylcoumarins. ChemistrySelect 2017, 2, 10628–10634. [Google Scholar] [CrossRef]
- Fathi, T.; An, N.D.; Schmitt, G.; Cerutti, E.; Laude, B. Regiochemistry of the cycloadditions of diphenylnitrilimine to coumarin, 3-ethoxycarbonyl and 3-acetyl coumarins. Tetrahedron 1988, 44, 4527–4536. [Google Scholar] [CrossRef]
- Ito, K.; Maruyama, J. 4-Diazomethylcoumarins and Related Stable Heteroaryldiazomethanes. Thermal Conversion into Condensed Pyrazoles. J. Heterocycl. Chem. 1988, 25, 1681–1687. [Google Scholar] [CrossRef]
- Ito, K.; Maruyama, J. A Facile Intramolecular Cyclization of 4-Diazomethylcoumarins. A Convenient Route to Benzopyrano[3,4-c]pyrazol-4(3H)-ones. Heterocycles 1984, 22, 1057. [Google Scholar] [CrossRef]
- Colotta, V.; Catarzi, D.; Varano, F.; Cecchi, L.; Filacchioni, G.; Martini, C.; Giusti, L.; Lucacchini, A. Tricyclic heteroaromatic systems. Synthesis and benzodiazepine receptor affinity of 2-substituted-1-benzopyrano[3,4-d]oxazol-4-ones, -thiazol-4-ones, and -imidazol-4-ones. IL Farm. 1998, 53, 375–381. [Google Scholar] [CrossRef]
- Beccalli, E.M.; Contini, A.; Trimarco, P. 3-Nitrocoumarin Amidines: A New Synthetic Strategy for Substituted [1]Benzopyrano[3,4-d]imidazol-4(3H)-ones. Eur. J. Org. Chem. 2003, 2003, 3976–3984. [Google Scholar] [CrossRef]
- Anwar, S.; Paul, S.B.; Majumdar, K.C.; Choudhury, S. Green, One-Pot, Multicomponent Synthesis of Fused-Ring 2-Aminothiazoles. Synth. Commun. 2014, 44, 3304–3313. [Google Scholar] [CrossRef]
- Belal, M.; Khan, A.T. Oxidative cross coupling reaction mediated by I2/H2O2: A novel approach for the construction of fused thiazole containing coumarin derivatives. RSC Adv. 2015, 5, 104155–104163. [Google Scholar] [CrossRef]
- Brownsort, P.A.; Paton, R.; Sutherland, A.G. Intramolecular cycloaddition reactions involving nitrile sulphides. Tetrahedron Lett. 1985, 26, 3727–3730. [Google Scholar] [CrossRef]
- Brownsort, P.A.; Michael Paton, A. Nitrile Sulphides. Part 7. Synthesis of [I ]Benzopyrano[4,3-c]Isothiazoles and Isothiazolo[4,3-c]Quinolines. J. Chem. Soc. Perkin Trans. 1987, 2339–2344. [Google Scholar] [CrossRef]
- Brownsort, P.A.; Paton, R.M.; Sutherland, A.G. Nitrile Sulphides. Part 1O. Intramolecular 1.3-Dipolar Cycloadditions. J. Chem. Soc. Perkin Trans. 1989, 1679–1686. [Google Scholar] [CrossRef]
- Fordyce, E.A.; Morrison, A.J.; Sharp, R.D.; Paton, R.M. Microwave-induced generation and reactions of nitrile sulfides: An improved method for the synthesis of isothiazoles and 1,2,4-thiadiazoles. Tetrahedron 2010, 66, 7192–7197. [Google Scholar] [CrossRef]
- Seo, B.; Kim, H.; Kim, Y.G.; Baek, Y.; Um, K.; Lee, P.H. Synthesis of Bicyclic Isothiazoles through an Intramolecular Rhodium-Catalyzed Transannulation of Cyanothiadiazoles. J. Org. Chem. 2017, 82, 10574–10582. [Google Scholar] [CrossRef] [PubMed]
- Checchi, S.; Pecori Vettori, L.; Vincieri, F. 4-Hydroxycoumarins. VIII. Substitution Reactions of 4-Chloro-3-Cyanocoumarins. Gazz. Chim. Ital. 1968, 98, 1488–1502. [Google Scholar]
- Lee, Y.R.; Suk, J.Y.; Kim, B.S. Rhodium(II)-catalyzed reactions of 3-diazo-2,4-chromenediones. First one-step synthesis of pterophyllin 2. Tetrahedron Lett. 1999, 40, 6603–6607. [Google Scholar] [CrossRef]
- Taber, D.F.; Ruckle, R.E.; Hennessy, M.J. Mesyl azide: A superior reagent for diazo transfer. J. Org. Chem. 1986, 51, 4077–4078. [Google Scholar] [CrossRef]
- Dallacker, F.; Kratzer, P.; Lipp, M. Derivate des 2.4-Pyronons und 4-Hydroxy-cumarins. Eur. J. Org. Chem. 1961, 643, 97–109. [Google Scholar] [CrossRef]
- Balalas, T.D.; Stratidis, G.; Papatheodorou, D.; Vlachou, E.-E.; Gabriel, C.; Hadjipavlou-Litina, D.J.; Litinas, K.E. One-pot Synthesis of 2-Substituted 4H-Chromeno[3,4-d]oxazol-4-ones from 4-Hydroxy-3-nitrocoumarin and Acids in the Presence of Triphenylphosphine and Phosphorus Pentoxide under Microwave Irradiation. SynOpen 2018, 2, 0105–0113. [Google Scholar] [CrossRef]
- Gammon, D.W.; Hunter, R.; Wilson, S.A. An efficient synthesis of 7-hydroxy-2,6-dimethylchromeno[3,4-d]oxazol-4-one—a protected fragment of novenamine. Tetrahedron 2005, 61, 10683–10688. [Google Scholar] [CrossRef]
- Ray, S.; Paul, S.K. Studies on Reaction of Hydroxycoumarin Compounds with Formamide. Cheminform 2005, 36. [Google Scholar] [CrossRef]
- Freedman, J.; Milwaukee, W. 3-Substituted-4H(1)Benzopyrano(3,4-d) Isoxazoles 1971. U.S. Patent 3,553,228, 5 January 1971. [Google Scholar]
- Sosnovskikh, V.Y.; Moshkin, V.S.; Kodess, M.I. A reinvestigation of the reactions of 3-substituted chromones with hydroxylamine. Unexpected synthesis of 3-amino-4H-chromeno[3,4-d]isoxazol-4-one and 3-(diaminomethylene)chroman-2,4-dione. Tetrahedron Lett. 2008, 49, 6856–6859. [Google Scholar] [CrossRef]
- Saloutin, V.; Skryabina, Z.; Bazyl’, I.; Kisil’, S. Interaction of 3-ethoxycarbonyl(carboxy)-substituted 5,6,7,8-tetrafluorochromones with N-nucleophiles: Synthesis of fluorocoumarins. J. Fluor. Chem. 1999, 94, 83–90. [Google Scholar] [CrossRef]
- Dean, F.M.; Park, B.K. Activating groups for the ring expansion of coumarin by diazoethane: Benzoyl, pivaloyl, arylsulphonyl, arylsulphinyl, and nitro. J. Chem. Soc. Perkin Trans. 1976, 1, 1260–1268. [Google Scholar] [CrossRef]
- Ito, K.; Hariya, J. Electrocyclization of 4-Azidocoumarins Leading to Benzopyrano[3,4-d]-1,2,3-triazol-4-ones. Heterocycles 1987, 26, 35. [Google Scholar] [CrossRef]
- D’Ambrosio, G.; Fringuelli, F.; Pizzo, F.; Vaccaro, L. TBAF-catalyzed [3 + 2]cycloaddition of TMSN3 to 3-nitrocoumarins under SFC: An effective green route to chromeno[3,4-d][1,2,3]triazol-4(3H)-ones. Green Chem. 2005, 7, 874–877. [Google Scholar] [CrossRef]
- Wang, T.; Hu, X.-C.; Huang, X.-J.; Li, X.-S.; Xie, J.-W. Efficient synthesis of functionalized 1,2,3-triazoles by catalyst-free 1,3-dipolar cycloaddition of nitroalkenes with sodium azide. J. Braz. Chem. Soc. 2012, 23, 1119–1123. [Google Scholar] [CrossRef][Green Version]
- Schwendt, G.; Glasnov, T. Intensified synthesis of [3,4-d]triazole-fused chromenes, coumarins, and quinolones. Mon. Für Chem. Chem. Mon. 2017, 148, 69–75. [Google Scholar] [CrossRef]
- Savel’Ev, V.L.; Samsonova, O.L.; Troitskaya, V.S.; Vinokurov, V.G.; Lezina, V.P.; Smirnov, L.D. Synthesis of 4H-[1]benzopyrano[3,4-c][1,2,5]thyadiazol-4-one and its reactions with some nucleophilic and electrophilic agents. Chem. Heterocycl. Compd. 1988, 24, 805–810. [Google Scholar] [CrossRef]

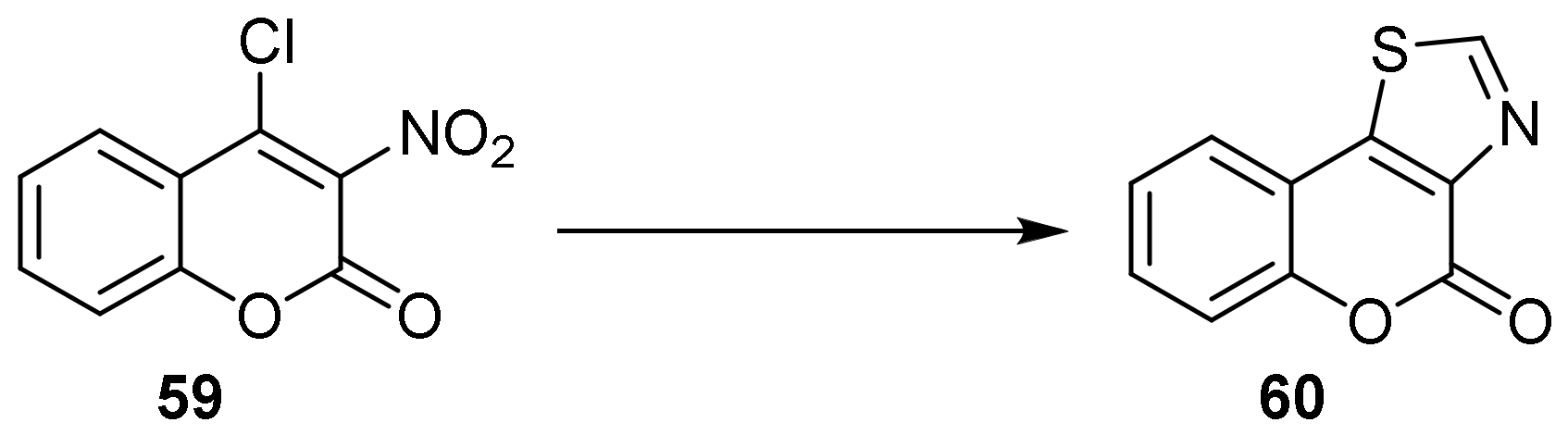


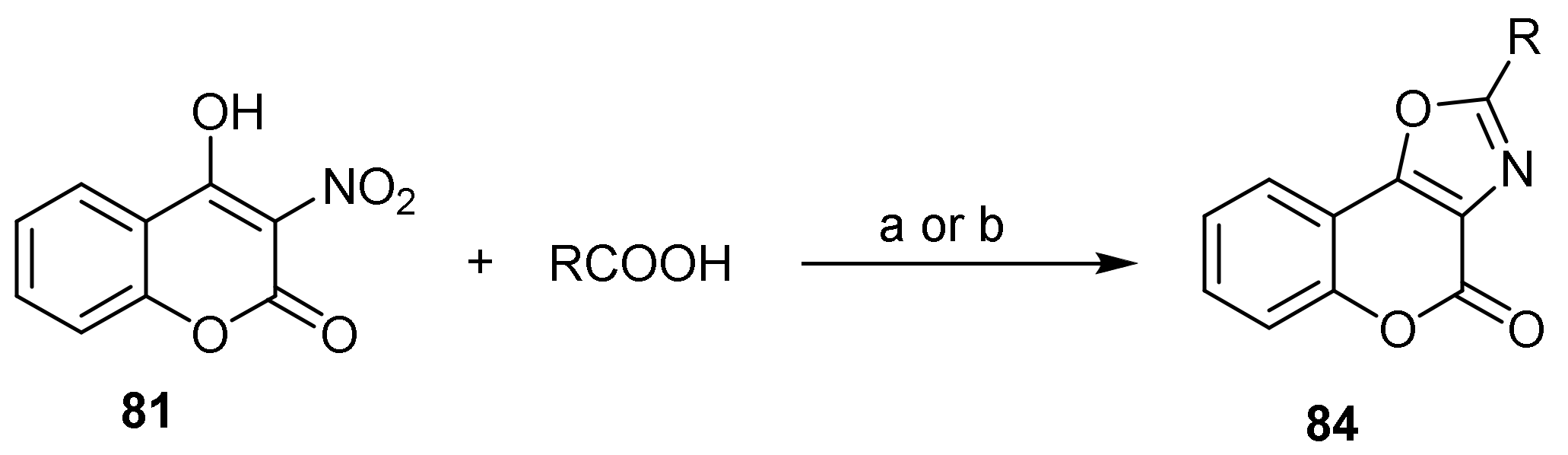



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Sawy, E.R.; Abdelwahab, A.B.; Kirsch, G. Synthetic Routes to Coumarin(Benzopyrone)-Fused Five-Membered Aromatic Heterocycles Built on the α-Pyrone Moiety. Part II: Five-Membered Aromatic Rings with Multi Heteroatoms. Molecules 2021, 26, 3409. https://doi.org/10.3390/molecules26113409
El-Sawy ER, Abdelwahab AB, Kirsch G. Synthetic Routes to Coumarin(Benzopyrone)-Fused Five-Membered Aromatic Heterocycles Built on the α-Pyrone Moiety. Part II: Five-Membered Aromatic Rings with Multi Heteroatoms. Molecules. 2021; 26(11):3409. https://doi.org/10.3390/molecules26113409
Chicago/Turabian StyleEl-Sawy, Eslam Reda, Ahmed Bakr Abdelwahab, and Gilbert Kirsch. 2021. "Synthetic Routes to Coumarin(Benzopyrone)-Fused Five-Membered Aromatic Heterocycles Built on the α-Pyrone Moiety. Part II: Five-Membered Aromatic Rings with Multi Heteroatoms" Molecules 26, no. 11: 3409. https://doi.org/10.3390/molecules26113409
APA StyleEl-Sawy, E. R., Abdelwahab, A. B., & Kirsch, G. (2021). Synthetic Routes to Coumarin(Benzopyrone)-Fused Five-Membered Aromatic Heterocycles Built on the α-Pyrone Moiety. Part II: Five-Membered Aromatic Rings with Multi Heteroatoms. Molecules, 26(11), 3409. https://doi.org/10.3390/molecules26113409






