The Use of Megamolecular Polysaccharide Sacran in Food and Biomedical Applications
Abstract
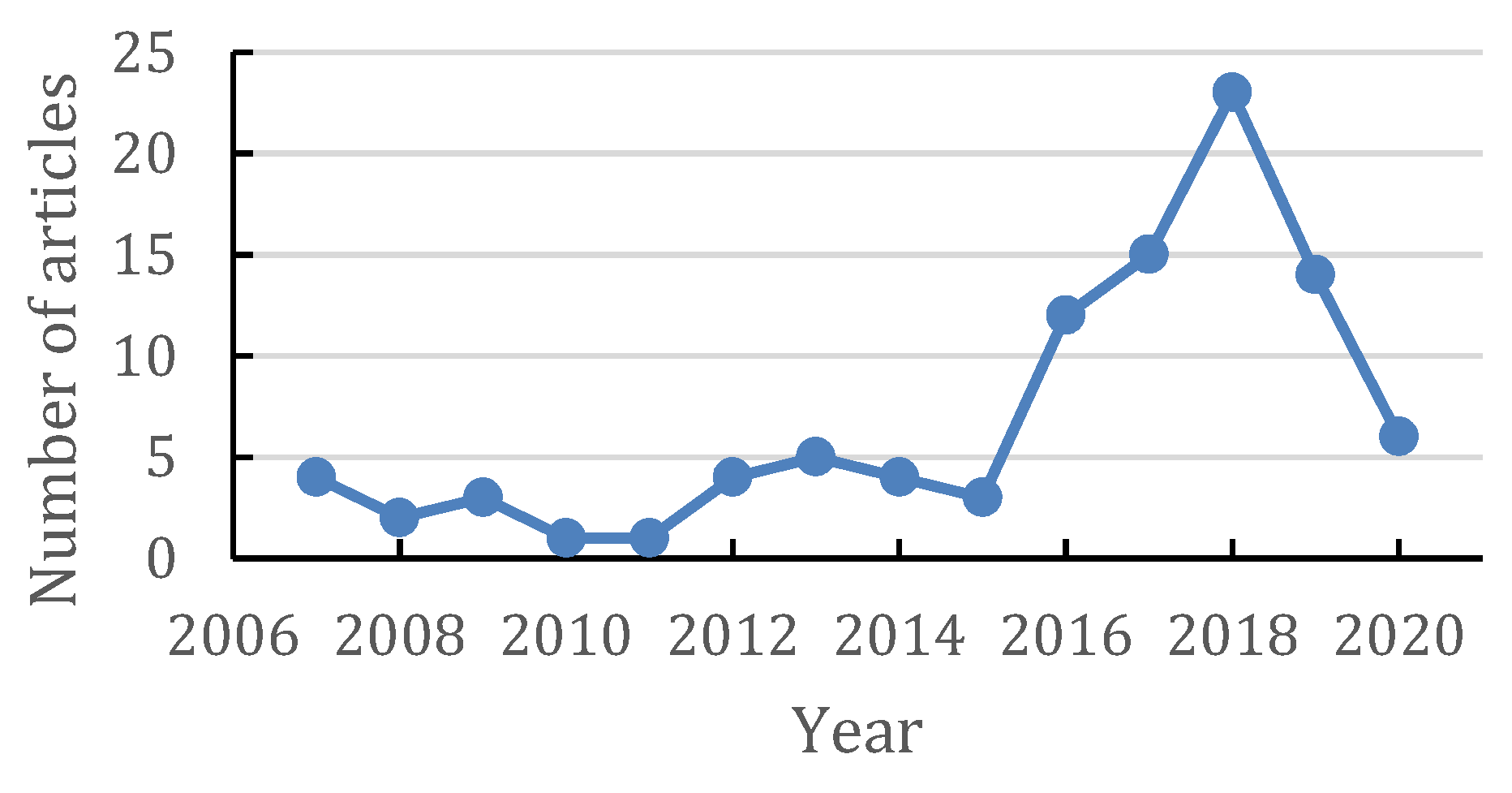
1. Introduction
2. Methodology
3. Aphanothece sacrum
3.1. Aphanothece sacrum
3.2. Characteristic of Aphanothece sacrum
3.3. Sacran
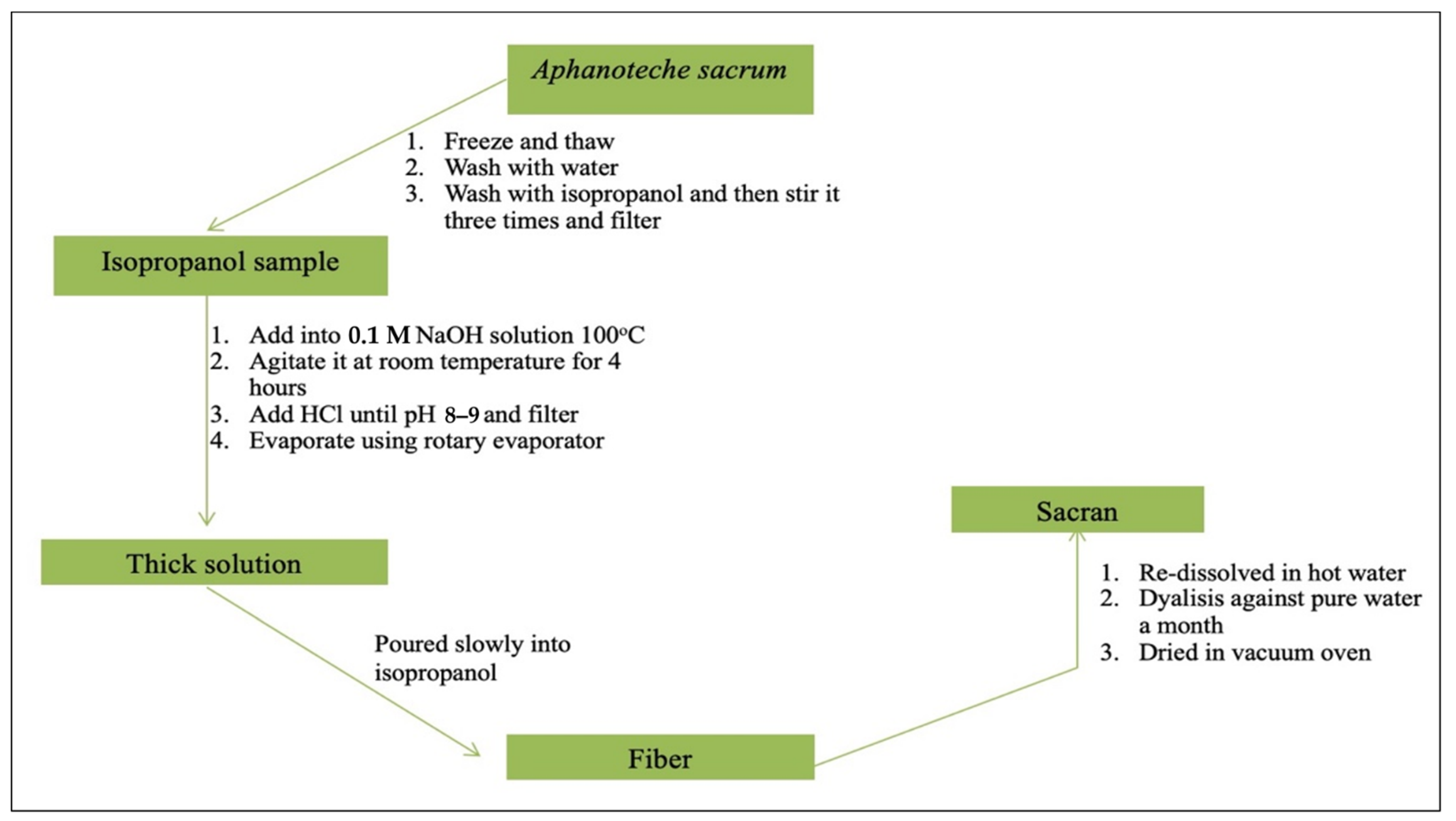
3.4. Extraction and Isolation of Sacran
3.5. Characteristics of Sacran
4. Application of Sacran as Traditional Food
5. Application of Sacran in Biomedical Fields
5.1. Cancer Treatment
5.2. Wound Dressing
5.3. Anti-Allergy
5.4. Anti-Inflammation
5.5. Other Application
6. Conclusions and Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Qi, X.; Wu, L.; Su, T.; Zhang, J.; Dong, W. Polysaccharide-based cationic hydrogels for dye adsorption. Colloids Surf. B Biointerfaces 2018, 170, 364–372. [Google Scholar] [CrossRef]
- Jayakumar, R.; Kumar, P.S.; Mohandas, A.; Lakshmanan, V.-K.; Biswas, R. Exploration of alginate hydrogel/nano zinc oxide composite bandages for infected wounds. Int. J. Nanomed. 2015, 10, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Rasib, S.Z.M.; Akil, H.M.; Khan, A.; Hamid, Z.A.A. Controlled release studies through chitosan-based hydrogel synthesized at different polymerization stages. Int. J. Biol. Macromol. 2019, 128, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.R.; Bartlett, S.L.; Weirich, C.A.; Hernandez, J. Automated subdaily sampling of cyanobacterial toxins on a buoy reveals new temporal patterns in toxin dynamics. Environ. Sci. Technol. 2019, 53, 5661–5670. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.R.; Beversdorf, L.J.; Weirich, C.A.; Bartlett, S.L. Cyanobacterial toxins of the laurentian great lakes, their toxicological effects, and numerical limits in drinking water. Mar. Drugs 2017, 15, 160. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, M.; Hess, W.R. Systems and synthetic biology for the biotechnological application of cyanobacteria. Curr. Opin. Biotechnol. 2018, 49, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.Z.; Mellor, S.B.; Vavitsas, K.; Wlodarczyk, A.; Gnanasekaran, T.; de Jesus, M.P.R.H.; King, B.; Bakowski, K.; Jensen, P.E. Extending the biosynthetic repertoires of cyanobacteria and chloroplasts. Plant J. 2016, 87, 87–102. [Google Scholar] [CrossRef]
- Ko, S.C.; Lee, H.J.; Choi, S.Y.; Choi, J.-I.; Woo, H.M. Bio-solar cell factories for photosynthetic isoprenoids production. Planta 2018, 249, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Ohki, K.; Kanesaki, Y.; Suzuki, N.; Okajima, M.; Kaneko, T.; Yoshikawa, S. Physiological properties and genetic analysis related to exopolysaccharide (EPS) production in the fresh-water unicellular cyanobacterium Aphanothece sacrum (Suizenji Nori). J. Gen. Appl. Microbiol. 2019, 65, 39–46. [Google Scholar] [CrossRef]
- Okajima-Kaneko, M.; Ono, M.; Kabata, K.; Kaneko, T. Extraction of novel sulfated polysaccharides from Aphanothece sacrum (Sur.) Okada, and its spectroscopic characterization. Pure Appl. Chem. 2007, 79, 2039–2046. [Google Scholar] [CrossRef]
- Igata, K.; Sakamaki, T.; Inutsuka, Y.; Higaki, Y.; Okajima, M.K.; Yamada, N.L.; Kaneko, T.; Takahara, A. Cationic polymer brush/giant polysaccharide sacran assembly: Structure and lubricity. Langmuir 2020, 36, 6494–6501. [Google Scholar] [CrossRef] [PubMed]
- Mantzouki, E.; Lürling, M.; Fastner, J.; Domis, L.D.S.; Wilk-Woźniak, E.; Koreivienė, J.; Seelen, L.; Teurlincx, S.; Verstijnen, Y.; Krztoń, W.; et al. Temperature effects explain continental scale distribution of cyanobacterial toxins. Toxins 2018, 10, 156. [Google Scholar] [CrossRef]
- Jaša, L.; Sadílek, J.; Kohoutek, J.; Straková, L.; Maršálek, B.; Babica, P. Application of passive sampling for sensitive time-integrative monitoring of cyanobacterial toxins microcystins in drinking water treatment plants. Water Res. 2019, 153, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Dydowiczova, A.; Ctverackova, L.; Jasa, L.; Trosko, J.E.; Blaha, L.; Babica, P. Assessment of hepatotoxic potential of cyanobacterial toxins using 3D in vitro model of adult human liver stem cells. Environ. Sci. Technol. 2018, 52, 10078–10088. [Google Scholar] [CrossRef]
- Dao, T.-S.; Vo, T.-M.-C.; Wiegand, C.; Bui, B.-T.; Dinh, K.V. Transgenerational effects of cyanobacterial toxins on a tropical micro-crustacean Daphnia lumholtzi across three generations. Environ. Pollut. 2018, 243, 791–799. [Google Scholar] [CrossRef]
- Dittmann, E.; Gugger, M.; Sivonen, K.; Fewer, D.P. Natural product biosynthetic diversity and comparative genomics of the cyanobacteria. Trends Microbiol. 2015, 23, 642–652. [Google Scholar] [CrossRef]
- Kaneko, T.; Okajima, M.; Tateyama, S. Structure and properties of sacran, one of supergiant polysaccharides, and its biomimetic functionalization. Nippon. GOMU KYOKAISHI 2014, 87, 146–152. [Google Scholar] [CrossRef]
- Gomes Ferreira, M. Study of Anti-Inflammatory Bioactivity of Cyanobacterial Strains Using Murine Macrophage RAW 264.7 Cells. Master’s Thesis, University of Porto, Porto, Portugal, 2016; pp. 265–267. [Google Scholar]
- Fujishiro, T.; Ogawa, T.; Matsuoka, M.; Nagahama, K.; Takeshima, Y.; Hagiwara, H. Establishment of a pure culture of the hitherto uncultured unicellular cyanobacterium aphanothece sacrum, and phylogenetic position of the organism. Appl. Environ. Microbiol. 2004, 70, 3338–3345. [Google Scholar] [CrossRef] [PubMed]
- Wada, K.; Kagamiyama, H.; Shin, M.; Matsubara, H. Ferredoxin from a blue-green alga, aphanothece sacrum (suringar) OKADA1. J. Biochem. 1974, 76, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Hase, T.; Wakabayashi, S.; Wada, K.; Matsubara, H. Amino acid sequence of aphanothece sacrum ferredoxin II (minor component). J. Biochem. 1978, 83, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Sugawa, H.; Shirakawa, J.-I.; Ohno, R.-I.; Kinoshita, S.; Ichimaru, K.; Arakawa, S.; Nagai, M.; Kabata, K.; Nagai, R. Aphanothece sacrum (Sur.) okada prevents cataractogenesis in type 1 diabetic mice. J. Nutr. Sci. Vitaminol. 2017, 63, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Wathoni, N.; Motoyama, K.; Higashi, T.; Okajima, M.; Kaneko, T.; Arima, H. Physically crosslinked-sacran hydrogel films for wound dressing application. Int. J. Biol. Macromol. 2016, 89, 465–470. [Google Scholar] [CrossRef]
- Okajima, M.K.; Miyazato, S.; Kaneko, T. Cyanobacterial megamolecule sacran efficiently forms LC gels with very heavy metal ions. Langmuir 2009, 25, 8526–8531. [Google Scholar] [CrossRef] [PubMed]
- Alcântara, A.C.S.; Darder, M.; Aranda, P.; Tateyama, S.; Okajima, M.K.; Kaneko, T.; Ogawa, M.; Ruiz-Hitzky, E. Clay-bionanocomposites with sacran megamolecules for the selective uptake of neodymium. J. Mater. Chem. A 2014, 2, 1391–1399. [Google Scholar] [CrossRef]
- Shikinaka, K.; Okeyoshi, K.; Masunaga, H.; Okajima, M.K.; Kaneko, T. Solution structure of cyanobacterial polysaccharide, sacran. Polymer 2016, 99, 767–770. [Google Scholar] [CrossRef]
- Doi, M.; Sagawa, Y.; Momose, S.; Tanaka, T.; Mizutani, T.; Okano, Y.; Masaki, H. Topical treatment with sacran, a sulfated polysaccharide from Aphanothece sacrum, improves corneocyte-derived parameters. J. Dermatol. 2017, 44, 1360–1367. [Google Scholar] [CrossRef]
- Yusof, F.A.A.; Yamaki, M.; Kawai, M.; Okajima, M.K.; Kaneko, T.; Mitsumata, T. Rheopectic behavior for aqueous solutions of megamolecular polysaccharide sacran. Biomolecules 2020, 10, 155. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Takada, A.; Ma, W.; Fujikawa, S.; Ariyoshi, M.; Igata, K.; Okajima, M.K.; Kaneko, T.; Takahara, A. Structure and properties of hybrid film fabricated by spin-assisted layer-by-layer assembly of sacran and imogolite nanotubes. Langmuir 2020, 36, 1718–1726. [Google Scholar] [CrossRef]
- Amornwachirabodee, K.; Okajima, M.K.; Kaneko, T. Uniaxial swelling in LC hydrogels formed by two-step cross-linking. Macromolecules 2015, 48, 8615–8621. [Google Scholar] [CrossRef]
- Okeyoshi, K.; Okajima, M.K.; Kaneko, T. Milliscale self-integration of megamolecule biopolymers on a drying gas–aqueous liquid crystalline interface. Biomacromolecules 2016, 17, 2096–2103. [Google Scholar] [CrossRef]
- Doi, M.; Sagawa, Y.; Mizutani, T.; Okano, Y.; Momose, S.; Tanaka, T.; Masaki, H. Possibilities of sacran-polyol complexes in skin care. J. Soc. Cosmet. Chem. Jpn. 2017, 51, 117–125. [Google Scholar] [CrossRef]
- Motoyama, K.; Tanida, Y.; Hata, K.; Hayashi, T.; Abu Hashim, I.I.; Higashi, T.; Ishitsuka, Y.; Kondo, Y.; Irie, T.; Kaneko, S.; et al. Anti-inflammatory effects of novel polysaccharide sacran extracted from cyanobacterium aphanothece sacrum in various inflammatory animal models. Biol. Pharm. Bull. 2016, 39, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, K.; Tanida, Y.; Hata, K.; Hayashi, T.; Higashi, T.; Ishitsuka, Y.; Kondo, Y.; Irie, T.; Kaneko, S.; Arima, H. Potential use of a megamolecular polysaccharide sacran as a hydrogel-based sustained release system. Chem. Pharm. Bull. 2014, 62, 636–641. [Google Scholar] [CrossRef]
- Okajima, M.K.; Le Nguyen, Q.T.; Nakamura, M.; Ogawa, T.; Kurata, H.; Kaneko, T. Double-metal complexation of heterogels containing cyanobacterial polysaccharides. J. Appl. Polym. Sci. 2012, 128, 676–683. [Google Scholar] [CrossRef]
- Okeyoshi, K.; Okajima, M.K.; Kaneko, T. Unidirectionally-oriented Membrane formation of supra-polysaccharides sacran and application to drug delivery system. Yakugaku Zasshi 2018, 138, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Sornkamnerd, S.; Okajima, M.K.; Matsumura, K.; Kaneko, T. Micropatterned cell orientation of cyanobacterial liquid-crystalline hydrogels. ACS Appl. Mater. Interfaces 2018, 10, 44834–44843. [Google Scholar] [CrossRef] [PubMed]
- Okajima, M.K.; Kumar, A.; Fujiwara, A.; Mitsumata, T.; Kaneko, D.; Ogawa, T.; Kurata, H.; Isoda, S.; Kaneko, T. Anionic complexes of MWCNT with supergiant cyanobacterial polyanions. Biopolymers 2013, 99, 1–9. [Google Scholar] [CrossRef]
- Wathoni, N.; Hasanah, A.N.; Mohammed, A.F.A.; Pratiwi, E.D.; Mahmudah, R. Accelerated wound healing ability of sacran hydrogel film by keratinocyte growth factor in alloxan-induced diabetic mice. Int. J. Appl. Pharm. 2018, 10, 57–61. [Google Scholar] [CrossRef][Green Version]
- Okajima, M.K.; Sornkamnerd, S.; Kaneko, T. Development of functional bionanocomposites using cyanobacterial polysaccharides. Chem. Rec. 2018, 18, 1167–1177. [Google Scholar] [CrossRef]
- Mitsumata, T.; Miura, T.; Takahashi, N.; Kawai, M.; Okajima, M.K.; Kaneko, T. Ionic state and chain conformation for aqueous solutions of supergiant cyanobacterial polysaccharide. Phys. Rev. E 2013, 87, 042607. [Google Scholar] [CrossRef]
- Okajima, M.K.; Nakamura, M.; Ogawa, T.; Kurata, H.; Mitsumata, T.; Kaneko, T. Spongy hydrogels of cyanobacterial polyanions mediate energy-saving electrolytic metal-refinement. Ind. Eng. Chem. Res. 2012, 51, 8704–8707. [Google Scholar] [CrossRef]
- Zhao, Y.; Hien, K.T.T.; Mizutani, G.; Rutt, H.N.; Amornwachirabodee, K.; Okajima, M.; Kaneko, T. Optical second-harmonic images of sacran megamolecule aggregates. J. Opt. Soc. Am. A 2017, 34, 146–152. [Google Scholar] [CrossRef]
- Ngatu, N.R.; Okajima, M.K.; Yokogawa, M.; Hirota, R.; Eitoku, M.; Muzembo, B.A.; Dumavibhat, N.; Takaishi, M.; Sano, S.; Kaneko, T.; et al. Anti-inflammatory effects of sacran, a novel polysaccharide from Aphanothece sacrum, on 2,4,6-trinitrochlorobenzene–induced allergic dermatitis in vivo. Ann. Allergy Asthma Immunol. 2012, 108, 117–122.e2. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Okajima, M.K. Super liquid crystalline polysaccharides produced by ultimately-ecological microreactors. Yakugaku Zasshi 2018, 138, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Wathoni, N. Design and Evaluation of Sacran/Cyclodextrin Hydrogel Films for Wound Dressing Materials Graduate School of Pharmaceutical Sciences Department of Physical Pharmaceutics Nasrul Wathoni. Ph.D. Thesis, Kumamoto University, Kumamoto, Japan, 2017. [Google Scholar]
- Budpud, K.; Okeyoshi, K.; Okajima, M.K.; Kaneko, T. Vapor-sensitive materials from polysaccharide fibers with self-assembling twisted microstructures. Small 2020, 16, e2001993. [Google Scholar] [CrossRef] [PubMed]
- Okajima, M.K.; Kaneko, D.; Mitsumata, T.; Kaneko, T.; Watanabe, J. Cyanobacteria that produce megamolecules with efficient self-orientations. Macromolecules 2009, 42, 3057–3062. [Google Scholar] [CrossRef]
- Okajima, M.K.; Le Nguyen, Q.T.; Tateyama, S.; Masuyama, H.; Tanaka, T.; Mitsumata, T.; Kaneko, T. Photoshrinkage in polysaccharide gels with trivalent metal ions. Biomacromolecules 2012, 13, 4158–4163. [Google Scholar] [CrossRef]
- Mitsumata, T. Negative thixotropic behavior for sacran aqueous solutions. Yakugaku Zasshi 2018, 138, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, Y.; Hien, K.T.T.; Mizutani, G.; Ito, N.; Rutt, H.N.; Okajima, M.; Kaneko, T. Electric field effect on optical second-harmonic generation of amphoteric megamolecule aggregates. J. Phys. Soc. Jpn. 2017, 86. [Google Scholar] [CrossRef]
- Okajima, M.K.; Higashi, T.; Asakawa, R.; Mitsumata, T.; Kaneko, D.; Kaneko, T.; Ogawa, T.; Kurata, H.; Isoda, S. Gelation behavior by the lanthanoid adsorption of the cyanobacterial extracellular polysaccharide. Biomacromolecules 2010, 11, 3172–3177. [Google Scholar] [CrossRef] [PubMed]
- Ogura, F.; Hayashi, K.; Lee, J.-B.; Kanekiyo, K.; Hayashi, T. Evaluation of an edible blue-green alga,aphanothece sacrum, for its inhibitory effect on replication of herpes simplex virus type 2 and influenza virus type A. Biosci. Biotechnol. Biochem. 2010, 74, 1687–1690. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Azuma, K.; Arima, H.; Kaneko, S.; Higashi, T.; Motoyama, K.; Michihara, A.; Shimizu, T.; Kadowaki, D.; Maruyama, T.; et al. Sacran, a sulfated polysaccharide, suppresses the absorption of lipids and modulates the intestinal flora in non-alcoholic steatohepatitis model rats. Life Sci. 2021, 268, 118991. [Google Scholar] [CrossRef] [PubMed]
- Arima, H.; Motoyama, K.; Higashi, T. Potential use of sacran for dermal and oral preparations. Yakugaku Zasshi 2019, 139, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Higashi, T.; Motoyama, K.; Jono, H.; Ando, Y.; Arima, H. In vitro and in vivo siRNA delivery to hepatocyte utilizing ternary complexation of lactosylated dendrimer/cyclodextrin conjugates, siRNA and low-molecular-weight sacran. Int. J. Biol. Macromol. 2018, 107, 1113–1121. [Google Scholar] [CrossRef]
- Ohyama, A.; Higashi, T.; Motoyama, K.; Arima, H. Ternary complexes of folate-PEG-appended dendrimer (G4)/α-cyclodextrin conjugate, siRNA and low-molecular-weight polysaccharide sacran as a novel tumor-selective siRNA delivery system. Int. J. Biol. Macromol. 2017, 99, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Wathoni, N.; Motoyama, K.; Higashi, T.; Okajima, M.; Kaneko, T.; Arima, H. Enhancing effect of γ-cyclodextrin on wound dressing properties of sacran hydrogel film. Int. J. Biol. Macromol. 2017, 94, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, K.; Higashi, T.; Okajima, M.K.; Kaneko, T.; Arima, H. Potential use of sacran hydrogels as wound dressing material. Yakugaku Zasshi 2018, 138, 517–520. [Google Scholar] [CrossRef]
- Wathoni, N.; Motoyama, K.; Higashi, T.; Okajima, M.; Kaneko, T.; Arima, H. Enhancement of curcumin wound healing ability by complexation with 2-hydroxypropyl-γ-cyclodextrin in sacran hydrogel film. Int. J. Biol. Macromol. 2017, 98, 268–276. [Google Scholar] [CrossRef]
- Wathoni, N.; Rusdiana, T.; Hasanah, A.N.; Muhtadi, A.; Pratiwi, E.D.; Mahmudah, R.; Mohammed, A.F.A.; Okajima, M.; Kaneko, T.; Arima, H. Sacran hydrogel film containing keratinocyte growth factor accelerates wound healing by stimulating fibroblast migration and re-epithelization. Chem. Pharm. Bull. 2019, 67, 849–854. [Google Scholar] [CrossRef]
- Goto, M.; Ifuku, S.; Azuma, K.; Arima, H.; Kaneko, S.; Iohara, D.; Hirayama, F.; Anraku, M. Preparation and evaluation of freeze dried surface-deacetylated chitin nanofiber/sacran pellets for use as an extended-release excipient. Int. J. Biol. Macromol. 2019, 124, 888–894. [Google Scholar] [CrossRef]
- Motoyama, K.; Tanida, Y.; Sakai, A.; Higashi, T.; Kaneko, S.; Arima, H. Anti-allergic effects of novel sulfated polysaccharide sacran on mouse model of 2,4-Dinitro-1-fluorobenzene-induced atopic dermatitis. Int. J. Biol. Macromol. 2018, 108, 112–118. [Google Scholar] [CrossRef]
- Ngatu, N.R.; Motoyama, K.; Nishimura, Y.; Okajima, M.K.; Hirota, R.; Higashi, T.; Lee, S.; Arima, H.; Ikeda, M.; Nojima, S.; et al. Anti-allergic and Profilaggrin (ProFLG)-mRNA expression modulatory effects of sacran. Int. J. Biol. Macromol. 2017, 105, 1532–1538. [Google Scholar] [CrossRef] [PubMed]
- Nlandu, N.R. Anti-allergic effects and immunomodulatory activity of sacran, a bioactive compound from river alga aphanothece sacrum. Evid.-Based Med. Public Health 2018, 2, 1–10. [Google Scholar] [CrossRef]
- Motoyama, K.; Higashi, T.; Ngatu, N.R.; Okajima, M.K.; Nishimura, Y.; Arima, H.; Kaneko, T. Sacran: Novel sulfated polysaccharide as anti-allergic skincare biomaterial for atopic dermatitis. In Occupational and Environmental Skin Disorders; Springer Science and Business Media LLC: Berlin, Germany, 2018; pp. 79–92. [Google Scholar]
- Fukushima, S.; Motoyama, K.; Tanida, Y.; Higashi, T.; Ishitsuka, Y.; Kondo, Y.; Irie, T.; Tanaka, T.; Ihn, H.; Arima, H. Clinical evaluation of novel natural polysaccharides sacran as a skincare material for atopic dermatitis patients. J. Cosmet. Dermatol. Sci. Appl. 2016, 6, 9–18. [Google Scholar] [CrossRef][Green Version]
- Arima, H.; Motoyama, K.; Higashi, T.; Fukushima, S.; Ihn, H. Anti-inflammatory effect of sacran on atopic dermatitis. Yakugaku Zasshi 2018, 138, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Doi, M.; Sagawa, Y.; Sasano, K.; Tanaka, T.; Mizutani, T.; Okano, Y.; Masaki, H.; Co, L.D.K.K. Protective effects of sacran, a natural polysaccharide, against adverse effects on the skin induced by tobacco smoke. J. Cosmet. Sci. 2019, 70, 17–31. [Google Scholar]
- Doi, M.; Sagawa, Y.; Tanaka, T.; Mizutani, T.; Okano, Y.; Masaki, H. Defensive effects of a unique polysaccharide, sacran, to protect keratinocytes against extracellular stimuli and its possible mechanism of action. Biol. Pharm. Bull. 2018, 41, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Masaki, H.; Doi, M. Function of sacran as an artificial skin barrier and the development of skincare products. Yakugaku Zasshi 2019, 139, 371–379. [Google Scholar] [CrossRef]
- Sornkamnerd, S.; Okajima, M.K.; Matsumura, K.; Kaneko, T. Surface-selective control of cell orientation on cyanobacterial liquid crystalline gels. ACS Omega 2018, 3, 6554–6559. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Izakovic, M.; Mazur, M.; Rhodes, C.J.; Telser, J. Role of oxygen radicals in DNA damage and cancer incidence. Mol. Cell. Biochem. 2004, 266, 37–56. [Google Scholar] [CrossRef]
- Saad, M.; Garbuzenko, O.B.; Minko, T. Co-delivery of siRNA and an anticancer drug for treatment of multidrug-resistant cancer. Nanomedicine 2008, 3, 761–776. [Google Scholar] [CrossRef]
- Deng, Z.J.; Morton, S.W.; Ben-Akiva, E.; Dreaden, E.C.; Shopsowitz, K.E.; Hammond, P.T. Layer-by-layer nanoparticles for systemic codelivery of an anticancer drug and siRNA for potential triple-negative breast cancer treatment. ACS Nano 2013, 7, 9571–9584. [Google Scholar] [CrossRef] [PubMed]
- Taratula, O.; Garbuzenko, O.B.; Chen, A.M.; Minko, T. Innovative strategy for treatment of lung cancer: Targeted nanotechnology-based inhalation co-delivery of anticancer drugs and siRNA. J. Drug Target. 2011, 19, 900–914. [Google Scholar] [CrossRef]
- Le, N.T.T.; Nguyen, T.N.Q.; Cao, V.D.; Hoang, D.T.; Ngo, V.C.; Hoang Thi, T.T. Recent progress and advances of multi-stimuli-responsive dendrimers in drug delivery for cancer treatment. Pharmaceutics 2019, 11, 591. [Google Scholar] [CrossRef]
- Wang, H.; Huang, Q.; Chang, H.; Xiao, J.; Cheng, Y. Stimuli-responsive dendrimers in drug delivery. Biomater. Sci. 2016, 4, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Okajima, M.K.; Nakamura, M.; Mitsumata, T.; Kaneko, T. Cyanobacterial polysaccharide gels with efficient rare-earth-metal sorption. Biomacromolecules 2010, 11, 1773–1778. [Google Scholar] [CrossRef]
- Talekar, Y.P.; Apte, K.G.; Paygude, S.V.; Tondare, P.R.; Parab, P.B. Studies on wound healing potential of polyherbal formulation using in vitro and in vivo assays. J. Ayurveda Integr. Med. 2017, 8, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Balqis, U.; Darmawi, C.D.I.; Salim, M.N. Angiogenesis activity of Jatropha curcas L. latex in cream formulation on wound healing in mice. Veter- World 2018, 11, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Sun, L.; Li, P.; Zhu, C.; Lin, Z. The wound dressings and their applications in wound healing and management. Health Sci. J. 2019, 13, 1–8. Available online: http://www.imedpub.com/ (accessed on 15 December 2020).
- Qu, J.; Zhao, X.; Liang, Y.; Zhang, T.; Ma, P.X.; Guo, B. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 2018, 183, 185–199. [Google Scholar] [CrossRef]
- Ito, W.; Yamaki, M.; Kawai, M.; Mitsumata, T.; Okajima, M.K.; Kaneko, T. Extraordinary swelling of hydrogels physically crosslinked by megamolecular chain sacran. Chem. Lett. 2016, 45, 339–340. [Google Scholar] [CrossRef]
- Okajima, M.K.; Bamba, T.; Kaneso, Y.; Hirata, K.; Fukusaki, E.; Kajiyama, S.; Kaneko, T. Supergiant ampholytic sugar chains with imbalanced charge ratio form saline ultra-absorbent hydrogels. Macromolecules 2008, 41, 4061–4064. [Google Scholar] [CrossRef]
- Sornkamnerd, S.; Okajima, M.K.; Kaneko, T. Tough and porous hydrogels prepared by simple lyophilization of LC gels. ACS Omega 2017, 2, 5304–5314. [Google Scholar] [CrossRef] [PubMed]
- Okajima-Kaneko, M.; Miyazato, S.; Kaneko, T. Chemically cross-linking effects on the sorption of heavy metal ions to hydrogels of cyanobacterial megamolecules, sacran. Trans. Mater. Res. Soc. Jpn. 2009, 34, 359–362. [Google Scholar] [CrossRef][Green Version]
- Yan, B.; Jiang, Z.; Yuan, J.; Li, M.; Zeng, J.; Tang, J.; Lu, Z.K.; Ding, H.; Xia, J.; Wang, Q.; et al. Effects and safety of herbal medicines among community-dwelling residents during COVID-19 pandemic: A large prospective, randomized controlled trial (RCT). Phytomedicine 2021, 85, 153403. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, L.; An, T.; Xian, M.; Luckanagul, J.A.; Su, Z.; Lin, Y.; Wang, Q. A hydrogen sulfide-releasing alginate dressing for effective wound healing. Acta Biomater. 2020, 104, 85–94. [Google Scholar] [CrossRef]
- Wathoni, N.; Rusdiana, T.; Hasanah, A.N.; Pratama, A.R.; Okajima, M.; Kaneko, T.; Mohammed, A.F.A.; Putera, B.W.; Arima, H. Epidermal growth factor in sacran hydrogel film accelerates fibroblast migration. J. Adv. Pharm. Technol. Res. 2020, 11, 74. [Google Scholar] [CrossRef]
- Li, H.; Cheng, F.; Wei, X.; Yi, X.; Tang, S.; Wang, Z.; Zhang, Y.S.; He, J.; Huang, Y. Injectable, self-healing, antibacterial, and hemostatic N,O-carboxymethyl chitosan/oxidized chondroitin sulfate composite hydrogel for wound dressing. Mater. Sci. Eng. C 2021, 118, 111324. [Google Scholar] [CrossRef]
- Ohashi-Doi, K.; Kito, H.; Du, W.; Nakazawa, H.; Ipsen, H.; Gudmann, P.; Lund, K. Bioavailability of house dust mite allergens in sublingual allergy tablets is highly dependent on the formulation. Int. Arch. Allergy Immunol. 2017, 174, 26–34. [Google Scholar] [CrossRef]
- Panaszek, B.; Liebhart, J. Sources of actions and efficacy of antiallergic drugs. Pharmacol. Rep. 2007, 59 (Suppl. 1), 111–122. [Google Scholar]
- Eichenfield, L.F.; Tom, W.L.; Berger, T.G.; Krol, A.; Paller, A.S.; Schwarzenberger, K.; Bergman, J.N.; Chamlin, S.L.; Cohen, D.E.; Cooper, K.D.; et al. Guidelines of care for the management of atopic dermatitis. J. Am. Acad. Dermatol. 2014, 71, 116–132. [Google Scholar] [CrossRef] [PubMed]
- Kabashima, K.; Furue, M.; Hanifin, J.M.; Pulka, G.; Wollenberg, A.; Galus, R.; Etoh, T.; Mihara, R.; Nakano, M.; Ruzicka, T. Nemolizumab in patients with moderate-to-severe atopic dermatitis: Randomized, phase II, long-term extension study. J. Allergy Clin. Immunol. 2018, 142, 1121–1130. [Google Scholar] [CrossRef]
- Megna, M.; Italian Adult Atopic Dermatitis Study Group; Patruno, C.; Balato, A.; Rongioletti, F.; Stingeni, L.; Balato, N. An Italian multicentre study on adult atopic dermatitis: Persistent versus adult-onset disease. Arch. Dermatol. Res. 2017, 309, 443–452. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef]
- Heratizadeh, A.; Werfel, T. Anti-inflammatory therapies in atopic dermatitis. Allergy 2016, 71, 1666–1675. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Yeo, I.J.; Han, J.H.; Suh, J.W.; Lee, H.P.; Hong, J.T. Anti-inflammatory effect of astaxanthin in phthalic anhydride-induced atopic dermatitis animal model. Exp. Dermatol. 2018, 27, 378–385. [Google Scholar] [CrossRef]
- Tsang, M.S.M.; Jiao, D.; Chan, B.C.L.; Hon, K.-L.; Leung, P.C.; Lau, C.B.S.; Wong, E.C.W.; Cheng, L.; Chan, C.K.M.; Lam, C.W.K.; et al. Anti-inflammatory activities of pentaherbs formula, berberine, gallic acid and chlorogenic acid in atopic dermatitis-like skin inflammation. Molecules 2016, 21, 519. [Google Scholar] [CrossRef]
- Wang, S.; Li, Q.; Wang, B.; Hou, Y.; Zhang, T. Recognition of different rough surface based highly sensitive silver nanowire-graphene flexible hydrogel skin. Ind. Eng. Chem. Res. 2019, 58, 21553–21561. [Google Scholar] [CrossRef]
- Higashi, T.; Motoyama, K.; Arima, H. Cyclodextrin-based drug carriers for low molecular weight drugs, proteins, and nucleic acids. In Methods in Molecular Biology; J.B. Metzler: Stuttgart, Germany, 2016; pp. 27–45. [Google Scholar]
- Chun, J.M.; Lee, A.Y.; Nam, J.Y.; Lee, M.Y.; Choe, M.S.; Lim, K.S.; Kim, C.; Kim, J.-S. Protective effects of Phlomis umbrosa extract on a monosodium iodoacetate–induced osteoarthritis model and prediction of molecular mechanisms using transcriptomics. Phytomedicine 2021, 81, 153429. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Iohara, D.; Kaneko, S.; Higashi, T.; Motoyama, K.; Arima, H.; Maruyama, T.; Uekama, K.; Hirayama, F.; Anraku, M.; et al. Sacran, a high-molecular weight polysaccharide inhibits renal injury and oxidative stress in chronic renal failure model rats. J. Nutr. Biol. 2018, 4, 267–275. [Google Scholar] [CrossRef][Green Version]

| Bioactivity | In Vivo Test | Ref. |
|---|---|---|
| Reduce body weight | Tested on male mice (160–180 g) for 8 weeks | [54] |
| Reduce oxidative stress | Tested on male mice (160–180 g) for 8 weeks | [54] |
| Painkiller and reduce gastric ulcers | Tested on mice induced with HCl/EtOH for gastric ulcer | [55] |
| Application in Biomedical Fields | Testing Object | Test Type | Ref. |
|---|---|---|---|
| Cancer Delivery | Polyamidoamine conjugates alpha-cyclodextrin and phosphate-polyethylene glycol with low-molecular-weight sacran for selective siRNA delivery | In Vitro and In Vivo | [56,57] |
| Wound Dress | Sacran hydrogel | In Vitro and In Vivo | [23,58,59] |
| -Cyclodextrin addition to sacran hydrogel film | In Vitro and In Vivo | [58] | |
| Curcumin addition to 2-hydroxypropil--cyclodextrin on hydrogel film | In Vitro and In Vivo | [60] | |
| Sacran hydrogel film with keratinocyte growth factor | In Vitro | [39] | |
| Epidermal growth factor (EGF) in sacran hydrogel film as the increased fibroblast migration | In Vitro | [61] | |
| HP-βCD complex in freeze dried Sac/SDACNF | In Vivo | [62] | |
| Anti-allergy | Topical sacran in mouse model induced by 2,4-dinitro-1-fluorobenzene | In Vivo | [63] |
| Topical sacran in mouse model induced by DME- | In Vivo | [64,65] | |
| Topical sacran for atopic dermatitis | In Vivo | [66] | |
| Anti-inflammation | Sacran for atopic dermatitis in mouse induced by 2,4-dinitro-1-fluorobenzene | In Vivo | [67,68] |
| Sacran for atopic dermatitis in mouse induced by 2,4-dinitro-6-fluorobenzene | In Vivo | [44] | |
| Sacran and carrageenan induced in TPA-induced mouse ear | In Vivo | [33] | |
| Others | Effectiveness of sacran on air pollution | In Vitro | [69] |
| Effectiveness of sacran to prevent skin evaporation | In Vitro | [70] | |
| Improve the maturation of corneocytes | Clinical study | [27,71] | |
| Genetic engineering | In Vitro | [37,72] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puluhulawa, L.E.; Joni, I.M.; Mohammed, A.F.A.; Arima, H.; Wathoni, N. The Use of Megamolecular Polysaccharide Sacran in Food and Biomedical Applications. Molecules 2021, 26, 3362. https://doi.org/10.3390/molecules26113362
Puluhulawa LE, Joni IM, Mohammed AFA, Arima H, Wathoni N. The Use of Megamolecular Polysaccharide Sacran in Food and Biomedical Applications. Molecules. 2021; 26(11):3362. https://doi.org/10.3390/molecules26113362
Chicago/Turabian StylePuluhulawa, Lisa Efriani, I Made Joni, Ahmed Fouad Abdelwahab Mohammed, Hidetoshi Arima, and Nasrul Wathoni. 2021. "The Use of Megamolecular Polysaccharide Sacran in Food and Biomedical Applications" Molecules 26, no. 11: 3362. https://doi.org/10.3390/molecules26113362
APA StylePuluhulawa, L. E., Joni, I. M., Mohammed, A. F. A., Arima, H., & Wathoni, N. (2021). The Use of Megamolecular Polysaccharide Sacran in Food and Biomedical Applications. Molecules, 26(11), 3362. https://doi.org/10.3390/molecules26113362







