In Search of Factors Determining Activity of Co3O4 Nanoparticles Dispersed in Partially Exfoliated Montmorillonite Structure
Abstract
1. Introduction
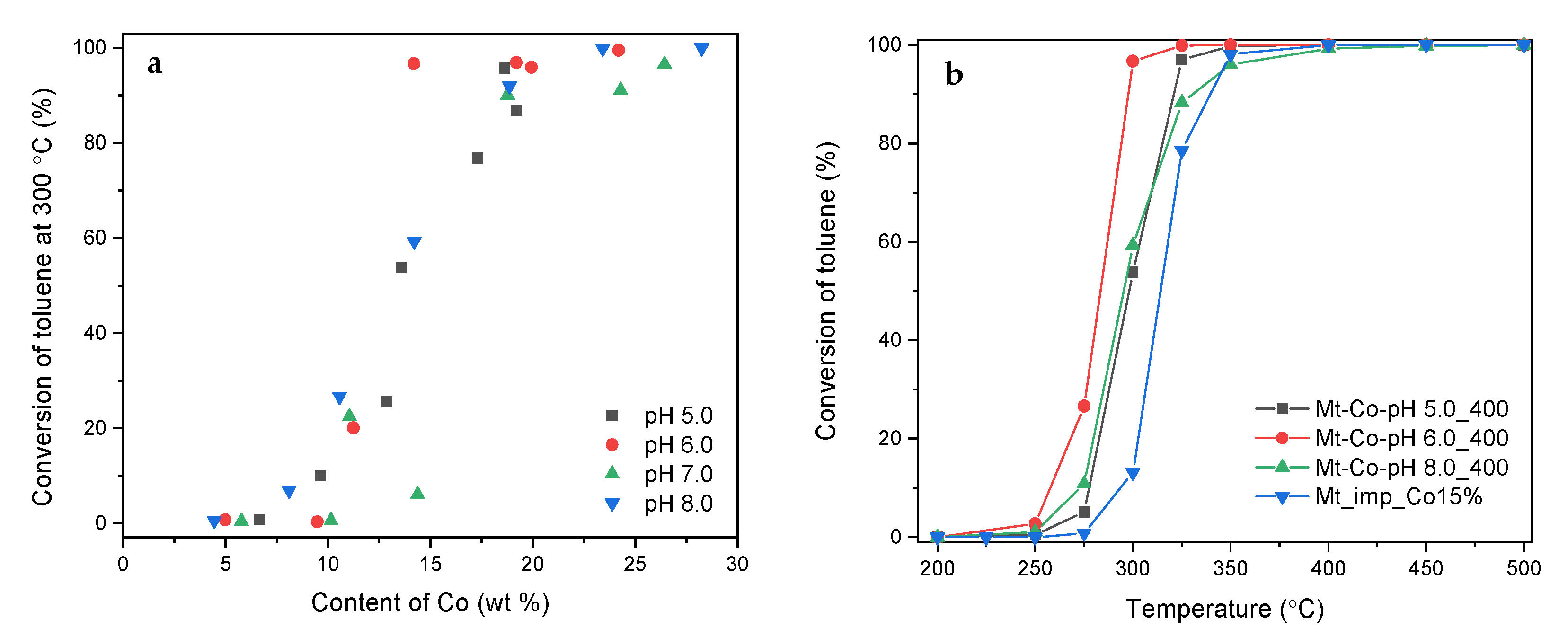
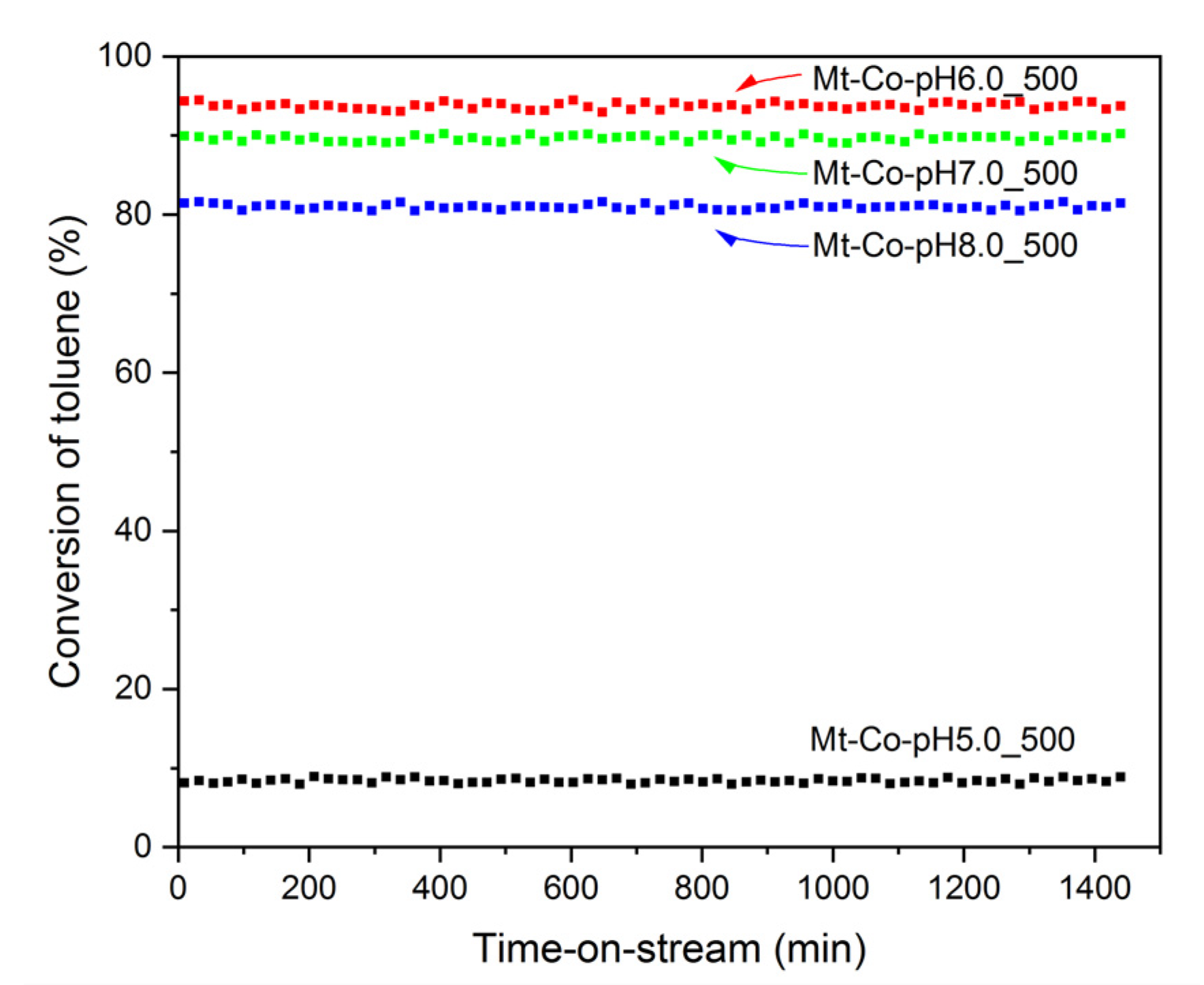
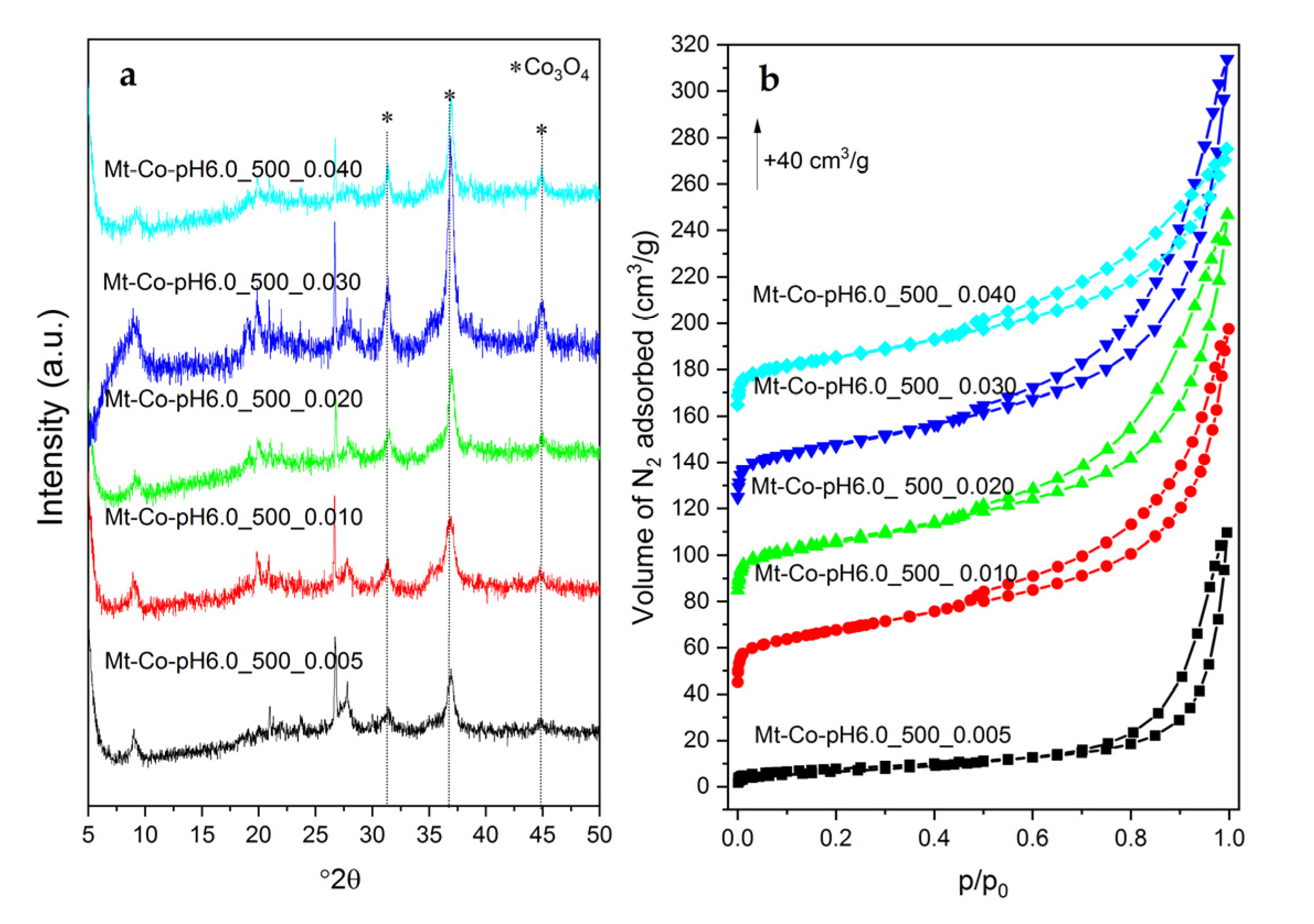
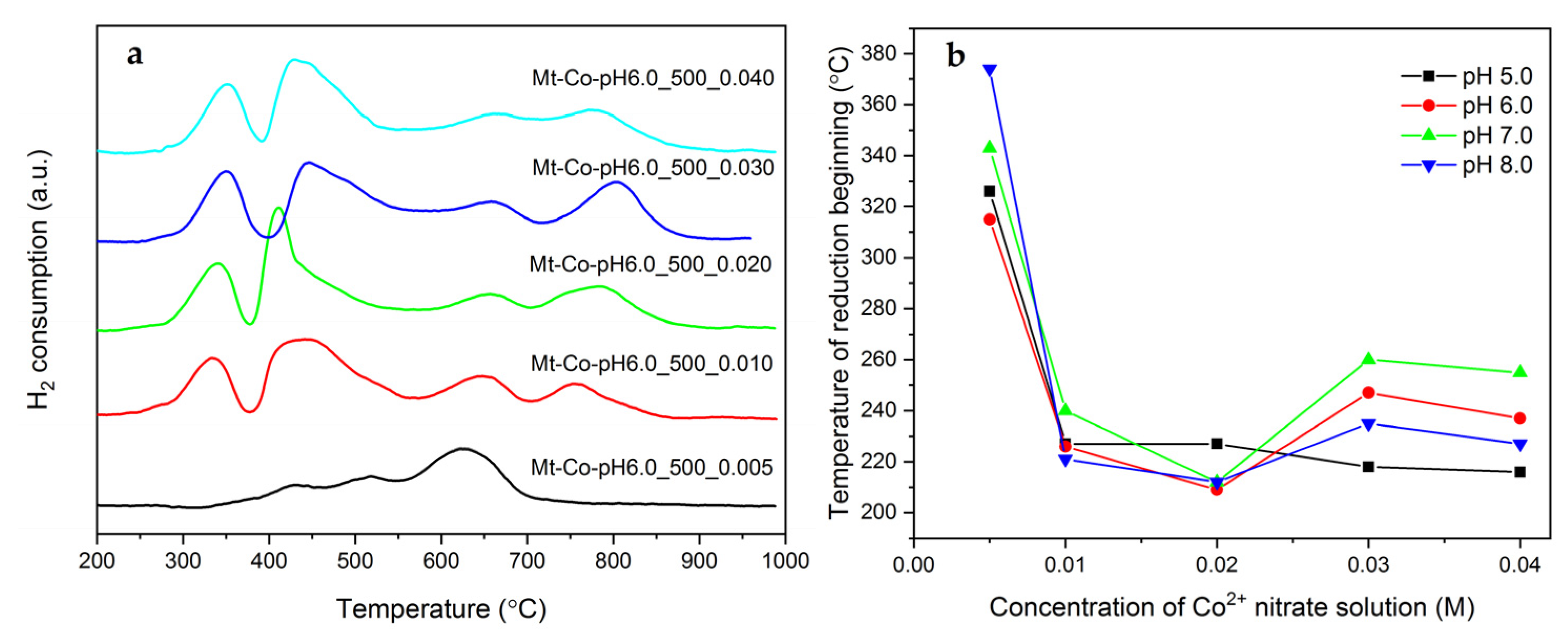
2. Results and Discussion
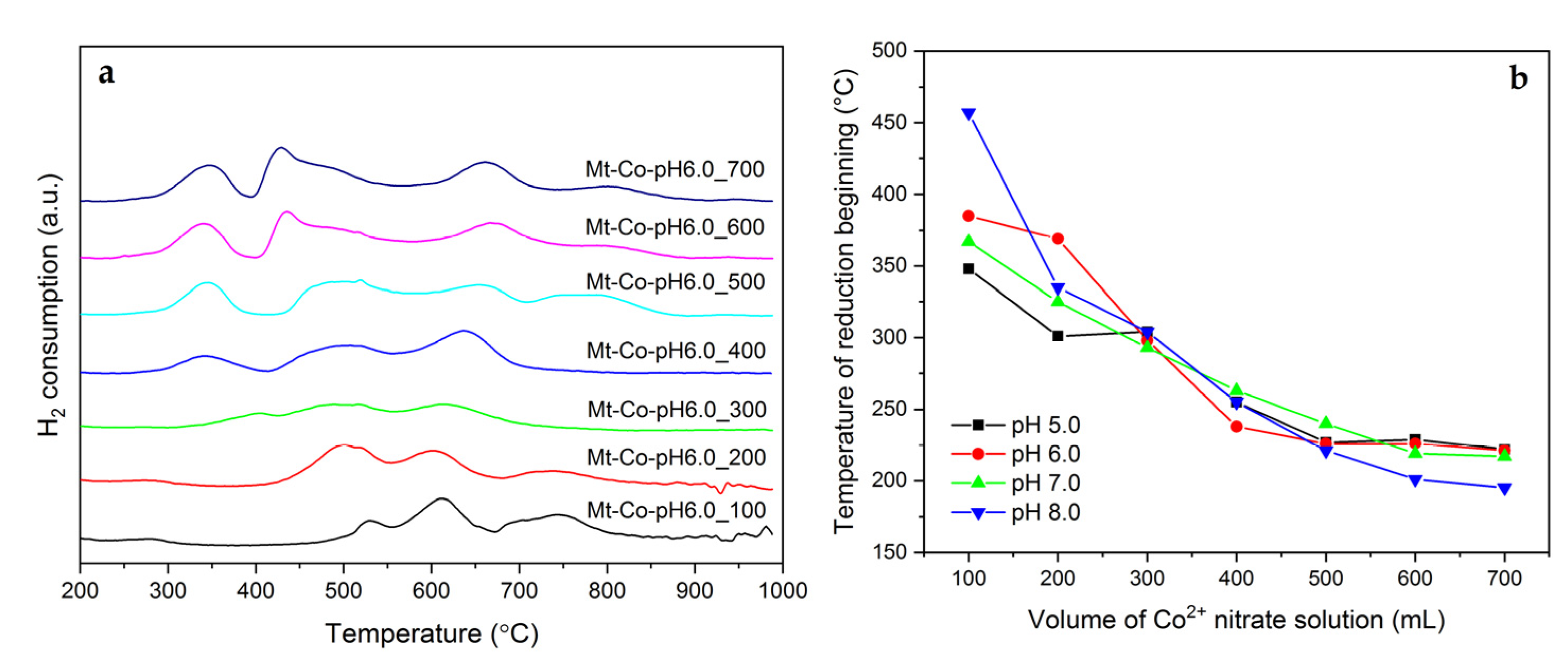
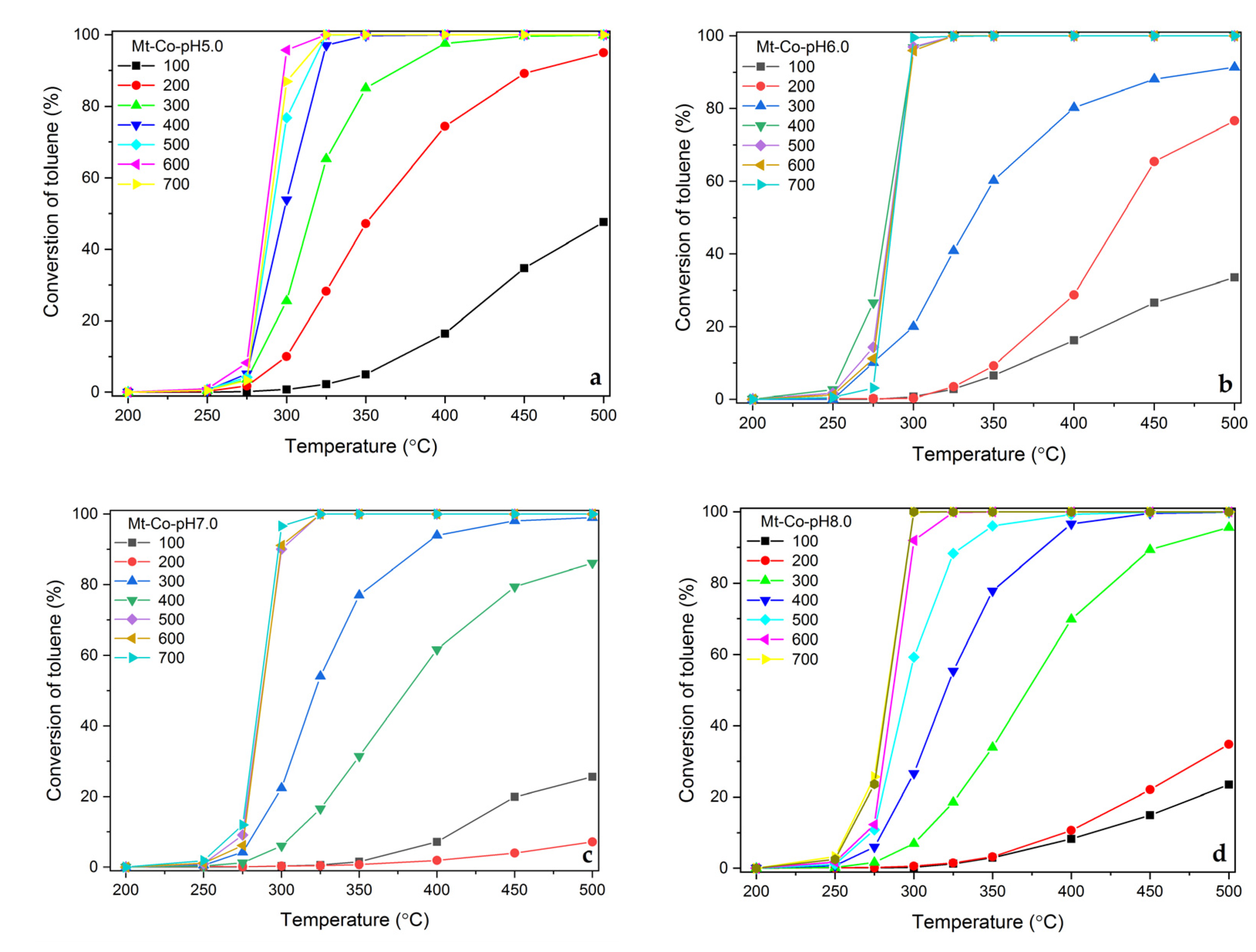
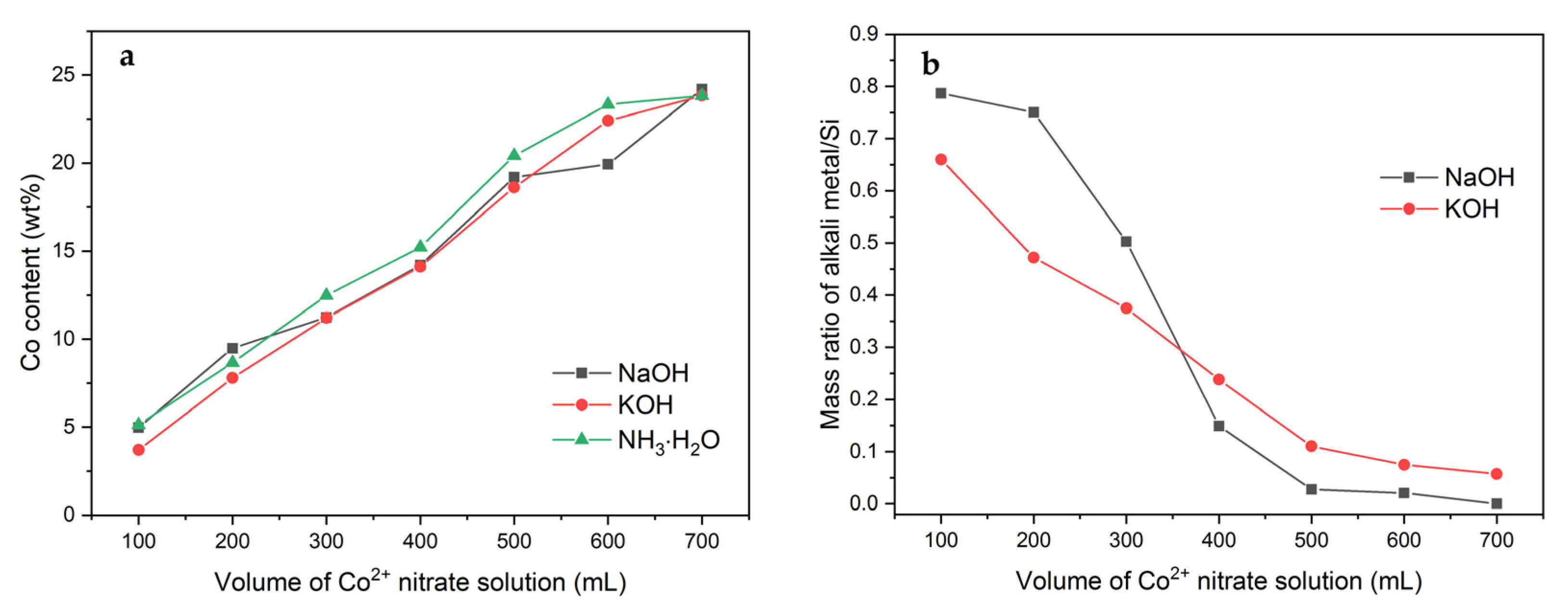
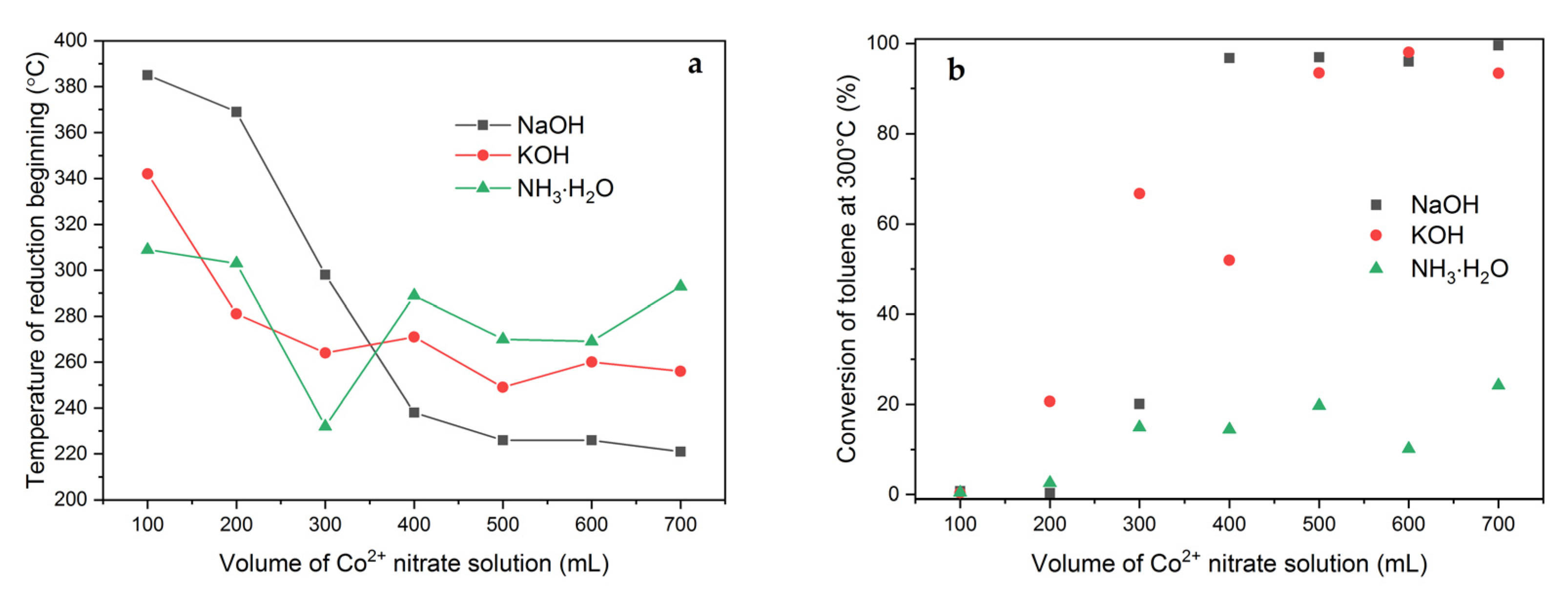
2.1. Effect of Volume of Co2+ Solution
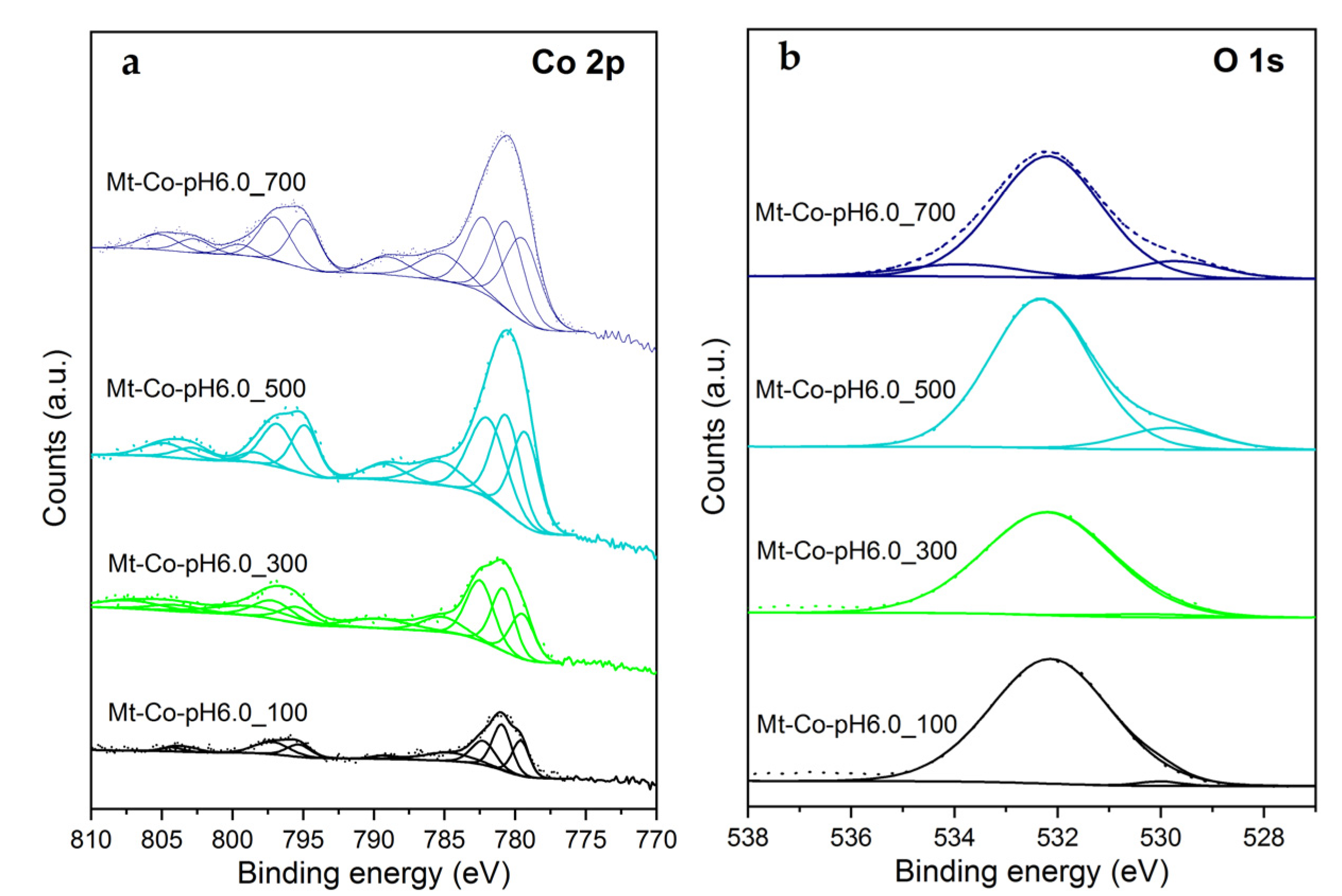
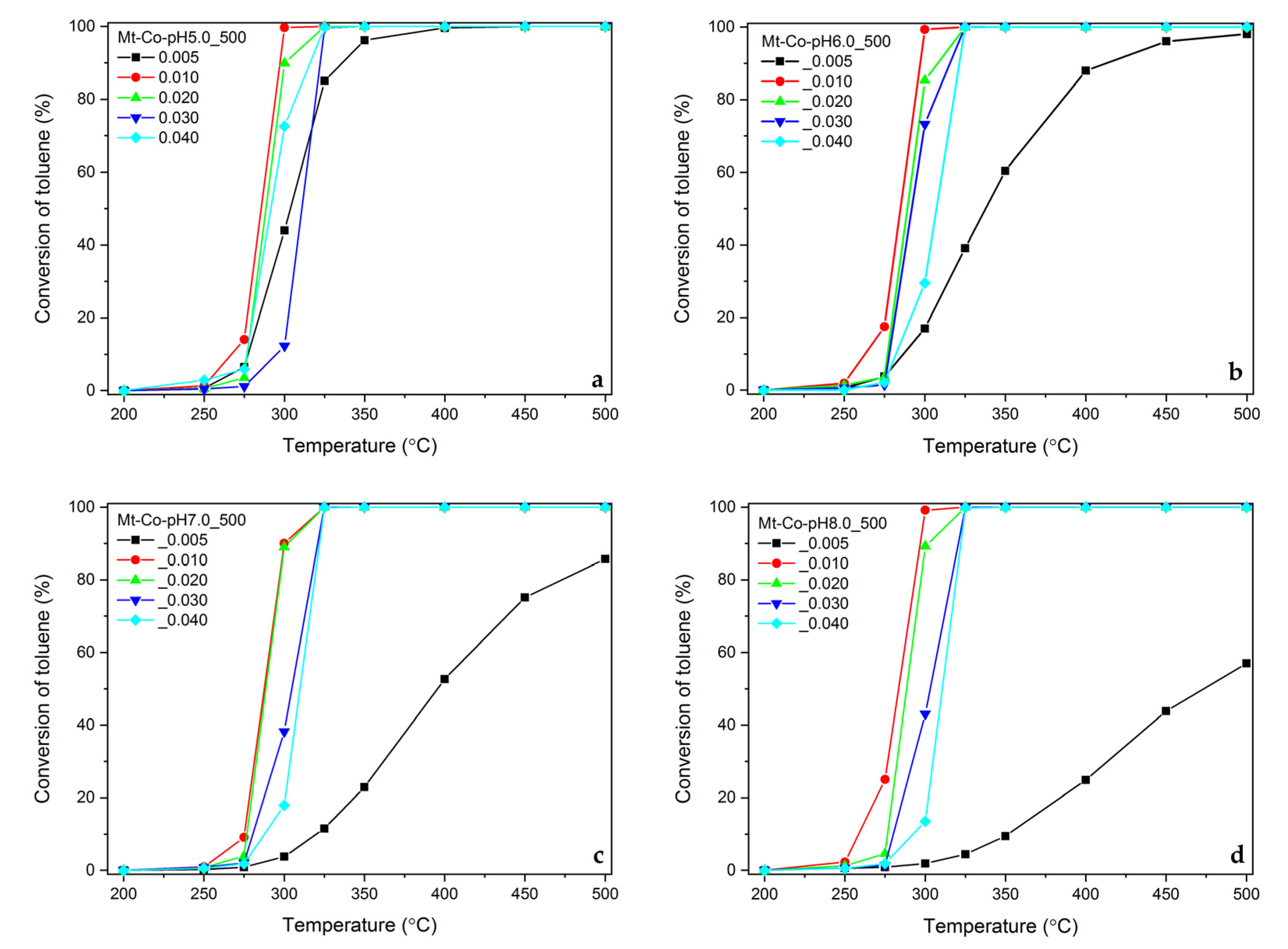
2.2. Effect of Concentration of Co2+ Solution
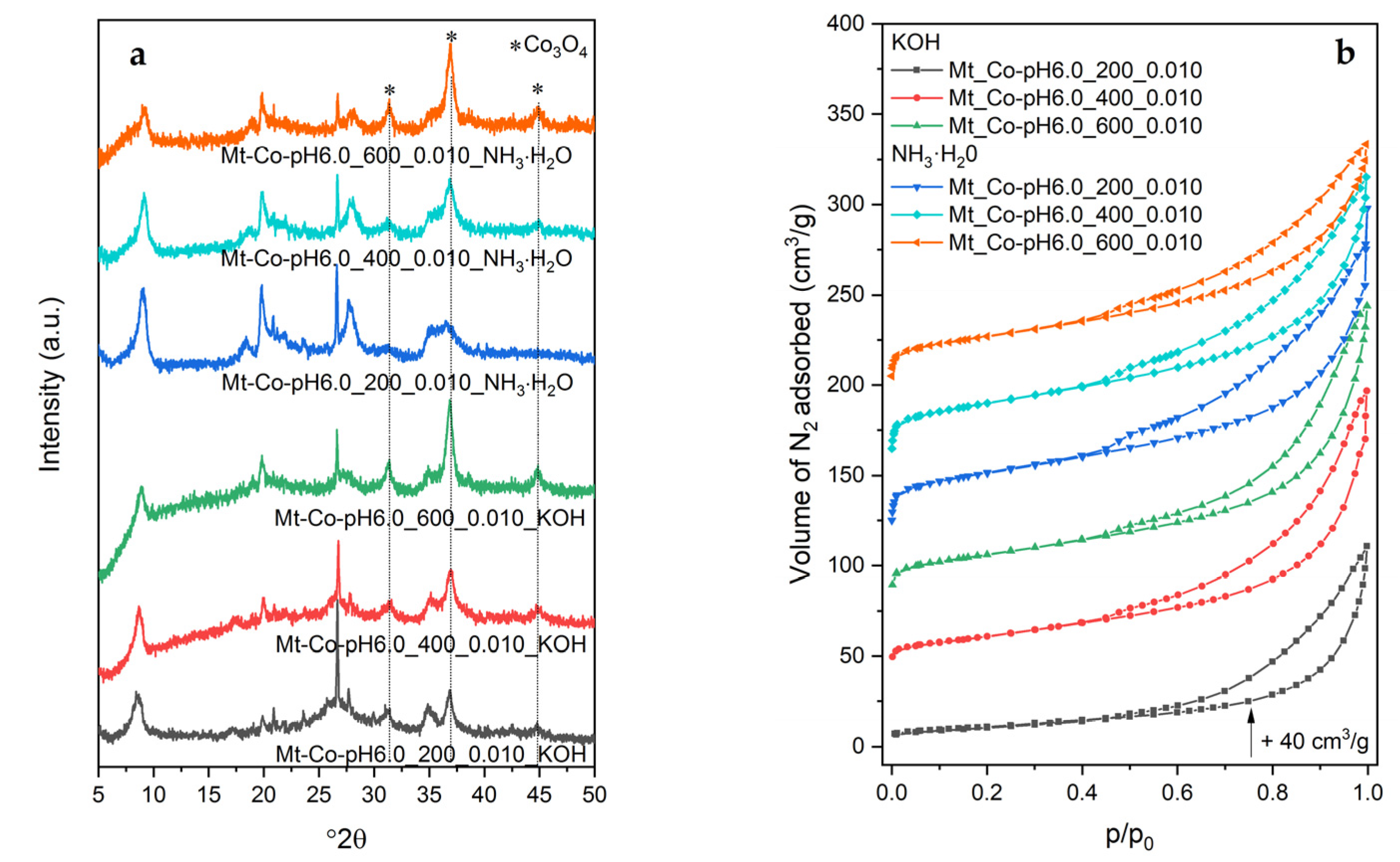
2.3. Effect of pH-Controlling Agent
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis
3.3. Characterization
3.4. Catalytic Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Karpińska, J.; Kotowska, U. Removal of Organic Pollution in the Water Environment. Water 2019, 11, 2017. [Google Scholar] [CrossRef]
- European Environment Agency. Industrial Waste Water Treatment—Pressures on Europe’s Environment 2018; EEA: Luxembourg, 2019.
- Mahugo-Santana, C.; Sosa-Ferrera, Z.; Torres-Padrón, M.E.; Santana-Rodríguez, J.J. Analytical methodologies for the determination of nitroimidazole residues in biological and environmental liquid samples: A review. Anal. Chim. Acta 2010, 665, 113–122. [Google Scholar] [CrossRef]
- Anku, W.W.; Mamo, A.M.; Govender, P.P. Phenolic Compounds in Water: Sources, Reactivity, Toxicity and Treatment Methods. In Phenolic Compounds—Natural Sources, Importance and Applications; Soto-Hernandez, M., Palma-Tenango, M., Del Rosario Garcia-Mateos, M., Eds.; InTech: London, UK, 2017. [Google Scholar]
- Kuśtrowski, P.; Rokicińska, A.; Kondratowicz, T. Abatement of Volatile Organic Compounds Emission as a Target for Various Human Activities Including Energy Production. Adv. Inorg. Chem. 2018, 72, 385–419. [Google Scholar]
- Santos, V.P.; Carabineiro, S.A.C.; Tavares, P.B.; Pereira, M.F.R.; Órfão, J.J.M.; Figueiredo, J.J. Oxidation of CO, ethanol and toluene over TiO2 supported noble metal catalysts. Appl. Catal. B Environ. 2010, 99, 198–205. [Google Scholar] [CrossRef]
- An, N.; Yu, Q.; Liu, G.; Li, S.; Jia, M.; Zhang, W. Complete oxidation of formaldehyde at ambient temperature over supported Pt/Fe2O3 catalysts prepared by colloid-deposition method. J. Hazard. Mater. 2011, 186, 1392–1397. [Google Scholar] [CrossRef]
- Zhao, S.; Hu, F.; Li, J. Hierarchical Core-Shell Al2O3@Pd-CoAlO Microspheres for Low-Temperature Toluene Combustion. ACS Catal. 2016, 6, 3433–3441. [Google Scholar] [CrossRef]
- Odoom-Wubah, T.; Li, Q.; Adilov, I.; Huang, J.; Li, Q. Towards efficient Pd/Mn3O4 catalyst with enhanced acidic sites and low temperature reducibility for Benzene abatement. Mol. Catal. 2019, 477, 110558. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C.; Chen, X.; Martynyuk, O.; Bogdanchikova, N.; Avalos-Borja, M.; Pestryakov, A.; Tavares, B.; Orfao, J.J.M.; Pereira, M.F.R.; Figueiredo, J.L. Gold supported on metal oxides for volatile organic compounds total oxidation. Catal. Today 2015, 244, 103–114. [Google Scholar] [CrossRef]
- Liotta, L.F.; Wu, H.; Pantaleo, G.; Venezia, A.M. Co3O4 nanocrystals and Co3O4–MOx binary oxides for CO, CH4 and VOC oxidation at low temperatures: A review. Catal. Sci. Technol. 2013, 3, 3085–3102. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, J.; Song, J.; Huang, S.; Yang, N.; Zhang, J.; Sun, Y.; Zhu, Y. Exploring the Effect of Co3O4 Nanocatalysts with Different Dimensional Architectures on Methane Combustion. Chem. Commun. 2016, 8, 540–545. [Google Scholar]
- Xie, X.W.; Li, Y.; Liu, Z.Q.; Haruta, M.; Shen, W.J. Low-temperature oxidation of CO catalysed by Co3O4 nanorods. Nature 2009, 458, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Z.; Zhao, Y.X.; Gao, C.G.; Liu, D.S. Preparation and catalytic performance of Co3O4 catalysts for low-temperature CO oxidation. Catal. Lett. 2007, 116, 136–142. [Google Scholar] [CrossRef]
- Hu, L.H.; Peng, Q.; Li, Y.D. Selective Synthesis of Co3O4 Nanocrystal with Different Shape and Crystal Plane Effect on Catalytic Property for Methane Combustion. J. Am. Chem. Soc. 2008, 130, 16136–16137. [Google Scholar] [CrossRef]
- Xue, W.J.; Wang, Y.F.; Li, P.; Liu, Z.T.; Hao, Z.P.; Ma, C.Y. Morphology effects of Co3O4 on the catalytic activity of Au/Co3O4 catalysts for complete oxidation of trace ethylene. Catal. Commun. 2011, 12, 1265–1268. [Google Scholar] [CrossRef]
- Ma, C.Y.; Mu, Z.; Li, J.J.; Jin, Y.G.; Cheng, J.; Lu, G.Q.; Hao, Z.P.; Qiao, S.Z. Macrocyclic Platforms for the Construction of Tetranuclear Oxo and Hydroxo Zinc Clusters. J. Am. Chem. Soc. 2010, 132, 2608–2613. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Yang, D.P.; Liu, A. Leaf-templated synthesis of 3D hierarchical porous cobalt oxide nanostructure as direct electrochemical biosensing interface with enhanced electrocatalysis. Biosens. Bioelectron. 2015, 63, 145–152. [Google Scholar] [CrossRef]
- Du, Y.C.; Meng, Q.; Wang, J.S.; Yan, J.; Fan, H.G.; Liu, Y.X.; Dai, H.X. Three dimensional mesoporous manganese oxides and cobalt oxides: High-efficiency catalysts for the removal of toluene and carbon monoxide. Microporous Mesoporous Mater. 2012, 162, 199–206. [Google Scholar] [CrossRef]
- García, T.; Agouram, S.; Sánchez-Royo, J.F.; Murillo, R.; Mastral, A.M.; Aranda, A.; Vázquez, I.; Dejoz, A.; Solsona, B. Deep oxidation of volatile organic compounds using ordered cobalt oxides prepared by a nanocasting route. Appl. Catal. A 2010, 386, 16–27. [Google Scholar] [CrossRef]
- Fu, X.P.; Shen, Q.K.; Shi, D.; Wu, K.; Jin, Z.; Wang, X.; Si, R.; Song, Q.S.; Jia, C.J.; Yan, C.H. Co3O4-Al2O3 mesoporous hollow spheres as efficient catalyst for Fischer-Tropsch synthesis. Appl. Catal. B Environ. 2017, 211, 176–187. [Google Scholar] [CrossRef]
- Menon, U.; Galvita, V.V.; Marin, G.B. Reaction network for the oxidation of toluene over CuO-CeO2/Al2O3. J. Catal. 2011, 283, 1–9. [Google Scholar] [CrossRef]
- Esmaeilirada, M.; Zabihi, M.; Shayegan, J.; Khorasheh, F. Oxidation of toluene in humid air by metal oxides supported on γ-alumina. J. Hazard. Mater. 2017, 333, 293–307. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Zhang, T.; Luo, Y.; Lan, Z.; Zhang, K.; Zuo, J.; Jiang, L.; Wang, R. Geometrical-Site-Dependent Catalytic Activity of Ordered Mesoporous Co-Based Spinel for Benzene Oxidation: In Situ DRIFTS Study Coupled with Raman and XAFS Spectroscopy. ACS Catal. 2017, 7, 1626–1636. [Google Scholar] [CrossRef]
- Rooke, J.C.; Barakat, T.; Finol, M.F.; Billemont, P.; De Weireld, G.; Li, Y.; Cousin, R.; Giraudon, J.-M.; Siffert, S.; Lamonier, J.-F.; et al. Influence of hierarchically porous niobium doped TiO2 supports in the total catalytic oxidation of model VOCs over noble metal nanoparticles. Appl. Catal. B 2013, 142, 149–160. [Google Scholar] [CrossRef]
- Liu, X.; Zeng, J.; Shi, W.; Wang, J.; Zhu, T.; Chena, Y. Catalytic oxidation of benzene over ruthenium–cobalt bimetallic catalysts and study of its mechanism. Catal. Sci. Technol. 2017, 7, 213–221. [Google Scholar] [CrossRef]
- Shi, Z.; Lan, L.; Li, Y.; Yang, Y.; Zhang, Q.; Wu, J.; Zhang, G.; Zhao, X. Co3O4/TiO2 Nanocomposite Formation Leads to Improvement in Ultraviolet-Visible-Infrared-Driven Thermocatalytic Activity Due to Photoactivation and Photocatalysis-Thermocatalysis Synergetic Effect. ACS Sustain. Chem. Eng. 2018, 6, 16503–16514. [Google Scholar] [CrossRef]
- Fiorenza, R.; Crisafulli, C.; Condorelli, G.G.; Lupo, F.; Scirè, S. Au-Ag/CeO2 and Au-Cu/CeO2 Catalysts for Volatile Organic Compounds Oxidation and CO Preferential Oxidation. Catal. Lett. 2015, 145, 1691–1702. [Google Scholar] [CrossRef]
- Konsolakis, M.; Carabineiro, S.A.C.; Marnellos, G.E.; Asad, M.F.; Soares, O.S.G.P.; Pereira, M.F.R.; Órfão, J.J.M.; Figueiredo, J.L. Effect of cobalt loading on the solid state properties and ethyl acetate oxidation performance of cobalt-cerium mixed oxides. J. Colloid Interface Sci. 2017, 496, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.F.; Zhou, Y.; Han, W.F.; Huang, H.F.; Chen, Y.F. Promoting effect of ZrO2 carrier on activity and thermal stability of CeO2-based oxides catalysts for toluene combustion. Appl. Catal. A 2013, 464, 101–108. [Google Scholar] [CrossRef]
- Xiang, Y.; Zhu, Y.; Zhu, C.; Zhu, M.; Xie, Q.; Chen, T. Co3O4/α-Fe2O3 catalyzed oxidative degradation of gaseous benzene: Preparation, characterization and its catalytic properties. Solid State Sci. 2019, 93, 79–86. [Google Scholar] [CrossRef]
- Zhu, D.; Huang, Y.; Cao, J.; Leed, S.C.; Chene, M.; Shen, Z. Cobalt nanoparticles encapsulated in porous nitrogen-doped carbon: Oxygen activation and efficient catalytic removal of formaldehyde at room temperature. Appl. Catal. B Environ. 2019, 258, 117981. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Kang, C.; Roh, C.; Shim, J.J. Supercritical CO2-Mediated synthesis of. CNT@Co3O4 nanocomposite and its application for energy storage. Ind. Eng. Chem. Res. 2016, 55, 7338–7343. [Google Scholar] [CrossRef]
- Xu, Q.Q.; Xu, G.; Yin, J.Z.; Wang, A.Q.; Ma, Y.L.; Gao, J.J. Preparation of superhighly dispersed Co3O4@SBA-15 with different morphologies in supercritical CO2 with the assistance of dilute acids. Ind. Eng. Chem. Res. 2014, 53, 10366–10371. [Google Scholar] [CrossRef]
- Rokicińska, A.; Drozdek, M.; Dudek, B.; Gil, B.; Michorczyk, P.; Brouri, D.; Dzwigaj, S.; Kuśtrowski, P. Cobalt-containing BEA zeolite for catalytic combustion of toluene. Appl. Catal. B Environ. 2017, 212, 59–67. [Google Scholar] [CrossRef]
- Blanch-Raga, N.; Palomares, A.E.; Martínez-Triguero, J.; Valencia, S. Cu and Co modified beta zeolite catalysts for the trichloroethylene oxidation. Appl. Catal. B Environ. 2016, 187, 90–97. [Google Scholar] [CrossRef]
- Giroir-Fendler, A.; Alves-Fortunato, M.; Richard, M.; Wang, C.; Díaz, J.A.; Gil, S.; Zhang, C.; Can, F.; Bion, N.; Guo, Y. Synthesis of oxide supported LaMnO3 perovskites to enhance yields in toluene combustion. Appl. Catal. B 2016, 180, 29–37. [Google Scholar] [CrossRef]
- Gennequin, C.; Barakat, T.; Tidahy, H.L.; Cousin, R.; Lamonier, J.F.; Aboukaïs, A.; Siffert, S. Use and observation of the hydrotalcite “memory effect” for VOC oxidation. Catal. Today 2010, 157, 191–197. [Google Scholar] [CrossRef]
- Aguilera, D.A.; Perez, A.; Molina, R.; Moreno, S. Cu-Mn and Co-Mn catalysts synthesized from hydrotalcites and their use in the oxidation of VOCs. Appl. Catal. B Environ. 2011, 104, 144–150. [Google Scholar] [CrossRef]
- Lin, L.-Y.; Bai, H. Salt-induced formation of hollow and mesoporous CoOx/SiO2 spheres and their catalytic behavior in toluene oxidation. RCS Adv. 2016, 6, 24304–24313. [Google Scholar] [CrossRef]
- Wang, K.; Cao, Y.; Hu, J.; Li, Y.; Xie, J.; Jia, D.J. Solvent-free Chemical Approach to Synthesize Various Morphological Co3O4 for CO Oxidation. ACS Appl. Mater. Interfaces 2017, 9, 16128–16137. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.M.; Zhen, M.; Jin, J.L.; Yong, G.J.; Jie, C.; Gao, Q.L.; Zheng, P.H.; Shi, Z.Q. Mesoporous Co3O4 and Au/Co3O4 Catalysts for Low-Temperature Oxidation of Trace Ethylene. J. Am. Chem. Soc. 2010, 132, 2608–2613. [Google Scholar]
- Wei, L.; Rui, L.; Xuejun, Z. Controllable synthesis of 3D hierarchical Co3O4 catalysts and their excellent catalytic performance for toluene combustion. Appl. Surf. Sci. 2020, 507, 145174. [Google Scholar]
- Ren, Q.; Mp, S.; Peng, R.; Feng, Z.; Zhang, M.; Chen, L.; Fu, M.; Wu, J.; Ye, D. Controllable synthesis of 3D hierarchical Co3O4 nanocatalysts with various morphologies for the catalytic oxidation of toluene. J. Mater. Chem. A 2018, 6, 498–509. [Google Scholar] [CrossRef]
- Li, Z.; Yang, D.-P.; Chen, Y.; Du, Z.; Guo, Y.; Huang, J.; Li, Q. eggshells to valuable Co3O4/CaCO3 materials as efficient catalysts for VOCs oxidation. Mol. Catal. 2020, 483, 110766. [Google Scholar] [CrossRef]
- Zhong, J.; Zeng, Y.; Chen, D.; Mo, S.; Zhang, M.; Fu, M.; Wu, J.; Su, Z.; Chen, P.; Ye, D. Toluene oxidation over Co3+ -rich spinel Co3O4: Evaluation of chemical and by-product species identified by in situ DRIFTS combined with PTR-TOF-MS. J. Hazard. Mater. 2020, 386, 121957. [Google Scholar] [CrossRef] [PubMed]
- Rokicińska, A.; Natkański, P.; Dudek, B.; Drozdek, M.; Lityńska-Dobrzyńska, L.; Kuśtrowski, P. Co3O4-pillared montmorillonite catalysts synthesized by hydrogel-assisted route for total oxidation of toluene. Appl. Catal. B Environ. 2016, 195, 59–68. [Google Scholar] [CrossRef]
- Amri, A.; Duan, X.F.; Yin, C.-Y.; Jiang, Z.-T.; Mahbubur Rahman, M.; Pryor, T. Solar absorptance of copper-cobalt oxide thin film coatings with nano-size, grain-like morphology: Optimization and synchrotron radiation XPS studies. Appl. Surf. Sci. 2013, 275, 127–135. [Google Scholar] [CrossRef]
- Iablokov, V.; Barbosa, R.; Pollefeyt, G.; Van Driessche, V.; Chenakin, S.; Kruse, N. Catalytic CO Oxidation over Well-Defined Cobalt Oxide Nanoparticles: Size-Reactivity Correlation. ACS Catal. 2015, 5, 5714–5718. [Google Scholar] [CrossRef]
- Pu, Z.; Zhou, H.; Zheng, Y.; Huang, W.; Li, X. Enhanced methane combustion over Co3O4 catalysts prepared by a facile precipitation method: Effect of aging time. Appl. Surf. Sci. 2017, 410, 14–21. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, Y.; Zhou, H.; Huang, W.; Pu, Z. Complete combustion of methane over Co3O4 catalysts: Influence of pH values. J. Alloys Compounds 2018, 734, 112–120. [Google Scholar] [CrossRef]
- Arnoldy, P.; Moulijn, J.A. Temperature-programmed reduction of CoOAI2O3 catalysts. J. Catal. 1985, 93, 38–54. [Google Scholar] [CrossRef]
- Ji, Y.; Zhao, Z.; Duan, A.; Jiang, G.; Liu, J. Comparative Study on the Formation and Reduction of Bulk and Al2O3-Supported Cobalt Oxides by H2-TPR Technique. J. Phys. Chem. C 2009, 113, 7186–7199. [Google Scholar] [CrossRef]
- Fischer, N.; Minnermann, M.; Baeumer, M.; van Steen, E.; Claeys, M. Metal Support Interactions in Co3O4/Al2O3 Catalysts Prepared from w/o Microemulsions. Catal. Lett. 2012, 142, 830–837. [Google Scholar] [CrossRef]

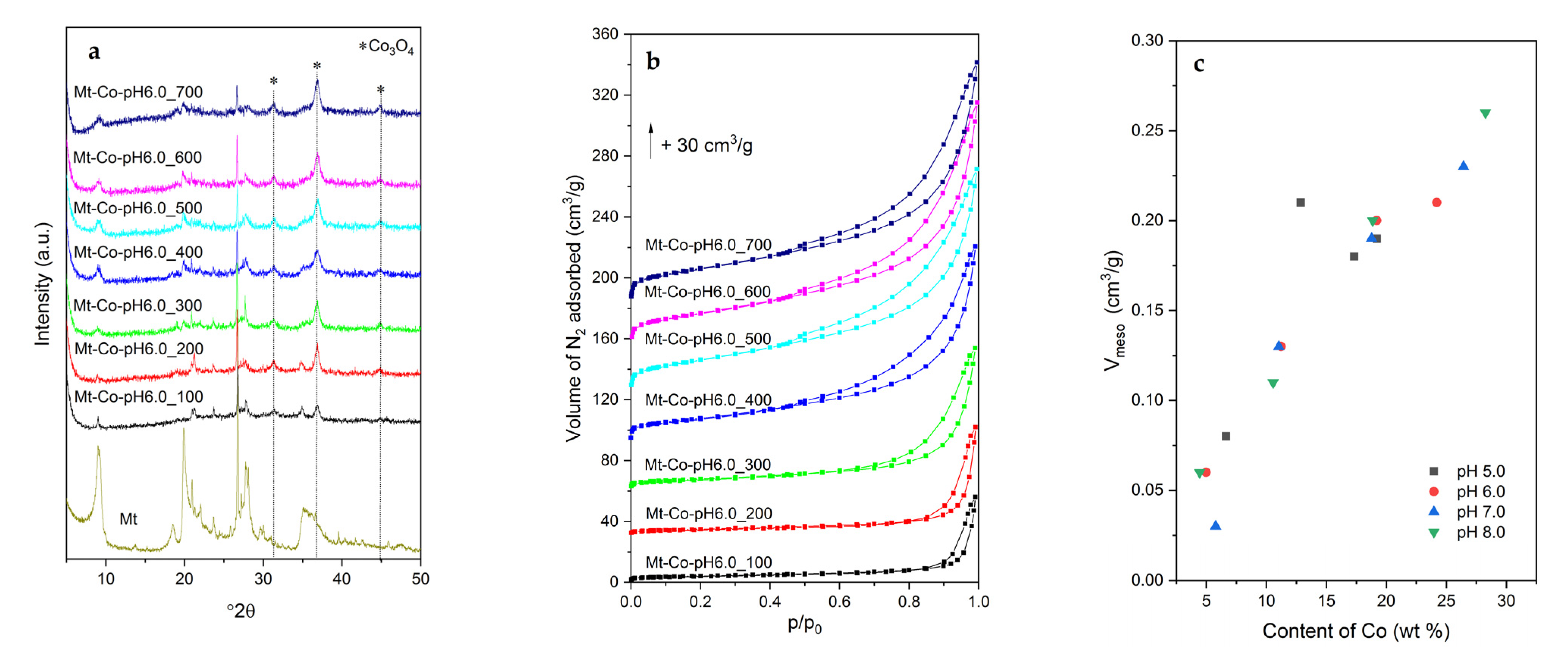


| Sample | Textural Parameters | |||
|---|---|---|---|---|
| SBET (m2/g) | Vmeso (cm3/g) | Vmicro (cm3/g) | Vtotal (cm3/g) | |
| Mt-Co-pH5.0_100 | 21 | 0.08 | 0.001 | 0.12 |
| Mt-Co-pH5.0_200 | 32 | 0.13 | 0.001 | 0.17 |
| Mt-Co-pH5.0_300 | 93 | 0.21 | 0.001 | 0.25 |
| Mt-Co-pH5.0_400 | 55 | 0.17 | 0.001 | 0.18 |
| Mt-Co-pH5.0_500 | 86 | 0.18 | 0.003 | 0.22 |
| Mt-Co-pH5.0_600 | 32 | 0.11 | 0.003 | 0.15 |
| Mt-Co-pH5.0_700 | 93 | 0.19 | 0.003 | 0.22 |
| Mt-Co-pH6.0_100 | 14 | 0.06 | 0.001 | 0.09 |
| Mt-Co-pH6.0_200 | 17 | 0.10 | 0.001 | 0.11 |
| Mt-Co-pH6.0_300 | 27 | 0.13 | 0.001 | 0.15 |
| Mt-Co-pH6.0_400 | 63 | 0.19 | 0.001 | 0.20 |
| Mt-Co-pH6.0_500 | 94 | 0.20 | 0.005 | 0.23 |
| Mt-Co-pH6.0_600 | 96 | 0.21 | 0.001 | 0.26 |
| Mt-Co-pH6.0_700 | 94 | 0.21 | 0.005 | 0.25 |
| Mt-Co-pH7.0_100 | 15 | 0.03 | 0.001 | 0.06 |
| Mt-Co-pH7.0_200 | 20 | 0.08 | 0.001 | 0.12 |
| Mt-Co-pH7.0_300 | 31 | 0.13 | 0.001 | 0.18 |
| Mt-Co-pH7.0_400 | 23 | 0.13 | 0.001 | 0.15 |
| Mt-Co-pH7.0_500 | 55 | 0.19 | 0.001 | 0.23 |
| Mt-Co-pH7.0_600 | 82 | 0.19 | 0.009 | 0.23 |
| Mt-Co-pH7.0_700 | 92 | 0.23 | 0.006 | 0.27 |
| Mt-Co-pH8.0_100 | 20 | 0.06 | 0.003 | 0.09 |
| Mt-Co-pH8.0_200 | 26 | 0.10 | 0.001 | 0.15 |
| Mt-Co-pH8.0_300 | 31 | 0.11 | 0.001 | 0.13 |
| Mt-Co-pH8.0_400 | 40 | 0.13 | 0.001 | 0.16 |
| Mt-Co-pH8.0_500 | 78 | 0.20 | 0.002 | 0.21 |
| Mt-Co-pH8.0_600 | 96 | 0.29 | 0.004 | 0.30 |
| Mt-Co-pH8.0_700 | 103 | 0.26 | 0.004 | 0.30 |
| Sample | Content (% at.) | Molar Ratio | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Si | Al | O2− | OH− | Na | Co | Co2+/Co3+ | Co/Si Bulk a | Co/Si Surface b | |
| Mt-Co-pH5.0 | |||||||||
| 100 | 17.61 | 5.83 | 2.71 | 52.01 | 16.26 | 5.58 | 1.5 | 0.28 | 0.32 |
| 300 | 20.93 | 6.97 | 7.14 | 48.64 | 10.74 | 5.59 | 1.2 | 0.53 | 0.27 |
| 500 | 22.68 | 7.70 | 2.34 | 59.16 | 2.76 | 5.34 | 1.7 | 0.69 | 0.24 |
| 700 | 22.38 | 7.15 | 3.02 | 58.72 | - | 8.74 | 1.7 | 0.79 | 0.39 |
| Mt-Co-pH6.0 | |||||||||
| 100 | 17.66 | 5.79 | 0.70 | 55.06 | 18.20 | 2.59 | 1.4 | 0.23 | 0.15 |
| 300 | 18.61 | 5.98 | 1.31 | 53.95 | 12.88 | 7.28 | 2.3 | 0.50 | 0.39 |
| 500 | 21.58 | 6.76 | 7.17 | 54.08 | - | 10.42 | 1.5 | 0.81 | 0.48 |
| 700 | 21.47 | 6.61 | 6.03 | 54.29 | - | 11.59 | 1.4 | 1.11 | 0.54 |
| Mt-Co-pH7.0 | |||||||||
| 100 | 14.43 | 4.04 | 1.50 | 53.74 | 22.93 | 3.36 | 1.2 | 0.31 | 0.23 |
| 300 | 18.88 | 5.71 | 5.59 | 48.97 | 12.81 | 8.04 | 1.8 | 0.47 | 0.43 |
| 500 | 19.65 | 5.01 | 13.02 | 42.87 | 6.56 | 12.90 | 1.1 | 0.84 | 0.66 |
| 700 | 21.22 | 6.27 | 9.43 | 50.72 | - | 12.38 | 1.1 | 1.29 | 0.58 |
| Mt-Co-pH8.0 | |||||||||
| 100 | 14.49 | 4.48 | 0.69 | 50.68 | 26.77 | 2.88 | 1.9 | 0.22 | 0.20 |
| 300 | 18.66 | 5.52 | 13.65 | 39.35 | 13.95 | 8.87 | 1.0 | 0.45 | 0.48 |
| 500 | 19.22 | 5.20 | 12.06 | 43.50 | 5.84 | 14.18 | 1.7 | 0.83 | 0.74 |
| 700 | 20.43 | 6.16 | 8.81 | 49.17 | 2.77 | 12.68 | 1.0 | 1.48 | 0.62 |
| Sample | Textural Parameters | |||
|---|---|---|---|---|
| SBET (m2/g) | Vmeso (cm3/g) | Vmicro (cm3/g) | Vtotal (cm3/g) | |
| Mt-Co-pH5.0_500_0.005 | 37 | 0.11 | 0.002 | 0.15 |
| Mt-Co-pH5.0_500_0.010 | 86 | 0.18 | 0.003 | 0.22 |
| Mt-Co-pH5.0_500_0.020 | 100 | 0.19 | 0.005 | 0.27 |
| Mt-Co-pH5.0_500_0.030 | 101 | 0.19 | 0.005 | 0.28 |
| Mt-Co-pH5.0_500_0.040 | 85 | 0.19 | 0.004 | 0.22 |
| Mt-Co-pH6.0_500_0.005 | 28 | 0.11 | 0.001 | 0.17 |
| Mt-Co-pH6.0_500_0.010 | 94 | 0.20 | 0.005 | 0.23 |
| Mt-Co-pH6.0_500_0.020 | 93 | 0.18 | 0.005 | 0.26 |
| Mt-Co-pH6.0_500_0.030 | 100 | 0.21 | 0.005 | 0.30 |
| Mt-Co-pH6.0_500_0.040 | 90 | 0.16 | 0.005 | 0.18 |
| Mt-Co-pH7.0_500_0.005 | 24 | 0.09 | 0.001 | 0.15 |
| Mt-Co-pH7.0_500_0.010 | 55 | 0.19 | 0.001 | 0.23 |
| Mt-Co-pH7.0_500_0.020 | 94 | 0.19 | 0.004 | 0.25 |
| Mt-Co-pH7.0_500_0.030 | 107 | 0.21 | 0.007 | 0.29 |
| Mt-Co-pH7.0_500_0.040 | 90 | 0.20 | 0.001 | 0.22 |
| Mt-Co-pH8.0_500_0.005 | 20 | 0.08 | 0.001 | 0.14 |
| Mt-Co-pH8.0_500_0.010 | 78 | 0.20 | 0.002 | 0.21 |
| Mt-Co-pH8.0_500_0.020 | 100 | 0.26 | 0.005 | 0.26 |
| Mt-Co-pH8.0_500_0.030 | 110 | 0.19 | 0.007 | 0.27 |
| Mt-Co-pH8.0_500_0.040 | 105 | 0.20 | 0.008 | 0.24 |
| Sample | Content (% at.) | Molar Ratio | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Si | Al | O2− | OH− | Na | Co | Co2+/Co3+ | Co/Si Bulk a | Co/Si Surface b | |
| Mt-Co-pH5.0_500 | |||||||||
| 0.005 | 21.23 | 6.78 | 2.34 | 59.16 | 9.51 | 4.88 | 1.5 | 0.65 | 0.23 |
| 0.010 | 22.68 | 7.70 | 4.24 | 57.21 | 2.76 | 5.34 | 1.7 | 0.69 | 0.24 |
| 0.020 | 20.40 | 5.52 | 9.89 | 47.60 | - | 16.58 | 1.2 | 1.00 | 0.81 |
| 0.030 | 20.07 | 5.27 | 7.57 | 49.84 | - | 17.24 | 1.8 | 1.12 | 0.86 |
| 0.040 | 21.92 | 6.67 | 6.45 | 54.50 | - | 10.47 | 1.7 | 1.22 | 0.48 |
| Mt-Co-pH6.0_500 | |||||||||
| 0.005 | 18.68 | 5.86 | 5.84 | 48.62 | 16.04 | 4.95 | 1.7 | 0.68 | 0.26 |
| 0.010 | 21.58 | 6.76 | 7.17 | 54.08 | - | 10.42 | 1.5 | 0.81 | 0.48 |
| 0.020 | 20.74 | 5.93 | 8.11 | 50.11 | - | 15.11 | 1.2 | 1.21 | 0.73 |
| 0.030 | 22.44 | 6.70 | 6.54 | 54.88 | - | 9.51 | 1.9 | 1.21 | 0.42 |
| 0.040 | 20.55 | 5.74 | 9.62 | 49.78 | - | 14.29 | 1.4 | 1.25 | 0.70 |
| Mt-Co-pH7.0_500 | |||||||||
| 0.005 | 17.55 | 5.18 | 4.28 | 49.32 | 18.27 | 5.40 | 2.3 | 0.70 | 0.31 |
| 0.010 | 19.65 | 5.01 | 13.02 | 42.87 | 6.56 | 12.90 | 1.1 | 0.84 | 0.66 |
| 0.020 | 22.53 | 7.18 | 9.74 | 51.55 | - | 9.07 | 1.1 | 1.45 | 0.40 |
| 0.030 | 19.88 | 5.29 | 9.03 | 48.76 | - | 17.05 | 1.3 | 1.65 | 0.86 |
| 0.040 | 19.46 | 5.46 | 8.24 | 49.52 | - | 17.34 | 1.3 | 1.40 | 0.89 |
| Mt-Co-pH8.0_500 | |||||||||
| 0.005 | 16.48 | 4.56 | 4.97 | 47.74 | 21.87 | 4.40 | 1.5 | 0.72 | 0.27 |
| 0.010 | 19.22 | 5.20 | 12.06 | 43.50 | 5.84 | 14.18 | 1.7 | 0.83 | 0.74 |
| 0.020 | 20.58 | 5.33 | 8.10 | 48.84 | - | 17.16 | 1.7 | 1.69 | 0.83 |
| 0.030 | 18.94 | 3.20 | 14.13 | 39.77 | - | 23.95 | 1.9 | 2.83 | 1.26 |
| 0.040 | 18.93 | 3.80 | 11.42 | 44.94 | - | 20.90 | 1.9 | 3.01 | 1.10 |
| Sample | Textural Parameters | |||
|---|---|---|---|---|
| SBET (m2/g) | Vmeso (cm3/g) | Vmicro (cm3/g) | Vtotal (cm3/g) | |
| KOH | ||||
| Mt-Co-pH6.0_100_0.010 | 27 | 0.08 | 0.001 | 0.09 |
| Mt-Co-pH6.0_200_0.010 | 39 | 0.11 | 0.002 | 0.11 |
| Mt-Co-pH6.0_300_0.010 | 51 | 0.14 | 0.001 | 0.14 |
| Mt-Co-pH6.0_400_0.010 | 75 | 0.16 | 0.001 | 0.17 |
| Mt-Co-pH6.0_500_0.010 | 91 | 0.15 | 0.005 | 0.18 |
| Mt-Co-pH6.0_600_0.010 | 94 | 0.17 | 0.005 | 0.19 |
| Mt-Co-pH6.0_700_0.010 | 98 | 0.21 | 0.005 | 0.21 |
| NH3·H2O | ||||
| Mt-Co-pH6.0_100_0.010 | 93 | 0.10 | 0.007 | 0.13 |
| Mt-Co-pH6.0_200_0.010 | 113 | 0.15 | 0.006 | 0.19 |
| Mt-Co-pH6.0_300_0.010 | 121 | 0.20 | 0.005 | 0.21 |
| Mt-Co-pH6.0_400_0.010 | 108 | 0.16 | 0.005 | 0.19 |
| Mt-Co-pH6.0_500_0.010 | 101 | 0.16 | 0.004 | 0.19 |
| Mt-Co-pH6.0_600_0.010 | 97 | 0.15 | 0.004 | 0.17 |
| Mt-Co-pH6.0_700_0.010 | 107 | 0.18 | 0.005 | 0.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rokicińska, A.; Berniak, T.; Drozdek, M.; Kuśtrowski, P. In Search of Factors Determining Activity of Co3O4 Nanoparticles Dispersed in Partially Exfoliated Montmorillonite Structure. Molecules 2021, 26, 3288. https://doi.org/10.3390/molecules26113288
Rokicińska A, Berniak T, Drozdek M, Kuśtrowski P. In Search of Factors Determining Activity of Co3O4 Nanoparticles Dispersed in Partially Exfoliated Montmorillonite Structure. Molecules. 2021; 26(11):3288. https://doi.org/10.3390/molecules26113288
Chicago/Turabian StyleRokicińska, Anna, Tomasz Berniak, Marek Drozdek, and Piotr Kuśtrowski. 2021. "In Search of Factors Determining Activity of Co3O4 Nanoparticles Dispersed in Partially Exfoliated Montmorillonite Structure" Molecules 26, no. 11: 3288. https://doi.org/10.3390/molecules26113288
APA StyleRokicińska, A., Berniak, T., Drozdek, M., & Kuśtrowski, P. (2021). In Search of Factors Determining Activity of Co3O4 Nanoparticles Dispersed in Partially Exfoliated Montmorillonite Structure. Molecules, 26(11), 3288. https://doi.org/10.3390/molecules26113288







