Milk Proteins—Their Biological Activities and Use in Cosmetics and Dermatology
Abstract
1. Introduction
| Component | Colostrum | Milk | ||||
|---|---|---|---|---|---|---|
| Bovine | Goat | Sheep | Bovine | Goat | Sheep | |
| Lactose | 3.6 | 3.39–4.24 a | 3.3 | 4.6 | 4.1 | 4.8; 4.9 b |
| Minerals | 0.9 | 0.85–0.9 a | 0.9 | 0.7 | 0.8 | 0.94; 1 b |
| Proteins | 7.1 | 3.53–5.69 a | 11.8 | 3.4 | 2.9 | 5.5 |
| Fats | 5.1 | 3.88–8.21 a | 13 | 3.7 | 4.5 | 6; 7.4 b |
2. Bioactive Proteins of Milk
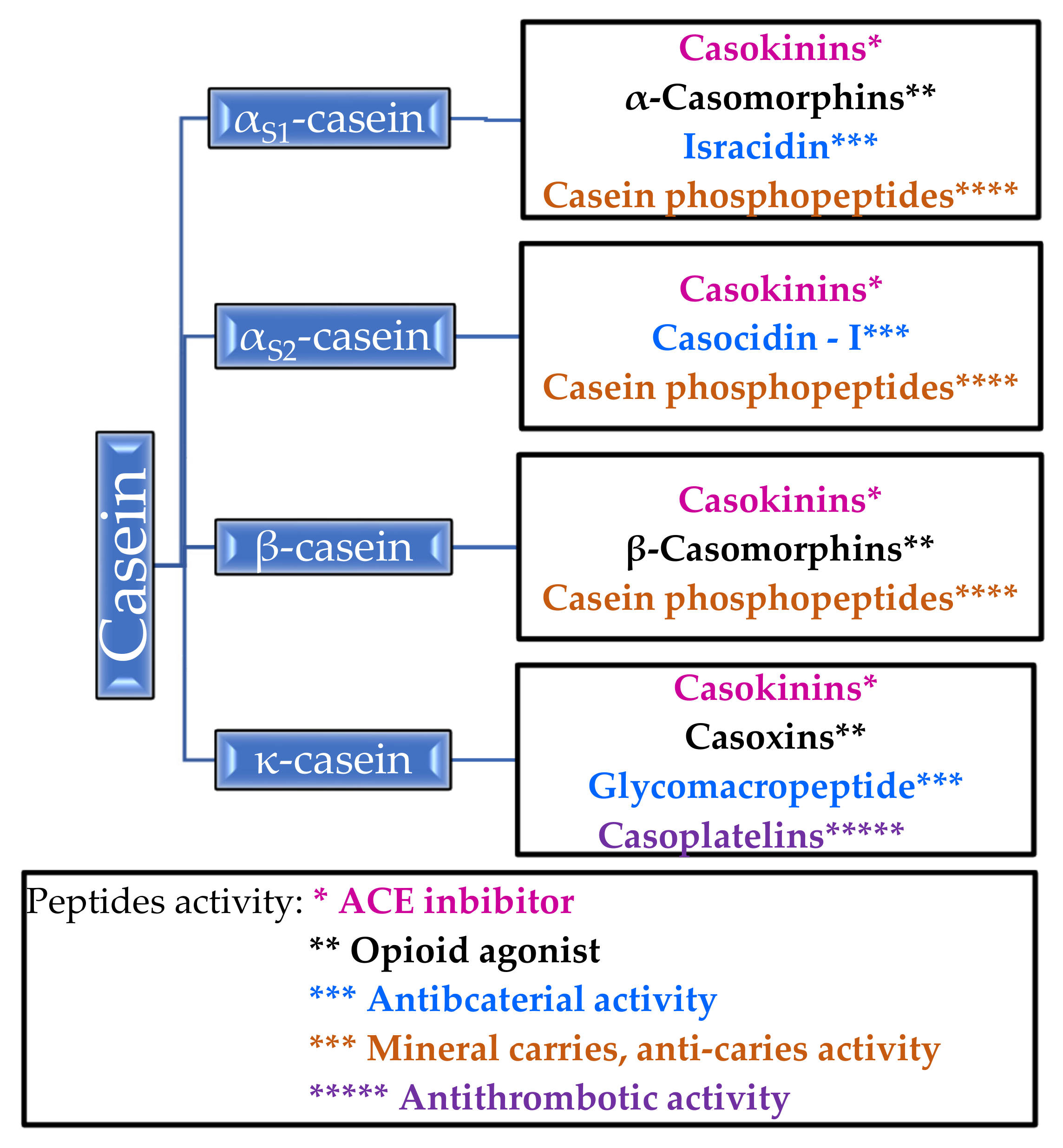
2.1. Casein
2.1.1. Biological Properties of Casein
| Proteins | Colostrum | Milk | [Ref] | ||||
|---|---|---|---|---|---|---|---|
| Bovine | Goat | Sheep | Bovine | Goat | Sheep | ||
| Casein (g/L) | 2.6 | n.d. | n.d. | * 2.7; 2.8 | 2.5 | 4.6 | [14], * [22] |
| κ-casein (%) | n.d. | n.d. | n.d. | * 12 | ** 20.4 | * 9.1–10.2 a | * [23,24], ** [22] |
| αS1-casein (%) | n.d. | n.d. | n.d. | * 37 | ** 5.6 | * 33.9–39.9 a | * [23,24], ** [22] |
| αS2-casein (%) | n.d. | n.d. | n.d. | * 10 | ** 19.2 | * 12–16.4 a | * [23,24], ** [22] |
| β-casein (%) | n.d. | n.d. | n.d. | * 35 | ** 54.8 | * 37–42.3 a | * [23,24], ** [22] |
| β-lactoglobulin (mg/mL) | * 7.9–30 | * 9.3–49.8 | ** 4–19 | *** 3.3 | **** 3.07 | **** 5.97 | * [25], ** [26], *** [14], **** [27] |
| α-lactalbumin (mg/mL) | * 3 | ** 2.77 | *** 1.5–2 | * 1.2 | **** 1.27 | **** 0.95 | * [14],** [25],*** [26],**** [27] |
| Immunoglobulins (g/L) | 20–150 | n.d. | n.d. | 0.5–1 | n.d. | n.d. | [14] |
| IgG | * 15–180 | ** 50–60 | *** 45–69 | * 0.35; ** 0.59 b | ** 0.1–0.4 | **** 0.35–1.62 | * [28,29,30,31,32,33,34], ** [22], *** [35], **** [36] |
| IgM | * 4.2; ** 5 b | * 1.6–5.2 | *** 1.3–21.20 | * 0.05 | * 0.01–0.04 | *** 0.2 | * [13,37],** [28,29,30,31,32,33,34],*** [38] |
| IgA | * 3.5; **3.9 b | ** 0.9–2.4 | *** 3.5 | ** 0.14 | ** 0.03–0.08 | *** 0.2 | * [28,29,30,31,32,33,34],** [13,37],*** [38] |
| Glycomacro-peptide (g/L) | 2.5 | n.d. | n.d. | 1.2 | n.d. | n.d. | [14] |
| Lactoferrin (g/L) | * 0.8; ***1.5–5 b | ** 0.38 | ** 0.74 | * 0.02–0.2; 0.02–0.75 b | * 0.098–0.15 | * 0.14 | * [39,40,41,42], ** [36], *** [43] |
| Lactoperoxidase (g/L) | * 0.02; *** 0.011–0.045 b | ** 0.062–0.204 | n.d. | * 0.03; *** 0.013–0.03 b | n.d. | n.d. | * [14], ** [8], *** [6] |
| Lysozyme (mg/L) | * 0.14–0.7 | n.d. | n.d. | ** 0.37–0.6 | ** 0.25 | ** 1–4 | * [44], ** [13] |
| Serum albumin (g/L) | * 1.3 | n.d. | n.d. | * 0.3 | ** 0.26–0.3 | ** 0.55–0.6 | * [14], ** [45] |
| Growth factors (µg/L) | 50 µg–40 mg/L | n.d. | n.d. | <1 µg–2 mg/L | n.d. | n.d. | [14] |
| IGF-I | * 0.049–2 a | n.d. | *** 0.199–0.265; ** 50–500 b | * <0.002–0.101 | **** 11–16.8 a | ** “low” | * [46,47,48,49,50,51,52,53,54,55,56,57,58],** [59],*** [60],**** [61,62,63] |
| IGF-II | ** 0.15–0.6 a | n.d. | n.d. | ** 0.002–0.1 a | * 106 | n.d. | * [62], ** [46,51,56] |
| EGF | * 0.004–0.008; 0.3242 b | n.d. | ** 1.7–2.3 | <0.002; 0.155 b | n.d. | ** <0.0008 | * [64,65], ** [66] |
| TGF-β1 | 0.0124–0.0426 | n.d. | n.d. | 0.0008–0.0035 | n.d. | n.d. | [49] |
| TGF-β2 | 0.15–1.15; 0.3 b | n.d. | n.d. | 0.013–0.07; 0.066 b | n.d. | n.d. | [48,67] |
2.1.2. Properties of Casein Peptides
2.1.3. Casein as Nanocarrier for Some Drugs
2.2. α-Lactalbumin
Biological Properties of α-Lactalbumin
2.3. β-Lactoglobulin
Biological Properties of β-Lactoglobulin
2.4. Lactoferrin
2.4.1. Biological Properties of Lactoferrin
2.5. Lactoperoxidase (LPO)
2.6. Immunoglobulins
2.7. Lysozyme
2.8. PRP (Proline-Rich Peptide)
2.9. Growth Factors
3. Milk- and Colostrum-Based Products in Cosmetics and Dermatology
3.1. Impact of Supplementation with Milk- and Colostrum-Based Products on Skin Conditions
| Product Used | Type of Disease or Healthy Skin (Number of Patients) | Result of the Study | [Ref.] |
|---|---|---|---|
| Topically applied milk-based products | |||
| bovine colostrum preparation (supported antibiotic therapy) | difficult-to-heal wounds caused by buttock erythematosus and by erosion erythema | significant improvement in wound healing | [174] |
| ointments containing 10% and 20% lactoferrin | moderate psoriatic plaque (n = 22) | improvement in elevation, redness, and scaling of psoriatic lesions | [114] |
| soap containing 5% Podolian cow milk | healthy skin | good cleansing and antibacterial properties | [175] |
| creams with skimmed donkey milk encapsulated in nanoliposomes | healthy skin (n = 15) | satisfactory moisturizing properties; antiaging effects | [176] |
| cream containing 30% horse colostrum | seborroic acne (n = 12) | complete skin regeneration | [177] |
| cream containing 20% horse colostrum and 10% horse milk (plus mint and benzocaine) | contact skin lesions (n = 5) | resolution of contact skin lesions and pain immediately after application | [177] |
| cream containing 20% horse colostrum and 10% horse milk (plus mint and benzocaine) | hyperthermia sunburn skin (n = 30) | immediate relief of pain and skin tension (within 24 h); the appearance of a normal tan, without any scale-off skin effect | [177] |
| cream containing 20% horse colostrum and 10% horse milk (plus mint and benzocaine) | 2° degree and 3° fire burns (n = 8) | rapid pain relief; rebuilding the epithelium in a week | [177] |
| emulsion with 20% horse colostrum | moderate atopic dermatitis (n = 7) | reduction in erythema and pruritus; softening, moisturizing, soothing, and anti-inflammatory effects | [177] |
| liposomal gel containing 20% horse colostrum | ulcerative skin lesions (n = 10) | improvement of skin healing and repair | [177] |
| cosmetic formulations based on a combination of horse colostrum and horse milk | healthy skin | antiaging, moisturizing, protective, tensio-distensive, tonic, smoothing, anti-irritant, emollient, bleaching, decongestant, and sebostatic effects | [177] |
| fermented (by lactic acid bacteria) horse colostrum | atopic dermatitis (atopy and psoriasis) | alleviating symptoms; moisturizing and anti-inflammatory effects | [178] |
| fermented colostrum | acne | improvement related to the antibacterial effect | [179] |
| formulations containing bovine or equine colostrum (plus hyaluronic acid or its salt and olive oil or vitamin E) | healthy skin of elderly volunteers | improvement elasticity and tension; moisturizing and antioxidant effects; reduction in skin sagging and liver spots | [180] |
| cosmetic formulation based on colostrum albumin (plus arbutin) | healthy skin with discoloration | whitening properties | [181] |
| Milk-based products used as supplements | |||
| fermented milk enriched with lactoferrin | acne vulgaris (n = 18) | reduction in inflammation, sebum content, and the severity of acne lesions | [90] |
| lactoferrin | mild to moderate common acne | overall improvement in acne lesions | [170] |
| capsules containing lactoferrin (plus vitamin E and zinc) | acne | reduction in the number of acne lesions, blackheads, and inflammatory changes; regulation of sebum secretion | [173] |
3.2. Influence of Milk or Milk-Derived Ingredients on Skin Cells In Vitro
3.3. Topical Applications of Milk or Colostrum Containing Products
4. Conclusions
Funding
Conflicts of Interest
References
- Jenness, R. Composition of Milk. Fundam. Dairy Chem. 1988, 1–38. [Google Scholar] [CrossRef]
- Audic, J.-L.; Chaufer, B.; Daufin, G. Non-Food Applications of Milk Components and Dairy Co-Products: A Review. Lait 2003, 83, 417–438. [Google Scholar] [CrossRef]
- Luisa, B.G. Handbook of Milk Composition; Elsevier: Amsterdam, The Netherlands, 1995; ISBN 978-0-08-053311-7. [Google Scholar]
- Ballard, O.; Morrow, A.L. Human Milk Composition: Nutrients and Bioactive Factors. Pediatric Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef]
- Vollmer, D.; West, V.; Lephart, E. Enhancing Skin Health: By Oral Administration of Natural Compounds and Minerals with Implications to the Dermal Microbiome. Int. J. Mol. Sci. 2018, 19, 3059. [Google Scholar] [CrossRef] [PubMed]
- McGrath, B.A.; Fox, P.F.; McSweeney, P.L.H.; Kelly, A.L. Composition and Properties of Bovine Colostrum: A Review. Dairy Sci. Technol. 2016, 96, 133–158. [Google Scholar] [CrossRef]
- Bergman, A.J.; Turner, C.W. The Composition of the Colostrum of the Dairy Goat. J. Dairy Sci. 1937, 20, 37–45. [Google Scholar] [CrossRef]
- Harjanti, D.W.; Ciptaningtyas, R.; Al-Baarri, A.N.; Kusumanti, E. Isolation and Identification of Lactoferrin and Lactoperoxidase from the Colostrum of Indonesian Ettawa Crossbred Goat. Adv. Sci. Lett. 2017, 23, 3321–3324. [Google Scholar] [CrossRef][Green Version]
- Rashida, K.; Ahmed, T.; Mirza, B. Comparative Analysis of Quality of Milk Collected from Buffalo, Cow, Goat and Sheep of Rawalpindi/Islamabad Region in Pakistan. Asian J. Plant Sci. 2004, 3. [Google Scholar] [CrossRef]
- Haenlain, G.F.W. Nutritional Value of Dairy Products of Ewe and Goat Milk; International Dairy Federation: Brussels, Belgium, 1996. [Google Scholar]
- Wendorff, W.L.; Haenlein, G.F.W. Sheep Milk—Composition and Nutrition. In Handbook of Milk of Non-Bovine Mammals; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 210–221. [Google Scholar] [CrossRef]
- Anifantakis, E.M. Comparison of the Physico-Chemical Properties of Ewes’ and Cows’ Milk; FIL-IDF. Secretariat General; International Dairy Federation: Belgium, Brussels, 1986. [Google Scholar]
- Park, Y.W.; Juárez, M.; Ramos, M.; Haenlein, G.F.W. Physico-Chemical Characteristics of Goat and Sheep Milk. Small Rumin. Res. 2007, 68, 88–113. [Google Scholar] [CrossRef]
- Park, Y.W. (Ed.) Bioactive Components in Milk and Dairy Products; Wiley-Blackwell: Ames, IA, USA, 2009; ISBN 978-0-8138-1982-2. [Google Scholar]
- Gobbetti, M.; Stepaniak, L.; De Angelis, M.; Corsetti, A.; Di Cagno, R. Latent Bioactive Peptides in Milk Proteins: Proteolytic Activation and Significance in Dairy Processing. Crit. Rev. Food Sci. Nutr. 2002, 42, 223–239. [Google Scholar] [CrossRef]
- Clare, D.A.; Swaisgood, H.E. Bioactive Milk Peptides: A Prospectus. J. Dairy Sci. 2000, 83, 1187–1195. [Google Scholar] [CrossRef]
- Cheema, M.; Hristov, A.N.; Harte, F.M. The Binding of Orally Dosed Hydrophobic Active Pharmaceutical Ingredients to Casein Micelles in Milk. J. Dairy Sci. 2017, 100, 8670–8679. [Google Scholar] [CrossRef]
- Horne, D.S. Analytical Methods | Light Scattering Techniques. In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J.W., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 133–140. ISBN 978-0-12-374407-4. [Google Scholar]
- Byun, J.S.; Lee, S.S. Effect of Soybeans and Sword Beans on Bone Metabolism in a Rat Model of Osteoporosis. Ann. Nutr. Metab. 2010, 56, 106–112. [Google Scholar] [CrossRef]
- Milan, A.M.; Waddington, R.J.; Embery, G. Fluoride Alters Casein Kinase II and Alkaline Phosphatase Activity in Vitro with Potential Implications for Dentine Mineralization. Arch. Oral Biol. 2001, 46, 343–351. [Google Scholar] [CrossRef]
- Meisel, H. Biochemical Properties of Bioactive Peptides Derived from Milk Proteins: Potential Nutraceuticals for Food and Pharmaceutical Applications. Livest. Prod. Sci. 1997, 50, 125–138. [Google Scholar] [CrossRef]
- Park, Y.; Haenlein, G.F.W.; Wendorff, W. Handbook of Milk of Non-Bovine Mammals, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017; ISBN 978-1-119-11027-9. [Google Scholar]
- Moatsou, G.; Samolada, M.; Katsabeki, A.; Anifantakis, E. Casein Fraction of Ovine Milk from Indigenous Greek Breeds. Lait 2004, 84, 285–296. [Google Scholar] [CrossRef]
- Al-Saadi, J.M.S.; Deeth, H.C. Preparation and Functional Properties of Protein Coprecipitate from Sheep Milk. Int. J. Dairy Technol. 2011, 64, 461–466. [Google Scholar] [CrossRef]
- Levieux, D.; Morgan, F.; Geneix, N.; Masle, I.; Bouvier, F. Caprine Immunoglobulin G, Beta-Lactoglobulin, Alpha-Lactalbumin and Serum Albumin in Colostrum and Milk during the Early Post Partum Period. J. Dairy Res. 2002, 69, 391–399. [Google Scholar] [CrossRef]
- Perez, M.D.; Sanchez, L.; Aranda, P.; Ena, J.M.; Oria, R.; Calvo, M. Synthesis and Evolution of Concentration of Beta-Lactoglobulin and Alpha-Lactalbumin from Cow and Sheep Colostrum and Milk throughout Early Lactation. Cell. Mol. Biol. 1990, 36, 205–212. [Google Scholar] [PubMed]
- Ruprichova, L.; Kralova, M.; Borkovcova, I.; Vorlova, L.; Bedanova, I. Determination of Whey Proteins in Different Types of Milk. Acta Veterinaria Brno 2014, 83, 67–72. [Google Scholar] [CrossRef]
- Feeney, S.; Morrin, S.T.; Joshi, L.; Hickey, R.M. The Role of Immunoglobulins from Bovine Colostrum and Milk in Human Health Promotion. In Novel Proteins for Food, Pharmaceuticals and Agriculture; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 291–314. ISBN 978-1-119-38533-2. [Google Scholar]
- Atkinson, D.J.; von Keyserlingk, M.A.G.; Weary, D.M. Benchmarking Passive Transfer of Immunity and Growth in Dairy Calves. J. Dairy Sci. 2017, 100, 3773–3782. [Google Scholar] [CrossRef]
- Roginski, H.; Fuquay, J.W.; Fox, P.F. Encyclopedia of Dairy Sciences; Academic Press: Cambridge, MA, USA, 2003; ISBN 978-0-12-227235-6. [Google Scholar]
- Stelwagen, K.; Carpenter, E.; Haigh, B.; Hodgkinson, A.; Wheeler, T.T. Immune Components of Bovine Colostrum and Milk. J. Anim. Sci. 2009, 87, 3–9. [Google Scholar] [CrossRef]
- Kehoe, S.I.; Jayarao, B.M.; Heinrichs, A.J. A Survey of Bovine Colostrum Composition and Colostrum Management Practices on Pennsylvania Dairy Farms1. J. Dairy Sci. 2007, 90, 4108–4116. [Google Scholar] [CrossRef] [PubMed]
- Mesmin, C.; Fenaille, F.; Becher, F.; Tabet, J.-C.; Ezan, E. Identification and Characterization of Apelin Peptides in Bovine Colostrum and Milk by Liquid Chromatography–Mass Spectrometry. J. Proteome Res. 2011, 10, 5222–5231. [Google Scholar] [CrossRef] [PubMed]
- Recio, I.; de la Fuente, M.A.; Juárez, M.; Ramos, M. Bioactive Components in Sheep Milk. In Bioactive Components in Milk and Dairy Products; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; pp. 83–104. ISBN 978-0-8138-2150-4. [Google Scholar]
- Tabatabaei, S.; Nikbakht, G.; Vatankhah, M.; Sharifi, H.; Alidadi, N. Variation in Colostral Immunoglobulin G Concentration in Fat Tailed Sheep and Evaluation of Methods for Estimation of Colostral Immunoglobulin Content. Acta Veterinaria Brno 2013, 82, 271–275. [Google Scholar] [CrossRef]
- Navarro, F.; Galan-Malo, P.; Pérez, M.D.; Abecia, J.-A.; Mata, L.; Calvo, M.; Sánchez, L. Lactoferrin and IgG Levels in Ovine Milk throughout Lactation: Correlation with Milk Quality Parameters. Small Rumin. Res. 2018, 168, 12–18. [Google Scholar] [CrossRef]
- Hernández-Castellano, L.E.; Almeida, A.M.; Renaut, J.; Argüello, A.; Castro, N. A Proteomics Study of Colostrum and Milk from the Two Major Small Ruminant Dairy Breeds from the Canary Islands: A Bovine Milk Comparison Perspective. J. Dairy Res. 2016, 83, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Farrell, H.M.; Jimenez-Flores, R.; Bleck, G.T.; Brown, E.M.; Butler, J.E.; Creamer, L.K.; Hicks, C.L.; Hollar, C.M.; Ng-Kwai-Hang, K.F.; Swaisgood, H.E. Nomenclature of the Proteins of Cows’ Milk—Sixth Revision. J. Dairy Sci. 2004, 87, 1641–1674. [Google Scholar] [CrossRef]
- Madureira, A.R.; Pereira, C.I.; Gomes, A.M.P.; Pintado, M.E.; Xavier Malcata, F. Bovine Whey Proteins—Overview on Their Main Biological Properties. Food Res. Int. 2007, 40, 1197–1211. [Google Scholar] [CrossRef]
- Smithers, G.W. Whey and Whey Proteins—From ‘Gutter-to-Gold’. Int. Dairy J. 2008, 18, 695–704. [Google Scholar] [CrossRef]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Lactoferrin: Structure, Function, Denaturation and Digestion. Crit. Rev. Food Sci. Nutr. 2019, 59, 580–596. [Google Scholar] [CrossRef]
- Mulder, A.M.; Connellan, P.A.; Oliver, C.J.; Morris, C.A.; Stevenson, L.M. Bovine Lactoferrin Supplementation Supports Immune and Antioxidant Status in Healthy Human Males. Nutr. Res. 2008, 28, 583–589. [Google Scholar] [CrossRef]
- Mehra, R.; Singh, R.; Nayan, V.; Buttar, H.S.; Kumar, N.; Kumar, S.; Bhardwaj, A.; Kaushik, R.; Kumar, H. Nutritional Attributes of Bovine Colostrum Components in Human Health and Disease: A Comprehensive Review. Food Biosci. 2021, 40, 100907. [Google Scholar] [CrossRef]
- McSweeney, P.L.H.; Fox, P.F. (Eds.) Advanced Dairy Chemistry: Volume 1A: Proteins: Basic Aspects, 4th ed.; Springer: Boston, MA, USA, 2013; ISBN 978-1-4614-4713-9. [Google Scholar]
- Hernández-Ledesma, B.; Ramos, M.; Gómez-Ruiz, J.Á. Bioactive Components of Ovine and Caprine Cheese Whey. Small Rumin. Res. 2011, 101, 196–204. [Google Scholar] [CrossRef]
- Blum, J.W.; Hammon, H. Colostrum Effects on the Gastrointestinal Tract, and on Nutritional, Endocrine and Metabolic Parameters in Neonatal Calves. Livest. Prod. Sci. 2000, 66, 151–159. [Google Scholar] [CrossRef]
- Campbell, P.G.; Baumrucker, C.R. Insulin-like Growth Factor-I and Its Association with Binding Proteins in Bovine Milk. J. Endocrinol. 1989, 120, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Elfstrand, L.; Lindmark-Månsson, H.; Paulsson, M.; Nyberg, L.; Åkesson, B. Immunoglobulins, Growth Factors and Growth Hormone in Bovine Colostrum and the Effects of Processing. Int. Dairy J. 2002, 12, 879–887. [Google Scholar] [CrossRef]
- Ginjala, V.; Pakkanen, R. Determination of Transforming Growth Factor-Β1 (TGF-Β1) and Insulin-Like Growth Factor 1 (IGF-1) in Bovine Colostrum Samples. J. Immunoass. 1998, 19, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Hadsell, D.L.; Baumrucker, C.R.; Kensinger, R.S. Effects of Elevated Blood Insulin-like Growth Factor-I (IGF-I) Concentration upon IGF-I in Bovine Mammary Secretions during the Colostrum Phase. J. Endocrinol. 1993, 137, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Malven, P.V.; Head, H.H.; Collier, R.J.; Buonomo, F.C. Periparturient Changes in Secretion and Mammary Uptake of Insulin and in Concentrations of Insulin and Insulin-like Growth Factors in Milk of Dairy Cows. J. Dairy Sci. 1987, 70, 2254–2265. [Google Scholar] [CrossRef]
- Marcotty, C.; Frankenne, F.; Van Beeumen, J.; Maghuin-Rogister, G.; Hennen, G. Insulin-like Growth Factor I (IGF-I) from Cow Colostrum: Purification and Characterization. Growth Regul. 1991, 1, 56–61. [Google Scholar]
- Oda, S.; Satoh, H.; Sugawara, T.; Matsunaga, N.; Kuhara, T.; Katoh, K.; Shoji, Y.; Nihei, A.; Ohta, M.; Sasaki, Y. Insulin-like Growth Factor-I, GH, Insulin and Glucagon Concentrations in Bovine Colostrum and in Plasma of Dairy Cows and Neonatal Calves around Parturition. Comp. Biochem. Physiol. Comp. Physiol. 1989, 94, 805–808. [Google Scholar] [CrossRef]
- Ontsouka, C.E.; Bruckmaier, R.M.; Blum, J.W. Fractionized Milk Composition during Removal of Colostrum and Mature Milk. J. Dairy Sci. 2003, 86, 2005–2011. [Google Scholar] [CrossRef]
- Sejrsen, K.; Pedersen, L.O.; Vestergaard, M.; Purup, S. Biological Activity of Bovine Milk: Contribution of IGF-I and IGF Binding Proteins. Livest. Prod. Sci. 2001, 70, 79–85. [Google Scholar] [CrossRef]
- Skaar, T.C.; Vega, J.R.; Pyke, S.N.; Baumrucker, C.R. Changes in Insulin-like Growth Factor-Binding Proteins in Bovine Mammary Secretions Associated with Pregnancy and Parturition. J. Endocrinol. 1991, 131, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Sparks, A.L.; Kirkpatrick, J.G.; Chamberlain, C.S.; Waldner, D.; Spicer, L.J. Insulin-like Growth Factor-I and Its Binding Proteins in Colostrum Compared to Measures in Serum of Holstein Neonates. J. Dairy Sci. 2003, 86, 2022–2029. [Google Scholar] [CrossRef]
- Vacher, P.-Y.; Blum, J.W. Age-Dependency of Insulin-like Growth Factor I, Insulin, Protein and Immunoglobulin Concentrations and Gamma-Glutamyl-Transferase Activity in First Colostrum of Dairy Cows. Milchwissenschaft 1993, 48, 423–426. [Google Scholar]
- Simmen, F.A.; Simmen, R.C.; Reinhart, G. Maternal and Neonatal Somatomedin C/Insulin-like Growth Factor-I (IGF-I) and IGF Binding Proteins during Early Lactation in the Pig. Dev. Biol. 1988, 130, 16–27. [Google Scholar] [CrossRef]
- Hall, D.G.; Holst, P.J.; Shutt, D.A. The Effect of Nutritional Supplements in Late Pregnancy on Ewe Colostrum Production Plasma Progesterone and IGF-1 Concentrations. Aust. J. Agric. Res. 1992, 43, 325–337. [Google Scholar] [CrossRef]
- Prosser, C.G.; Royle, C.; Fleet, I.R.; Mepham, T.B. The Galactopoietic Effect of Bovine Growth Hormone in Goats Is Associated with Increased Concentrations of Insulin-like Growth Factor-I in Milk and Mammary Tissue. J. Endocrinol. 1991, 128, 457–463. [Google Scholar] [CrossRef]
- Prosser, C.G.; Fleet, I.R.; Davis, A.J.; Heap, R.B. Mechanism of Secretion of Plasma Insulin-like Growth Factor-I into Milk of Lactating Goats. J. Endocrinol. 1991, 131, 459–466. [Google Scholar] [CrossRef]
- Prosser, C.G.; Fleet, I.R.; Corps, A.N.; Froesch, E.R.; Heap, R.B. Increase in Milk Secretion and Mammary Blood Flow by Intra-Arterial Infusion of Insulin-like Growth Factor-I into the Mammary Gland of the Goat. J. Endocrinol. 1990, 126, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Yagi, H.; Suzuki, S.; Noji, T.; Nagashima, K.; Kuroume, T. Epidermal Growth Factor in Cow’s Milk and Milk Formulas. Acta Paediatr. 1986, 75, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Iacopetta, B.J.; Grieu, F.; Horisberger, M.; Sunahara, G.I. Epidermal Growth Factor in Human and Bovine Milk. Acta Paediatr. 1992, 81, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Gow, C.B.; Singleton, D.J.; Silvapulle, M.J.; Moore, G.P.M. Lack of Effect of Epidermal Growth Factor Treatment in Late-Pregnant Ewes on Subsequent Lactation. J. Dairy Res. 1991, 58, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pakkanen, R. Determination of Transforming Growth Factor-Beta 2 (TGF-Beta 2) in Bovine Colostrum Samples. J. Immunoass. 1998, 19, 23–37. [Google Scholar] [CrossRef]
- Gobbetti, M.; Minervini, F.; Rizzello, C.G. Angiotensin I-Converting-Enzyme-Inhibitory and Antimicrobial Bioactive Peptides. Int. J. Dairy Technol. 2004, 57, 173–188. [Google Scholar] [CrossRef]
- Gobbetti, M.; Minervini, F.; Rizzello, C.G. Bioactive Peptides in Dairy Products. In Handbook of Food Products Manufacturing; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 489–517. ISBN 978-0-470-11355-4. [Google Scholar]
- FitzGerald, R.J.; Meisel, H. Milk Protein-Derived Peptide Inhibitors of Angiotensin-I-Converting Enzyme. Br. J. Nutr. 2000, 84, 33–37. [Google Scholar] [CrossRef]
- Brody, E.P. Biological Activities of Bovine Glycomacropeptide. Br. J. Nutr. 2000, 84, S39–S46. [Google Scholar] [CrossRef]
- Manso, M. Casein Macropeptides from Cheese Whey: Physicochemical, Biological, Nutritional, and Technological Features for Possible Uses. Food Rev. Int. 2004, 20, 329–355. [Google Scholar] [CrossRef]
- Song, J.; Gao, J.; Du, M.; Mao, X. Casein Glycomacropeptide Hydrolysates Ameliorate Hepatic Insulin Resistance of C57BL/6J Mice Challenged with High-Fat Diet. J. Funct. Foods 2018, 45, 190–198. [Google Scholar] [CrossRef]
- Li, T.; Gao, D.; Du, M.; Cheng, X.; Mao, X. Casein Glycomacropeptide Hydrolysates Inhibit PGE2 Production and COX2 Expression in LPS-Stimulated RAW 264.7 Macrophage Cells via Akt Mediated NF-ΚB and MAPK Pathways. Available online: https://pubmed-1ncbi-1nlm-1nih-1gov-12ik8q6jo0903.han.cib.umed.lodz.pl/29666854/ (accessed on 27 May 2020).
- Yuan, Q.; Zhan, B.; Chang, R.; Du, M.; Mao, X. Antidiabetic Effect of Casein Glycomacropeptide Hydrolysates on High-Fat Diet and STZ-Induced Diabetic Mice via Regulating Insulin Signaling in Skeletal Muscle and Modulating Gut Microbiota. Nutrients 2020, 12, 220. [Google Scholar] [CrossRef]
- Semo, E.; Kesselman, E.; Danino, D.; Livney, Y.D. Casein Micelle as a Natural Nano-Capsular Vehicle for Nutraceuticals. Food Hydrocoll. 2007, 21, 936–942. [Google Scholar] [CrossRef]
- Ma, J.; An, W.; Xu, Q.; Fan, Q.; Wang, Y. Antibacterial Casein-Based ZnO Nanocomposite Coatings with Improved Water Resistance Crafted via Double in Situ Route. Prog. Org. Coat. 2019, 134, 40–47. [Google Scholar] [CrossRef]
- Cheng, H.; Dong, H.; Liang, L. A Comparison of β-Casein Complexes and Micelles as Vehicles for Trans-/Cis-Resveratrol. Food Chem. 2020, 330, 127209. [Google Scholar] [CrossRef]
- Razmi, M.; Divsalar, A.; Saboury, A.A.; Izadi, Z.; Haertlé, T.; Mansuri-Torshizi, H. Beta-Casein and Its Complexes with Chitosan as Nanovehicles for Delivery of a Platinum Anticancer Drug. Colloids Surf. B Biointerfaces 2013, 112, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Chatterton, D.E.W.; Smithers, G.; Roupas, P.; Brodkorb, A. Bioactivity of β-Lactoglobulin and α-Lactalbumin—Technological Implications for Processing. Int. Dairy J. 2006, 16, 1229–1240. [Google Scholar] [CrossRef]
- Markus, C.R.; Olivier, B.; De Haan, E.H. Whey Protein Rich in α-Lactalbumin Increases the Ratio of Plasma Tryptophan to the Sum of the Other Large Neutral Amino Acids and Improves Cognitive Performance in Stress-Vulnerable Subjects. Am. J. Clin. Nutr. 2002, 75, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Nurgali, K.; Mishra, V.K. Food Proteins as Source of Opioid Peptides—A Review. Curr. Med. Chem. 2016, 23, 893–910. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Daliri, E.B.-M.; Kwami Ofosu, F.; Yeon, S.-J.; Oh, D.-H. Food-Derived Opioid Peptides in Human Health: A Review. Int. J. Mol. Sci. 2020, 21, 8825. [Google Scholar] [CrossRef]
- Pellegrini, A.; Thomas, U.; Bramaz, N.; Hunziker, P. Isolation and Identification of Three Bactericidal Domains in the Bovine α-Lactalbumin Molecule. Biochim. Biophys. Acta 1999, 1426, 439–448. [Google Scholar] [CrossRef]
- Mok, K.H.; Pettersson, J.; Orrenius, S.; Svanborg, C. HAMLET, Protein Folding, and Tumor Cell Death. Biochem. Biophys. Res. Commun. 2007, 354, 1–7. [Google Scholar] [CrossRef]
- Mossberg, A.-K.; Hun Mok, K.; Morozova-Roche, L.A.; Svanborg, C. Structure and Function of Human α-Lactalbumin Made Lethal to Tumor Cells (HAMLET)-Type Complexes. FEBS J. 2010, 277, 4614–4625. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, L.; Leijonhufvud, I.; Aronsson, A.; Mossberg, A.-K.; Svanborg, C. Treatment of Skin Papillomas with Topical Alpha-Lactalbumin-Oleic Acid. N. Engl. J. Med. 2004, 350, 2663–2672. [Google Scholar] [CrossRef] [PubMed]
- Mossberg, A.-K.; Wullt, B.; Gustafsson, L.; Månsson, W.; Ljunggren, E.; Svanborg, C. Bladder Cancers Respond to Intravesical Instillation of HAMLET (Human Alpha-Lactalbumin Made Lethal to Tumor Cells). Int. J. Cancer 2007, 121, 1352–1359. [Google Scholar] [CrossRef] [PubMed]
- Creamer, L.K.; Harris, D.P. Relationship between Milk Protein Polymorphism and Physico-Chemical Properties; New Z.D.R.I.; International Dairy Federation: Brussels, Belgium, 1997. [Google Scholar]
- Kim, J.; Ko, Y.; Park, Y.-K.; Kim, N.-I.; Ha, W.-K.; Cho, Y. Dietary Effect of Lactoferrin-Enriched Fermented Milk on Skin Surface Lipid and Clinical Improvement of Acne Vulgaris. Nutrition 2010, 26, 902–909. [Google Scholar] [CrossRef]
- Bogahawaththa, D.; Chandrapala, J.; Vasiljevic, T. Thermal Denaturation of Bovine β-Lactoglobulin in Different Protein Mixtures in Relation to Antigenicity. Int. Dairy J. 2019, 91, 89–97. [Google Scholar] [CrossRef]
- Liu, H.C.; Chen, W.L.; Mao, S.J.T. Antioxidant Nature of Bovine Milk Beta-Lactoglobulin. J. Dairy Sci. 2007, 90, 547–555. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; Dávalos, A.; Bartolomé, B.; Amigo, L. Preparation of Antioxidant Enzymatic Hydrolysates from α-Lactalbumin and β-Lactoglobulin. Identification of Active Peptides by HPLC-MS/MS. J. Agric. Food Chem. 2005, 53, 588–593. [Google Scholar] [CrossRef]
- McIntosh, G.H.; Royle, P.J.; Le Leu, R.K.; Regester, G.O.; Johnson, M.A.; Grinsted, R.L.; Kenward, R.S.; Smithers, G.W. Whey Proteins as Functional Food Ingredients? Int. Dairy J. 1998, 8, 425–434. [Google Scholar] [CrossRef]
- Wanders, D.; Hobson, K.; Ji, X. Methionine Restriction and Cancer Biology. Nutrients 2020, 12, 684. [Google Scholar] [CrossRef]
- Pan, Y.; Shiell, B.; Wan, J.; Coventry, M.J.; Michalski, W.P.; Leeb, A.; Roginski, H. The Molecular Characterisation and Antimicrobial Properties of Amidated Bovine β-Lactoglobulin. Int. Dairy J. 2007, 17, 1450–1459. [Google Scholar] [CrossRef]
- Oevermann, A.; Engels, M.; Thomas, U.; Pellegrini, A. The Antiviral Activity of Naturally Occurring Proteins and Their Peptide Fragments after Chemical Modification. Antivir. Res. 2003, 59, 23–33. [Google Scholar] [CrossRef]
- Czosnykowska-Łukacka, M.; Orczyk-Pawiłowicz, M.; Broers, B.; Królak-Olejnik, B. Lactoferrin in Human Milk of Prolonged Lactation. Nutrients 2019, 11, 2350. [Google Scholar] [CrossRef]
- Rahman, M.; Kim, W.-S.; Kumura, H.; Shimazaki, K. Bovine Lactoferrin Region Responsible for Binding to Bifidobacterial Cell Surface Proteins. Biotechnol. Lett. 2009, 31, 863–868. [Google Scholar] [CrossRef]
- Steijns, J.M.; van Hooijdonk, A.C.M. Occurrence, Structure, Biochemical Properties and Technological Characteristics of Lactoferrin. Br. J. Nutr. 2000, 84, 11–17. [Google Scholar] [CrossRef]
- Bobbarala, V. A Search for Antibacterial Agents; InTech: Rijeka, Croatia, 2012; ISBN 978-953-51-0724-8. [Google Scholar]
- Baker, E.N.; Baker, H.M. Molecular Structure, Binding Properties and Dynamics of Lactoferrin. Cell. Mol. Life Sci. 2005, 62, 2531. [Google Scholar] [CrossRef]
- Legrand, D.; Pierce, A.; Elass, E.; Carpentier, M.; Mariller, C.; Mazurier, J. Lactoferrin Structure and Functions. Bioact. Compon. Milk 2008, 163–194. [Google Scholar] [CrossRef]
- Orsi, N. The Antimicrobial Activity of Lactoferrin: Current Status and Perspectives. Biometals 2004, 17, 189–196. [Google Scholar] [CrossRef]
- Żelechowska, P.; Agier, J.; Brzezińska-Błaszczyk, E. Endogenous Antimicrobial Factors in the Treatment of Infectious Diseases. Cent. Eur. J. Immunol. 2016, 41, 419–425. [Google Scholar] [CrossRef]
- Zimecki, M.; Spiegel, K.; Właszczyk, A.; Kübler, A.; Kruzel, M.L. Lactoferrin Increases the Output of Neutrophil Precursors and Attenuates the Spontaneous Production of TNF-Alpha and IL-6 by Peripheral Blood Cells. Arch. Immunol. Exp. 1999, 47, 113–118. [Google Scholar]
- Yamauchi, K.; Wakabayashi, H.; Hashimoto, S.; Teraguchi, S.; Hayasawa, H.; Tomita, M. Effects of Orally Administered Bovine Lactoferrin on the Immune System of Healthy Volunteers. Adv. Exp. Med. Biol. 1998, 443, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H.; Yamauchi, K.; Takase, M. Lactoferrin Research, Technology and Applications. Int. Dairy J. 2006, 16, 1241–1251. [Google Scholar] [CrossRef]
- Van der Strate, B.W.; Beljaars, L.; Molema, G.; Harmsen, M.C.; Meijer, D.K. Antiviral Activities of Lactoferrin. Antivir. Res. 2001, 52, 225–239. [Google Scholar] [CrossRef]
- Van der Kraan, M.I.A.; Groenink, J.; Nazmi, K.; Veerman, E.C.I.; Bolscher, J.G.M.; Nieuw Amerongen, A.V. Lactoferrampin: A Novel Antimicrobial Peptide in the N1-Domain of Bovine Lactoferrin. Peptides 2004, 25, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Superti, F.; Ammendolia, M.G.; Valenti, P.; Seganti, L. Antirotaviral Activity of Milk Proteins: Lactoferrin Prevents Rotavirus Infection in the Enterocyte-like Cell Line HT-29. Med. Microbiol. Immunol. 1997, 186, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Siqueiros-Cendón, T.; Arévalo-Gallegos, S.; Iglesias-Figueroa, B.F.; García-Montoya, I.A.; Salazar-Martínez, J.; Rascón-Cruz, Q. Immunomodulatory Effects of Lactoferrin. Acta Pharm. Sin. 2014, 35, 557–566. [Google Scholar] [CrossRef]
- Seganti, L.; Di Biase, A.M.; Marchetti, M.; Pietrantoni, A.; Tinari, A.; Superti, F. Antiviral Activity of Lactoferrin towards Naked Viruses. Biometals 2004, 17, 295–299. [Google Scholar] [CrossRef]
- Saraceno, R.; Gramiccia, T.; Chimenti, S.; Valenti, P.; Pietropaoli, M.; Bianchi, L. Topical Lactoferrin Can Improve Stable Psoriatic Plaque. G. Ital. Dermatol. Venereol. 2014, 149, 335–340. [Google Scholar]
- Superti, F. Lactoferrin from Bovine Milk: A Protective Companion for Life. Nutrients 2020, 12, 2562. [Google Scholar] [CrossRef]
- Drago-Serrano, M.E.; de la Garza-Amaya, M.; Luna, J.S.; Campos-Rodríguez, R. Lactoferrin-Lipopolysaccharide (LPS) Binding as Key to Antibacterial and Antiendotoxic Effects. Int. Immunopharmacol. 2012, 12, 1–9. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Uchida, K.; Yamauchi, K.; Teraguchi, S.; Hayasawa, H.; Yamaguchi, H. Lactoferrin given in Food Facilitates Dermatophytosis Cure in Guinea Pig Models. J. Antimicrob. Chemother. 2000, 46, 595–602. [Google Scholar] [CrossRef]
- Fernandes, K.E.; Carter, D.A. The Antifungal Activity of Lactoferrin and Its Derived Peptides: Mechanisms of Action and Synergy with Drugs against Fungal Pathogens. Front. Microbiol. 2017, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Leboffe, L.; Giansanti, F.; Antonini, G. Antifungal and Antiparasitic Activities of Lactoferrin. Anti Infect. Agents Med. Chem. 2009, 8, 114–127. [Google Scholar] [CrossRef]
- Berlutti, F.; Pantanella, F.; Natalizi, T.; Frioni, A.; Paesano, R.; Polimeni, A.; Valenti, P. Antiviral Properties of Lactoferrin—A Natural Immunity Molecule. Molecules 2011, 16, 6992–7018. [Google Scholar] [CrossRef]
- Redwan, E.M.; Uversky, V.N.; El-Fakharany, E.M.; Al-Mehdar, H. Potential Lactoferrin Activity against Pathogenic Viruses. Comptes Rendus Biol. 2014, 337, 581–595. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; Frevert, U. Dual Interaction of the Malaria Circumsporozoite Protein with the Low Density Lipoprotein Receptor-Related Protein (LRP) and Heparan Sulfate Proteoglycans. J. Exp. Med. 1996, 184, 1699–1711. [Google Scholar] [CrossRef] [PubMed]
- Cirioni, O.; Giacometti, A.; Barchiesi, F.; Scalise, G. Inhibition of Growth of Pneumocystis Carinii by Lactoferrins Alone and in Combination with Pyrimethamine, Clarithromycin and Minocycline. J. Antimicrob. Chemother. 2000, 46, 577–582. [Google Scholar] [CrossRef] [PubMed]
- León-Sicairos, N.; Reyes-López, M.; Ordaz-Pichardo, C.; de la Garza, M. Microbicidal Action of Lactoferrin and Lactoferricin and Their Synergistic Effect with Metronidazole in Entamoeba Histolytica. Biochem. Cell Biol. 2006, 84, 327–336. [Google Scholar] [CrossRef]
- Weinberg, G.A. Iron Chelators as Therapeutic Agents against Pneumocystis Carinii. Antimicrob. Agents Chemother. 1994, 38, 997–1003. [Google Scholar] [CrossRef]
- Jenssen, H.; Hancock, R. Antimicrobial Properties of Lactoferrin. Biochimie 2009, 91, 19–29. [Google Scholar] [CrossRef]
- Superti, F.; Berlutti, F.; PAESANO, R. Structure and Activity of Lactoferrin—A Multi Functional Protective Agent for Human Health. Iron Metab. Dis. 2008, 8, 1–32. [Google Scholar]
- Andersen, J.H.; Jenssen, H.; Gutteberg, T.J. Lactoferrin and Lactoferricin Inhibit Herpes Simplex 1 and 2 Infection and Exhibit Synergy When Combined with Acyclovir. Antivir. Res. 2003, 58, 209–215. [Google Scholar] [CrossRef]
- Hendrixson, D.R.; Qiu, J.; Shewry, S.C.; Fink, D.L.; Petty, S.; Baker, E.N.; Plaut, A.G.; St Geme, J.W. Human Milk Lactoferrin Is a Serine Protease That Cleaves Haemophilus Surface Proteins at Arginine-Rich Sites. Mol. Microbiol. 2003, 47, 607–617. [Google Scholar] [CrossRef]
- Singh, H.; Ye, A.; Horne, D. Structuring Food Emulsions in the Gastrointestinal Tract to Modify Lipid Digestion. Prog. Lipid Res. 2009, 48, 92–100. [Google Scholar] [CrossRef]
- Diarra, M.S.; Petitclerc, D.; Lacasse, P. Effect of Lactoferrin in Combination with Penicillin on the Morphology and the Physiology of Staphylococcus Aureus Isolated from Bovine Mastitis. J. Dairy Sci. 2002, 85, 1141–1149. [Google Scholar] [CrossRef]
- Ishii, K.; Takamura, N.; Shinohara, M.; Wakui, N.; Shin, H.; Sumino, Y.; Ohmoto, Y.; Teraguchi, S.; Yamauchi, K. Long-Term Follow-up of Chronic Hepatitis C Patients Treated with Oral Lactoferrin for 12 Months. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2003, 25, 226–233. [Google Scholar] [CrossRef]
- Andersen, J.H.; Jenssen, H.; Sandvik, K.; Gutteberg, T.J. Anti-HSV Activity of Lactoferrin and Lactoferricin Is Dependent on the Presence of Heparan Sulphate at the Cell Surface. J. Med. Virol. 2004, 74, 262–271. [Google Scholar] [CrossRef]
- Berkhout, B.; Floris, R.; Recio, I.; Visser, S. The Antiviral Activity of the Milk Protein Lactoferrin against the Human Immunodeficiency Virus Type 1. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2004, 17, 291–294. [Google Scholar] [CrossRef]
- Brock, J.H. The Physiology of Lactoferrin. Biochem. Cell Biol. 2002, 80, 1–6. [Google Scholar] [CrossRef]
- Firestein, G.S.; Stanford, S.M. Mechanisms of Inflammation and Tissue Repair. In Goldman’s Cecil Medicine, 26th ed.; Goldman, L., Schafer, A., Eds.; Elsevier: Alpharetta, GA, USA, 2019; Chapter 42; pp. 212–216. ISBN 978-0-323-55087-1. [Google Scholar]
- Larkins, N. Potential Implications of Lactoferrin as a Therapeutic Agent. Am. J. Vet. Res. 2005, 66, 739–742. [Google Scholar] [CrossRef]
- Fox, F.; Kelly, A.L. Indigenous Enzymes in Milk: Overview and Historical Aspects—Part 1|Request PDF. Int. Dairy J. 2006, 16, 500–516. [Google Scholar] [CrossRef]
- Garcia, H.S.; López-Hernandez, A.; Hill, C.G. 4.47—Enzyme Technology—Dairy Industry Applications. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Academic Press: Burlington, VT, USA, 2011; pp. 567–574. ISBN 978-0-08-088504-9. [Google Scholar]
- Özer, B. Natural Anti-Microbial Systems | Lactoperoxidase and Lactoferrin. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Oxford, UK, 2014; pp. 930–935. ISBN 978-0-12-384733-1. [Google Scholar]
- Pakkanen, R.; Aalto, J. Growth Factors and Antimicrobial Factors of Bovine Colostrum. Int. Dairy J. 1997, 7, 285–297. [Google Scholar] [CrossRef]
- Seifu, E.; Buys, E.; Donkin, E. Significance of the Lactoperoxidase System in the Dairy Industry and Its Potential Applications: A Review. Trends Food Sci. Technol. 2005, 16, 137–154. [Google Scholar] [CrossRef]
- Shin, K.; Hayasawa, H.; Lönnerdal, B. Inhibition of Escherichia Coli Respiratory Enzymes by the Lactoperoxidase-Hydrogen Peroxide-Thiocyanate Antimicrobial System. J. Appl. Microbiol. 2001, 90, 489–493. [Google Scholar] [CrossRef]
- Belding, M.E.; Klebanoff, S.J.; Ray, C.G. Peroxidase-Mediated Virucidal Systems. Science 1970, 167, 195–196. [Google Scholar] [CrossRef]
- Purdy, M.A.; Tenovuo, J.; Pruitt, K.M.; White, W.E. Effect of Growth Phase and Cell Envelope Structure on Susceptibility of Salmonella Typhimurium to the Lactoperoxidase-Thiocyanate-Hydrogen Peroxide System. Infect. Immun. 1983, 39, 1187–1195. [Google Scholar] [CrossRef]
- El-Fakharany, E.M.; Abd-Elhamid, A.I.; El-Deeb, N.M. Preparation and Characterization of Novel Nanocombination of Bovine Lactoperoxidase with Dye Decolorizing and Anti-Bacterial Activity. Sci. Rep. 2019, 9, 8530. [Google Scholar] [CrossRef]
- Hurley, W.L.; Theil, P.K. Perspectives on Immunoglobulins in Colostrum and Milk. Nutrients 2011, 3, 442–474. [Google Scholar] [CrossRef]
- Czosnykowska-Łukacka, M.; Lis-Kuberka, J.; Królak-Olejnik, B.; Orczyk-Pawiłowicz, M. Changes in Human Milk Immunoglobulin Profile During Prolonged Lactation. Front. Pediatr. 2020, 8. [Google Scholar] [CrossRef]
- Gapper, L.W.; Copestake, D.E.J.; Otter, D.E.; Indyk, H.E. Analysis of Bovine Immunoglobulin G in Milk, Colostrum and Dietary Supplements: A Review. Anal. Bioanal. Chem. 2007, 389, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, H. Milk-Derived Bioactive Peptides: From Science to Applications. J. Funct. Foods 2009, 1, 177–187. [Google Scholar] [CrossRef]
- Chiang, B.H.; Su, C.K.; Tsai, G.J.; Tsao, G.T. Egg White Lysozyme Purification by Ultrafiltration and Affinity Chromatography. J. Food Sci. 2006, 58, 303–306. [Google Scholar] [CrossRef]
- Cegielska-Radziejewska, R.; Lesnierowski, G.; Kijowski, J. Properties and Application of Egg White Lysozyme and Its Modified Preparations—A Review. Pol. J. Food Nutr. Sci. 2008, 58, 5–10. [Google Scholar]
- Benkerroum, N. Antimicrobial Activity of Lysozyme with Special Relevance to Milk. Afr. J. Biotechnol. 2008, 7. [Google Scholar] [CrossRef]
- Clementi, E.A.; Wilhelm, K.R.; Schleucher, J.; Morozova-Roche, L.A.; Hakansson, A.P. A Complex of Equine Lysozyme and Oleic Acid with Bactericidal Activity against Streptococcus Pneumoniae. PLoS ONE 2013, 8, e80649. [Google Scholar] [CrossRef]
- Tripathy, N.; Ahmad, R.; Bang, S.H.; Min, J.; Hahn, Y.-B. Tailored Lysozyme–ZnO Nanoparticle Conjugates as Nanoantibiotics. Chem. Commun. 2014, 50, 9298–9301. [Google Scholar] [CrossRef] [PubMed]
- Janusz, M.; Woszczyna, M.; Lisowski, M.; Kubis, A.; Macała, J.; Gotszalk, T.; Lisowski, J. Ovine Colostrum Nanopeptide Affects Amyloid Beta Aggregation. FEBS Lett. 2009, 583, 190–196. [Google Scholar] [CrossRef]
- Kruzel, M.L.; Janusz, M.; Lisowski, J.; Fischleigh, R.V.; Georgiades, J.A. Towards an Understanding of Biological Role of Colostrinin Peptides. J. Mol. Neurosci. 2001, 17, 379–389. [Google Scholar] [CrossRef]
- Boldogh, I.; Aguilera-Aguirre, L.; Bacsi, A.; Choudhury, B.K.; Saavedra-Molina, A.; Kruzel, M. Colostrinin Decreases Hypersensitivity and Allergic Responses to Common Allergens. Int. Arch. Allergy Immunol. 2008, 146, 298–306. [Google Scholar] [CrossRef]
- Leszek, J.; Inglot, A.D.; Janusz, M.; Byczkiewicz, F.; Kiejna, A.; Georgiades, J.; Lisowski, J. Colostrinin Proline-Rich Polypeptide Complex from Ovine Colostrum--a Long-Term Study of Its Efficacy in Alzheimer’s Disease. Med. Sci. Monit. 2002, 8, PI93–PI96. [Google Scholar] [PubMed]
- Mailloux, A.W.; Zhang, L.; Moscinski, L.; Bennett, J.M.; Yang, L.; Yoder, S.J.; Bloom, G.; Wei, C.; Wei, S.; Sokol, L.; et al. Fibrosis and Subsequent Cytopenias Are Associated with Basic Fibroblast Growth Factor-Deficient Pluripotent Mesenchymal Stromal Cells in Large Granular Lymphocyte Leukemia. Available online: https://pubmed.ncbi.nlm.nih.gov/24014875/ (accessed on 8 July 2020).
- Fiore, M.; Chaldakov, G.N.; Aloe, L. Nerve Growth Factor as a Signaling Molecule for Nerve Cells and Also for the Neuroendocrine-Immune Systems. Rev. Neurosci. 2009, 20, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Galazios, G.; Papazoglou, D.; Tsikouras, P.; Kolios, G. Vascular Endothelial Growth Factor Gene Polymorphisms and Pregnancy. J. Matern. Fetal. Neonatal. Med. 2009, 22, 371–378. [Google Scholar] [CrossRef]
- Gauthier, S.F.; Pouliot, Y.; Maubois, J.-L. Growth Factors from Bovine Milk and Colostrum: Composition, Extraction and Biological Activities. Lait 2006, 86, 99–125. [Google Scholar] [CrossRef]
- Mehra, R.; Marnila, P.; Korhonen, H. Milk Immunoglobulins for Health Promotion. Int. Dairy J. 2006, 16, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Struff, W.; Sprotte, G. Bovine Colostrum as a Biologic in Clinical Medicine: A Review—Part II: Clinical Studies. Int. J. Clin. Pharmacol. Ther. 2008, 46, 211–225. [Google Scholar] [CrossRef]
- Triantafillidis, J.K.; Merikas, E.; Georgopoulos, F. Current and Emerging Drugs for the Treatment of Inflammatory Bowel Disease. Drug Des. Dev. Ther. 2011, 5, 185–210. [Google Scholar] [CrossRef]
- Song, Y.; Pimentel, C.; Walters, K.; Boller, L.; Ghiasvand, S.; Liu, J.; Staley, K.J.; Berdichevsky, Y. Neuroprotective Levels of IGF-1 Exacerbate Epileptogenesis after Brain Injury. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Playford, R.J.; Macdonald, C.E.; Johnson, W.S. Colostrum and Milk-Derived Peptide Growth Factors for the Treatment of Gastrointestinal Disorders. Am. J. Clin. Nutr. 2000, 72, 5–14. [Google Scholar] [CrossRef]
- Bhora, F.Y.; Dunkin, B.J.; Batzri, S.; Aly, H.M.; Bass, B.L.; Sidawy, A.N.; Harmon, J.W. Effect of Growth Factors on Cell Proliferation and Epithelialization in Human Skin. J. Surg. Res. 1995, 59, 236–244. [Google Scholar] [CrossRef]
- Mueller, E.A.; Trapp, S.; Frentzel, A.; Kirch, W.; Brantl, V. Efficacy and Tolerability of Oral Lactoferrin Supplementation in Mild to Moderate Acne Vulgaris: An Exploratory Study. Curr. Med. Res. Opin. 2011, 27, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Poulin, Y.; Pouliot, Y.; Lamiot, E.; Aattouri, N.; Gauthier, S. Safety and Efficacy of a Milk-Derived Extract in the Treatment of Plaque Psoriasis:An Open-Label Study. J. Cutan. Med. Surg. 2006, 9, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Satoh, T.; Wakabayashi, H.; Yamauchi, K.; Abe, F.; Nomura, Y. Oral Administration of Bovine Lactoferrin Attenuates Ultraviolet B-Induced Skin Photodamage in Hairless Mice. J. Dairy Sci. 2014, 97, 651–658. [Google Scholar] [CrossRef]
- Chan, H.; Chan, G.; Santos, J.; Dee, K.; Co, J.K. A Randomized, Double-Blind, Placebo-Controlled Trial to Determine the Efficacy and Safety of Lactoferrin with Vitamin E and Zinc as an Oral Therapy for Mild to Moderate Acne Vulgaris. Int. J. Dermatol. 2017, 56, 686–690. [Google Scholar] [CrossRef]
- Pukacka, M.; Pukacki, P.; Żaba, R.; Adamski, Z.; Mrozewicz, B. Use of Bovine Colostrum in Dermatology in the Department of Dermatology and Venerology of Poznan. Dermatol. Prakt. 2015, 7, 61–64. [Google Scholar]
- Cosentino, C.; Elshafie, H.S.; Labella, C.; D’Adamo, C.; Pecora, G.; Musto, M.; Paolino, R.; Camele, I.; Freschi, P. Study on the Protective Effect of an Innovative Cow Milk-Based Product against Some Human Skin-Bacterial Pathogens. J. Biol. Res. 2018, 91. [Google Scholar] [CrossRef]
- Kocic, H.; Stankovic, M.; Tirant, M.; Lotti, T.; Arsic, I. Favorable Effect of Creams with Skimmed Donkey Milk Encapsulated in Nanoliposomes on Skin Physiology. Dermatol. Ther. 2020, 33, e13511. [Google Scholar] [CrossRef]
- Gobbi, R.M. Pharmaceutical and Dermocosmetic Compositions Containing Equine Colostrum. U.S. Patent No. 5750149, 12 May 1998. [Google Scholar]
- Kwak, T.; Kim, K.I.; Kim, J.H.; Jung, M.G.; An, Y.; Park, S.J. Composition for Improving Atopic Skin of Fermented Product of Colostrum. KR Patent No. 20200034217A, 31 March 2020. [Google Scholar]
- Kwak, T.; Kim, K.; Kim, J.H.; Jung, M.G.; Seo, S.A.; Seo, H.R. A Cosmetic Composition of Fermented Colostrum Product for Anti-Acne. KR Patent No. 20190060556A, 3 June 2019. [Google Scholar]
- Gobbi, R.M. Cosmetic Compositions Containing Hyaluronic Acid and Colostrum. WO Patent 2007009790A1, 25 January 2007. [Google Scholar]
- Park, S.M.; Lee, Y. HWhitening Functional Cosmetics Using Colostrum and Arbutin and Preparation Method Thereof. KR Patent 100619289B1, 12 April 2007. [Google Scholar]
- Torre, C.; Jeusette, I.; Serra, M.; Brazis, P.; Puigdemont, A. Bovine Colostrum Increases Proliferation of Canine Skin Fibroblasts. J. Nutr. 2006, 136, 2058S–2060S. [Google Scholar] [CrossRef] [PubMed]
- Zava, S.; Barello, C.; Pessione, A.; Garoffo, L.P.; Fattori, P.; Montorfano, G.; Conti, A.; Giunta, C.; Pessione, E.; Berra, B.; et al. Mare’s Colostrum Globules Stimulate Fibroblast Growth in Vitro: A Biochemical Study. J. Med. Food 2009, 12, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Amiot, J.; Germain, L.; Turgeon, S.; Lemay, M.; Ory-Salam, C.; Auger, F.A. Peptides from Milk Protein Hydrolysates to Improve the Growth of Human Keratinocytes in Culture. Int. Dairy J. 2004, 14, 619–626. [Google Scholar] [CrossRef]
- Kocic, H.; Langerholc, T.; Kostic, M.; Stojanovic, S.; Najman, S.; Krstic, M.; Nesic, I.; Godic, A.; Wollina, U. The Regenerative Potential of Donkey and Human Milk on the Redox-Sensitive and Proliferative Signaling Pathways of Skin Fibroblasts. Oxidative Med. Cell. Longev. 2020, 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, D.; Maresca, V.; Flori, E.; Mastrofrancesco, A.; Picardo, M.; Cardinali, G. Bovine Colostrum Induces the Differentiation of Human Primary Keratinocytes. FASEB J. 2020. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, E.; Mros, S.; McConnell, M.; Cabral, J.D.; Ali, A. Melt-Electrowriting with Novel Milk Protein/PCL Biomaterials for Skin Regeneration. Biomed. Mater. 2019, 14. [Google Scholar] [CrossRef] [PubMed]
- Javanmardi, S.; Safari, I.; Aghaz, F.; Khazaei, M. Wound Healing Activities of Gundelia Tournefortii L Extract and Milk-Cream Ointment on Second-Degree Burns of Rat Skin. Int. J. Low. Extrem. Wounds 2020, 1534734620921589. [Google Scholar] [CrossRef] [PubMed]
- Kocić, H.D.; Arsić, I.; Šmelcerović, A.; Knežević, D.; Godić, A.D. Process for Producing Cream and Serum with Partly Skimmed Donkey’s Milk and L-Arginine Encapsulated in Phospholipid Nanospheres. RS Patent 20160289A1, 31 December 2018. [Google Scholar]
| Kind of Activity | Mechanism of Action | [Ref.] |
|---|---|---|
| Antibacterial | - Reducing the concentration of iron ions that are necessary to bacterial growth and proliferation (chelation of iron via LF) - Interacting with lipoteichoic acid (LTA) of the cell walls of G(+) bacteria, disintegrating them and increasing their permeability | [102,115,116] |
| - Binding to lipopolysaccharide (LPS) of the walls of G(−) bacteria and disintegrating them. | ||
| Antifungal | - Damaging cell membranes of fungi and altering their permeability | [117,118,119] |
| - Sequestration of iron | ||
| - Membrane destabilization | ||
| Antiviral | - Blocking the host’s cell surface receptors due to the LF’s affinity for glycosaminoglycans- Direct interacting with capsid or viral envelope proteins | [113,120,121] |
| Antiparasitic | - Targets the host cell entry | [122,123,124,125,126] |
| - Sequestration of iron- Probably linked to sequestration of iron | ||
| - Acts additively or synergistically with the antiparasitic compounds used in therapy | ||
| Antioxidant | - Inhibiting the propagation of hydroxyl radicals by sequestering cationic iron and copper | [109,115,127] |
| Anticancer | - Reducing the production of tumor necrosis factor (TNF)-α in cell cultures | [104] |
| Immunomodulatory | - Stimulating the phagocytic activity of multinucleated leukocytes | [98,103,104] |
| - Reducing the production of interleukin (IL) -6 in cell cultures | ||
| - T-cell maturation | ||
| - Stimulation of NK (natural killer cells) cells | ||
| - Reducing pro-inflammatory cytokines |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazimierska, K.; Kalinowska-Lis, U. Milk Proteins—Their Biological Activities and Use in Cosmetics and Dermatology. Molecules 2021, 26, 3253. https://doi.org/10.3390/molecules26113253
Kazimierska K, Kalinowska-Lis U. Milk Proteins—Their Biological Activities and Use in Cosmetics and Dermatology. Molecules. 2021; 26(11):3253. https://doi.org/10.3390/molecules26113253
Chicago/Turabian StyleKazimierska, Kinga, and Urszula Kalinowska-Lis. 2021. "Milk Proteins—Their Biological Activities and Use in Cosmetics and Dermatology" Molecules 26, no. 11: 3253. https://doi.org/10.3390/molecules26113253
APA StyleKazimierska, K., & Kalinowska-Lis, U. (2021). Milk Proteins—Their Biological Activities and Use in Cosmetics and Dermatology. Molecules, 26(11), 3253. https://doi.org/10.3390/molecules26113253







