Xylochemical Synthesis and Biological Evaluation of Shancigusin C and Bletistrin G ‡
Abstract
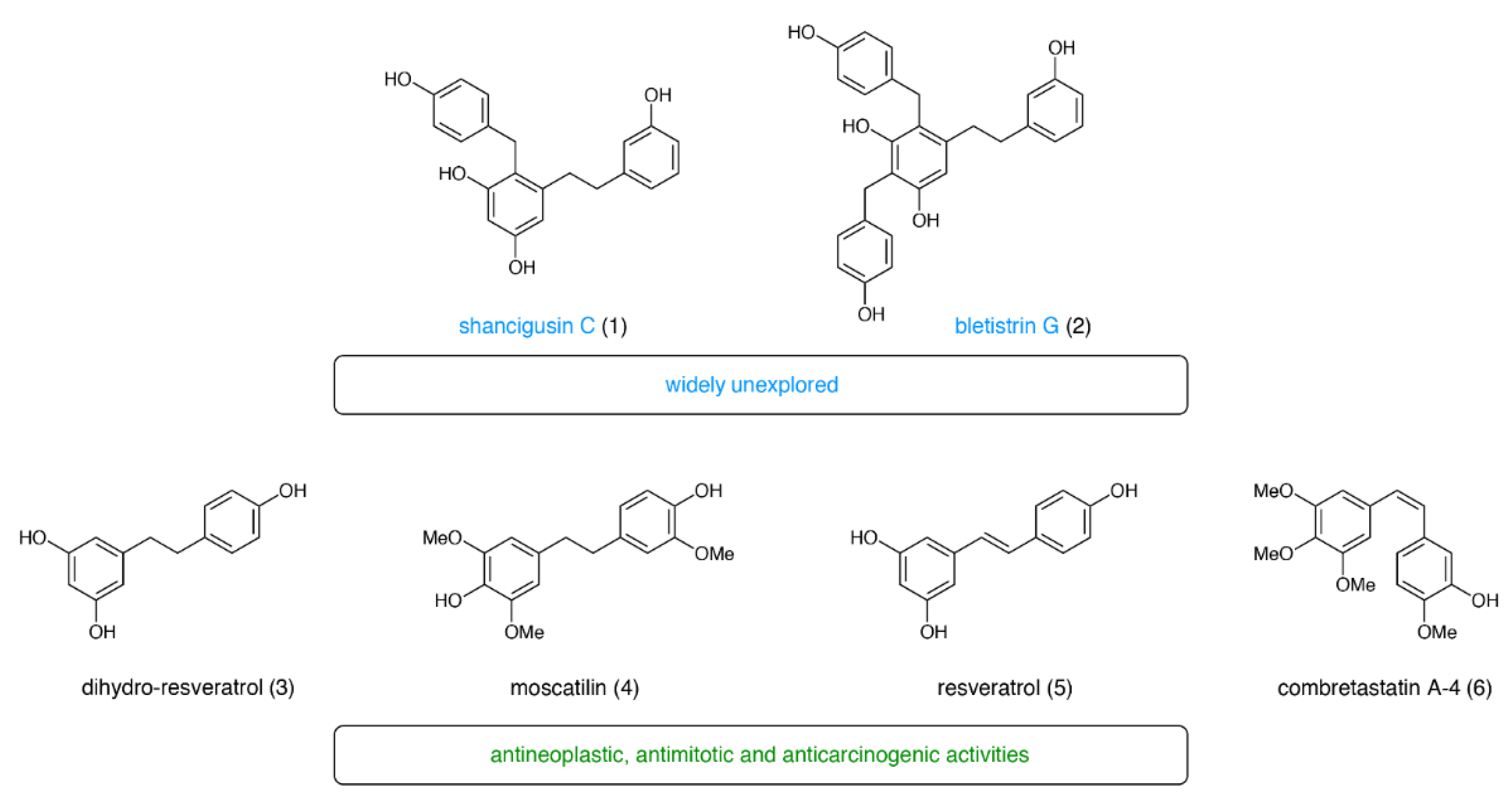
1. Introduction
2. Results
2.1. Structure Elucidation of Bletistrin G (2)
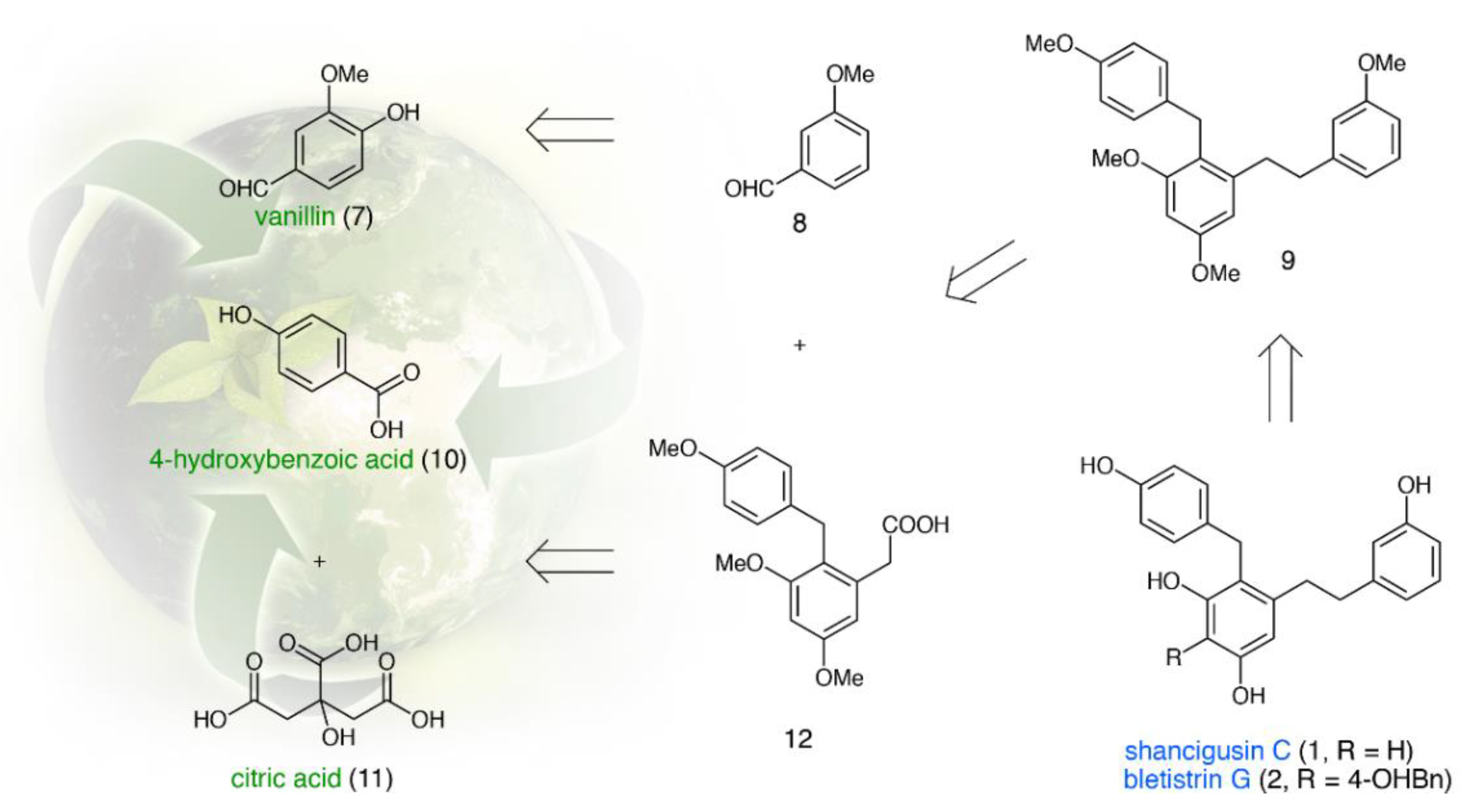
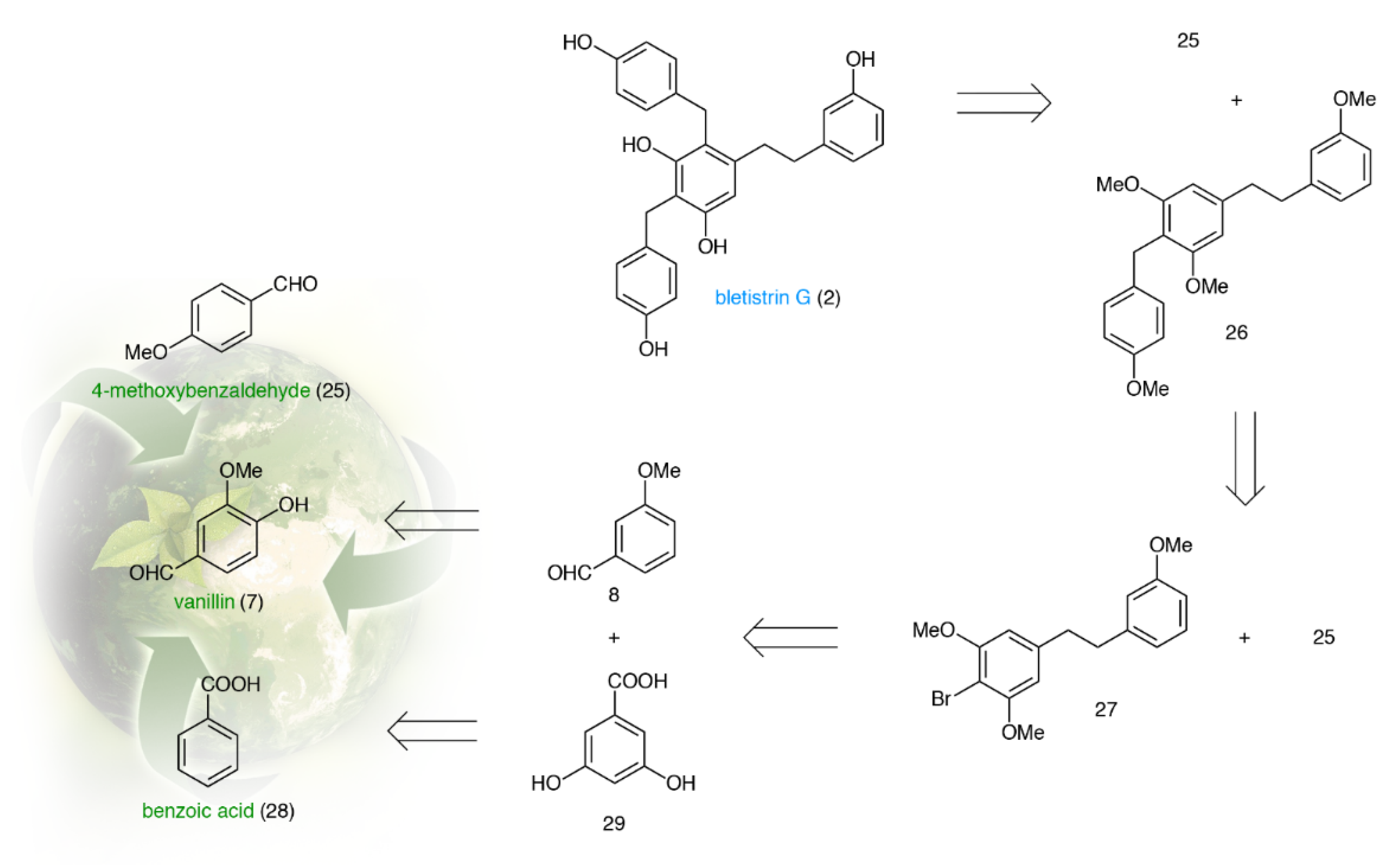
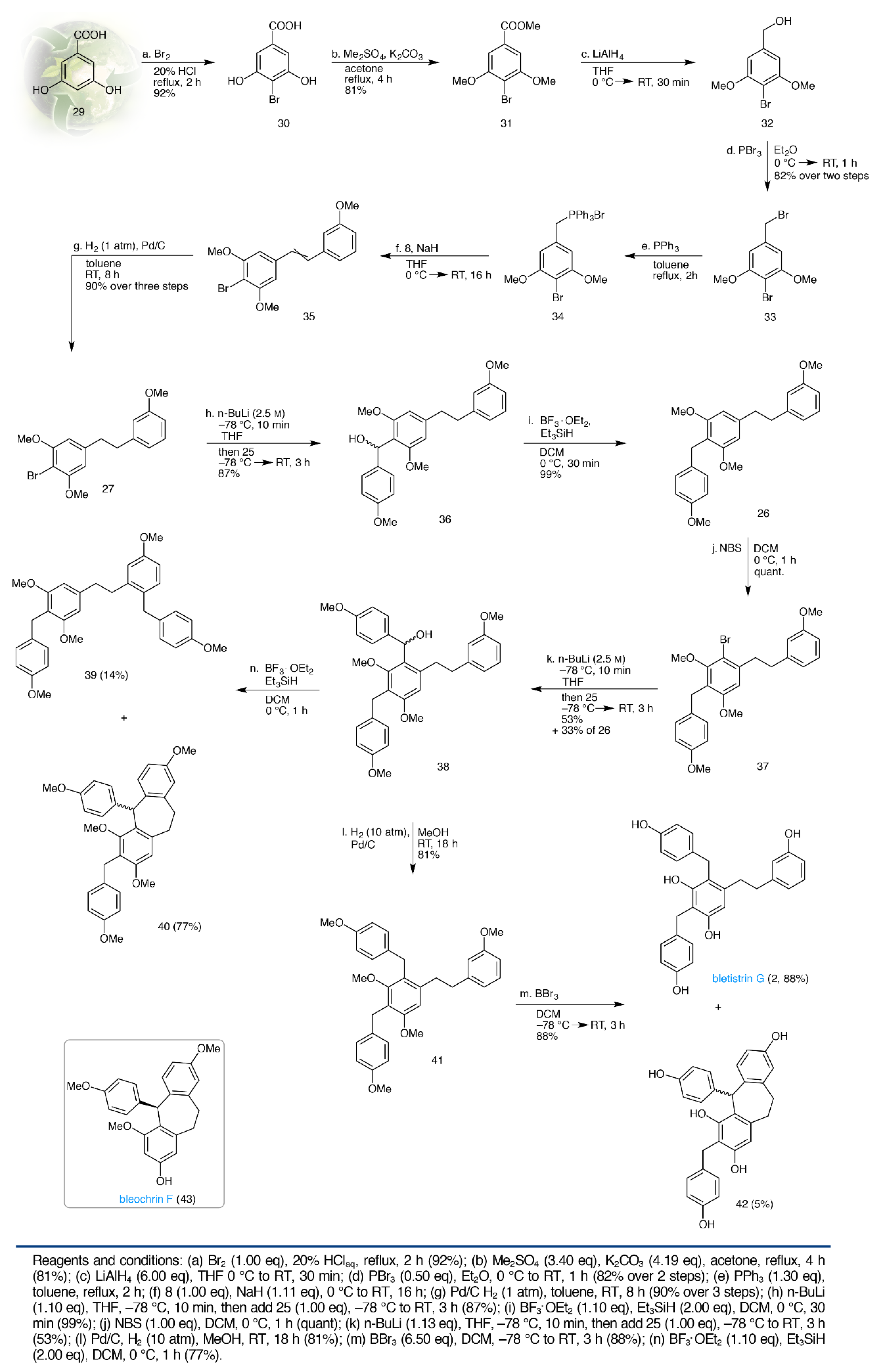
2.2. Total Synthesis of Shancigusin C (1)
2.3. Total Synthesis of Bletistrin G (2)
2.4. Biological Results
3. Discussion
4. Materials and Methods
4.1. Biological Part
4.2. Biological Evaluation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Teoh, E.S. Medicinal Orchids of Asia; Springer: Basel, Switzerland, 2016; pp. 614–615. [Google Scholar]
- Yamaki, M.; Honda, C. The stilbenoids from Dendrobium plicatile. Phytochemistry 1996, 43, 207–208. [Google Scholar] [CrossRef]
- Ye, Q.; Zhao, W. New alloaromadendrane, cadinene and cyclocopacamphane type sesquiterpene derivatives and bibenzyls from Dendrobium nobile. Planta Med. 2002, 68, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.G.; Yu, H.; Lian, X. Isolation of stilbenoids and lignans from Dendrobium hongdie. Trop. J. Pharm. Res. 2015, 14, 2055–2059. [Google Scholar] [CrossRef][Green Version]
- Zhang, X.; Xu, J.K.; Wang, J.; Wang, N.L.; Kurihara, H.; Kitanaka, S.; Yao, X.S. Bioactive bibenzyl derivatives and fluorenones from Dendrobium nobile. J. Nat. Prod. 2007, 70, 24–28. [Google Scholar] [CrossRef]
- Dong, H.L.; Wang, C.L.; Guo, S.X.; Yang, J.S. New bibenzyl derivatives from the tubers of Pleione yunnanensis. Chem. Pharm. Bull. 2009, 57, 513–515. [Google Scholar] [CrossRef]
- Jiang, S.; Wan, K.; Lou, H.Y.; Yi, P.; Zhang, N.; Zhou, M.; Song, Z.Q.; Wang, W.; Wu, M.K.; Pan, W.D. Antibacterial bibenzyl derivatives from the tubers of Bletilla striata. Phytochemistry 2019, 162, 216–223. [Google Scholar] [CrossRef]
- Sut, S.; Maggi, F.; Dall’Acqua, S. Bioactive secondary metabolites from Orchids (Orchidaceae). Chem. Biodivers. 2017, 14, e1700172. [Google Scholar] [CrossRef] [PubMed]
- Pettit, G.R.; Cragg, G.M.; Herald, D.L.; Schmidt, J.M.; Lohavanijaya, P. Isolation and structure of crombretastatin. Can. J. Chem. 1982, 60, 1374–1376. [Google Scholar] [CrossRef]
- Pettit, G.R.; Singh, S.B.; Niven, M.L.; Hamel, E.; Schmidt, J.M. Isolation, structure, and synthesis of combretastatins A-1 and B-1, potent new inhibitors of microtubule assembly, derived from Combretum caffrum. J. Nat. Prod. 1987, 50, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Pettit, G.R.; Cragg, G.M.; Singh, S.B. Antineoplastic agents, 122. Constituents of Combretum caffrum. J. Nat. Prod. 1987, 50, 386–391. [Google Scholar] [CrossRef]
- Lin, C.M.; Singh, S.B.; Chu, P.S.; Dempcy, R.O.; Schmidt, J.M.; Pettit, G.R.; Hamel, E. Interactions of tubulin with potent natural and synthetic analogs of the antimitotic agent combretastatin: A structure-activity study. Mol. Pharmacol. 1988, 34, 200–208. [Google Scholar] [PubMed]
- Pettit, G.R.; Singh, S.B.; Schmidt, J.M.; Nixen, M.L.; Hamel, E.; Lin, C.M. Isolation, structure, synthesis, and antimitotic properties of combretastatins b-3 and b-4 from Combretum caffrum. J. Nat. Prod. 1988, 51, 517–527. [Google Scholar] [CrossRef]
- Ferrigni, N.R.; McLaughlin, J.L.; Powell, R.G.; Smith, C.R. Use of potato disc and brine shrimp bioassays to detect activity and isolate piceatannol as the antileukemic principle from the seeds of Euphorbia lagascae. J. Nat. Prod. 1984, 47, 347–352. [Google Scholar] [CrossRef]
- Gill, M.T.; Bajaj, R.; Chang, C.J.; Nichols, D.E.; McLaughlin, J.L. 3,3′,5′-tri-o-methylpiceatannol and 4,3′,5′-tri-o-methylpiceatannol: Improvements over piceatannol in bioactivity. J. Nat. Prod. 1987, 50, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Cushman, M.; Nagarathnam, D.; Gopal, D.; Chakraborti, A.K.; Lin, C.M.; Hamel, E. Synthesis and evaluation of stilbene and dihydrostilbene derivatives as potential anticancer agents that inhibit tubulin polymerization. J. Med. Chem. 1991, 34, 2579–2588. [Google Scholar] [CrossRef]
- Lin, C.M.; Ho, H.H.; Pettit, G.R.; Hamel, E. Antimitotic natural products combretastatin a-4 and combretastatin a-2: Studies on the mechanism of their inhibition of the binding of colchicine to tubulin. Biochemistry 1989, 28, 6984–6991. [Google Scholar] [CrossRef]
- Tsang, S.W.; Guan, Y.F.; Wang, J.; Bian, Z.X.; Zhang, H.J. Inhibition of pancreatic oxidative damage by stilbene derivative dihydro-resveratrol: Implication for treatment of acute pancreatitis. Sci. Rep. 2016, 6, 22859. [Google Scholar] [CrossRef]
- Hernández-Romero, Y.; Acevedo, L.; de Los Ángeles Sánchez, M.; Shier, W.T.; Abbas, H.K.; Mata, R. Phytotoxic activity of bibenzyl derivatives from the Orchid Epidendrum rigidum. J. Agric. Food Chem. 2005, 53, 6276–6280. [Google Scholar] [CrossRef]
- Cardile, V.; Avola, R.; Graziano, A.C.E.; Russo, A. Moscatilin, a bibenzyl derivative from the Orchid Dendrobium loddigesii, induces apoptosis in melanoma cells. Chem. Biol. Interact. 2020, 323, 109075. [Google Scholar] [CrossRef]
- Wu, X.Q.; Li, W.; Chen, J.X.; Zhai, J.W.; Xu, H.Y.; Ni, L.; Wu, S.S. Chemical constituents and biological activity profiles on Pleione (Orchidaceae). Molecules 2019, 24, 3195. [Google Scholar] [CrossRef] [PubMed]
- Opatz, T.; Kauhl, U. Totalsynthese und Strukturaufklärung des (–)-Hymenosetins und Verwandter 3-Decalinoyltetramsäuren: Strukturaufklärung Biologisch Aktiver Naturstoffe. Ph.D. Thesis, Johannes Gutenberg University, Mainz, Germany, 2018. [Google Scholar]
- Kühlborn, J.; Danner, A.K.; Frey, H.; Iyer, R.; Arduengo, A.J.; Opatz, T. Examples of xylochemistry: Colorants and polymers. Green Chem. 2017, 19, 3780–3786. [Google Scholar] [CrossRef]
- Stubba, D.; Lahm, G.; Geffe, M.; Runyon, J.W.; Arduengo, A.J., III; Opatz, T. Xylochemistry—Making natural products entirely from wood. Angew. Chem. Int. Ed. 2015, 54, 14187–14189. [Google Scholar] [CrossRef]
- Goheen, D.W. Chemicals from wood and other biomass. Part I: Future supply of organic chemicals. J. Chem. Educ. 1981, 58, 465–468. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Holmbom, B.; Salmi, T.; Murzin, D.Y. Recent progress in synthesis of fine and specialty chemicals from wood and other biomass by heterogeneous catalytic processes. Catal. Rev. 2007, 49, 197–340. [Google Scholar] [CrossRef]
- Hoseyini, M.; Asefi, N.; Mozaffari, M. Production of citric acid from apple pomace by using surface culture method. Agric. J. 2011, 6, 226–230. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Brar, S.K.; Verma, M.; Tyagi, R.D. Enhanced solid-state citric acid bio-production using apple pomace waste through surface response methodology. J. Appl. Microbiol. 2011, 110, 1045–1055. [Google Scholar] [CrossRef]
- Harris, E.E.; D’Ianni, J.; Adkins, H. Reaction of hardwood lignin with hydrogen. J. Am. Chem. Soc. 1938, 60, 1467–1470. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, S.; Li, F.; Cao, X.; Sun, R. Production of vanillin from lignin: The relationship between β-O-4 linkages and vanillin yield. Ind. Crops Prod. 2018, 116, 116–121. [Google Scholar] [CrossRef]
- Smith, D.C.C. p-Hydroxybenzoate groups in the lignin of aspen (Populus tremula). J. Chem. Soc. 1955, 2347–2351. [Google Scholar] [CrossRef]
- Guyot, J.; Simon, L.J. Action de l’anhydride sulfurique et de l’oleum sur l’alcool methylique. Preparation du sulfate dimethylique. C. R. Hebd. Seances Acad. Sci. 1919, 169, 795–797. [Google Scholar]
- Theilacker, W.; Schmid, W. Zur Konstitution der Triacylmethane. II. Über das bicyclo-[2,2,2]-octantrion-(2,6,7). Liebigs Ann. 1950, 570, 15–33. [Google Scholar] [CrossRef]
- Elzner, S.; Schmidt, D.; Schollmeyer, D.; Erkel, G.; Anke, T.; Kleinert, H.; Förstermann, U.; Kunz, H. Inhibitors of inducible No synthase expression: Total synthesis of (S)-curvularin and its ring homologues. ChemMedChem 2008, 3, 924–939. [Google Scholar] [CrossRef] [PubMed]
- Weston, A.W.; Suter, C.M. 3,5-dihydroxybenzoic acid. Org. Synth. 1941, 21, 27. [Google Scholar]
- Deno, N.C.; Peterson, H.J.; Saines, G.S. The hydride-transfer reaction. Chem. Rev. 1960, 60, 7–14. [Google Scholar] [CrossRef]
- Gillet, J.P.; Efferth, T.; Remacle, J. Chemotherapy-induced resistance by ATP-binding cassette transporter genes. Biochim. Biophys. Acta 2007, 1775, 237–262. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Konkimalla, V.B.; Wang, Y.F.; Sauerbrey, A.; Meinhardt, S.; Zintl, F.; Mattern, J.; Volm, M. Prediction of broad spectrum resistance of tumors towards anticancer drugs. Clin. Cancer Res. 2008, 14, 2405–2412. [Google Scholar] [CrossRef]
- Kimmig, A.; Gekeler, V.; Neumann, M.; Frese, G.; Handgretinger, R.; Kardos, G.; Diddens, H.; Niethammer, D. Susceptibility of multidrug-resistant human leukemia cell cines to human interleukin 2-activated killer cells. Cancer Res. 1990, 50, 6793–6799. [Google Scholar]
- Efferth, T.; Sauerbrey, A.; Olbrich, A.; Gebhart, E.; Rauch, P.; Weber, H.O.; Hengstler, J.G.; Halatsch, M.-E.; Volm, M.; Tew, K.D.; et al. Molecular modes of action of artesunate in tumor cell lines. Mol. Pharmacol. 2003, 64, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Kadioglu, O.; Cao, J.; Kosyakova, N.; Mrasek, K.; Liehr, T.; Efferth, T. Genomic and transcriptomic profiling of resistant CEM/ADR-5000 and sensitive CCRF-CEM leukaemia cells for unravelling the full complexity of multi-factorial multidrug resistance. Sci. Rep. 2016, 6, 36754. [Google Scholar] [CrossRef]
- O’Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the alamar blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef]
- Mahmoud, N.; Saeed, M.E.M.; Sugimoto, Y.; Klinger, A.; Fleischer, E.; Efferth, T. Putative molecular determinants mediating sensitivity or resistance towards carnosic acid tumor cell responses. Phytomedicine 2020, 77, 153271. [Google Scholar] [CrossRef]
- Mbaveng, A.T.; Chi, G.F.; Bonsou, I.N.; Abdelfatah, S.; Tamfu, A.N.; Yeboah, E.M.O.; Kuete, V.; Efferth, T. N-Acetylglycoside of oleanolic acid (aridanin) displays promising cytotoxicity towards human and animal cancer cells, inducing apoptotic, ferroptotic and necroptotic cell death. Phytomedicine 2020, 76, 153261. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geske, L.; Kauhl, U.; Saeed, M.E.M.; Schüffler, A.; Thines, E.; Efferth, T.; Opatz, T. Xylochemical Synthesis and Biological Evaluation of Shancigusin C and Bletistrin G. Molecules 2021, 26, 3224. https://doi.org/10.3390/molecules26113224
Geske L, Kauhl U, Saeed MEM, Schüffler A, Thines E, Efferth T, Opatz T. Xylochemical Synthesis and Biological Evaluation of Shancigusin C and Bletistrin G. Molecules. 2021; 26(11):3224. https://doi.org/10.3390/molecules26113224
Chicago/Turabian StyleGeske, Leander, Ulrich Kauhl, Mohamed E. M. Saeed, Anja Schüffler, Eckhard Thines, Thomas Efferth, and Till Opatz. 2021. "Xylochemical Synthesis and Biological Evaluation of Shancigusin C and Bletistrin G" Molecules 26, no. 11: 3224. https://doi.org/10.3390/molecules26113224
APA StyleGeske, L., Kauhl, U., Saeed, M. E. M., Schüffler, A., Thines, E., Efferth, T., & Opatz, T. (2021). Xylochemical Synthesis and Biological Evaluation of Shancigusin C and Bletistrin G. Molecules, 26(11), 3224. https://doi.org/10.3390/molecules26113224






