Copper-Catalyzed Synthesis of Axially Chiral Biaryls with Diaryliodonium Salts as Arylation Reagents
Abstract
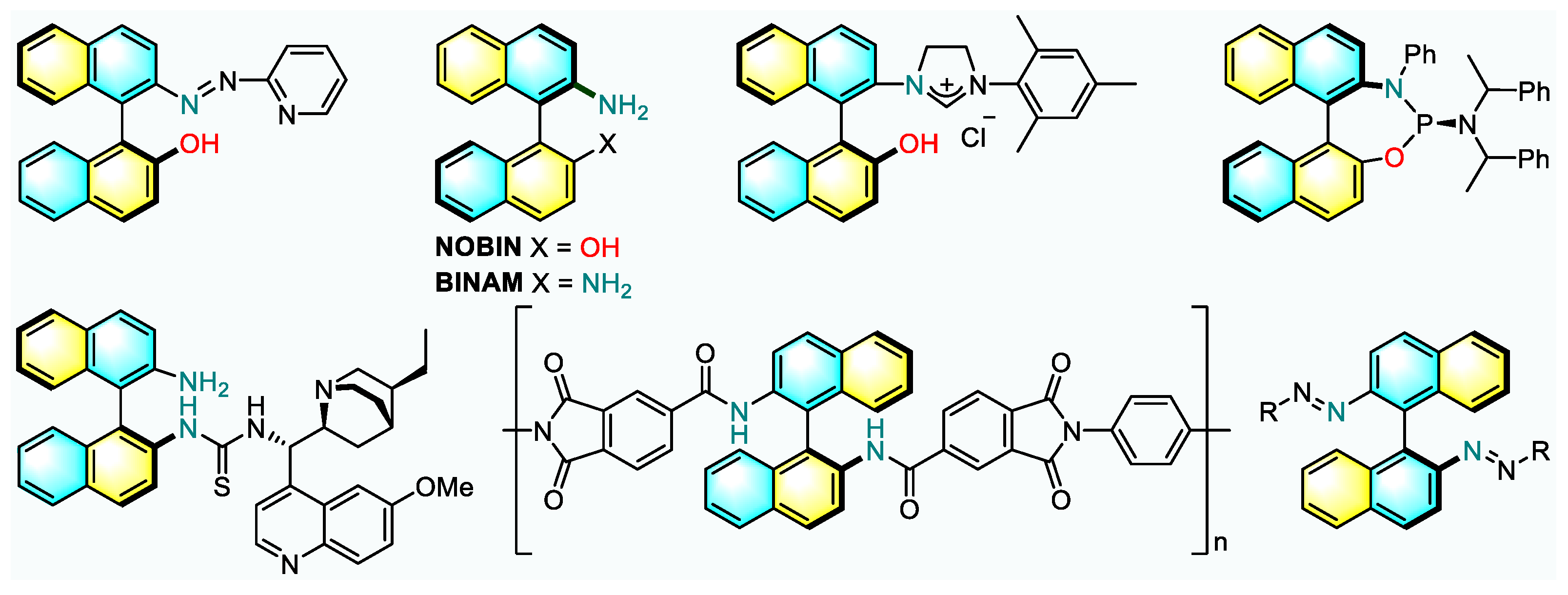
1. Introduction
2. Results and Discussion
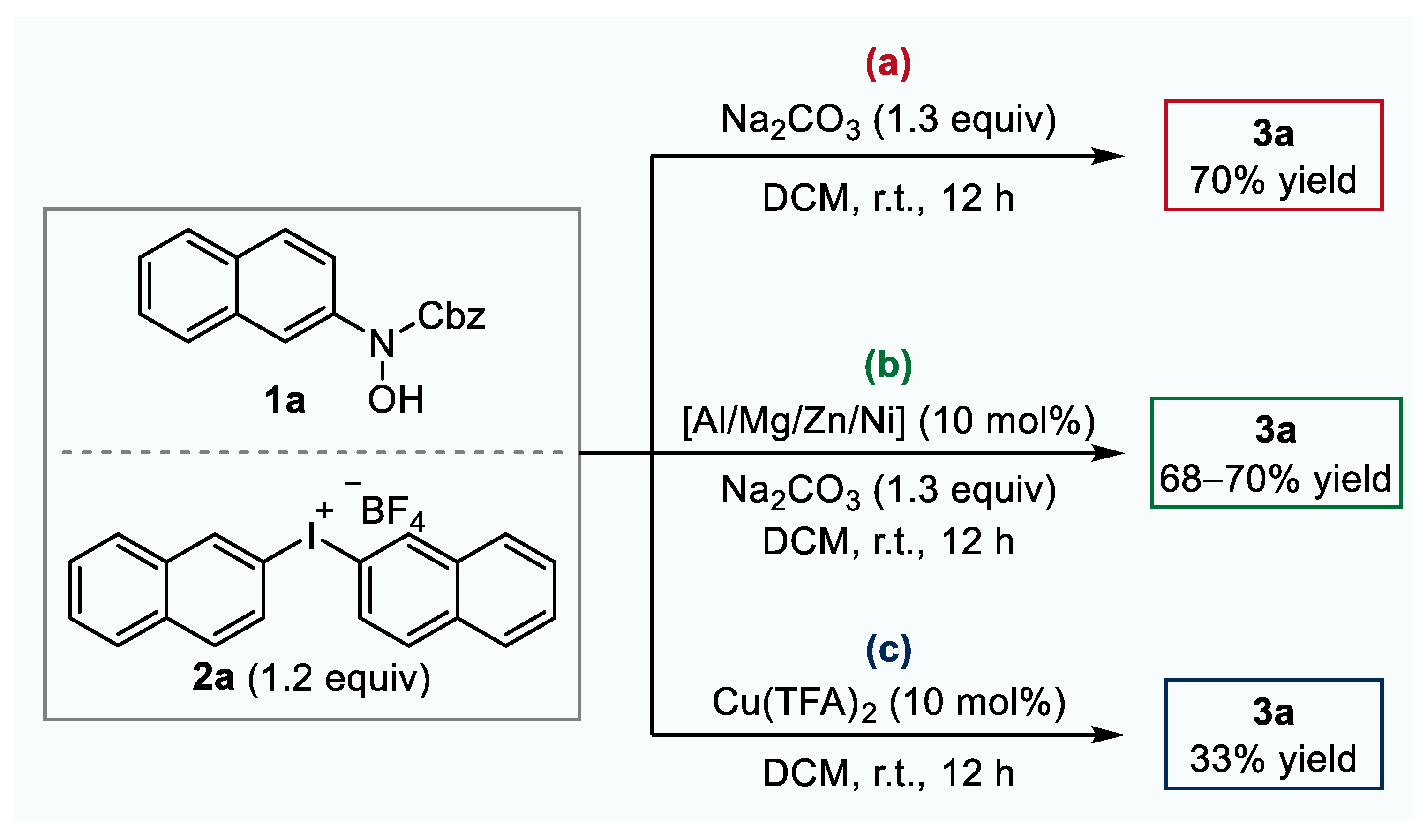
2.1. Optimization of Reaction Conditions
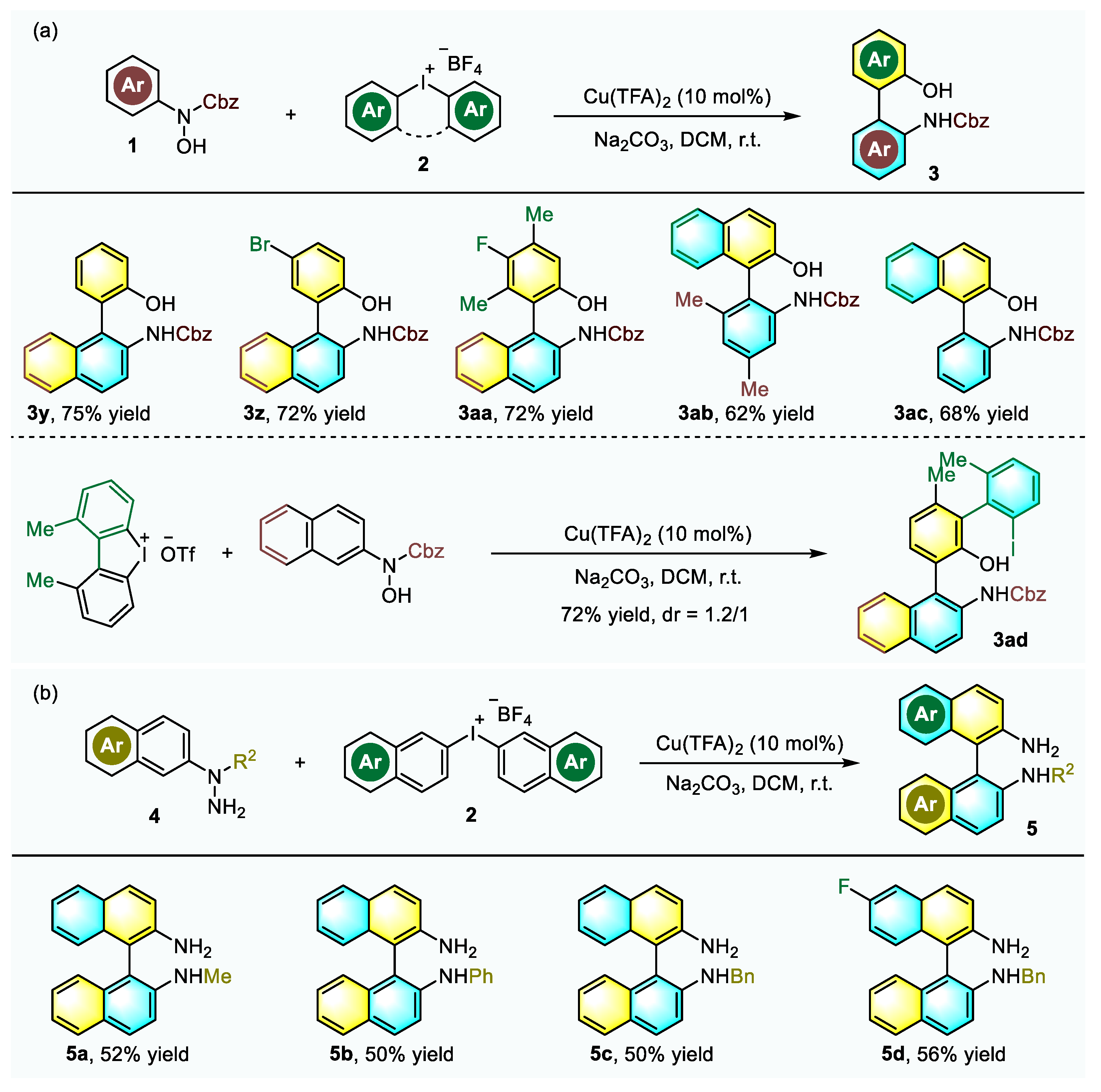
2.2. Substrate Scope
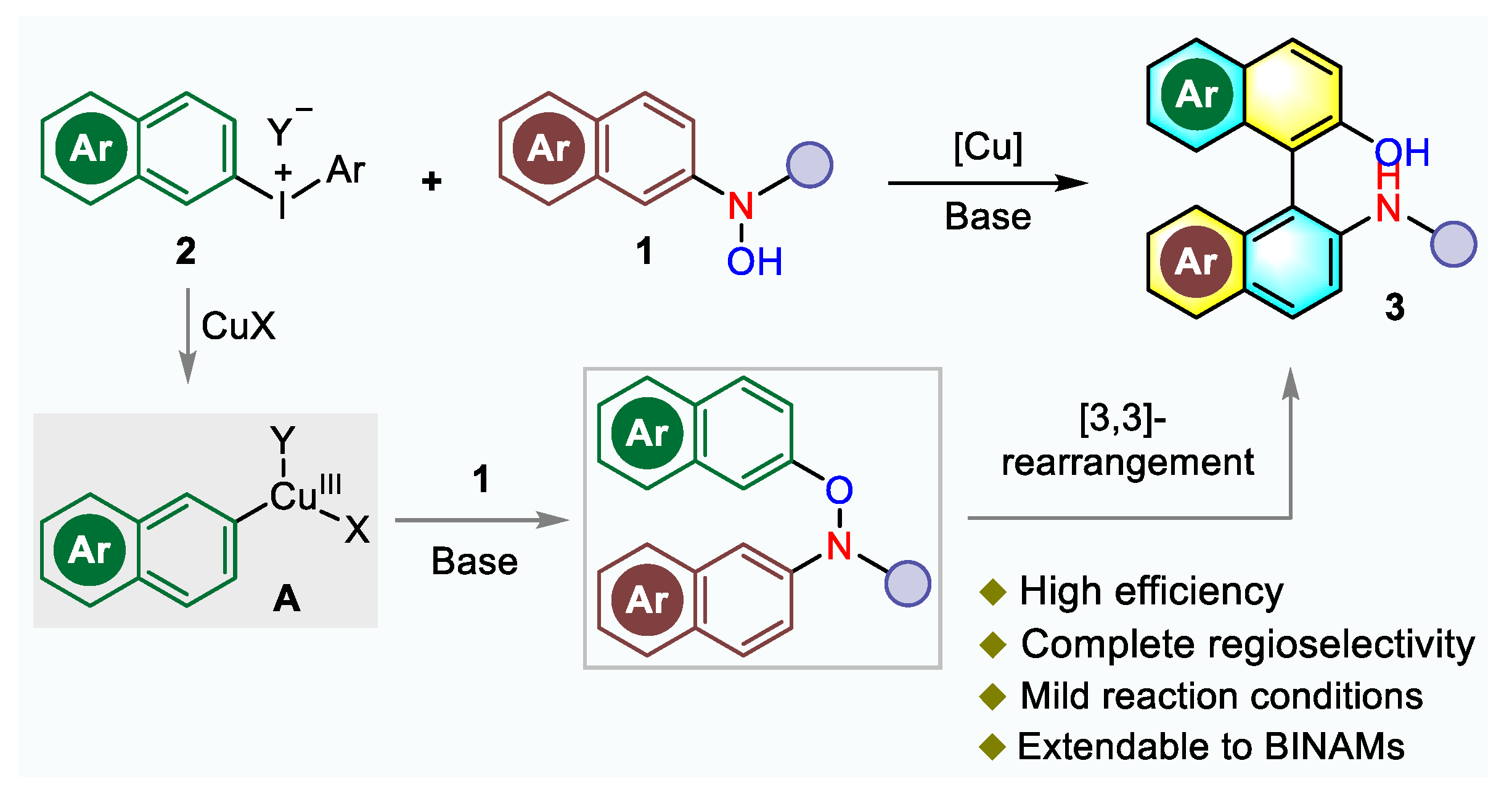
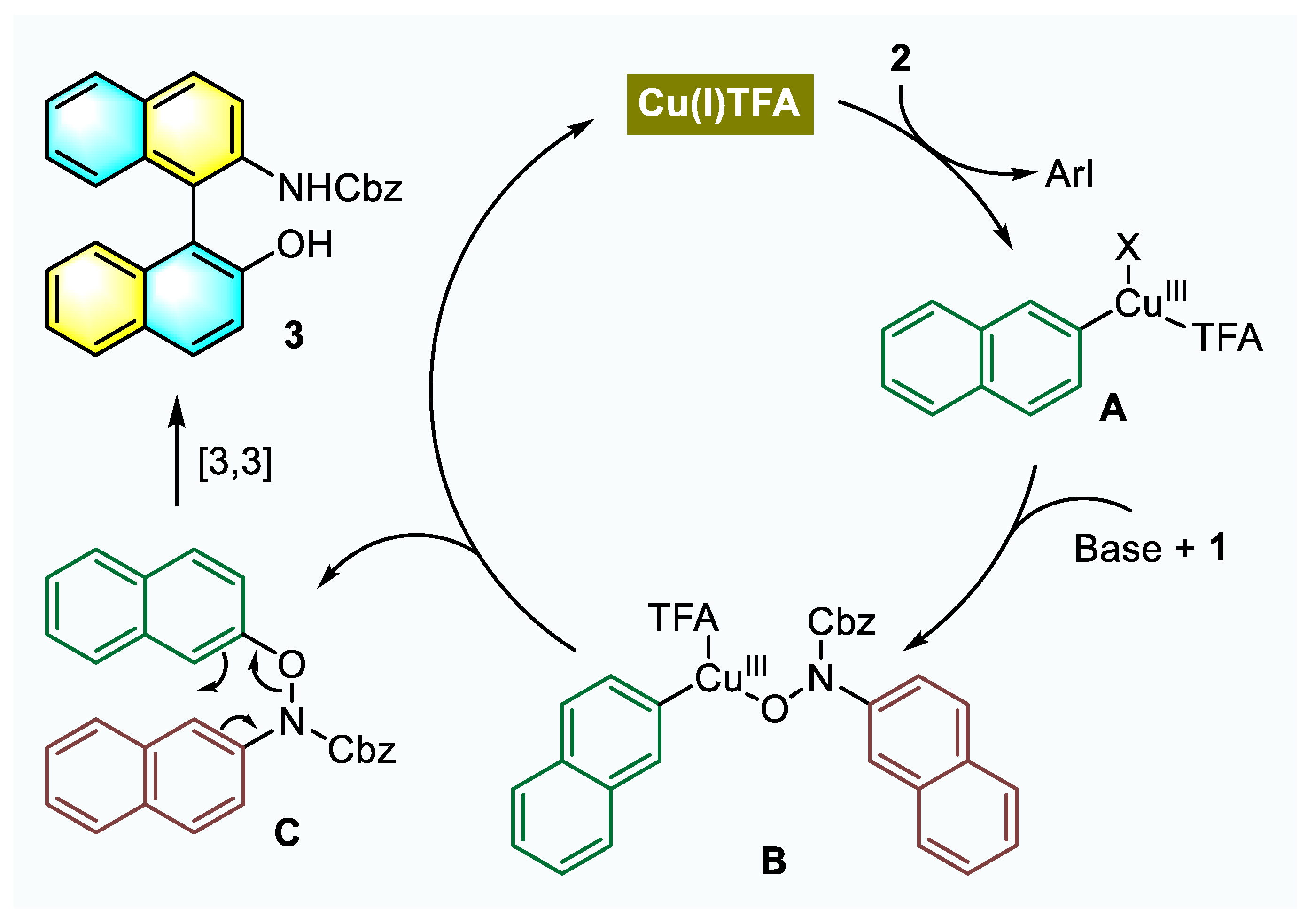
2.3. Control Experiments and Plausible Mechanism
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kočovský, P.; Vyskočil, Š.; Smrčina, M. Non-Symmetrically Substituted 1,1‘-Binaphthyls in Enantioselective Catalysis. Chem. Rev. 2003, 103, 3213–3246. [Google Scholar] [CrossRef]
- Ding, K.; Li, X.; Ji, B.; Guo, H.; Kitamura, M. Ten Years of Research on NOBIN Chemistry. Curr. Org. Synth. 2005, 2, 499–545. [Google Scholar] [CrossRef]
- Ding, K.; Guo, H.; Li, X.; Yuan, Y.; Wang, Y. Synthesis of NOBIN Derivatives for Asymmetric Catalysis. Top. Catal. 2005, 35, 105–116. [Google Scholar] [CrossRef]
- Zhou, Q.-L. (Ed.) Privileged Chiral Ligands and Catalysts; Wiley VCH: Weinheim, Germany, 2011; pp. 1–462. ISBN 978-3-527-32704-1. [Google Scholar]
- Carreira, E.M.; Lee, W.; Singer, R.A. Catalytic, Enantioselective Acetone Aldol Additions with 2-Methoxypropene. J. Am. Chem. Soc. 1995, 117, 3649–3650. [Google Scholar] [CrossRef]
- Van Veldhuizen, J.J.; Garber, S.B.; Kingsbury, J.S.; Hoveyda, A.H. A Recyclable Chiral Ru Catalyst for Enantioselective Olefin Metathesis. Efficient Catalytic Asymmetric Ring-Opening/Cross Metathesis in Air. J. Am. Chem. Soc. 2002, 124, 4954–4955. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, X. A Hybrid Phosphorus Ligand for Highly Enantioselective Asymmetric Hydroformylation. J. Am. Chem. Soc. 2006, 128, 7198–7202. [Google Scholar] [CrossRef]
- Uraguchi, D.; Kinoshita, N.; Ooi, T. Catalytic Asymmetric Protonation of α-Amino Acid-Derived Ketene Disilyl Acetals UsingP-Spiro Diaminodioxaphosphonium Barfates as Chiral Proton. J. Am. Chem. Soc. 2010, 132, 12240–12242. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Candeias, N.R.; Barbas, C.F., III. Construction of Bispirooxindoles Containing Three Quaternary Stereocentres in a Cascade Using a Single Multifunctional Organocatalyst. Nat. Chem. 2011, 3, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Vallavoju, N.; Selvakumar, S.; Jockusch, S.; Sibi, M.P.; Sivaguru, J. Enantioselective Organo-Photocatalysis Mediated by Atropisomeric Thiourea Derivatives. Angew. Chem. Int. Ed. 2014, 53, 5604–5608. [Google Scholar] [CrossRef] [PubMed]
- Lipshutz, B.H.; Buzard, D.; Olsson, C.; Noson, K. A modular route to nonracemic cyclo-NOBINs. Preparation of the parent ligand for homo- and heterogeneous catalysis. Tetrahedron 2004, 60, 4443–4449. [Google Scholar] [CrossRef]
- Li, Q.; Green, L.; Venkataraman, N.; Shiyanovskaya, I.; Khan, A.; Urbas, A.; Doane, J.W. Reversible Photoswitchable Axially Chiral Dopants with High Helical Twisting Power. J. Am. Chem. Soc. 2007, 129, 12908–12909. [Google Scholar] [CrossRef] [PubMed]
- Ritter, N.; Senkovska, I.; Kaskel, S.; Weber, J. Towards Chiral Microporous Soluble Polymers-Binaphthalene-Based Polyi-mides. Macromol. Rapid Commun. 2011, 32, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Singer, R.A.; Buchwald, S.L. Preparation of 2-Amino-2′-hydroxy-1,1’-binaphthyl and N-arylated 2-Amino-1,1’-binaphthyl Derivatives via Palladium-Catalyzed Amination. Tetrahedron Lett. 1999, 40, 1095–1098. [Google Scholar] [CrossRef]
- Patel, D.; Breitbach, Z.S.; Woods, R.M.; Lim, Y.; Wang, A.; Foss, F.W.; Armstrong, D.W. Gram Scale Conversion ofR-BINAM toR-NOBIN. J. Org. Chem. 2016, 81, 1295–1299. [Google Scholar] [CrossRef]
- Chang, X.; Zhang, Q.; Guo, C. Switchable Smiles Rearrangement for Enantioselective O-Aryl Amination. Org. Lett. 2019, 21, 4915–4918. [Google Scholar] [CrossRef]
- Körber, K.; Tang, W.; Hu, X.; Zhang, X. A Practical Synthesis of 2-Amino-2′-hydroxy-1,1′-binaphthyl (NOBIN). Tetrahedron Lett. 2002, 43, 7163–7165. [Google Scholar] [CrossRef]
- Smrcina, M.; Lorenc, M.; Hanuš, V.; Kocovsky, P. A Facile Synthesis of 2-Amino-2?-hydroxy-1,1?-binaphthyl and 2,2?-Diamino-1,1?-binaphthyl by Oxidative Coupling Using Copper(II) Chloride. Synlett 1991, 1991, 231–232. [Google Scholar] [CrossRef]
- Smrcina, M.; Lorenc, M.; Hanus, V.; Sedmera, P.; Kocovsky, P. Synthesis of enantiomerically pure 2,2’-dihydroxy-1,1’-binaphthyl, 2,2’-diamino-1,1’-binaphthyl, and 2-amino-2’-hydroxy-1,1’-binaphthyl. Comparison of processes operating as diastereoselective crystallization and as second order asymmetric transformation. J. Org. Chem. 1992, 57, 1917–1920. [Google Scholar] [CrossRef]
- Smrcina, M.; Polakova, J.; Vyskocil, S.; Kocovsky, P. Synthesis of enantiomerically pure binaphthyl derivatives. Mechanism of the enantioselective, oxidative coupling of naphthols and designing a catalytic cycle. J. Org. Chem. 1993, 58, 4534–4538. [Google Scholar] [CrossRef]
- Smrcina, M.; Vyskocil, S.; Maca, B.; Polasek, M.; Claxton, T.A.; Abbott, A.P.; Kocovsky, P. Selective Cross-Coupling of 2-Naphthol and 2-Naphthylamine Derivatives. A Facile Synthesis of 2,2’,3-Trisubstituted and 2,2’,3,3’-Tetrasubstituted 1,1’-Binaphthyls. J. Org. Chem. 1994, 59, 2156–2163. [Google Scholar] [CrossRef]
- Ding, K.; Xu, Q.; Wang, Y.; Liu, J.; Yu, Z.; Du, B.; Wu, Y.; Koshima, H.; Matsuura, T. Novel two-phase oxidative cross-coupling of the two-component molecular crystal of 2-naphthol and 2-naphthylamine. Chem. Commun. 1997, 693–694. [Google Scholar] [CrossRef]
- Singer, R.A.; Brock, J.R.; Carreira, E.M. Synthesis of A Tridentate Ligand for Use in TiIV-Catalyzed Acetate Aldol Addition Reactions. Helv. Chim. Acta 2003, 86, 1040–1044. [Google Scholar] [CrossRef]
- Zhao, X.-J.; Li, Z.-H.; Ding, T.-M.; Tian, J.-M.; Tu, Y.-Q.; Wang, A.-F.; Xie, Y.-Y. Enantioselective Synthesis of 3,3′-Disubstituted 2-Amino-2′-hydroxy-1,1′-binaphthyls by Copper-Catalyzed Aerobic Oxidative Cross-Coupling. Angew. Chem. Int. Ed. 2021, 60, 7061–7065. [Google Scholar] [CrossRef]
- Zhang, J.-W.; Jiang, F.; Chen, Y.-H.; Xiang, S.-H.; Tan, B. Synthesis of Structurally Diversified BINOLs and NOBINs via Palladium-Catalyzed C-H Arylation with Diazoquinones. Sci. China Chem. 2021. [Google Scholar] [CrossRef]
- Sheradsky, T.; Avramovici-Grisaru, S. Hydrolytic cleavages of 7-nitro-2-phenyl-1,2-benzisoxazol-3-one. Evidence for the occurrence of a benzidine-like rearrangement of N,O-diphenylhydroxylamines. J. Heterocycl. Chem. 1980, 17, 189–190. [Google Scholar] [CrossRef]
- Gao, H.; Ess, D.H.; Yousufuddin, M.; Kürti, L. Transition-Metal-Free Direct Arylation: Synthesis of Halogenated 2-Amino-2′-hydroxy-1,1′-biaryls and Mechanism by DFT Calculations. J. Am. Chem. Soc. 2013, 135, 7086–7089. [Google Scholar] [CrossRef]
- De, C.K.; Pesciaioli, F.; List, B. Catalytic Asymmetric Benzidine Rearrangement. Angew. Chem. Int. Ed. 2013, 52, 9293–9295. [Google Scholar] [CrossRef]
- Li, G.-Q.; Gao, H.; Keene, C.; Devonas, M.; Ess, D.H.; Kürti, L. Organocatalytic Aryl-Aryl Bond Formation: An Atro-poselective [3,3]-Rearrangement Approach to BINAM Derivatives. J. Am. Chem. Soc. 2013, 135, 7414–7417. [Google Scholar] [CrossRef]
- Yanagi, T.; Otsuka, S.; Kasuga, Y.; Fujimoto, K.; Murakami, K.; Nogi, K.; Yorimitsu, H.; Osuka, A. Metal-Free Approach to Biaryls from Phenols and Aryl Sulfoxides by Temporarily Sulfur-Tethered Regioselective C–H/C–H Coupling. J. Am. Chem. Soc. 2016, 138, 14582–14585. [Google Scholar] [CrossRef] [PubMed]
- Hori, M.; Guo, J.-D.; Yanagi, T.; Nogi, K.; Sasamori, T.; Yorimitsu, H. Sigmatropic Rearrangements of Hyperva-lent-Iodine-Tethered Intermediates for the Synthesis of Biaryls. Angew. Chem. Int. Ed. 2018, 57, 4663–4667. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Liu, F.; Wang, L.; Yuan, H.; Feng, L.; Kürti, L.; Gao, H. Cascade Approach to Highly Functionalized Biaryls by a Nucleophilic Aromatic Substitution with Arylhydroxylamines. Org. Lett. 2019, 21, 2894–2898. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Du, Y.; Liu, F.; Guo, L.; Sun, Q.; Feng, L.; Gao, H. Tandem Approach to NOBIN Analogues from Arylhydrox-ylamines and Diaryliodonium Salts via [3,3]-Sigmatropic Rearrangement. Chem. Commun. 2020, 56, 8226–8229. [Google Scholar] [CrossRef]
- Zhang, J.; Qi, L.; Li, S.; Xiang, S.; Tan, B. Direct Construction of NOBINs via Domino Arylation and Sigmatropic Rearrangement Reactions. Chin. J. Chem. 2020, 38, 1503–1514. [Google Scholar] [CrossRef]
- Deprez, N.R.; Sanford, M.S. Reactions of Hypervalent Iodine Reagents with Palladium: Mechanisms and Applications in Organic Synthesis. Inorg. Chem. 2007, 46, 1924–1935. [Google Scholar] [CrossRef]
- Phipps, R.J.; Gaunt, M. A Meta-Selective Copper-Catalyzed C-H Bond Arylation. Science 2009, 323, 1593–1597. [Google Scholar] [CrossRef] [PubMed]
- Duong, H.A.; Gilligan, R.E.; Cooke, M.L.; Phipps, R.J.; Gaunt, M. Copper(II)-Catalyzed meta-Selective Direct Arylation of α-Aryl Carbonyl Compounds. Angew. Chem. Int. Ed. 2010, 50, 463–466. [Google Scholar] [CrossRef]
- Ghosh, M.K.; Rzymkowski, J.; Kalek, M. Transition-Metal-Free Aryl-Aryl Cross-Coupling: C-H Arylation of 2-Naphthols with Diaryliodonium Salts. Chem. Eur. J. 2019, 25, 9619–9623. [Google Scholar] [CrossRef]
- Phipps, R.J.; Grimster, N.P.; Gaunt, M. Cu(II)-Catalyzed Direct and Site-Selective Arylation of Indoles Under Mild Conditions. J. Am. Chem. Soc. 2008, 130, 8172–8174. [Google Scholar] [CrossRef]
- Aradi, K.; Tóth, B.L.; Tolnai, G.L.; Novák, Z. Diaryliodonium Salts in Organic Syntheses: A Useful Compound Class for Novel Arylation Strategies. Synlett 2016, 27, 1456–1485. [Google Scholar] [CrossRef]
- Qi, L.-W.; Mao, J.-H.; Zhang, J.; Tan, B. Organocatalytic asymmetric arylation of indoles enabled by azo groups. Nat. Chem. 2017, 10, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.-W.; Li, S.; Xiang, S.-H.; Wang, J.J.; Tan, B. Asymmetric construction of atropisomeric biaryls via a redox neutral cross-coupling strategy. Nat. Catal. 2019, 2, 314–323. [Google Scholar] [CrossRef]
- Ding, W.-Y.; Yu, P.; An, Q.-J.; Bay, K.L.; Xiang, S.-H.; Li, S.; Chen, Y.; Houk, K.N.; Tan, B. DFT-Guided Phosphor-ic-Acid-Catalyzed Atroposelective Arene Functionalization of Nitrosonaphthalene. Chemistry 2020, 6, 2046–2059. [Google Scholar] [CrossRef]
- Yan, S.; Xia, W.; Li, S.; Song, Q.; Xiang, S.-H.; Tan, B. Michael Reaction Inspired Atroposelective Construction of Axially Chiral Biaryls. J. Am. Chem. Soc. 2020, 142, 7322–7327. [Google Scholar] [CrossRef] [PubMed]
- Phipps, R.J.; McMurray, L.; Ritter, S.; Duong, H.A.; Gaunt, M.J. Copper-Catalyzed Alkene Arylation with Diaryliodonium Salts. J. Am. Chem. Soc. 2012, 134, 10773–10776. [Google Scholar] [CrossRef]
- Allen, A.E.; Macmillan, D.W.C. Enantioselective α-Arylation of Aldehydes via the Productive Merger of Iodonium Salts and Organocatalysis. J. Am. Chem. Soc. 2011, 133, 4260–4263. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.S.; Simonovich, S.P.; Jamison, C.R.; Macmillan, D.W.C. Enantioselective α-Arylation of Carbonyls via Cu(I)-Bisoxazoline Catalysis. J. Am. Chem. Soc. 2011, 133, 13782–13785. [Google Scholar] [CrossRef]
- Zhu, S.; MacMillan, D.W.C. Enantioselective Copper-Catalyzed Construction of Aryl Pyrroloindolines via an Aryla-tion-Cyclization Cascade. J. Am. Chem. Soc. 2012, 134, 10815–10818. [Google Scholar] [CrossRef] [PubMed]
- Ribas, X.; Jackson, D.A.; Donnadieu, B.; Mahía, J.; Parella, T.; Xifra, R.; Hedman, B.; Hodgson, K.O.; Llobet, A.; Stack, T.D.P. Aryl C-H Activation by CuII To Form an Organometallic Aryl-CuIII Species: A Novel Twist on Copper Disproportionation. Angew. Chem. Int. Ed. 2002, 41, 2991–2994. [Google Scholar] [CrossRef]
- Yao, B.; Wang, D.-X.; Huang, Z.-T.; Wang, M.-X. Room-temperature aerobic formation of a stable aryl-Cu(III) complex and its reactions with nucleophiles: Highly efficient and diverse arene C-H functionalizations of azacalix[1]arene[3]pyridine. Chem. Commun. 2009, 20, 2899–2901. [Google Scholar] [CrossRef]
- Ribas, X.; Calle, C.; Poater, A.; Casitas, A.; Gómez, L.; Xifra, R.; Parella, T.; Benet-Buchholz, J.; Schweiger, A.; Mitrikas, G.; et al. Facile C-H Bond Cleavage via a Proton-Coupled Electron Transfer Involving a C-H···CuII Interaction. J. Am. Chem. Soc. 2010, 132, 12299–12306. [Google Scholar] [CrossRef]


| Entry | Variation from the Optimized Conditions | Yield (%) b |
|---|---|---|
| 1 | none | 98 |
| 2 | toluene instead of DCM | 80 |
| 3 | THF instead of DCM | 90 |
| 4 | MeCN instead of DCM | 88 |
| 5 | EA instead of DCM | 86 |
| 6 | Cu(OAc)2 instead of Cu(TFA)2 | 89 |
| 7 | Cu(OTf)2 instead of Cu(TFA)2 | 82 |
| 8 | CuOTf instead of Cu(TFA)2 | 89 |
| 9 | CuI instead of Cu(TFA)2 | 86 |
| 10 | K2CO3 instead of Na2CO3 | 93 |
| 11 | Cs2CO3 instead of Na2CO3 | 90 |
| 12 | NaOH instead of Na2CO3 | 75 |
| 13 | NaOtBu instead of Na2CO3 | 66 |
| 14 | Et3N instead of Na2CO3 | 73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.-W.; Xiang, S.-H.; Li, S.; Tan, B. Copper-Catalyzed Synthesis of Axially Chiral Biaryls with Diaryliodonium Salts as Arylation Reagents. Molecules 2021, 26, 3223. https://doi.org/10.3390/molecules26113223
Zhang J-W, Xiang S-H, Li S, Tan B. Copper-Catalyzed Synthesis of Axially Chiral Biaryls with Diaryliodonium Salts as Arylation Reagents. Molecules. 2021; 26(11):3223. https://doi.org/10.3390/molecules26113223
Chicago/Turabian StyleZhang, Ji-Wei, Shao-Hua Xiang, Shaoyu Li, and Bin Tan. 2021. "Copper-Catalyzed Synthesis of Axially Chiral Biaryls with Diaryliodonium Salts as Arylation Reagents" Molecules 26, no. 11: 3223. https://doi.org/10.3390/molecules26113223
APA StyleZhang, J.-W., Xiang, S.-H., Li, S., & Tan, B. (2021). Copper-Catalyzed Synthesis of Axially Chiral Biaryls with Diaryliodonium Salts as Arylation Reagents. Molecules, 26(11), 3223. https://doi.org/10.3390/molecules26113223





