Thermal and Mechanical Properties of Esterified Lignin in Various Polymer Blends
Abstract
1. Introduction
2. Results and Discussion
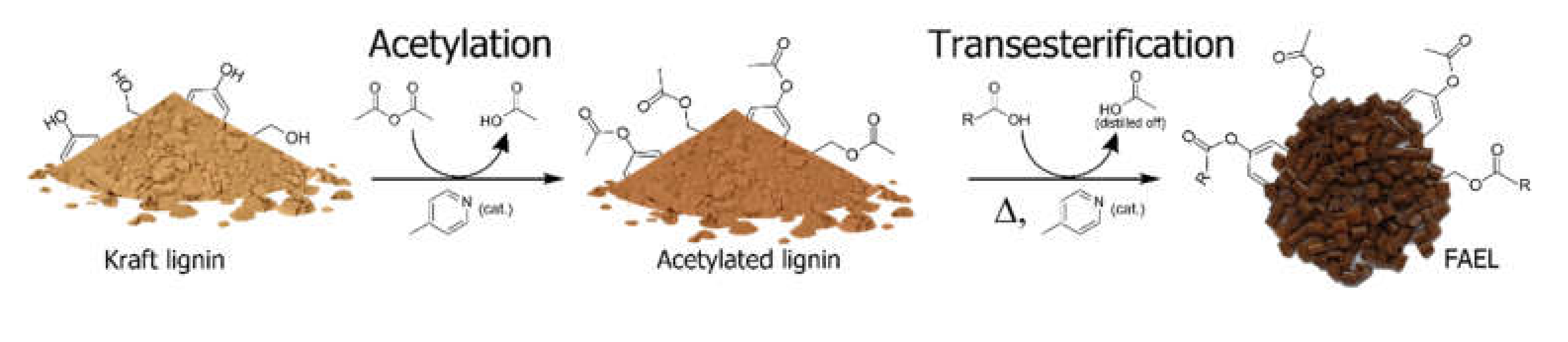
2.1. Preparation of FAELs
2.2. Analysis of FAELs
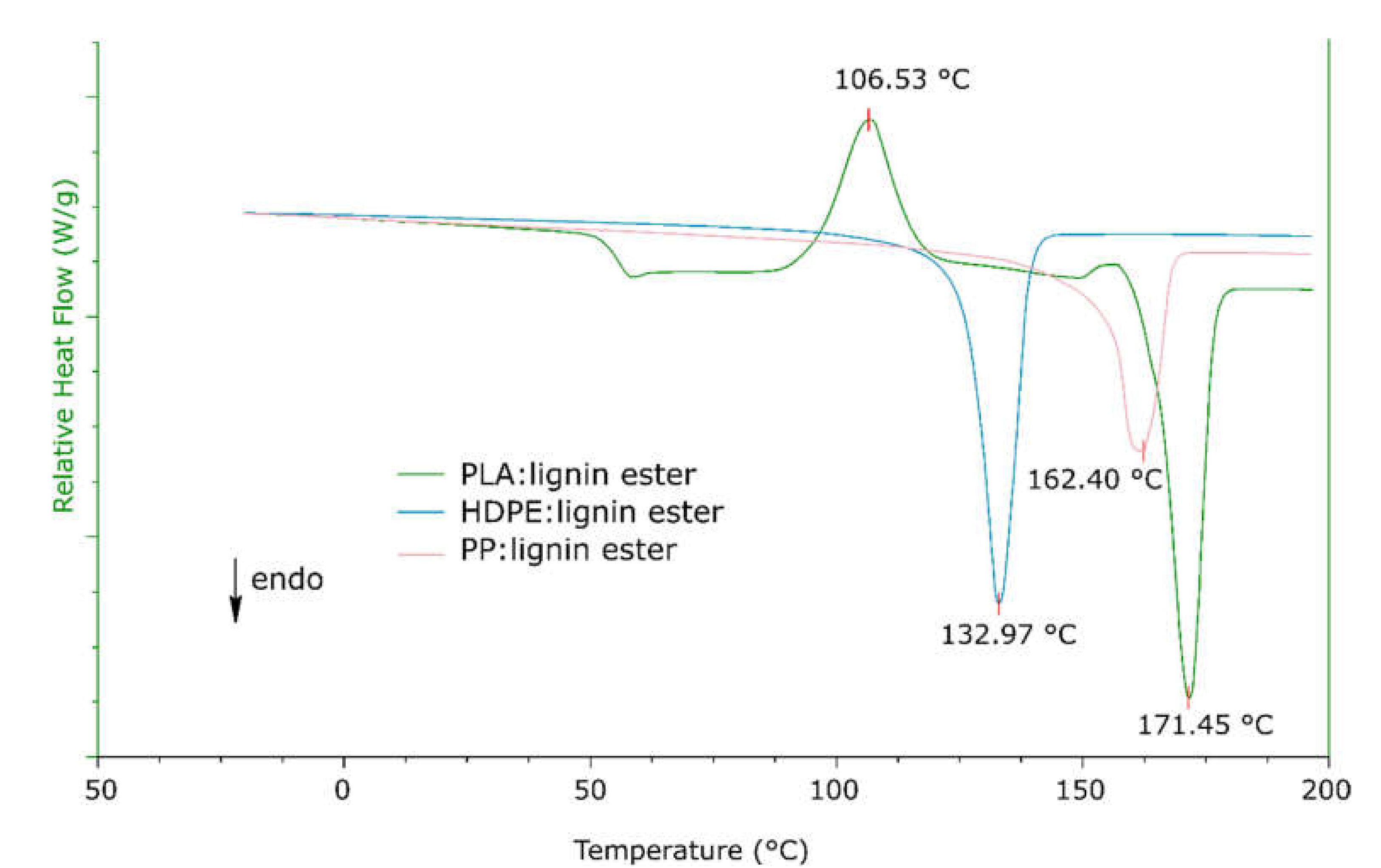
2.3. Thermal Properties
2.4. Blending of FAELs with Polymeric Matrices
2.5. Rheological Properties
2.6. Mechanical Properties
3. Materials and Methods
3.1. Materials
3.2. Analysis
3.3. Synthetic Procedures
3.3.1. Preparation of Fatty Acid Ester of Lignin (FAEL)
3.3.2. Blend Preparation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- PlasticsEurope Plastics–the Facts 2019 An Analysis of European Plastics Production, Demand and Waste Data. Available online: https://www.plasticseurope.org/en/resources/publications/1804-plastics-facts-2019 (accessed on 31 October 2020).
- Chalmin, P. The History of Plastics: From the Capitol to the Tarpeian Rock. Field Actions Sci. Rep. J. Field Actions 2019, 6–11. [Google Scholar]
- Weiss, M.; Haufe, J.; Carus, M.; Brandão, M.; Bringezu, S.; Hermann, B.; Patel, M.K. A Review of the Environmental Impacts of Biobased Materials. J. Ind. Ecol. 2012, 16, S169–S181. [Google Scholar] [CrossRef]
- Hong, S.-H.; Park, J.H.; Kim, O.Y.; Hwang, S.-H. Preparation of Chemically Modified Lignin-Reinforced PLA Biocomposites and Their 3D Printing Performance. Polymers 2021, 13, 667. [Google Scholar] [CrossRef] [PubMed]
- Ribca, I.; Jawerth, M.E.; Brett, C.J.; Lawoko, M.; Schwartzkopf, M.; Chumakov, A.; Roth, S.V.; Johansson, M. Exploring the Effects of Different Cross-Linkers on Lignin-Based Thermoset Properties and Morphologies. ACS Sustain. Chem. Eng. 2021, 9, 1692–1702. [Google Scholar] [CrossRef]
- Abejón, R.; Pérez-Acebo, H.; Clavijo, L. Alternatives for Chemical and Biochemical Lignin Valorization: Hot Topics from a Bibliometric Analysis of the Research Published During the 2000–2016 Period. Processes 2018, 6, 98. [Google Scholar] [CrossRef]
- Brewster, J. Economic Impact of Modern Kraft Recovery Boilers. 2007, p. 6. Available online: http://www.paptac.ca/ssp-members/1A_2_Brewster.pdf (accessed on 27 May 2021).
- Axelsson, E.; Olsson, M.R.; Berntsson, T. Increased Capacity in Kraft Pulp Mills: Lignin Separation and Reduced Steam Demand Compared with Recovery Boiler Upgrade. Nord. Pulp Pap. Res. J. 2006, 21, 485–492. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Washington, DC Office of Pollution, Prevention, and Toxics. In Proceedings of the International Symposium on Pollution Prevention in the Manufacture of Pulp and Paper: Opportunites & Barriers, Washington, DC, USA, 18–20 August 1992; Available online: https://nepis.epa.gov (accessed on 25 May 2021).
- Tomani, P. The Lignoboost Process. Cell. Chem. Technol. 2010, 44, 53–58. [Google Scholar]
- First LignoBoost Plants Producing Large Volumes of Kraft Lignin to the Market Place. Available online: https://www.valmet.com/media/articles/up-and-running/new-technology/PEERS1stLignoBoostPlants/ (accessed on 31 October 2020).
- Crestini, C.; Lange, H.; Sette, M.; Argyropoulos, D.S. On the Structure of Softwood Kraft Lignin. Green Chem. 2017, 19, 4104–4121. [Google Scholar] [CrossRef]
- Giummarella, N.; Lindén, P.A.; Areskogh, D.; Lawoko, M. Fractional Profiling of Kraft Lignin Structure: Unravelling Insights on Lignin Reaction Mechanisms. ACS Sustain. Chem. Eng. 2020, 8, 1112–1120. [Google Scholar] [CrossRef]
- Liu, L.; Qian, M.; Song, P.; Huang, G.; Yu, Y.; Fu, S. Fabrication of Green Lignin-Based Flame Retardants for Enhancing the Thermal and Fire Retardancy Properties of Polypropylene/Wood Composites. ACS Sustain. Chem. Eng. 2016, 4, 2422–2431. [Google Scholar] [CrossRef]
- Sen, S.; Patil, S.; Argyropoulos, D.S. Thermal Properties of Lignin in Copolymers, Blends, and Composites: A Review. Green Chem. 2015, 17, 4862–4887. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Chemical Modification of Lignins: Towards Biobased Polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Kai, D.; Tan, M.J.; Chee, P.L.; Chua, Y.K.; Yap, Y.L.; Loh, X.J. Towards Lignin-Based Functional Materials in a Sustainable World. Green Chem. 2016, 18, 1175–1200. [Google Scholar] [CrossRef]
- Toriz, G.; Denes, F.; Young, R.A. Lignin-Polypropylene Composites. Part 1: Composites from Unmodified Lignin and Polypropylene. Polym. Compos. 2002, 23, 806–813. [Google Scholar] [CrossRef]
- Maldhure, A.V.; Ekhe, J.D.; Deenadayalan, E. Mechanical Properties of Polypropylene Blended with Esterified and Alkylated Lignin. J. Appl. Polym. Sci. 2012, 125, 1701–1712. [Google Scholar] [CrossRef]
- Dehne, L.; Vila, C.; Saake, B.; Schwarz, K.U. Esterification of Kraft Lignin as a Method to Improve Structural and Mechanical Properties of Lignin-Polyethylene Blends. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- An, L.; Si, C.; Wang, G.; Choi, C.S.; Yu, Y.H.; Bae, J.H.; Lee, S.M.; Kim, Y.S. Efficient and Green Approach for the Esterification of Lignin with Oleic Acid Using Surfactant-Combined Microreactors in Water. BioResources 2020, 15, 89–104. [Google Scholar]
- Atifi, S.; Miao, C.; Hamad, W.Y. Surface Modification of Lignin for Applications in Polypropylene Blends. J. Appl. Polym. Sci. 2017, 134, 45103. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Y.; Hu, H.; Huang, Z.; Qin, Y.; Shen, F.; Huang, A.; Feng, Z. Effect of Lignin Esters on Improving the Thermal Properties of Poly(Vinyl Chloride). J. Appl. Polym. Sci. 2019, 136, 47176. [Google Scholar] [CrossRef]
- Thielemans, W.; Wool, R.P. Lignin Esters for Use in Unsaturated Thermosets: Lignin Modification and Solubility Modeling. Biomacromolecules 2005, 6, 1895–1905. [Google Scholar] [CrossRef]
- Di Francesco, D.; Dahlstrand, C.; Löfstedt, J.; Orebom, A.; Verendel, J.; Carrick, C.; Håkansson, Å.; Eriksson, S.; Rådberg, H.; Wallmo, H.; et al. Debottlenecking a Pulp Mill by Producing Biofuels from Black Liquor in Three Steps. ChemSusChem 2021. [Google Scholar] [CrossRef] [PubMed]
- Löfstedt, J.; Dahlstrand, C.; Orebom, A.; Meuzelaar, G.; Sawadjoon, S.; Galkin, M.V.; Agback, P.; Wimby, M.; Corresa, E.; Mathieu, Y.; et al. Green Diesel from Kraft Lignin in Three Steps. ChemSusChem 2016, 9, 1392–1396. [Google Scholar] [CrossRef] [PubMed]
- Laurichesse, S.; Huillet, C.; Avérous, L. Original Polyols Based on Organosolv Lignin and Fatty Acids: New Bio-Based Building Blocks for Segmented Polyurethane Synthesis. Green Chem. 2014, 16, 3958–3970. [Google Scholar] [CrossRef]
- Tamminen, T.; Ropponen, J.; Hult, E.-L.; Poppius-Levlin, K. Functionalized Lignin and Method of Producing the Same. U.S. Patent 201,161,544,303, 7 October 2011. [Google Scholar]
- Hesse, M.S.; Meier, H.; Zech, B. Spectroscopic Methods in Organic Chemistry (Translated by A. Linden and M. Murray). VIII and 365 Pp., 221 Fig., 100 Tab., Hard Cover: DM 168,–/SFr 149,–/ÖS 1226; ISBN 3 13 106 0611; Georg-Thieme Verlag Stuttgart–New York 1997; (New York ISBN 0 86577 6687). Starch Stärke 1997, 49, 257–258. [Google Scholar] [CrossRef]
- Shuhua, W.; Qiaoli, X.; Fen, L.; Jinming, D.; Husheng, J.; Bingshe, X. Preparation and Properties of Cellulose-Based Carbon Microsphere/Poly(Lactic Acid) Composites. J. Compos. Mater. 2014, 48, 1297–1302. [Google Scholar] [CrossRef]
- Cuiffo, M.A.; Snyder, J.; Elliott, A.M.; Romero, N.; Kannan, S.; Halada, G.P. Impact of the Fused Deposition (FDM) Printing Process on Polylactic Acid (PLA) Chemistry and Structure. Appl. Sci. 2017, 7, 579. [Google Scholar] [CrossRef]
- De Kort, G.W.; Bouvrie, L.H.C.; Rastogi, S.; Wilsens, C.H.R.M. Thermoplastic PLA-LCP Composites: A Route toward Sustainable, Reprocessable, and Recyclable Reinforced Materials. ACS Sustain. Chem. Eng. 2020, 8, 624–631. [Google Scholar] [CrossRef]




| PP-FAEL/PP | HDPE-FAEL/HDPE | PLA-FAEL/PLA 1 | |
|---|---|---|---|
| Stress at yield | 0.85 | 0.96 | 0.99 |
| Strain at yield | 0.50 | 0.75 | 0.88 |
| Young’s modulus | 1.09 | 1.05 | 0.93 |
| Impact strength | 0.93 | 0.39 | 0.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orebom, A.; Di Francesco, D.; Shakari, P.; Samec, J.S.M.; Pierrou, C. Thermal and Mechanical Properties of Esterified Lignin in Various Polymer Blends. Molecules 2021, 26, 3219. https://doi.org/10.3390/molecules26113219
Orebom A, Di Francesco D, Shakari P, Samec JSM, Pierrou C. Thermal and Mechanical Properties of Esterified Lignin in Various Polymer Blends. Molecules. 2021; 26(11):3219. https://doi.org/10.3390/molecules26113219
Chicago/Turabian StyleOrebom, Alexander, Davide Di Francesco, Patrick Shakari, Joseph S. M. Samec, and Clara Pierrou. 2021. "Thermal and Mechanical Properties of Esterified Lignin in Various Polymer Blends" Molecules 26, no. 11: 3219. https://doi.org/10.3390/molecules26113219
APA StyleOrebom, A., Di Francesco, D., Shakari, P., Samec, J. S. M., & Pierrou, C. (2021). Thermal and Mechanical Properties of Esterified Lignin in Various Polymer Blends. Molecules, 26(11), 3219. https://doi.org/10.3390/molecules26113219






