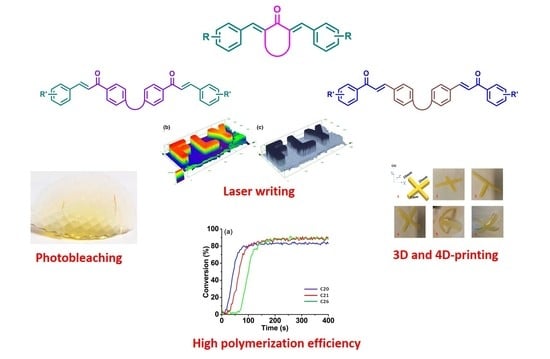
Recent Advances in bis-Chalcone-Based Photoinitiators of Polymerization: From Mechanistic Investigations to Applications
Abstract
1. Introduction
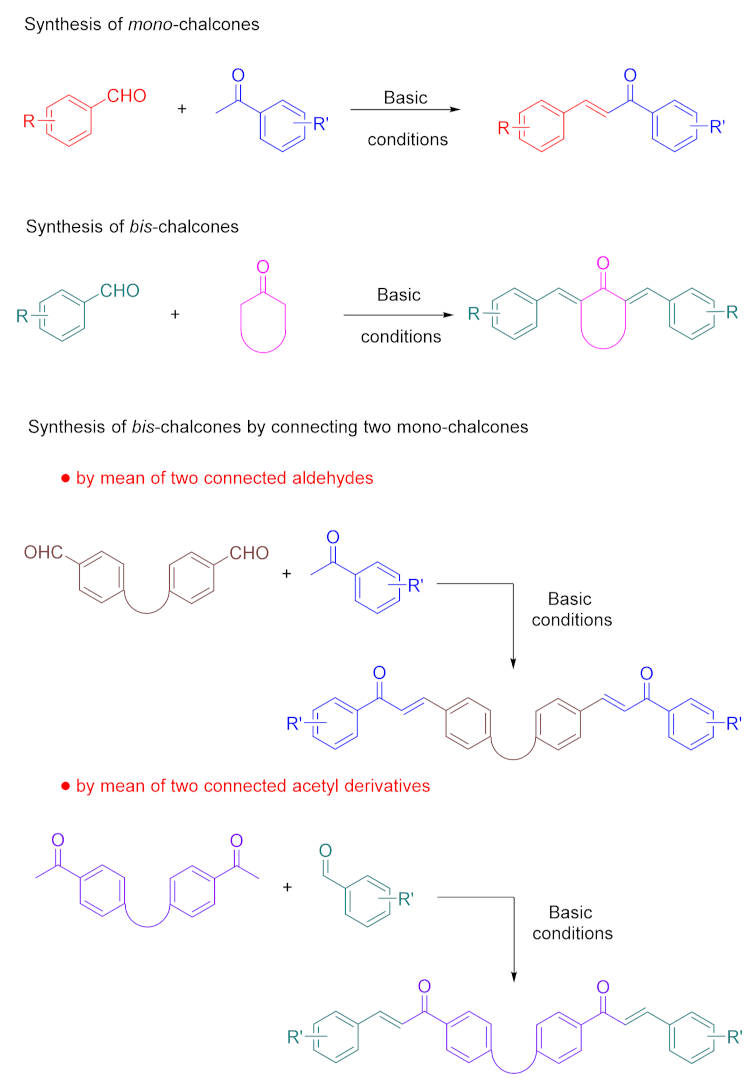
2. The Different Synthetic Routes to bis-Chalcones
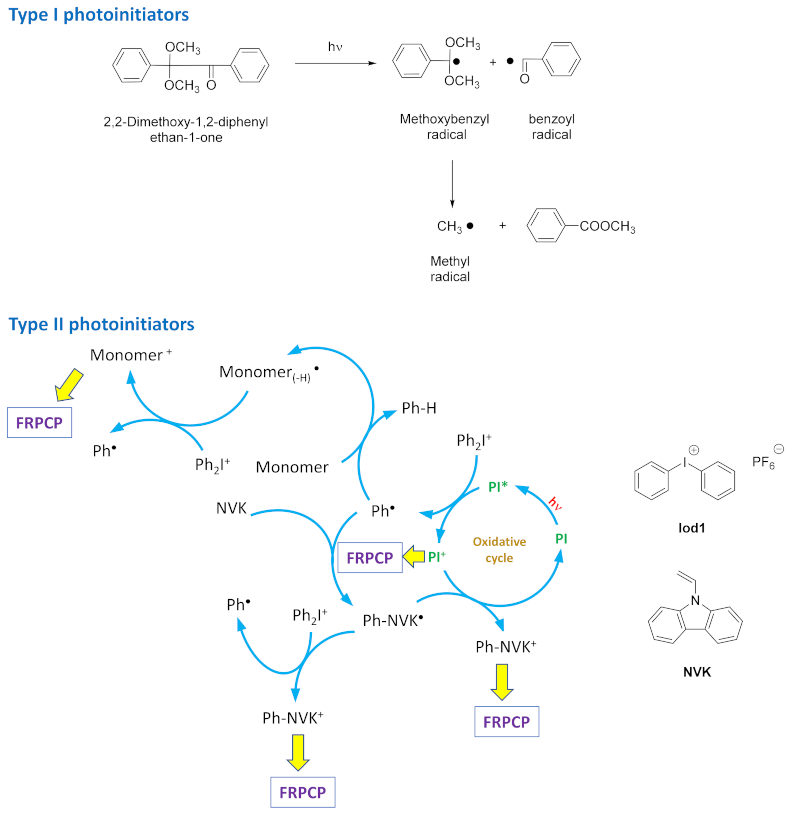
3. Bis-Chalcones as Photoinitiators
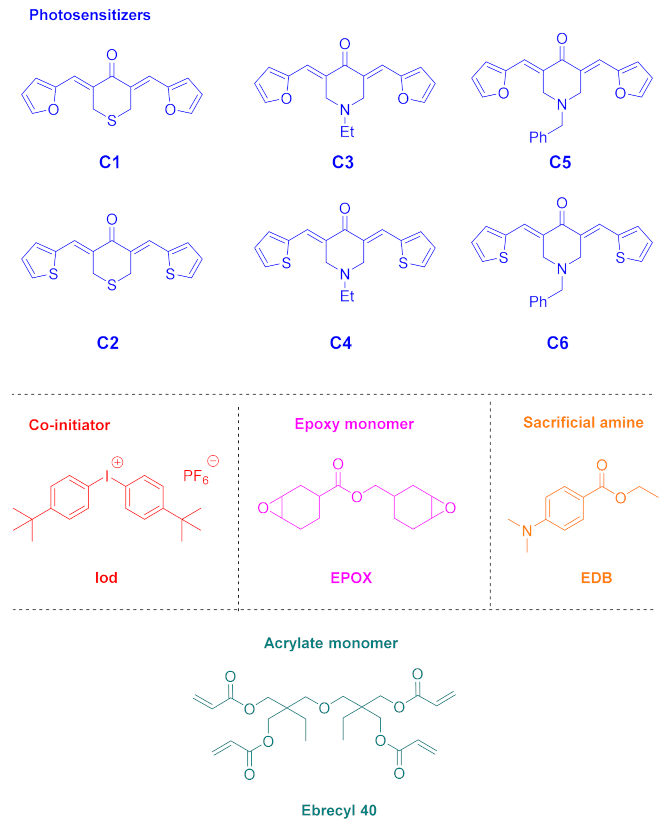
3.1. Bis-Chalcones Based on Cyclic Aliphatic Ketones
3.2. Bis-Chalcones Based on Two Connected Chalcones
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hatton, F.L. Recent advances in RAFT polymerization of monomers derived from renewable resources. Polym. Chem. 2020, 11, 220–229. [Google Scholar] [CrossRef]
- Nothling, M.D.; Fu, Q.; Reyhani, A.; Allison-Logan, S.; Jung, K.; Zhu, J.; Kamigaito, M.; Boyer, C.; Qiao, G.G. Progress and Perspectives Beyond Traditional RAFT Polymerization. Adv. Sci. 2020, 7, 2001656. [Google Scholar] [CrossRef]
- Tian, X.; Ding, J.; Zhang, B.; Qiu, F.; Zhuang, X.; Chen, Y. Recent Advances in RAFT Polymerization: Novel Initiation Mechanisms and Optoelectronic Applications. Polymers 2018, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Audran, G.; Bagryanskaya, E.G.; Marque, S.R.A.; Postnikov, P. New Variants of Nitroxide Mediated Polymerization. Polymers 2020, 12, 1481. [Google Scholar] [CrossRef] [PubMed]
- Sciannamea, V.; Jérôme, R.; Detrembleur, C. In-Situ Nitroxide-Mediated Radical Polymerization (NMP) Processes: Their Understanding and Optimization. Chem. Rev. 2008, 108, 1104–1126. [Google Scholar] [CrossRef]
- Audran, G.; Bagryanskaya, E.; Edeleva, M.; Marque, S.R.A.; Parkhomenko, D.; Tretyakov, E.; Zhivetyeva, S. Coordination-Initiated Nitroxide-Mediated Polymerization (CI-NMP). Aust. J. Chem. 2018, 71, 334–340. [Google Scholar] [CrossRef]
- Shao, J.; Huang, Y.; Fan, Q. Visible light initiating systems for photopolymerization: Status, development and challenges. Polym. Chem. 2014, 5, 4195–4210. [Google Scholar] [CrossRef]
- Park, H.K.; Shin, M.; Kim, B.; Park, J.W.; Lee, H. A visible light-curable yet visible wavelength-transparent resin for stereolithography 3D printing. NPG Asia Mater. 2018, 10, 82–89. [Google Scholar] [CrossRef]
- Yoshino, F.; Yoshida, A. Effects of blue-light irradiation during dental treatment. Jpn. Dent. Sci. Rev. 2018, 54, 160–168. [Google Scholar] [CrossRef]
- Garra, P.; Dietlin, C.; Morlet-Savary, F.; Dumur, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Redox two-component initiated free radical and cationic polymerizations: Concepts, reactions and applications. Prog. Polym. Sci. 2019, 94, 33–56. [Google Scholar] [CrossRef]
- Fouassier, J.-P.; Allonas, X.; Burget, D. Photopolymerization reactions under visible lights: Principle, mechanisms and examples of applications. Prog. Org. Coat. 2003, 47, 16–36. [Google Scholar] [CrossRef]
- Fiedor, P.; Pilch, M.; Szymaszek, P.; Chachaj-Brekiesz, A.; Galek, M.; Orty, J. Photochemical Study of a New Bimolecular Photoinitiating System for Vat Photopolymerization 3D Printing Techniques under Visible Light. Catalysts 2020, 10, 284. [Google Scholar] [CrossRef]
- Mendes-Felipe, C.; Oliveira, J.; Etxebarria, I.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. State-of-the-art and future challenges of UV curable polymer-based smart materials for printing technologies. Adv. Mater. Technol. 2019, 4, 1800618. [Google Scholar] [CrossRef]
- Shukla, V.; Bajpai, M.; Singh, D.K.; Singh, M.; Shukla, R. Review of basic chemistry of UV-curing technology. Pigm. Resin Technol. 2004, 33, 272–279. [Google Scholar] [CrossRef]
- Chen, M.; Zhong, M.; Johnson, J.A. Light-controlled radical polymerization: Mechanisms, methods, and applications. Chem. Rev. 2016, 116, 10167–10211. [Google Scholar] [CrossRef] [PubMed]
- Crivello, J.V.; Reichmanis, E. Photopolymer materials and processes for advanced technologies. Chem. Mater. 2014, 26, 533–548. [Google Scholar] [CrossRef]
- Bonardi, A.-H.; Dumur, F.; Grant, T.M.; Noirbent, G.; Gigmes, D.; Lessard, B.H.; Fouassier, J.-P.; Lalevée, J. High performance near infrared (NIR) photoinitiating systems operating under low light intensity and in presence of oxygen. Macromolecules 2018, 51, 1314–1324. [Google Scholar] [CrossRef]
- Scanone, A.C.; Casado, U.; Schroeder, W.F.; Hoppe, C.E. Visible-light photopolymerization of epoxy-terminated poly(dimethylsiloxane) blends: Influence of the cycloaliphatic monomer content on the curing behavior and network properties. Eur. Polym. J. 2020, 134, 109841. [Google Scholar] [CrossRef]
- Garra, P.; Bonardi, A.-H.; Baralle, A.; Al Mousawi, A.; Bonardi, F.; Dietlin, C.; Morlet-Savary, F.; Fouassier, J.-P.; Lalevée, J. Monitoring photopolymerization reactions through thermal imaging: A unique tool for the real-time follow-up of thick samples, 3D Printing, and composites. J. Polym. Sci. A Polym. Chem. 2018, 56, 889–899. [Google Scholar] [CrossRef]
- Sun, K.; Pigot, C.; Chen, H.; Nechab, M.; Gigmes, D.; Morlet-Savary, F.; Graff, B.; Liu, S.; Xiao, P.; Dumur, F.; et al. Free radical photopolymerization and 3D printing using newly developed dyes: Indane-1,3-dione and 1H-cyclopentanaphthalene-1,3-dione derivatives as photoinitiators in three-component systems. Catalysts 2020, 10, 463. [Google Scholar] [CrossRef]
- Lalevée, J.; Mokbel, H.; Fouassier, J.-P. Recent developments of versatile photoinitiating systems for cationic ring opening polymerization operating at any wavelengths and under low light intensity sources. Molecules 2015, 20, 7201–7221. [Google Scholar] [CrossRef] [PubMed]
- Tehfe, M.-A.; Louradour, F.; Lalevée, J.; Fouassier, J.-P. Photopolymerization reactions: On the way to a green and sustainable chemistry. Appl. Sci. 2013, 3, 490–514. [Google Scholar] [CrossRef]
- Podsiadły, R.; Maruszewska, A.; Michalski, R.; Marcinek, A.; Kolinska, J. Naphthoylene benzimidazolone dyes as electron transfer photosensitizers for iodonium salt induced cationic photopolymerizations. Dyes Pigm. 2012, 95, 252–259. [Google Scholar] [CrossRef]
- Zhang, Z.; Corrigan, N.; Bagheri, A.; Jin, J.; Boyer, C. A versatile 3D and 4D printing system through photocontrolled raft polymerization. Angew. Chem. 2019, 131, 18122–18131. [Google Scholar] [CrossRef]
- Bagheri, A.; Engel, K.A.; Anderson Bainbridge, C.W.; Xu, J.; Boyer, C.; Jin, J. 3D printing of polymeric materials based on photo-RAFT polymerization. Polym. Chem. 2020, 11, 641–647. [Google Scholar] [CrossRef]
- Bagheri, A.; Jin, J. Photopolymerization in 3D Printing. ACS Appl. Polym. Mater. 2019, 1, 593–611. [Google Scholar] [CrossRef]
- Aduba, D.C., Jr.; Margaretta, E.D.; Marnot, A.E.C.; Heifferon, K.V.; Surbey, W.R.; Chartrain, N.A.; Whittington, A.R.; Long, T.E.; Williams, C.B. Vat photopolymerization 3D printing of acid-cleavable PEG-methacrylate networks for biomaterial applications. Mater. Today Commun. 2019, 19, 204–211. [Google Scholar] [CrossRef]
- Lin, J.-T.; Cheng, D.-C.; Chen, K.-T.; Liu, H.-W. Dual-wavelength (UV and blue) controlled photopolymerization confinement for 3D-printing: Modeling and analysis of measurements. Polymers 2019, 11, 1819. [Google Scholar] [CrossRef]
- Jasinski, F.; Zetterlund, P.B.; Braun, A.M.; Chemtob, A. Photopolymerization in dispersed systems. Prog. Polym. Sci. 2018, 84, 47–88. [Google Scholar] [CrossRef]
- Noè, C.; Hakkarainen, M.; Sangermano, M. Cationic UV-Curing of Epoxidized Biobased Resins. Polymers 2021, 13, 89. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, C.; Zhang, R.; Liu, R.; Liu, J. Low volume shrinkage photopolymerization system using hydrogen-bond-based monomers. Prog. Org. Coat. 2019, 137, 105308. [Google Scholar] [CrossRef]
- Khudyakov, I.V.; Legg, J.C.; Purvis, M.B.; Overton, B.J. Kinetics of Photopolymerization of Acrylates with Functionality of 1-6. Ind. Eng. Chem. Res. 1999, 38, 3353–3359. [Google Scholar] [CrossRef]
- Noirbent, G.; Dumur, F. Photoinitiators of polymerization with reduced environmental impact: Nature as an unlimited and renewable source of dyes. Eur. Polym. J. 2021, 142, 110109. [Google Scholar] [CrossRef]
- Zhao, H.; Sha, J.; Wang, X.; Jiang, Y.; Chen, T.; Wu, T.; Chen, X.; Ji, H.; Gao, Y.; Xie, L.; et al. Spatiotemporal control of polymer brush formation through photoinduced radical polymerization regulated by DMD light modulation. Lab Chip 2019, 19, 2651–2662. [Google Scholar] [CrossRef] [PubMed]
- Xi, W.; Peng, H.; Aguirre-Soto, A.; Kloxin, C.J.; Stansbury, J.W.; Bowman, C.N. Spatial and Temporal Control of Thiol-Michael Addition via Photocaged Superbase in Photopatterning and Two-Stage Polymer Networks Formation. Macromolecules 2014, 47, 6159–6165. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Contal, E.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Novel highly efficient organophotocatalysts: Truxene-acridine-1,8-diones as photoinitiators of polymerization. Macromol. Chem. Phys. 2013, 214, 2189–2201. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Tehfe, M.-A.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Difunctional acridinediones as photoinitiators of polymerization under UV and visible lights: Structural effects. Polymer 2013, 54, 3458–3466. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Tehfe, M.-A.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Acridinediones: Effect of substituents on their photoinitiating abilities in radical and cationic photopolymerization. Macromol. Chem. Phys. 2013, 214, 2276–2282. [Google Scholar] [CrossRef]
- Zhang, J.; Campolo, D.; Dumur, F.; Xiao, P.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. The carbazole-bound ferrocenium salt as a specific cationic photoinitiator upon near-UV and visible LEDs (365–405 nm). Polym. Bull. 2016, 73, 493–507. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Dumur, F.; Toufaily, J.; Hamieh, T.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Carbazole scaffold based photoinitiators/photoredox catalysts for new LED projector 3D printing resins. Macromolecules 2017, 50, 2747–2758. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Magaldi Lara, D.; Noirbent, G.; Dumur, F.; Toufaily, J.; Hamieh, T.; Bui, T.-T.; Goubard, F.; Graff, B.; Gigmes, D.; et al. Carbazole derivatives with thermally activated delayed fluorescence property as photoinitiators/photoredox catalysts for LED 3D printing technology. Macromolecules 2017, 50, 4913–4926. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Garra, P.; Dumur, F.; Bui, T.-T.; Goubard, F.; Toufaily, J.; Hamieh, T.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; et al. Novel Carbazole Skeleton-Based Photoinitiators for LED Polymerization and LED Projector 3D Printing. Molecules 2018, 22, 2143. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Arar, A.; Ibrahim-Ouali, M.; Duval, S.; Dumur, F.; Garra, P.; Toufaily, J.; Hamieh, T.; Graff, B.; Gigmes, D.; et al. Carbazole-based compounds as photoinitiators for free radical and cationic polymerization upon near visible light illumination. Photochem. Photobiol. Sci. 2018, 17, 578–585. [Google Scholar] [CrossRef]
- Abdallah, M.; Magaldi, D.; Hijazi, A.; Graff, B.; Dumur, F.; Fouassier, J.-P.; Bui, T.-T.; Goubard, F.; Lalevée, J. Development of new high performance visible light photoinitiators based on carbazole scaffold and their applications in 3D printing and photocomposite synthesis. J. Polym. Sci. A Polym. Chem. 2019, 57, 2081–2092. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Contal, E.; Graff, B.; Gigmes, D.; Morlet-Savary, F.; Fouassier, J.-P.; Lalevée, J. New insights in radical and cationic polymerizations upon visible light exposure: Role of novel photoinitiator systems based on the pyrene chromophore. Polym. Chem. 2013, 4, 1625–1634. [Google Scholar] [CrossRef]
- Telitel, S.; Dumur, F.; Faury, T.; Graff, B.; Tehfe, M.-A.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. New core-pyrene π-structure organophotocatalysts usable as highly efficient photoinitiators. Beilstein J. Org. Chem. 2013, 9, 877–890. [Google Scholar] [CrossRef] [PubMed]
- Uchida, N.; Nakano, H.; Igarashi, T.; Sakurai, T. Nonsalt 1-(arylmethyloxy)pyrene photoinitiators capable of initiating cationic polymerization. J. Appl. Polym. Sci. 2014, 131, 40510. [Google Scholar] [CrossRef]
- Mishra, A.; Daswal, S. 1-(Bromoacetyl)pyrene, a novel photoinitiator for the copolymerization of styrene and methylmethacrylate. Rad. Phys. Chem. 2006, 75, 1093–1100. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Graff, B.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Design of new Type I & Type II photoinitiators possessing highly coupled pyrene-ketone moieties. Polym. Chem. 2013, 4, 2313–2324. [Google Scholar]
- Dumur, F. Recent advances on pyrene-based photoinitiators of polymerization. Eur. Polym. J. 2020, 126, 109564. [Google Scholar] [CrossRef]
- Lalevée, J.; Peter, M.; Dumur, F.; Gigmes, D.; Blanchard, N.; Tehfe, M.-A.; Morlet-Savary, F.; Fouassier, J.-P. Subtle ligand effects in oxidative photocatalysis with iridium complexes: Application to photopolymerization. Chem. Eur. J. 2011, 17, 15027–15031. [Google Scholar] [CrossRef]
- Lalevée, J.; Tehfe, M.-A.; Dumur, F.; Gigmes, D.; Blanchard, N.; Morlet-Savary, F.; Fouassier, J.-P. Iridium photocatalysts in free radical photopolymerization under visible lights. ACS Macro Lett. 2012, 1, 286–290. [Google Scholar] [CrossRef]
- Lalevée, J.; Dumur, F.; Mayer, C.R.; Gigmes, D.; Nasr, G.; Tehfe, M.-A.; Telitel, S.; Morlet-Savary, F.; Graff, B.; Fouassier, J.-P. Photopolymerization of N-vinylcarbazole using visible-light harvesting iridium complexes as photoinitiators. Macromolecules 2012, 45, 4134–4141. [Google Scholar] [CrossRef]
- Telitel, S.; Dumur, F.; Telitel, S.; Soppera, O.; Lepeltier, M.; Guillaneuf, Y.; Poly, J.; Morlet-Savary, F.; Fioux, P.; Fouassier, J.-P.; et al. Photoredox catalysis using a new iridium complex as an efficient toolbox for radical, cationic and controlled polymerizations under soft blue to green lights. Polym. Chem. 2015, 6, 613–624. [Google Scholar] [CrossRef]
- Telitel, S.; Dumur, F.; Lepeltier, M.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Photoredox process induced polymerization reactions: Iridium complexes for panchromatic photo-initiating systems. C. R. Chim. 2016, 19, 71–78. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Lepeltier, M.; Dumur, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Structural effects in the iridium complex series: Photoredox catalysis and photoinitiation of polymerization reactions under visible lights. Macromol. Chem. Phys. 2017, 218, 1700192. [Google Scholar] [CrossRef]
- Dumur, F.; Nasr, G.; Wantz, G.; Mayer, C.R.; Dumas, E.; Guerlin, A.; Miomandre, F.; Clavier, G.; Bertin, D.; Gigmes, D. Cationic iridium complex for the design of soft salt-based phosphorescent OLEDs and color-tunable Light-Emitting Electrochemical Cells. Org. Electron. 2011, 12, 1683–1694. [Google Scholar] [CrossRef]
- Nasr, G.; Guerlin, A.; Dumur, F.; Beouch, L.; Dumas, E.; Clavier, G.; Miomandre, F.; Goubard, F.; Gigmes, D.; Bertin, D.; et al. Iridium (III) soft salts from dinuclear cationic and mononuclear anionic complexes for OLEDs devices. Chem. Commun. 2011, 47, 10698–10700. [Google Scholar] [CrossRef] [PubMed]
- Dumur, F.; Bertin, D.; Gigmes, D. Iridium (III) complexes as promising emitters for solid-state light-emitting electrochemical cells (LECs). Int. J. Nanotechnol. 2012, 9, 377–395. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Zhang, J.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. Copper complexes in radical photoinitiating systems: Applications to free radical and cationic polymerization under visible lights. Macromolecules 2014, 47, 3837–3844. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Zhang, J.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Copper complexes: The effect of ligands on their photoinitiation efficiencies in radical polymerization reactions under visible light. Polym. Chem. 2014, 5, 6350–6357. [Google Scholar] [CrossRef]
- Xiao, P.; Zhang, J.; Campolo, D.; Dumur, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Copper and iron complexes as visible-light-sensitive photoinitiators of polymerization. J. Polym. Sci. A Polym. Chem. 2015, 53, 2673–2684. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Kermagoret, A.; Versace, D.-L.; Toufaily, J.; Hamieh, T.; Graff, B.; Dumur, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Copper photoredox catalysts for polymerization upon near UV or visible light: Structure/reactivity/ efficiency relationships and use in LED projector 3D printing resins. Polym. Chem. 2017, 8, 568–580. [Google Scholar] [CrossRef]
- Garra, P.; Kermagoret, A.; Al Mousawi, A.; Dumur, F.; Gigmes, D.; Morlet-Savary, F.; Dietlin, C.; Fouassier, J.-P.; Lalevée, J. New copper(I) complex based initiating systems in redox polymerization and comparison with the amine/benzoyl peroxide reference. Polym. Chem. 2017, 8, 4088–4097. [Google Scholar] [CrossRef]
- Garra, P.; Dumur, F.; Morlet-Savary, F.; Dietlin, C.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Mechanosynthesis of a copper complex for redox initiating systems with a unique near infrared light activation. J. Polym. Sci. A Polym. Chem. 2017, 55, 3646–3655. [Google Scholar] [CrossRef]
- Mokbel, H.; Anderson, D.; Plenderleith, R.; Dietlin, C.; Morlet-Savary, F.; Dumur, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Copper PhotoRedox Catalyst “G1”: A New High Performance Photoinitiator for Near-UV and Visible LEDs. Polym. Chem. 2017, 8, 5580–5592. [Google Scholar] [CrossRef]
- Garra, P.; Dumur, F.; Al Mousawi, A.; Graff, B.; Gigmes, D.; Morlet-Savary, F.; Dietlin, C.; Fouassier, J.-P.; Lalevée, J. Mechanosynthesized Copper (I) complex based initiating systems for redox polymerization: Towards upgraded oxidizing and reducing agents. Polym. Chem. 2017, 8, 5884–5896. [Google Scholar] [CrossRef]
- Garra, P.; Carré, M.; Dumur, F.; Morlet-Savary, F.; Dietlin, C.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Copper-based (photo) redox initiating systems as highly efficient systems for interpenetrating polymer network preparation. Macromolecules 2018, 51, 679–688. [Google Scholar] [CrossRef]
- Garra, P.; Dumur, F.; Nechab, M.; Morlet-Savary, F.; Dietlin, C.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Stable copper acetylacetonate-based oxidizing agents in redox (NIR photoactivated) polymerization: An opportunity for one pot grafting from approach and example on a 3D printed object. Polym. Chem. 2018, 9, 2173–2182. [Google Scholar] [CrossRef]
- Mokbel, H.; Anderson, D.; Plenderleith, R.; Dietlin, C.; Morlet-Savary, F.; Dumur, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Simultaneous initiation of radical and cationic polymerization reactions using the “G1” copper complex as photoRedox catalyst: Applications of free radical/cationic hybrid photo-polymerization in the composites and 3D printing fields. Prog. Org. Coat. 2019, 132, 50–61. [Google Scholar] [CrossRef]
- Launay, V.; Caron, A.; Noirbent, G.; Gigmes, D.; Dumur, F.; Lalevée, J. NIR dyes as innovative tools for reprocessing/recycling of plastics: Benefits of the photothermal activation in the near-infrared range. Adv. Funct. Mater. 2021, 31, 2006324. [Google Scholar] [CrossRef]
- Bonardi, A.; Bonardi, F.; Noirbent, G.; Dumur, F.; Dietlin, C.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Different NIR dye scaffolds for polymerization reactions under NIR light. Polym. Chem. 2019, 10, 6505–6514. [Google Scholar] [CrossRef]
- Giacoletto, N.; Ibrahim-Ouali, M.; Dumur, F. Recent advances on squaraine-based photoinitiators of polymerization. Eur. Polym. J. 2021, 150, 110427. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Winkel, A.; Eisenburger, M.; Menzel, H. Carboxylated camphorquinone as visible-light photoinitiator for biomedical application: Synthesis, characterization, and application. Arab. J. Chem. 2016, 9, 745–754. [Google Scholar] [CrossRef]
- Santini, A.; Gallegos, I.T.; Felix, C.M. Photoinitiators in dentistry: A review. Prim. Dent. J. 2013, 2, 30–33. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Frigoli, M.; Graff, B.; Morlet-Savary, F.; Wantz, G.; Bock, H.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. Perylene derivatives as photoinitiators in blue light sensitive cationic or radical curable films and panchromatic thiol-ene polymerizable films. Eur. Polym. J. 2014, 53, 215–222. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Green light induced cationic ring opening polymerization reactions: Perylene-3,4:9,10-bis(dicarboximide) as efficient photosensitizers. Macromol. Chem. Phys. 2013, 214, 1052–1060. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Red-light-induced cationic photopolymerization: Perylene derivatives as efficient photoinitiators. Macromol. Rapid Commun. 2013, 34, 1452–1458. [Google Scholar] [CrossRef]
- Mokbel, H.; Toufaily, J.; Hamieh, T.; Dumur, F.; Campolo, D.; Gigmes, D.; Fouassier, J.-P.; Ortyl, J.; Lalevee, J. Specific cationic photoinitiators for Near UV and visible LEDs: Iodonium vs. ferrocenium structures. J. Appl. Polym. Sci. 2015, 132, 42759. [Google Scholar] [CrossRef]
- Villotte, S.; Gigmes, D.; Dumur, F.; Lalevée, J. Design of Iodonium Salts for UV or Near-UV LEDs for Photoacid Generator and Polymerization Purposes. Molecules 2020, 25, 149. [Google Scholar] [CrossRef]
- Zivic, N.; Bouzrati-Zerrelli, M.; Villotte, S.; Morlet-Savary, F.; Dietlin, C.; Dumur, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. A novel naphthalimide scaffold based iodonium salt as a one-component photoacid/photoinitiator for cationic and radical polymerization under LED exposure. Polym. Chem. 2016, 7, 5873–5879. [Google Scholar] [CrossRef]
- Liu, S.; Chen, H.; Zhang, Y.; Sun, K.; Xu, Y.; Morlet-Savary, F.; Graff, B.; Noirbent, G.; Pigot, C.; Brunel, D.; et al. Monocomponent photoinitiators based on benzophenone-carbazole structure for led photoinitiating systems and application on 3D printing. Polymers 2020, 12, 1394. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Dumur, F.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Variations on the benzophenone skeleton: Novel high-performance blue light sensitive photoinitiating systems. Macromolecules 2013, 46, 7661–7667. [Google Scholar] [CrossRef]
- Zhang, J.; Frigoli, M.; Dumur, F.; Xiao, P.; Ronchi, L.; Graff, B.; Morlet-Savary, F.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. Design of novel photoinitiators for radical and cationic photopolymerizations under Near UV and Visible LEDs (385, 395 and 405 nm). Macromolecules 2014, 47, 2811–2819. [Google Scholar] [CrossRef]
- Liu, S.; Brunel, D.; Noirbent, G.; Mau, A.; Chen, H.; Morlet-Savary, F.; Graff, B.; Gigmes, D.; Xiao, P.; Dumur, F.; et al. New multifunctional benzophenone-based photoinitiators with high migration stability and their application in 3D printing. Mater. Chem. Front. 2021, 5, 1982–1994. [Google Scholar] [CrossRef]
- Liu, S.; Brunel, D.; Sun, K.; Zhang, Y.; Chen, H.; Xiao, P.; Dumur, F.; Lalevée, J. Novel photoinitiators based on benzophenone-triphenylamine hybrid structure for led photopolymerization. Macromol. Rapid Commun. 2020, 41, 2000460. [Google Scholar] [CrossRef]
- Liu, S.; Brunel, D.; Sun, K.; Xu, Y.; Morlet-Savary, F.; Graff, B.; Xiao, P.; Dumur, F.; Lalevée, J. A mono-component bifunctional benzophenone-carbazole type II photoinitiator for led photoinitiating systems. Polym. Chem. 2020, 11, 3551–3556. [Google Scholar] [CrossRef]
- Mokbel, H.; Dumur, F.; Lalevée, J. On demand NIR activated photopolyaddition reactions. Polym. Chem. 2020, 11, 4250–4259. [Google Scholar] [CrossRef]
- Mokbel, H.; Graff, B.; Dumur, F.; Lalevée, J. NIR sensitizer operating under long wavelength (1064 nm) for free radical photopolymerization processes. Macromol. Rapid Commun. 2020, 41, 2000289. [Google Scholar] [CrossRef]
- Zhang, J.; Zivic, N.; Dumur, F.; Guo, C.; Li, Y.; Xiao, P.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Panchromatic photoinitiators for radical, cationic and thiol-ene polymerization reactions: A search in the diketopyrrolopyrrole or indigo dye series. Mater. Today Commun. 2015, 4, 101–108. [Google Scholar] [CrossRef]
- Xiao, P.; Hong, W.; Li, Y.; Dumur, F.; Graff, B.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. Diketopyrrolopyrrole dyes: Structure/reactivity/efficiency relationship in photoinitiating systems upon visible lights. Polymer 2014, 55, 746–751. [Google Scholar] [CrossRef]
- Xiao, P.; Hong, W.; Li, Y.; Dumur, F.; Graff, B.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. Green light sensitive diketopyrrolopyrrole derivatives used in versatile photoinitiating systems for photopolymerizations. Polym. Chem. 2014, 5, 2293–2300. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Dumur, F.; Garra, P.; Toufaily, J.; Hamieh, T.; Goubard, F.; Bui, T.-T.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; et al. Azahelicenes as visible light photoinitiators for cationic and radical polymerization: Preparation of photo-luminescent polymers and use in high performance LED projector 3D printing resins. J. Polym. Sci. A Polym. Chem. 2017, 55, 1189–1199. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Schmitt, M.; Dumur, F.; Ouyang, J.; Favereaud, L.; Dorcet, V.; Vanthuyne, N.; Garra, P.; Toufaily, J.; Hamieh, T.; et al. Visible light chiral photoinitiator for radical polymerization and synthesis of polymeric films with strong chiroptical activity. Macromolecules 2018, 51, 5628–5637. [Google Scholar] [CrossRef]
- Bonardi, A.-H.; Zahouily, S.; Dietlin, C.; Graff, B.; Morlet-Savary, F.; Ibrahim-Ouali, M.; Gigmes, D.; Hoffmann, N.; Dumur, F.; Lalevée, J. New 1,8-naphthalimide derivatives as photoinitiators for free radical polymerization upon visible light. Catalysts 2019, 9, 637. [Google Scholar] [CrossRef]
- Zhang, J.; Zivic, N.; Dumur, F.; Xiao, P.; Graff, B.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. Naphthalimide-tertiary amine derivatives as blue-light-sensitive photoinitiators. ChemPhotoChem 2018, 2, 481–489. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Zhang, J.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Naphthalimide derivatives: Substituent effects on the photoinitiating ability in polymerizations under near UV, purple, white and blue LEDs (385 nm, 395 nm, 405 nm, 455 nm or 470 nm). Macromol. Chem. Phys. 2015, 216, 1782–1790. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Zhang, J.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Naphthalimide-phthalimide derivative based photoinitiating systems for polymerization reactions under blue lights. J. Polym. Sci. A Polym. Chem. 2015, 53, 665–674. [Google Scholar] [CrossRef]
- Zhang, J.; Zivic, N.; Dumur, F.; Xiao, P.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. A benzophenone-naphthalimide derivative as versatile photoinitiator for near UV and visible lights. J. Polym. Sci. A Polym. Chem. 2015, 53, 445–451. [Google Scholar] [CrossRef]
- Zhang, J.; Zivic, N.; Dumur, F.; Xiao, P.; Graff, B.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. N-[2-(dimethylamino)ethyl]-1,8-naphthalimide derivatives as photoinitiators under LEDs. Polym. Chem. 2018, 9, 994–1003. [Google Scholar] [CrossRef]
- Zivic, N.; Zhang, J.; Bardelang, D.; Dumur, F.; Xiao, P.; Jet, T.; Versace, D.-L.; Dietlin, C.; Morlet-Savary, F.; Graff, B.; et al. Novel naphthalimideamine based photoinitiators operating under violet and blue LEDs and usable for various polymerization reactions and synthesis of hydrogels. Polym. Chem. 2016, 7, 418–429. [Google Scholar] [CrossRef]
- Zhang, J.; Dumur, F.; Xiao, P.; Graff, B.; Bardelang, D.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Structure design of naphthalimide derivatives: Towards versatile photo-initiators for near UV/Visible LEDs, 3D printing and water-soluble photoinitiating systems. Macromolecules 2015, 48, 2054–2063. [Google Scholar] [CrossRef]
- Zhang, J.; Zivic, N.; Dumur, F.; Xiao, P.; Graff, B.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. UV violet-blue LED induced polymerizations: Specific photoinitiating systems at 365, 385, 395 and 405 nm. Polymer 2014, 55, 6641–6648. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Blue light sensitive dyes for various photopolymerization reactions: Naphthalimide and naphthalic anhydride derivatives. Macromolecules 2014, 47, 601–608. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Frigoli, M.; Tehfe, M.-A.; Graff, B.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. Naphthalimide based methacrylated photoinitiators in radical and cationic photopolymerization under visible light. Polym. Chem. 2013, 4, 5440–5448. [Google Scholar] [CrossRef]
- Noirbent, G.; Dumur, F. Recent advances on naphthalic anhydrides and 1,8-naphthalimide-based photoinitiators of polymerization. Eur. Polym. J. 2020, 132, 109702. [Google Scholar] [CrossRef]
- Rahal, M.; Mokbel, H.; Graff, B.; Pertici, V.; Gigmes, D.; Toufaily, J.; Hamieh, T.; Dumur, F.; Lalevée, J. Naphthalimide-Based dyes as photoinitiators under visible light irradiation and their applications: Photocomposite synthesis, 3D printing and polymerization in water. ChemPhotoChem 2021, 5, 476–490. [Google Scholar] [CrossRef]
- Chen, H.; Noirbent, G.; Sun, K.; Brunel, D.; Gigmes, D.; Morlet-Savary, F.; Zhang, Y.; Liu, S.; Xiao, P.; Dumur, F.; et al. Photoinitiators derived from natural product scaffolds: Mono-chalcones in three-component photoinitiating systems and their applications in 3D printing. Polym. Chem. 2020, 11, 4647–4659. [Google Scholar] [CrossRef]
- Tang, L.; Nie, J.; Zhu, X. A high-performance phenyl-free LED photoinitiator for cationic or hybrid photopolymerization and its application in LED cationic 3D printing. Polym. Chem. 2020, 11, 2855–2863. [Google Scholar] [CrossRef]
- Xu, Y.; Noirbent, G.; Brunel, D.; Ding, Z.; Gigmes, D.; Graff, B.; Xiao, P.; Dumur, F.; Lalevée, J. Allyloxy ketones as efficient photoinitiators with high migration stability in free radical polymerization and 3D printing. Dyes Pigm. 2021, 185, 108900. [Google Scholar] [CrossRef]
- Sun, K.; Chen, H.; Zhang, Y.; Morlet-Savary, F.; Graff, B.; Xiao, P.; Dumur, F.; Lalevée, J. High-performance sunlight induced polymerization using novel push-pull dyes with high light absorption properties. Eur. Polym. J. 2021, 151, 110410. [Google Scholar] [CrossRef]
- Chen, H.; Noirbent, G.; Liu, S.; Brunel, D.; Graff, B.; Gigmes, D.; Zhang, Y.; Sun, K.; Morlet-Savary, F.; Xiao, P.; et al. Bis-chalcone derivatives derived from natural products as near-UV/visible light sensitive photoinitiators for 3D/4D printing. Mater. Chem. Front. 2021, 5, 901–916. [Google Scholar] [CrossRef]
- Xu, Y.; Ding, Z.; Zhu, H.; Graff, B.; Xiao, P.; Dumur, F.; Lalevée, F. Design of ketone derivatives as highly efficient photoinitiators for free radical and cationic photopolymerizations and application in 3D printing of composites. J. Polym. Sci. 2021. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Y.; Sun, K.; Graff, B.; Xiao, P.; Dumur, F.; Lalevée, J. Design of photoinitiating systems based on the chalcone-anthracene scaffold for led cationic photopolymerization and application in 3D Printing. Eur. Polym. J. 2021, 147, 110300. [Google Scholar] [CrossRef]
- Zhang, J.; Campolo, D.; Dumur, F.; Xiao, P.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. Iron complexes as photoinitiators for radical and cationic polymerization through photoredox catalysis processes. J. Polym. Sci. A Polym. Chem. 2015, 53, 42–49. [Google Scholar] [CrossRef]
- Telitel, S.; Dumur, F.; Campolo, D.; Poly, J.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Iron complexes as potential photocatalysts for controlled radical photopolymerizations: A tool for modifications and patterning of surfaces. J. Polym. Sci. A Polym. Chem. 2016, 54, 702–713. [Google Scholar] [CrossRef]
- Zhang, J.; Campolo, D.; Dumur, F.; Xiao, P.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. Visible-light-sensitive photoredox catalysis by iron complexes: Applications in cationic and radical polymerization reactions. J. Polym. Sci. A Polym. Chem. 2016, 54, 2247–2253. [Google Scholar] [CrossRef]
- Zhang, J.; Campolo, D.; Dumur, F.; Xiao, P.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. Novel iron complexes in visible-light-sensitive photoredox catalysis: Effect of ligands on their photoinitiation efficiencies. ChemCatChem 2016, 8, 2227–2233. [Google Scholar] [CrossRef]
- Zhang, J.; Dumur, F.; Horcajada, P.; Livage, C.; Xiao, P.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. Iron-based metal-organic frameworks (MOF) as photocatalysts for radical and cationic polymerizations under near UV and visible LEDs (385–405 nm). Macromol. Chem. Phys. 2016, 217, 2534–2540. [Google Scholar] [CrossRef]
- Dumur, F. Recent advances on ferrocene-based photoinitiating systems. Eur. Polym. J. 2021, 147, 110328. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Xiao, P.; Graff, B.; Morlet-Savary, F.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. New chromone based photoinitiators for polymerization reactions upon visible lights. Polym. Chem. 2013, 4, 4234–4244. [Google Scholar] [CrossRef]
- You, J.; Fu, H.; Zhao, D.; Hu, T.; Nie, J.; Wang, T. Flavonol dyes with different substituents in photopolymerization. J. Photochem. Photobiol. A Chem. 2020, 386, 112097. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Garra, P.; Schmitt, M.; Toufaily, J.; Hamieh, T.; Graff, B.; Fouassier, J.-P.; Dumur, F.; Lalevée, J. 3-Hydroxyflavone and N-phenyl-glycine in high performance photo-initiating systems for 3D printing and photocomposites synthesis. Macromolecules 2018, 51, 4633–4641. [Google Scholar] [CrossRef]
- Karaca, N.; Ocala, N.; Arsua, N.; Jockusch, S. Thioxanthone-benzothiophenes as photoinitiator for free radical polymerization. J. Photochem. Photobiol. A Chem. 2016, 331, 22–28. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, X.; Xiong, Y.; Yang, J.; Tang, H. Thioxanthone based one-component polymerizable visible light photoinitiator for free radical polymerization. RSC Adv. 2016, 6, 66098–66107. [Google Scholar] [CrossRef]
- Qiu, J.; Wei, J. Thioxanthone photoinitiator containing polymerizable N-aromatic maleimide for photopolymerization. J. Polym. Res. 2014, 21, 559. [Google Scholar] [CrossRef]
- Dadashi-Silab, S.; Aydogan, C.; Yagci, Y. Shining a light on an adaptable photoinitiator: Advances in photopolymerizations initiated by thioxanthones. Polym. Chem. 2015, 6, 6595–6615. [Google Scholar] [CrossRef]
- Zhang, J.; Lalevée, J.; Zhao, J.; Graff, B.; Stenzel, M.H.; Xiao, P. Dihydroxyanthraquinone derivatives: Natural dyes as blue-light-sensitive versatile photoinitiators of photopolymerization. Polym. Chem. 2016, 7, 7316–7324. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Poriel, C.; Dumur, F.; Toufaily, J.; Hamieh, T.; Fouassier, J.-P.; Lalevée, J. Zinc tetraphenylporphyrin as high performance visible-light photoinitiator of cationic photosensitive resins for LED projector 3D printing applications. Macromolecules 2017, 50, 746–753. [Google Scholar] [CrossRef]
- Noirbent, G.; Xu, Y.; Bonardi, A.-H.; Gigmes, D.; Lalevée, J.; Dumur, F. Metalated Porphyrins as versatile visible light and NIR photoinitiators of polymerization. Eur. Polym. J. 2020, 139, 110019. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Lalevée, J.; Dumur, F.; Telitel, S.; Gigmes, D.; Contal, E.; Bertin, D.; Fouassier, J.-P. Zinc-based metal complexes as new photocatalysts in polymerization initiating systems. Eur. Polym. J. 2013, 49, 1040–1049. [Google Scholar] [CrossRef]
- Abdallah, M.; Le, H.; Hijazi, A.; Graff, B.; Dumur, F.; Bui, T.-T.; Goubard, F.; Fouassier, J.-P.; Lalevée, J. Acridone derivatives as high performance visible light photoinitiators for cationic and radical photosensitive resins for 3D printing technology and for low migration photopolymer property. Polymer 2018, 159, 47–58. [Google Scholar] [CrossRef]
- Zhang, J.; Dumur, F.; Bouzrati, M.; Xiao, P.; Dietlin, C.; Morlet-Savary, F.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Novel panchromatic photopolymerizable matrices: N,N’-dibutyl-quinacridone as an efficient and versatile photoinitiator. J. Polym. Sci. A Polym. Chem. 2015, 53, 1719–1727. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Zein-Fakih, A.; Lalevée, J.; Dumur, F.; Gigmes, D.; Graff, B.; Morlet-Savary, F.; Hamieh, T.; Fouassier, J.-P. New pyridinium salts as versatile compounds for dye sensitized photo-polymerization. Eur. Polym. J. 2013, 49, 567–574. [Google Scholar] [CrossRef]
- Xiao, P.; Frigoli, M.; Dumur, F.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Julolidine or fluorenone based push-pull dyes for polymerization upon soft polychromatic visible light or green light. Macromolecules 2014, 47, 106–112. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Graff, B.; Morlet-Savary, F.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. New push-pull dyes derived from Michler’s ketone for polymerization reactions upon visible lights. Macromolecules 2013, 46, 3761–3770. [Google Scholar] [CrossRef]
- Mokbel, H.; Dumur, F.; Mayer, C.R.; Morlet-Savary, F.; Graff, B.; Gigmes, D.; Toufaily, J.; Hamieh, T.; Fouassier, J.-P.; Lalevée, J. End capped polyenic structures as visible light sensitive photoinitiators for polymerization of vinylethers. Dyes Pigm. 2014, 105, 121–129. [Google Scholar] [CrossRef]
- Garra, P.; Brunel, D.; Noirbent, G.; Graff, B.; Morlet-Savary, F.; Dietlin, C.; Sidorkin, V.F.; Dumur, F.; Duché, D.; Gigmes, D.; et al. Ferrocene-based (photo)redox polymerization under long wavelengths. Polym. Chem. 2019, 10, 1431–1441. [Google Scholar] [CrossRef]
- Telitel, S.; Dumur, F.; Kavalli, T.; Graff, B.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. The 1,3-bis(dicyanomethylidene)-indane skeleton as a (photo) initiator in thermal ring opening polymerization at RT and radical or cationic photopolymerization. RSC Adv. 2014, 4, 15930–15936. [Google Scholar] [CrossRef]
- Mokbel, H.; Dumur, F.; Graff, B.; Mayer, C.R.; Gigmes, D.; Toufaily, J.; Hamieh, T.; Fouassier, J.-P.; Lalevée, J. Michler’s ketone as an interesting scaffold for the design of high-performance dyes in photoinitiating systems upon visible lights. Macromol. Chem. Phys. 2014, 215, 783–790. [Google Scholar] [CrossRef]
- Sun, K.; Liu, S.; Pigot, S.; Brunel, D.; Graff, B.; Nechab, M.; Gigmes, D.; Morlet-Savary, F.; Zhang, Y.; Xiao, P.; et al. Novel push-pull dyes derived from 1H-cyclopenta[b]naphthalene-1,3(2H)-dione as versatile photoinitiators for photopolymerization and their related applications: 3D-printing and fabrication of photocomposites. Catalysts 2020, 10, 1196. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Graff, B.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Push–pull (thio)barbituric acid derivatives in dye photosensitized radical and cationic polymerization reactions under 457/473 nm laser beams or blue LEDs. Polym. Chem. 2013, 4, 3866–3875. [Google Scholar] [CrossRef]
- Dumur, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Organic Electronics: An El Dorado in the quest of new photoCatalysts as photoinitiators of polymerization. Acc. Chem. Res. 2016, 49, 1980–1989. [Google Scholar] [CrossRef]
- Sun, K.; Liu, S.; Chen, H.; Morlet-Savary, F.; Graff, B.; Pigot, C.; Nechab, M.; Xiao, P.; Dumur, F.; Lalevée, J. N-ethylcarbazole-1-allylidene-based push-pull dyes as efficient light harvesting photoinitiators for sunlight induced polymerization. Eur. Polym. J. 2021, 147, 110331. [Google Scholar] [CrossRef]
- Dumur, F. Recent advances on visible light photoinitiators of polymerization based on indane-1,3-dione and related derivatives. Eur. Polym. J. 2021, 143, 110178. [Google Scholar] [CrossRef]
- Abdallah, M.; Bui, T.-T.; Goubard, F.; Theodosopoulou, D.; Dumur, F.; Hijazi, A.; Fouassier, J.-P.; Lalevée, J. Phenothiazine derivatives as photoredox catalysts for cationic and radical photosensitive resins for 3D printing technology and photocomposites synthesis. Polym. Chem. 2019, 10, 6145–6156. [Google Scholar] [CrossRef]
- Abdallah, M.; Hijazi, A.; Graff, B.; Fouassier, J.-P.; Rodeghiero, G.; Gualandi, A.; Dumur, F.; Cozzi, P.G.; Lalevée, J. Coumarin derivatives as high performance visible light photoinitiators/photoredox catalysts for photosensitive resins for 3D printing technology, photopolymerization in water and photocomposites synthesis. Polym. Chem. 2019, 10, 872–884. [Google Scholar] [CrossRef]
- Li, Z.; Zou, X.; Zhu, G.; Liu, X.; Liu, R. Coumarin-Based Oxime Esters: Photobleachable and Versatile Unimolecular Initiators for Acrylate and Thiol-Based Click Photopolymerization under Visible Light-Emitting Diode Light Irradiation. ACS Appl. Mater. Interf. 2018, 10, 16113–16123. [Google Scholar] [CrossRef]
- Abdallah, M.; Dumur, F.; Hijazi, A.; Rodeghiero, G.; Gualandi, A.; Cozzi, P.G.; Lalevée, J. Keto-coumarin scaffold for photo-initiators for 3D printing and photo-composites. J. Polym. Sci. 2020, 58, 1115–1129. [Google Scholar] [CrossRef]
- Abdallah, M.; Hijazi, A.; Dumur, F.; Lalevée, J. Coumarins as powerful photosensitizers for the cationic polymerization of epoxy-silicones under near-UV and visible light and applications for 3D printing technology. Molecules 2020, 25, 2063. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, Q.; Gao, P.; Chi, B.; Nie, J.; He, Y. Photopolymerization of coumarin-containing reversible photoresponsive materials based on wavelength selectivity. Ind. Eng. Chem. Res. 2019, 58, 2970–2975. [Google Scholar] [CrossRef]
- Rahal, M.; Mokbel, H.; Graff, B.; Toufaily, J.; Hamieh, T.; Dumur, F.; Lalevée, J. Mono vs. difunctional coumarin as photoinitiators in photocomposite synthesis and 3D printing. Catalysts 2020, 10, 1202. [Google Scholar] [CrossRef]
- Abdallah, M.; Hijazi, A.; Cozzi, P.G.; Gualandi, A.; Dumur, F.; Lalevée, J. Boron compounds as additives for the cationic polymerization using coumarin derivatives in epoxy-silicones. Macromol. Chem. Phys. 2021, 222, 2000404. [Google Scholar] [CrossRef]
- Guit, J.; Tavares, M.B.L.; Hul, J.; Ye, C.; Loos, K.; Jager, J.; Folkersma, R.; Voet, V.S.D. Photopolymer Resins with Biobased Methacrylates Based on Soybean Oil for Stereolithography. ACS Appl. Polym. Mater. 2020, 2, 949–957. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Thirion, D.; Fagour, S.; Vacher, S.; Sallenave, X.; Morlet-Savary, F.; Graff, B.; Fouassier, J.-P.; Gigmes, D.; et al. Multicolor photoinitiators for radical and cationic polymerization: Mono vs. poly functional thiophene derivatives. Macromolecules 2013, 46, 6786–6793. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Ali, S.; Akram, M.Y.; Nie, J.; Zhu, X. The effect of polyethylene glycoldiacrylate complexation on type II photoinitiator and promotion for visible light initiation system. J. Photochem. Photobiol. A Chem. 2019, 384, 112037. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Li, Y.; Li, R.; Nie, J.; Zhu, X. In situ monitoring of photopolymerization by photoinitiator with luminescence characteristics. J. Photochem. Photobiol. A Chem. 2020, 389, 112225. [Google Scholar] [CrossRef]
- Li, J.; Hao, Y.; Zhong, M.; Tang, L.; Nie, J.; Zhu, X. Synthesis of furan derivative as LED light photoinitiator: One-pot, low usage, photobleaching for light color 3D printing. Dyes Pigm. 2019, 165, 467–473. [Google Scholar] [CrossRef]
- Xu, Y.; Noirbent, G.; Brunel, D.; Ding, Z.; Gigmes, D.; Graff, B.; Xiao, P.; Dumur, F.; Lalevée, J. Novel ketone derivatives based photoinitiating systems for free radical polymerization under mild conditions and 3D printing. Polym. Chem. 2020, 11, 5767–5777. [Google Scholar] [CrossRef]
- Arikawa, H.; Takahashi, H.; Kanie, T.; Ban, S. Effect of various visible light photoinitiators on the polymerization and color of light-activated resins. Dent. Mater. J. 2009, 28, 454–460. [Google Scholar] [CrossRef]
- Tomal, W.; Ortyl, J. Water-Soluble Photoinitiators in Biomedical Applications. Polymers 2020, 12, 1073. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Graff, B.; Clément, J.-L.; Gigmes, D.; Morlet-Savary, F.; Fouassier, J.-P.; Lalevée, J. New Cleavable Photoinitiator Architecture with Huge Molar Extinction Coefficients for Polymerization in the 340−450 nm Range. Macromolecules 2013, 46, 736–746. [Google Scholar] [CrossRef]
- Xiao, P.; Zhang, J.; Dumur, F.; Tehfe, M.-A.; Morlet-Savary, F.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Visible light sensitive photoinitiating systems: Recent progress in cationic and radical photopolymerization reactions under soft conditions. Prog. Polym. Sci. 2015, 41, 32–66. [Google Scholar] [CrossRef]
- Kabatc, J.; Iwinska, K.; Balcerak, A.; Kwiatkowska, D.; Skotnicka, A.; Czechband, Z.; Bartkowiak, M. Onium salts improve the kinetics of photopolymerization of acrylate activated with visible light. RSC Adv. 2020, 10, 24817–24829. [Google Scholar] [CrossRef]
- Crivello, J.V. The Discovery and Development of Onium Salt Cationic Photoinitiators. J. Polym. Sci. A Polym. Chem. 1999, 37, 4241–4254. [Google Scholar] [CrossRef]
- Crivello, J.V.; Lam, J.H.W. Diaryliodonium Salts. A New Class of Photoinitiators for Cationic Polymerization. Macromolecules 1977, 10, 1307–1315. [Google Scholar] [CrossRef]
- Topa, M.; Ortyl, J. Moving Towards a Finer Way of Light-Cured Resin-Based Restorative Dental Materials: Recent Advances in Photoinitiating Systems Based on Iodonium Salts. Materials 2020, 13, 4093. [Google Scholar] [CrossRef] [PubMed]
- Lalevée, J.; Telitel, S.; Xiao, P.; Lepeltier, M.; Dumur, F.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.-P. Metal and metal free photocatalysts: Mechanistic approach and application as photoinitiators of photopolymerization. Beilstein J. Org. Chem. 2014, 10, 863–876. [Google Scholar] [CrossRef]
- Fouassier, J.-P.; Lalevée, J. Photochemical Production of Interpenetrating Polymer Networks; Simultaneous Initiation of Radical and Cationic Polymerization Reactions. Polymers 2014, 6, 2588–2610. [Google Scholar] [CrossRef]
- Tomal, W.; Chachaj-Brekiesz, A.; Popielarz, R.; Ortyl, J. Multifunctional biphenyl derivatives as photosensitisers in various types of photopolymerization processes, including IPN formation, 3D printing of photocurable multiwalled carbon nanotubes (MWCNTs) fluorescent composites. RSC Adv. 2020, 10, 32162–32182. [Google Scholar] [CrossRef]
- Lalevée, J.; Morlet-Savary, F.; Dietlin, C.; Graff, B.; Fouassier, J.-P. Photochemistry and Radical Chemistry under Low Intensity Visible Light Sources: Application to Photopolymerization Reactions. Molecules 2014, 19, 15026–15041. [Google Scholar] [CrossRef]
- Andrzejewska, E.; Grajek, K. Recent advances in photo-induced free-radical polymerization. MOJ Poly. Sci. 2017, 1, 58–60. [Google Scholar] [CrossRef][Green Version]
- Corrigan, N.; Shanmugam, S.; Xu, J.; Boyer, C. Photocatalysis in organic and polymer synthesis. Chem. Soc. Rev. 2016, 45, 6165–6212. [Google Scholar] [CrossRef]
- Lalevée, J.; Tehfe, M.-A.; Morlet-Savary, F.; Graff, B.; Dumur, F.; Gigmes, D.; Blanchard, N.; Fouassier, J.-P. Photoredox catalysis for polymerization reactions. Chimia 2012, 66, 439–441. [Google Scholar] [CrossRef]
- Bonardi, A.-H.; Dumur, F.; Noirbent, G.; Lalevée, J.; Gigmes, D. Organometallic vs organic photoredox catalysts for photocuring reactions in the visible region. Beilstein J. Org. Chem. 2018, 14, 3025–3046. [Google Scholar] [CrossRef]
- Banoth, R.K.; Thatikonda, A. A review on natural chalcones an update. Int. J. Pharm. Sci. Res. 2020, 11, 546–555. [Google Scholar]
- Morita, Y.; Takagi, K.; Fukuchi-Mizutani, M.; Ishiguro, K.; Tanaka, Y.; Nitasaka, E.; Nakayama, M.; Saito, N.; Kagami, T.; Hoshino, A.; et al. A chalcone isomerase-like protein enhances flavonoid production and flower pigmentation. Plant J. 2014, 78, 294–304. [Google Scholar] [CrossRef]
- Davies, K.M.; Bloor, S.J.; Spiller, G.B.; Deroles, S.C. Production of yellow colour in flowers: Redirection of flavonoid biosynthesis in Petunia. Plant J. 1998, 13, 259–266. [Google Scholar] [CrossRef]
- Rammohan, A.; Reddy, J.S.; Sravya, G.; Rao, C.N.; Zyryanov, G.V. Chalcone synthesis, properties and medicinal applications: A review. Environ. Chem. Lett. 2020, 18, 433–458. [Google Scholar] [CrossRef]
- Phan, T.P.; Teo, K.Y.; Liu, Z.-Q.; Tsai, J.-K.; Tay, M.G. Application of unsymmetrical bis-chalcone compounds in dye sensitized solar cell. Chem. Data Coll. 2019, 22, 100256. [Google Scholar] [CrossRef]
- Rajakumar, P.; Thirunarayanan, A.; Raja, S.; Ganesan, S.; Maruthamuthu, P. Photophysical properties and dye-sensitized solar cell studies on thiadiazole–triazole–chalcone dendrimers. Tetrahedron Lett. 2012, 53, 1139–1143. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, A.S.; Agarwal, N.K.; Shahband, P.A.; Shrivastav, P.S. Self-assembled blue-light emitting materials for their liquid crystalline and OLED applications: From a simple molecular design to supramolecular materials. Mol. Syst. Des. Eng. 2020, 5, 1691–1705. [Google Scholar] [CrossRef]
- Zhang, Y.-P.; Wang, B.-X.; Yang, Y.-S.; Liang, C.; Yang, C.; Chai, H.-L. Synthesis and self-assembly of chalcone-based organogels. Coll. Surf. A 2019, 577, 449–455. [Google Scholar] [CrossRef]
- Chen, S.; Qin, C.; Jin, M.; Pan, H.; Wan, D. Novel chalcone derivatives with large conjugation structures as photosensitizers for versatile photopolymerization. J. Polym. Sci. 2021, 59, 578–593. [Google Scholar] [CrossRef]
- Nam, S.W.; Kang, S.H.; Chang, J.Y. Synthesis and Photopolymerization of Photoreactive Mesogens Based on Chalcone. Macromol. Res. 2007, 15, 74–81. [Google Scholar] [CrossRef]
- Yadav, G.D.; Wagh, D.P. Claisen-Schmidt Condensation using Green Catalytic Processes: A Critical Review. ChemistrySelect 2020, 5, 9059–9085. [Google Scholar] [CrossRef]
- Qian, H.; Liu, D. Synthesis of Chalcones via Claisen-Schmidt Reaction Catalyzed by SulfonicAcid-Functional Ionic Liquids. Ind. Eng. Chem. Res. 2011, 50, 1146–1149. [Google Scholar] [CrossRef]
- Rafiee, E.; Rahimi, F. A green approach to the synthesis of chalcones via Claisen-Schmidt condensation reaction using cesium salts of 12-tungstophosphoric acid as a reusable nanocatalyst. Monatsh. Chem. 2013, 144, 361–367. [Google Scholar] [CrossRef]
- Reza Sazegar, M.; Mahmoudian, S.; Mahmoudi, A.; Triwahyono, S.; Abdul Jalil, A.; Mukti, R.R.; Nazirah Kamarudine, N.H.; Ghoreishi, M.K. Catalyzed Claisen–Schmidt reaction by protonated aluminate mesoporous silica nanomaterial focused on the (E)-chalcone synthesis as a biologically active compound. RSC Adv. 2016, 6, 11023–11031. [Google Scholar] [CrossRef]
- Narendera, T.; Venkateswarlu, K.; Vishnu Nayak, B.; Sarkar, S. A new chemical access for 30-acetyl-40-hydroxychalcones usingborontrifluoride–etherate via a regioselective Claisen-Schmidt condensation and its application in the synthesis of chalcone hybrids. Tetrahedron Lett. 2011, 52, 5794–5798. [Google Scholar] [CrossRef]
- Rozmer, Z.; Perjési, P. Naturally occurring chalcones and their biological activities. Phytochem. Rev. 2016, 15, 87–120. [Google Scholar] [CrossRef]
- Shivani, T.; Bhavesh, S. A review: Chemical and biological activity of chalcones with their metal complex. Asian J. Biomed. Pharmaceut. Sci. 2020, 10, 6–13. [Google Scholar] [CrossRef]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Anand, A.; Kumar, V. Recent developments in biological activities of chalcones: A mini review. Eur. J. Med. Chem. 2014, 85, 758–777. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.-C.; Lee, Y.; Min, D.; Jung, M.; Oh, S. Practical Synthesis of Chalcone Derivatives and Their Biological Activities. Molecules 2017, 22, 1872. [Google Scholar] [CrossRef] [PubMed]
- Tehfe, M.-A.; Dumur, F.; Xiao, P.; Delgove, M.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Chalcone derivatives as highly versatile photoinitiators for radical, cationic, thiol-ene and IPN polymerization reactions upon visible lights. Polym. Chem. 2014, 5, 382–390. [Google Scholar] [CrossRef]
- Xu, Y.; Noirbent, G.; Brunel, D.; Liu, F.; Gigmes, D.; Sun, K.; Zhang, Y.; Liu, S.; Morlet-Savary, F.; Xiao, P.; et al. Ketone derivatives as photoinitiators for both radical and cationic photopolymerizations under visible LED and application in 3D printing. Eur. Polym. J. 2020, 132, 109737. [Google Scholar] [CrossRef]
- Gajda, M.; Rybakiewicz, R.; Cieplak, M.; Zołek, T.; Maciejewska, D.; Gilant, E.; Rudzki, P.J.; Grab, K.; Kutner, A.; Borowicza, P.; et al. Low-oxidation-potential thiophene-carbazole monomers for electro-oxidative molecular imprinting: Selective chemosensing of aripiprazole. Biosens. Bioelectron. 2020, 169, 112589. [Google Scholar] [CrossRef]
- Akoudad, S.; Frère, P.; Mercier, N.; Roncali, J. Low Oxidation Potential Tetrathiafulvalene Analogues Based on 3,4-Dialkoxythiophene—Conjugating Spacers. J. Org. Chem. 1999, 64, 4267–4272. [Google Scholar] [CrossRef]
- Czichy, M.; Zhylitskaya, H.; Zassowski, P.; Navakouski, M.; Chulkin, P.; Janasik, P.; Lapkowski, M.; Stępień, M. Electrochemical Polymerization of Pyrrole−Perimidine Hybrids: Low-Band-Gap Materials with High n-Doping Activity. J. Phys. Chem. C 2020, 124, 14350–14362. [Google Scholar] [CrossRef]
- Lee, T.Y.; Guymon, C.A.; Sonny Jönsson, E.; Hoyle, C.E. The effect of monomer structure on oxygen inhibition of (meth)acrylates photopolymerization. Polymer 2004, 45, 6155–6162. [Google Scholar] [CrossRef]
- Belon, C.; Allonas, X.; Croutxé-Barghorn, C.; Lalevée, J. Overcoming the oxygen inhibition in the photopolymerization of acrylates: A study of the beneficial effect of triphenylphosphine. J. Polym. Sci. A Polym. Chem. 2010, 48, 2462–2469. [Google Scholar] [CrossRef]
- Ligon, S.C.; Husár, B.; Wutzel, H.; Holman, R.; Liska, R. Strategies to Reduce Oxygen Inhibition in Photoinduced Polymerization. Chem. Rev. 2014, 114, 557–589. [Google Scholar] [CrossRef]
- Pierrel, J.; Ibrahim, A.; Croutxé-Barghorn, C.; Allonas, X. Effect of the oxygen affected layer in multilayered photopolymers. Polym. Chem. 2017, 8, 4596–4602. [Google Scholar] [CrossRef]
- Lee, J.H.; Prud’homme, R.K.; Aksay, I.A. Cure depth in photopolymerization: Experiments and theory. J. Mater. Res. 2001, 16, 3536. [Google Scholar] [CrossRef]
- Garra, P.; Dietlin, C.; Morlet-Savary, F.; Dumur, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Photopolymerization of thick films and in shadow areas: A review for the access to composites. Polym. Chem. 2017, 8, 7088–7101. [Google Scholar] [CrossRef]
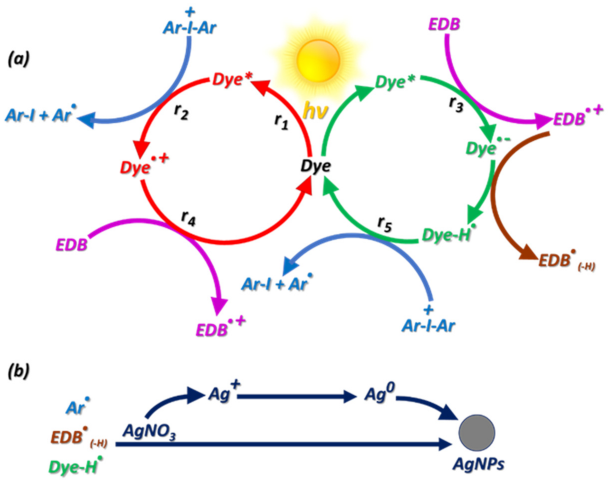
- Chen, H.; Noirbent, G.; Liu, S.; Zhang, Y.; Sun, K.; Morlet-Savary, F.; Gigmes, D.; Xiao, P.; Dumur, F.; Lalevée, J. In-situ generation of Ag nanoparticles during photopolymerization by using newly developed dyes-based three-component photoinitiating systems and their shape change behavior. J. Polym. Sci. 2021, 59, 843–859. [Google Scholar] [CrossRef]
- Chen, H.; Noirbent, G.; Zhang, Y.; Sun, K.; Liu, S.; Brunel, D.; Gigmes, D.; Graff, B.; Morlet-Savary, F.; Xiao, P.; et al. Photopolymerization and 3D/4D applications using newly developed dyes: Search around the natural chalcone scaffold in photoinitiating systems. Dyes Pigm. 2021, 188, 109213. [Google Scholar] [CrossRef]
- Corakci, B.; Hacioglu, S.O.; Toppare, L.; Bulut, U. Long wavelength photosensitizers in photoinitiated cationic polymerization: The effect of quinoxaline derivatives on photopolymerization. Polymer 2013, 54, 3182–3187. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Nie, J.; Zhu, X. Visible light and water-soluble photoinitiating system based on the charge transfer complex for free radical photopolymerization. J. Photochem. Photobiol. A Chem. 2020, 402, 112803. [Google Scholar] [CrossRef]
- Liska, R. Photoinitiators with functional groups. V. New water-soluble photoinitiators containing carbohydrate residues and copolymerizable derivatives thereof. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 1504–1518. [Google Scholar] [CrossRef]
- Lima, C.G.S.; Lima, T.D.; Duarte, M.; Jurberg, I.D.; Paixao, M.W. Organic synthesis enabled by light-irradiation of eda complexes: Theoretical background and synthetic applications. ACS Catal. 2016, 6, 1389–1407. [Google Scholar] [CrossRef]
- Kaya, K.; Kreutzer, J.; Yagci, Y. A Charge-Transfer Complex of Thioxanthonephenacyl Sulfonium Salt as a Visible-Light Photoinitiator for Free Radical and Cationic Polymerizations. ChemPhotoChem 2019, 3, 1187–1192. [Google Scholar] [CrossRef]
- Wang, D.; Garra, P.; Lakhdar, S.; Graff, B.; Fouassier, J.-P.; Mokbel, H.; Abdallah, M.; Lalevée, J. Charge Transfer Complexes as Dual Thermal and Photochemical Polymerization Initiators for 3D Printing and Composites Synthesis. ACS Appl. Polym. Mater. 2019, 3, 561–570. [Google Scholar] [CrossRef]
- Wang, D.; Kaya, K.; Garra, P.; Fouassier, J.-P.; Graff, B.; Yagci, Y.; Lalevée, J. Sulfonium salt-based charge transfer complexes as dual thermal and photochemical polymerization initiators for composites and 3D printing. Polym. Chem. 2019, 10, 4690–4698. [Google Scholar] [CrossRef]
- Garra, P.; Fouassier, J.-P.; Lakhdar, S.; Yagci, Y.; Lalevée, J. Visible light photoinitiating systems by charge transfer complexes: Photochemistry without dyes. Prog. Polym. Sci. 2020, 107, 101277. [Google Scholar] [CrossRef]
- Tasdelen, M.A.; Lalevée, J.; Yagci, Y. Photoinduced free radical promoted cationic polymerization 40 years after its discovery. Polym. Chem. 2020, 11, 1111–1121. [Google Scholar] [CrossRef]
- Garra, P.; Caron, A.; Al Mousawi, A.; Graff, B.; Morlet-Savary, F.; Dietlin, C.; Yagci, Y.; Fouassier, J.-P.; Lalevée, J. Photochemical, Thermal Free Radical, and Cationic Polymerizations Promoted by Charge Transfer Complexes: Simple Strategy for the Fabrication of Thick Composites. Macromolecules 2018, 51, 7872–7880. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, P. 3D printing of photopolymers. Polym. Chem. 2018, 9, 1530–1540. [Google Scholar] [CrossRef]
- Baralle, A.; Garra, P.; Graff, B.; Morlet-Savary, F.; Dietlin, C.; Fouassier, J.-P.; Lakhdar, S.; Lalevée, J. Iodinated Polystyrene for Polymeric Charge Transfer Complexes: Toward High-Performance Near-UV and Visible Light Macrophotoinitiators. Macromolecules 2019, 52, 3448–3453. [Google Scholar] [CrossRef]
- Han, W.; Fu, H.; Xue, T.; Liu, T.; Wang, Y.; Wang, T. Facilely prepared blue-green light sensitive curcuminoids with excellent bleaching properties as high performance photosensitizers in cationic and free radical photopolymerization. Polym. Chem. 2018, 9, 1787–1798. [Google Scholar] [CrossRef]
- Ebner, C.; Mitterer, J.; Eigruber, P.; Stieger, S.; Riess, G.; Kern, W. Ultra-High Through-Cure of (Meth)Acrylate Copolymers via Photofrontal Polymerization. Polymers 2020, 12, 1291. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Soppera, O.; Plain, J.; Jradi, S.; Sun, X.W.; Demir, H.V.; Yang, X.; Deeb, C.; Gray, S.K.; Wiederrecht, G.P. Plasmon-based photopolymerization: Near-field probing, advanced photonic nanostructures and nanophotochemistry. J. Opt. 2014, 16, 114002. [Google Scholar] [CrossRef]
- Chen, H.; Noirbent, G.; Zhang, Y.; Brunel, D.; Gigmes, D.; Liu, S.; Sun, K.; Morlet-Savary, F.; Graff, B.; Xiao, P.; et al. Novel D-π-A and A-π-D-π-A three-component photoinitiating systems based on carbazole/triphenylamino based chalcones and application in 3D and 4D Printing. Polym. Chem. 2020, 11, 6512–6528. [Google Scholar] [CrossRef]
- Jeong, H.Y.; Woo, B.H.; Kim, N.; Jun, Y.C. Multicolor 4D printing of shape-memory polymers for light-induced selective heating and remote actuation. Sci. Rep. 2020, 10, 6258. [Google Scholar] [CrossRef]
- Bajpai, A.; Baigent, A.; Raghav, S.; Brádaigh, C.O.; Koutsos, V.; Radacsi, N. 4D Printing: Materials, Technologies, and Future Applications in the Biomedical Field. Sustainability 2020, 12, 10628. [Google Scholar] [CrossRef]
- Chu, H.; Yang, W.; Sun, L.; Cai, S.; Yang, R.; Liang, W.; Yu, H.; Liu, L. 4D Printing: A Review on Recent Progresses. Micromachines 2020, 11, 796. [Google Scholar] [CrossRef]
- Lee, A.Y.; An, J.; Chua, C.K. Two-Way 4D Printing: A Review on the Reversibility of 3D-Printed Shape Memory Materials. Engineering 2017, 3, 663–674. [Google Scholar] [CrossRef]
- Keneth, E.S.; Lieberman, R.; Rednor, M.; Scalet, G.; Auricchio, F.; Magdassi, S. Multi-Material 3D Printed Shape Memory Polymer with Tunable Melting and Glass Transition Temperature Activated by Heat or Light. Polymers 2020, 12, 710. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, F.; Ge, Q. Multimaterial direct 4D printing of high stiffness structures with large bending curvature. Ext. Mech. Lett. 2021, 42, 10112. [Google Scholar]
- Zhang, Y.; Zhang, Y.; Josien, L.; Salomon, J.-P.; Simon-Masseron, A.; Lalevée, J. Photopolymerization of Zeolite/Polymer-Based Composites: Toward 3D and 4D Printing Applications. ACS Appl. Polym. Mater. 2021, 3, 400–409. [Google Scholar] [CrossRef]
- Inverardi, N.; Pandini, S.; Bignotti, F.; Scalet, G.; Marconi, S.; Auricchio, F. Sequential Motion of 4D Printed Photopolymers with Broad Glass Transition. Macromol. Mater. Eng. 2020, 305, 1900370. [Google Scholar] [CrossRef]




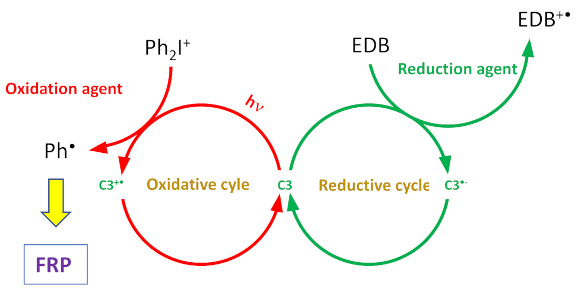
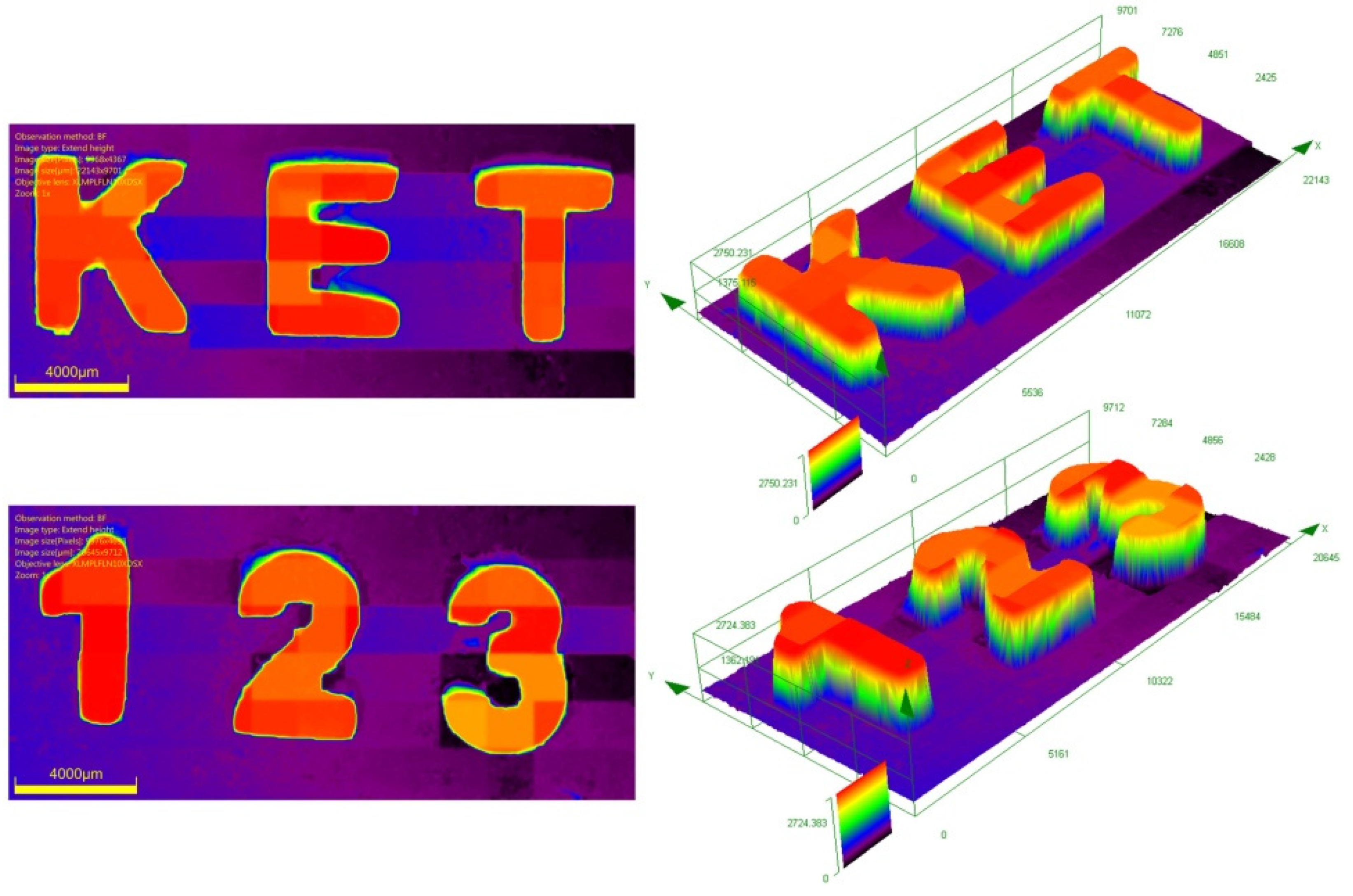
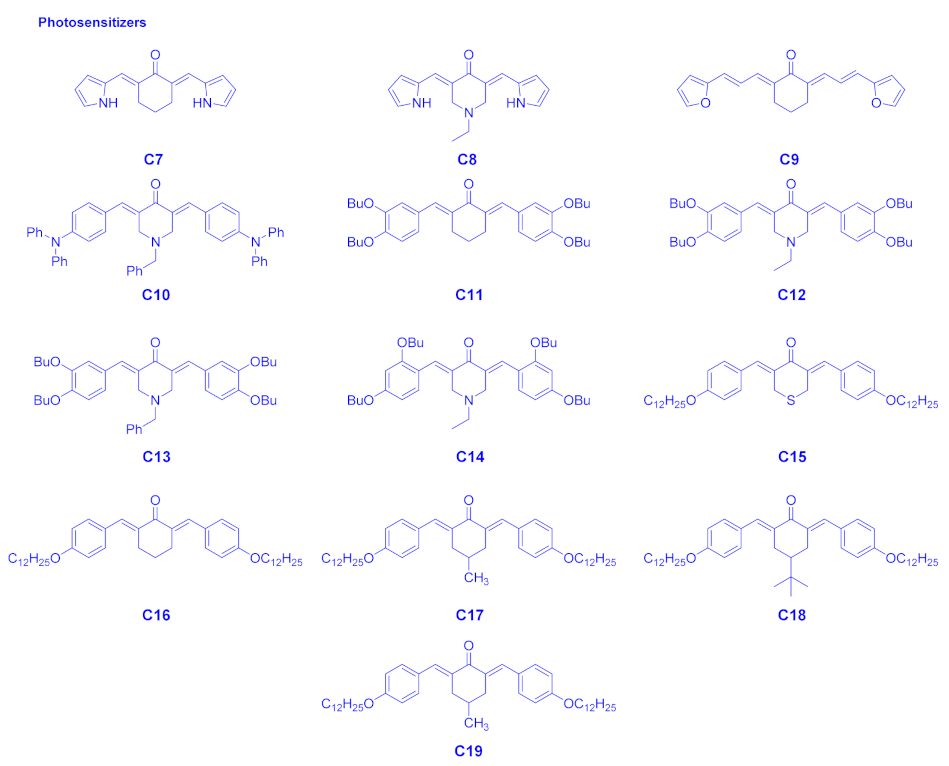
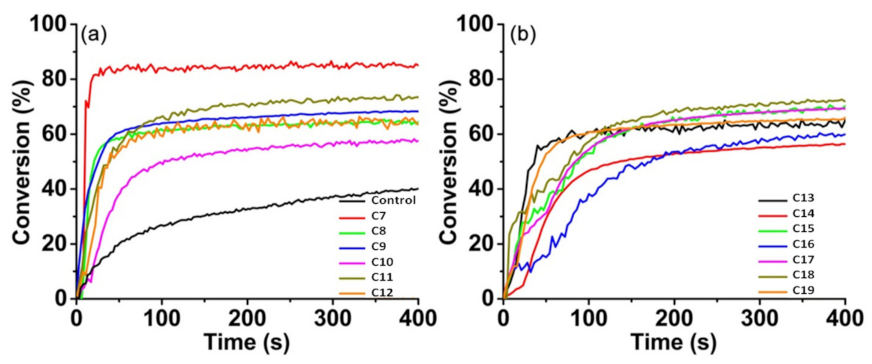
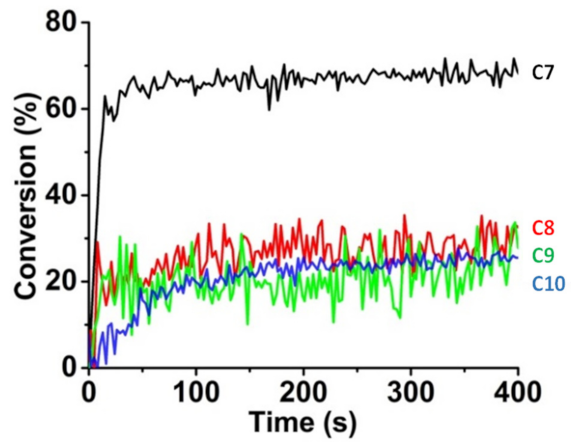
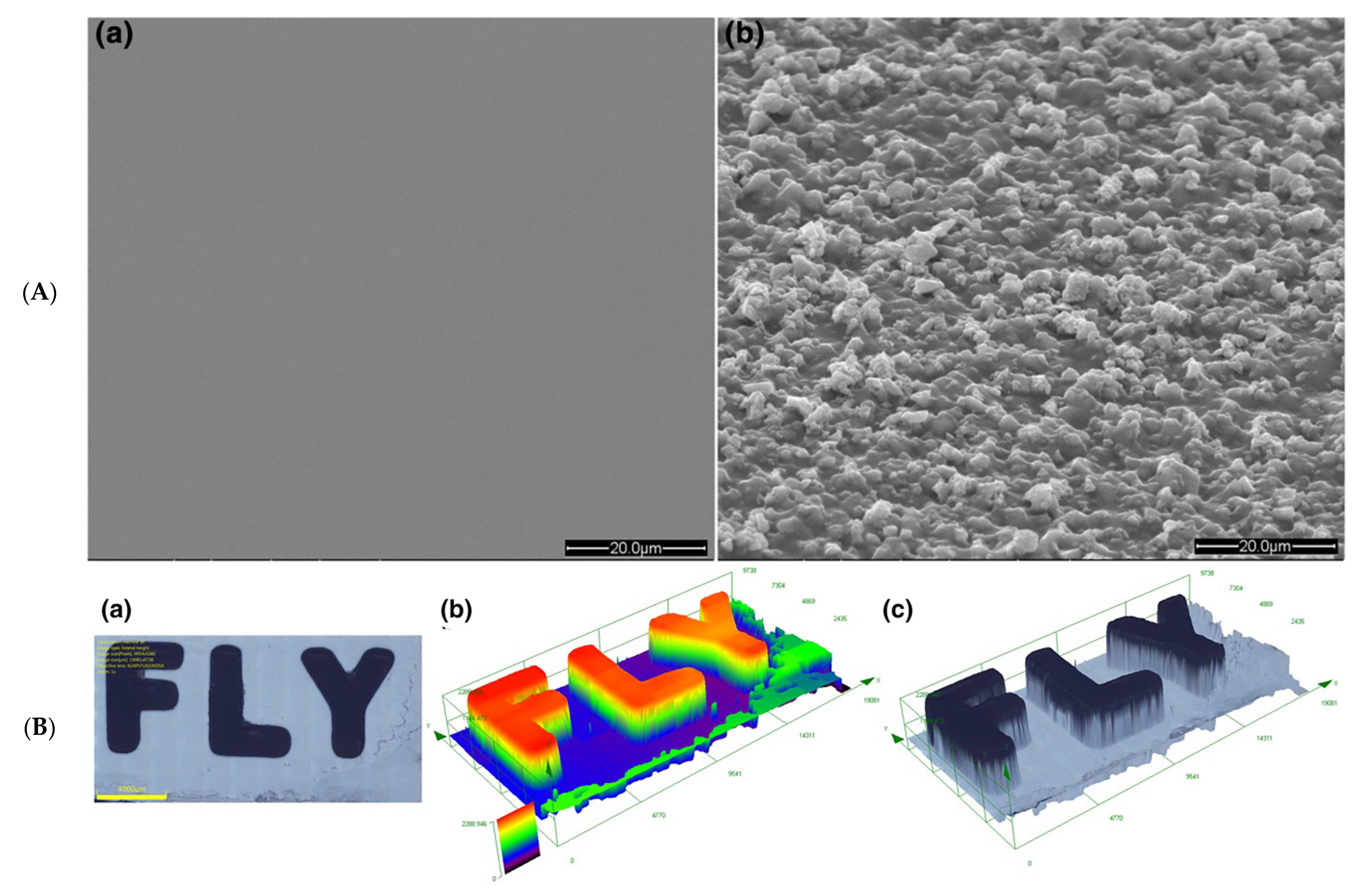
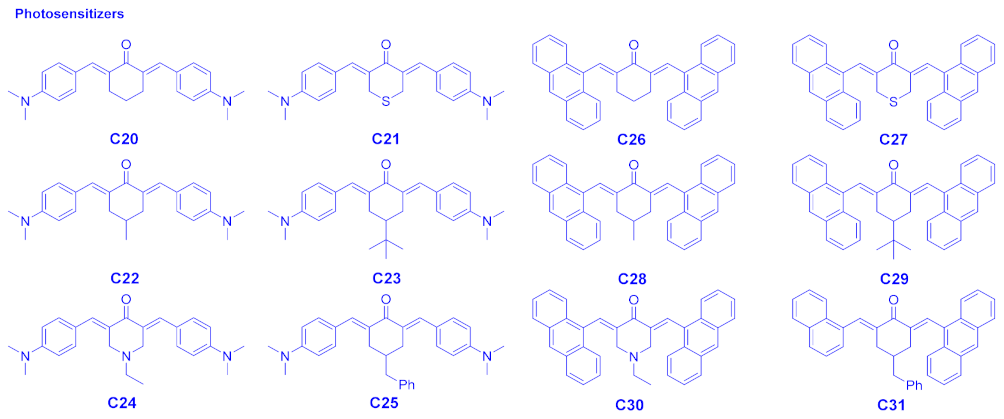


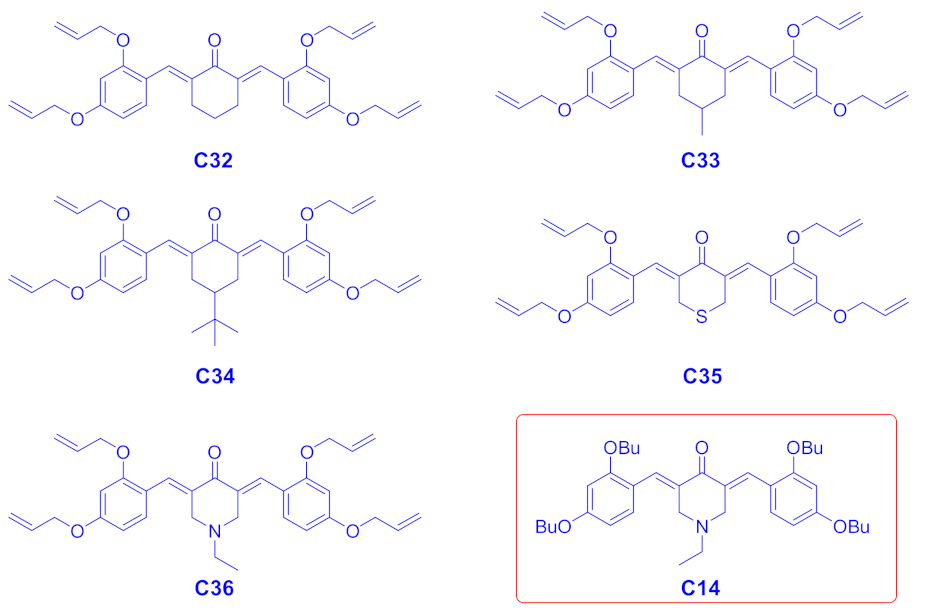

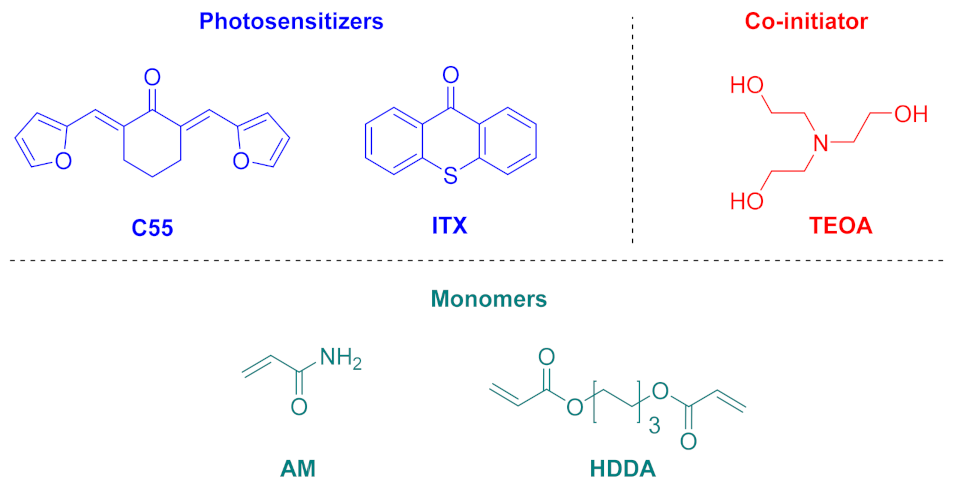
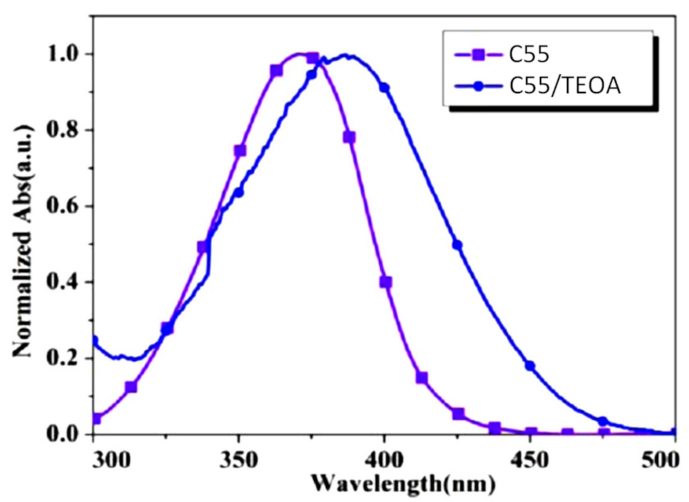
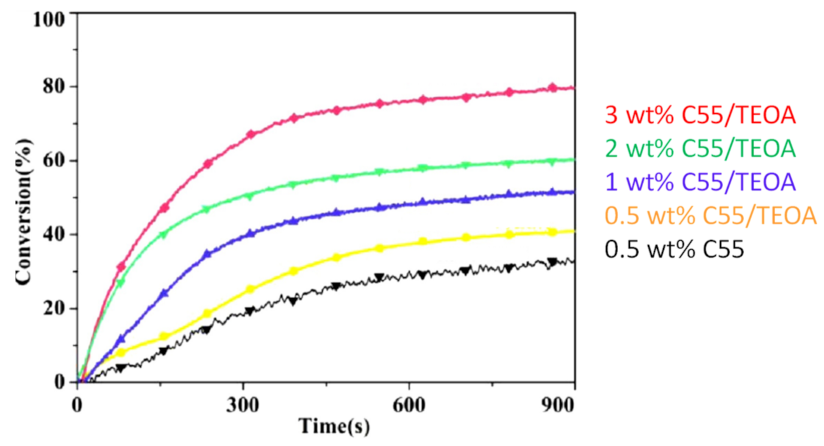

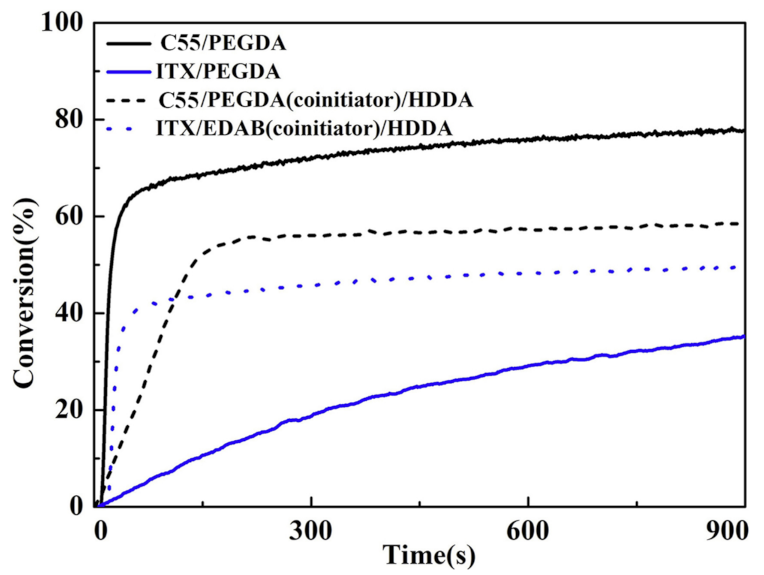



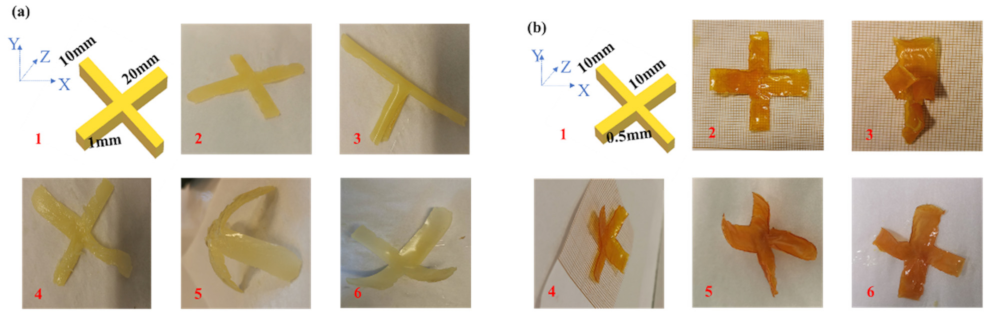
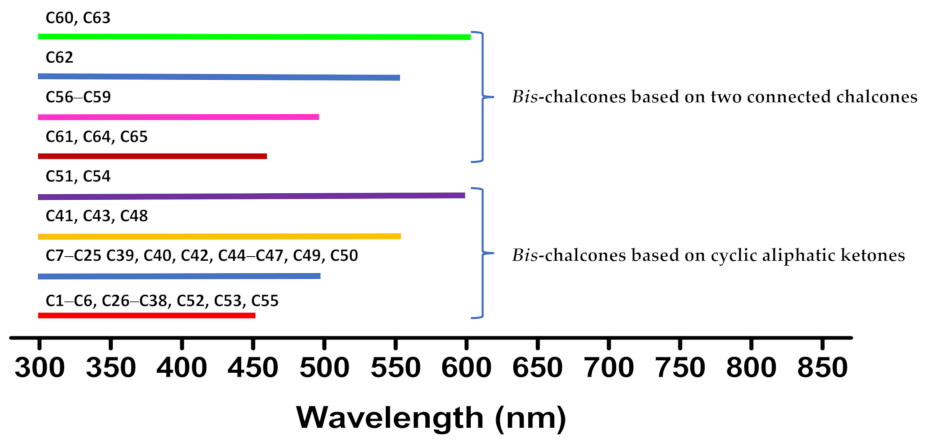
| Compounds | λmax (nm) | εmax (M−1·cm−1) | ε@405 nm (M−1·cm−1) |
|---|---|---|---|
| C1 | 368 | 29,230 | 9740 |
| C2 | 365 | 25,020 | 7980 |
| C3 | 370 | 34,920 | 11,690 |
| C4 | 368 | 34,200 | 10,130 |
| C5 | 372 | 31,470 | 11,950 |
| C6 | 370 | 29,750 | 10,280 |
| Compounds | C1 | C2 | C3 | C4 | C5 | C6 |
|---|---|---|---|---|---|---|
| FCs (thick films) | ~30% | ~24% | ~94% | ~24% | ~90% | ~25% |
| FCs (thin films) | ~55% | ~67% | ~55% | ~81% | ~59% | ~71% |
| Compounds | λmax (nm) | εmax (M−1·cm−1) | ε405 nm (M−1·cm−1) |
|---|---|---|---|
| C7 | 416 | 18,800 | 17,900 |
| C8 | 405 | 36,200 | 36,200 |
| C9 | 405 | 37,700 | 37,700 |
| C10 | 434 | 30,800 | 23,000 |
| C11 | 366 | 21,700 | 11,100 |
| C12 | 375 | 25,200 | 15,500 |
| C13 | 375 | 22,000 | 14,500 |
| C14 | 375 | 23,400 | 15,800 |
| C15 | 350 | 16,500 | 3100 |
| C16 | 350 | 2800 | 900 |
| C17 | 355 | 23,600 | 5300 |
| C18 | 355 | 12,900 | 2700 |
| C19 | 360 | 20,200 | 5700 |
| Photoinitiating Systems | FCs |
|---|---|
| Amine/Iod | |
| without chalcone | 39% |
| C7 | 85% |
| C8 | 64% |
| C9 | 68% |
| C10 | 56% |
| C11 | 73% |
| C12 | 64% |
| C13 | 63% |
| C14 | 56% |
| C15 | 69% |
| C16 | 60% |
| C17 | 69% |
| C18 | 72% |
| C19 | 66% |
| Compounds | λmax (nm) | εmax (M−1 cm−1) | ε405 nm (M−1 cm−1) |
|---|---|---|---|
| C20 | 430 | 41,600 | 31,900 |
| C21 | 430 | 45,300 | 35,000 |
| C22 | 431 | 49,900 | 38,400 |
| C23 | 430 | 40,000 | 31,900 |
| C24 | 436 | 49,200 | 34,200 |
| C25 | 440 | 43,300 | 30,000 |
| C26 | 250 | 81,800 | 5400 |
| C27 | 250 | 113,300 | 7400 |
| C28 | 250 | 149,600 | 9900 |
| C29 | 250 | 178,400 | 11,500 |
| C30 | 250 | 175,900 | 12,000 |
| C31 | 250 | 129,500 | 7800 |
| FCs (in thick) | C20 | C21 | C22 | C23 | C24 | C25 |
| ~84% | ~90% | ~84% | ~88% | ~86% | ~83% | |
| C26 | C27 | C28 | C29 | C30 | C31 | |
| ~90% | ~41% | ~68% | ~81% | ~41% | ~53% | |
| FCs (in thin) | C20 | C21 | C22 | C23 | C24 | C25 |
| ~43% | ~67% | ~56% | ~58% | ~63% | ~76% | |
| C26 | C27 | C28 | C29 | C30 | C31 | |
| ~66% | ~68% | ~60% | ~74% | ~73% | ~76% |
| Chalcone-Based Dyes | λmax (nm) | εmax (M−1·cm−1) | ε@405nm (M−1·cm−1) | ε@470nm (M−1·cm−1) |
|---|---|---|---|---|
| C37 | 396 | 41,980 | 37,050 | 270 |
| C38 | 392 | 40,840 | 33,590 | 110 |
| C39 | 400 274 | 41,770 69,250 | 41,610 | 2,720 |
| C40 | 399 | 42,500 | 41,580 | 1,280 |
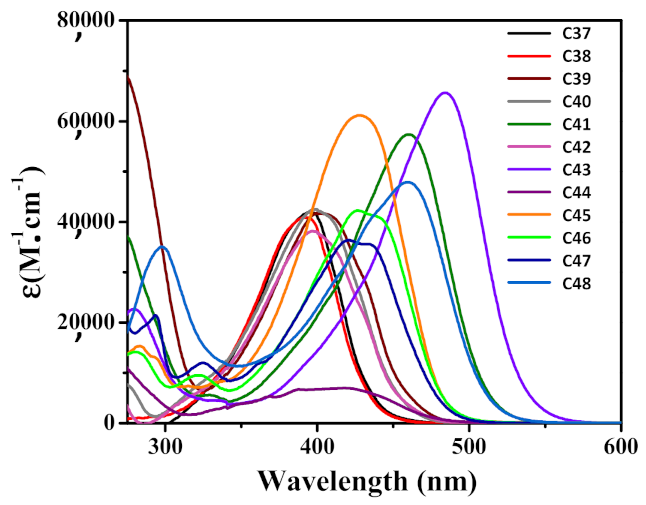
| C41 | 460 274 | 57,400 37,300 | 23,700 | 53,446 |
| C42 | 397 | 38,150 | 36,700 | 1,710 |
| C43 | 485 278 | 65,670 22,680 | 16,200 | 59,370 |
| C44 | 418 268 | 6,970 12,890 | 6800 | 1,600 |
| C45 | 428 283 | 61,130 15,340 | 50,310 | 16,540 |
| C46 | 427 280 | 42,062 14,160 | 32,270 | 14,380 |
| C47 | 421 236 | 36,350 46,810 | 30,560 | 10,160 |
| C48 | 460 298 | 47,620 35,060 | 25,300 | 44,400 |
| Chalcones | λmax (nm) | εmax (M−1·cm−1) | ε@375nm (M−1·cm−1) | ε@405nm (M−1·cm−1) | PEG-Diacrylate Conversion | EPOX Conversion | |
|---|---|---|---|---|---|---|---|
| 375 nm | 405 nm | 375 nm | |||||
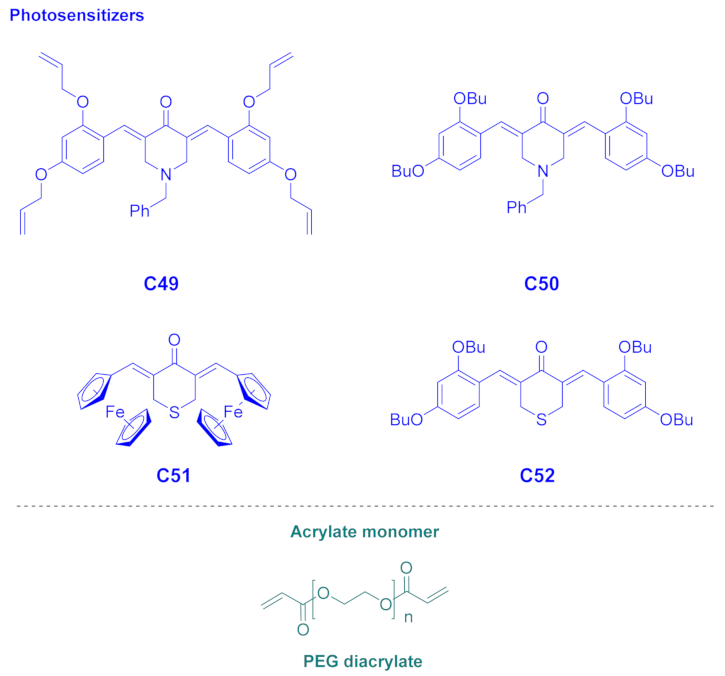
| C49 | 370 | 21,900 | 21,800 | 12,740 | 77 | 60 | 70 |
| C50 | 380 | 28,200 | 28,000 | 19,730 | 89 | 79 | 72 |
| C51 | 332 511 | 18,200 4940 | 5800 | 4420 | 60 | 42 | 66 |
| C52 | 369 | 4980 | 4800 | 2550 | 82 | 42 | 74 |
| blank 1 | - | - | - | - | 49 | 49 | - |
| blank 2 | - | - | - | - | - | - | 45 |
| Compounds | λmax (nm) | εmax(M−1·cm−1) | ε@405nm (M−1·cm−1) |
|---|---|---|---|
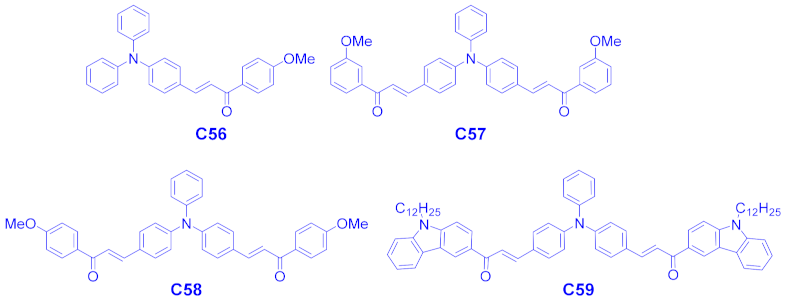
| C56 | 405 | 18,740 | 18,740 |
| C57 | 430 | 7990 | 6760 |
| C58 | 428 | 8540 | 7200 |
| C59 | 430 | 10,500 | 9020 |
| Chalcones | Chalcone/Iod/EDB | Chalcone/Iod | ||
|---|---|---|---|---|
| Thin Films | Thick Molds | Thin Films | Thick Molds | |
| C56 | 71% | 92% | 82% | 94% |
| C57 | 58% | 53% | 77% | 52% |
| C58 | 85% | 17% | 93% | 21% |
| C59 | 85% | 77% | 93% | 92% |
| blank | 50% | 93% | ||
| Chalcones | λmax (nm) | εmax(M−1·cm−1) | ε@405nm (M−1·cm−1) | ε@375nm (M−1·cm−1) |
|---|---|---|---|---|
| C60 | 347 | 23,100 | 6800 | 16,500 |
| C61 | 364 | 22,700 | 10,070 | 21,500 |
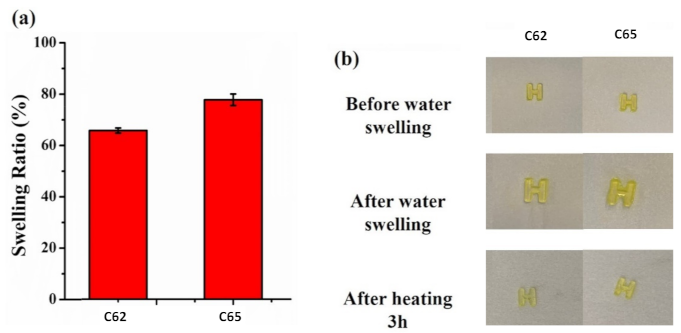
| C62 | 430 | 38,900 | 26,420 | 8500 |
| C63 | 330 | 19,800 | 2960 | 4000 |
| C64 | 370 | 24,600 | 13,670 | 24,000 |
| C65 | 350 | 49,300 | 2220 | 24,500 |
| LED@375 nm | |||||||
| Chalcones | C60 | C61 | C62 | C63 | C64 | C65 | Blank |
| FCs | 86 | 95 | 80 | 64 | 89 | 82 | 49 |
| LED@405 nm | |||||||
| Chalcones | C60 | C61 | C62 | C63 | C64 | C65 | Blank |
| FCs | 62 | 83 | 95 | 53 | 91 | 62 | 49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giacoletto, N.; Dumur, F. Recent Advances in bis-Chalcone-Based Photoinitiators of Polymerization: From Mechanistic Investigations to Applications. Molecules 2021, 26, 3192. https://doi.org/10.3390/molecules26113192
Giacoletto N, Dumur F. Recent Advances in bis-Chalcone-Based Photoinitiators of Polymerization: From Mechanistic Investigations to Applications. Molecules. 2021; 26(11):3192. https://doi.org/10.3390/molecules26113192
Chicago/Turabian StyleGiacoletto, Nicolas, and Frédéric Dumur. 2021. "Recent Advances in bis-Chalcone-Based Photoinitiators of Polymerization: From Mechanistic Investigations to Applications" Molecules 26, no. 11: 3192. https://doi.org/10.3390/molecules26113192
APA StyleGiacoletto, N., & Dumur, F. (2021). Recent Advances in bis-Chalcone-Based Photoinitiators of Polymerization: From Mechanistic Investigations to Applications. Molecules, 26(11), 3192. https://doi.org/10.3390/molecules26113192