Scale-Up Preparation of Crocins I and II from Gardeniajasminoides by a Two-Step Chromatographic Approach and Their Inhibitory Activity Against ATP Citrate Lyase
Abstract
1. Introduction
2. Results and Discussion
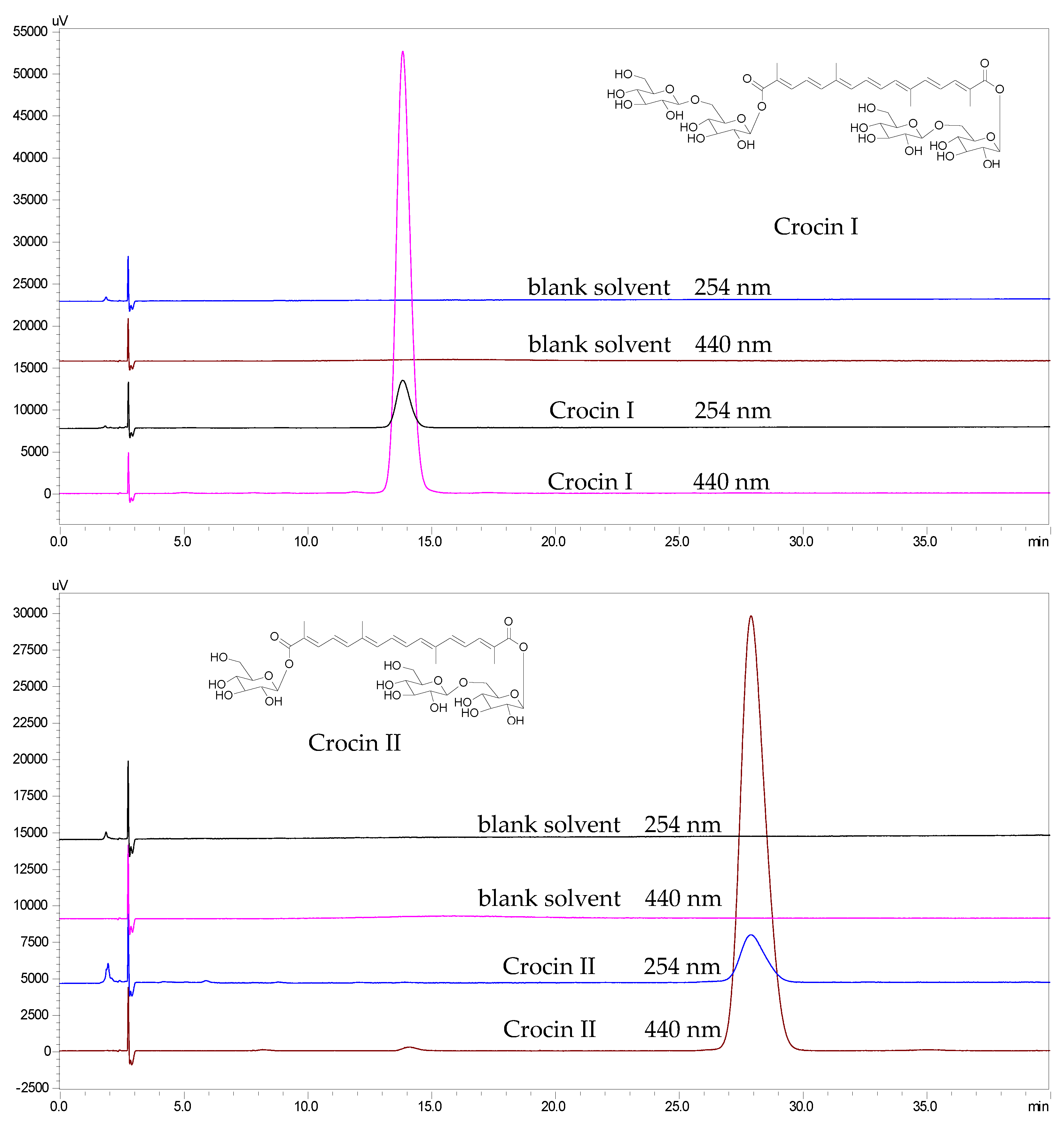
2.1. The Identification of the Two Target Compounds
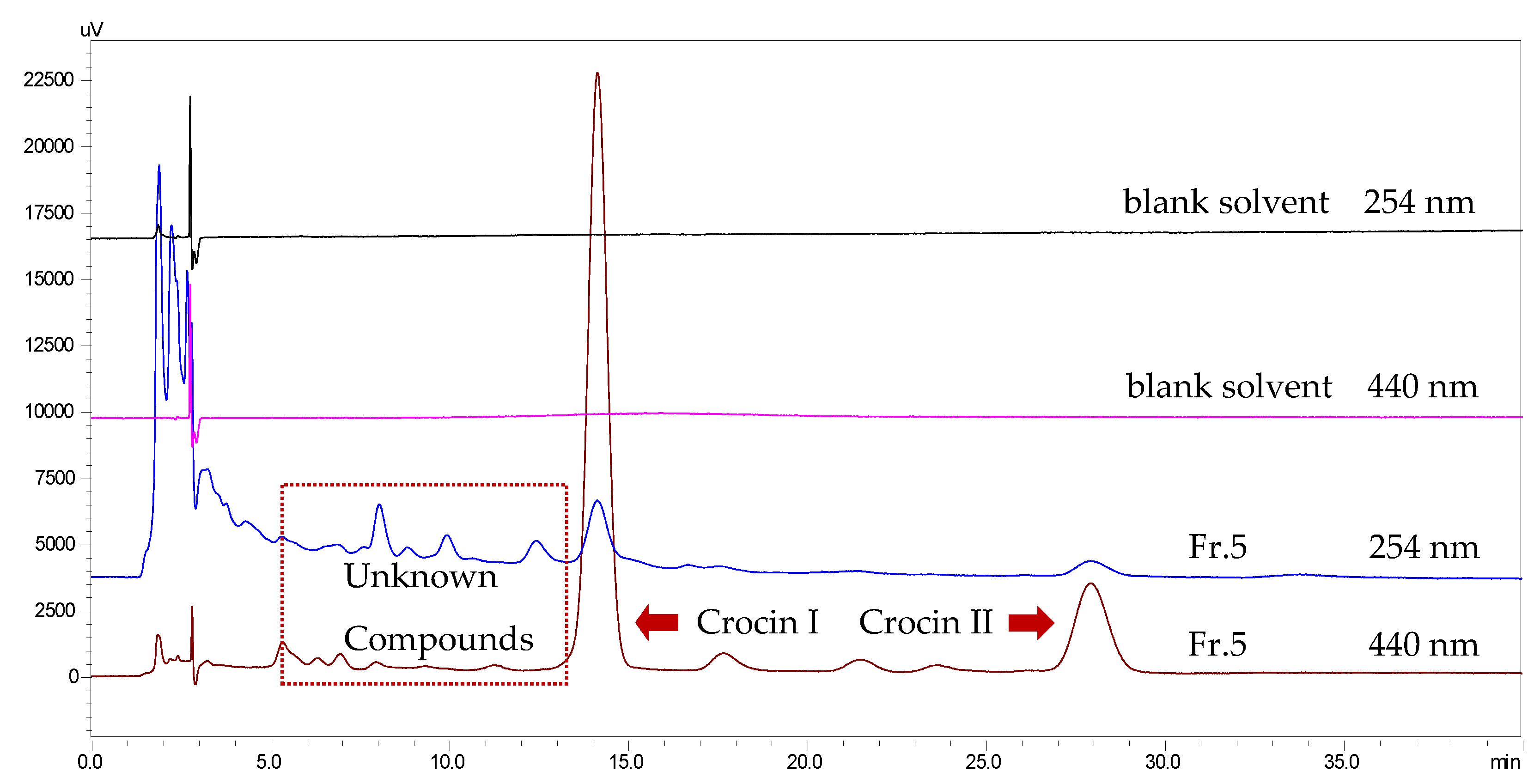
2.2. The Key Steps in the Selection of Fractionation Approach
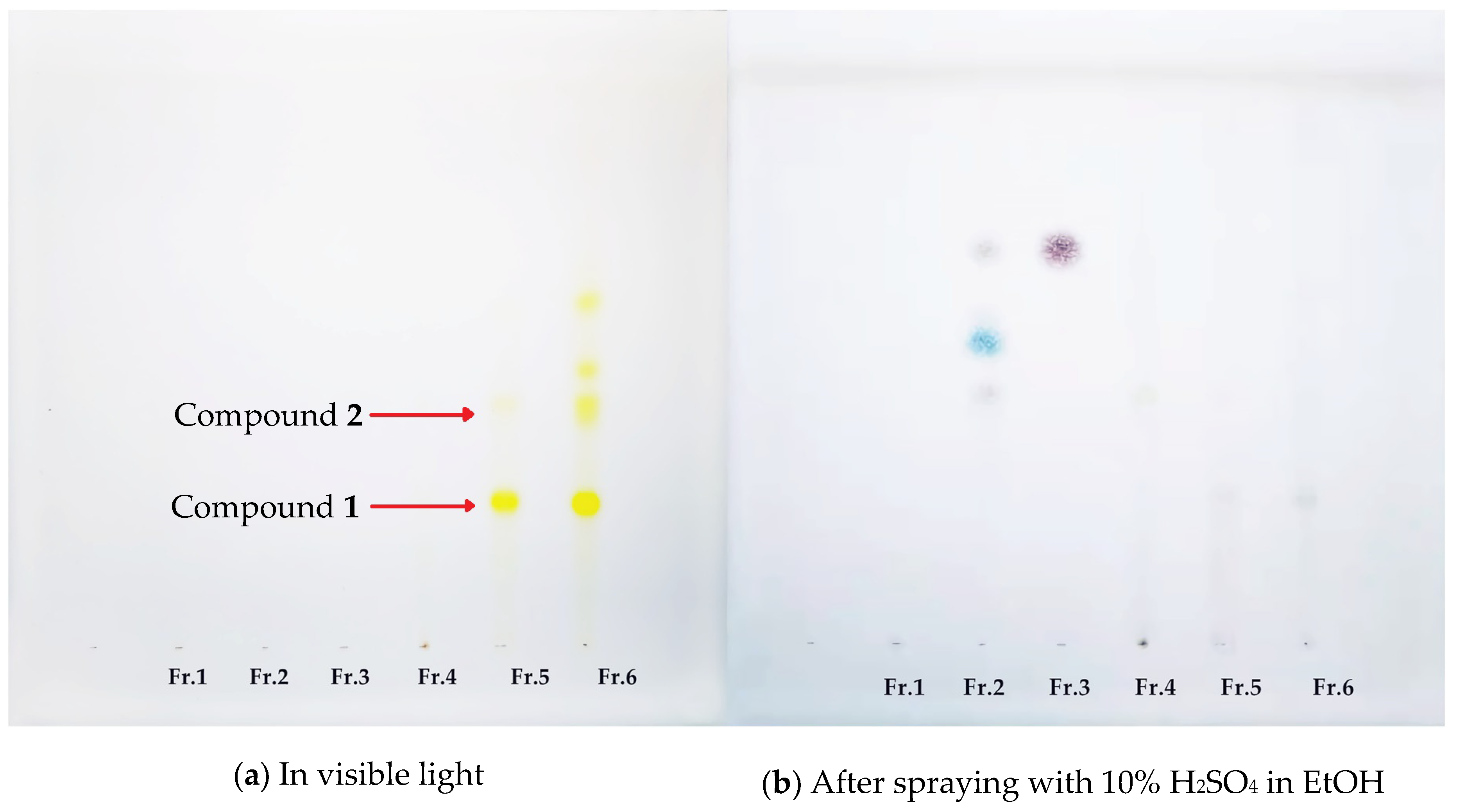
2.3. The TLC Results of the Eluents from the Macroporous Resin Column Chromatography
2.4. Selection of the Two-Phase Solvents by Settling Time and Separation Factors
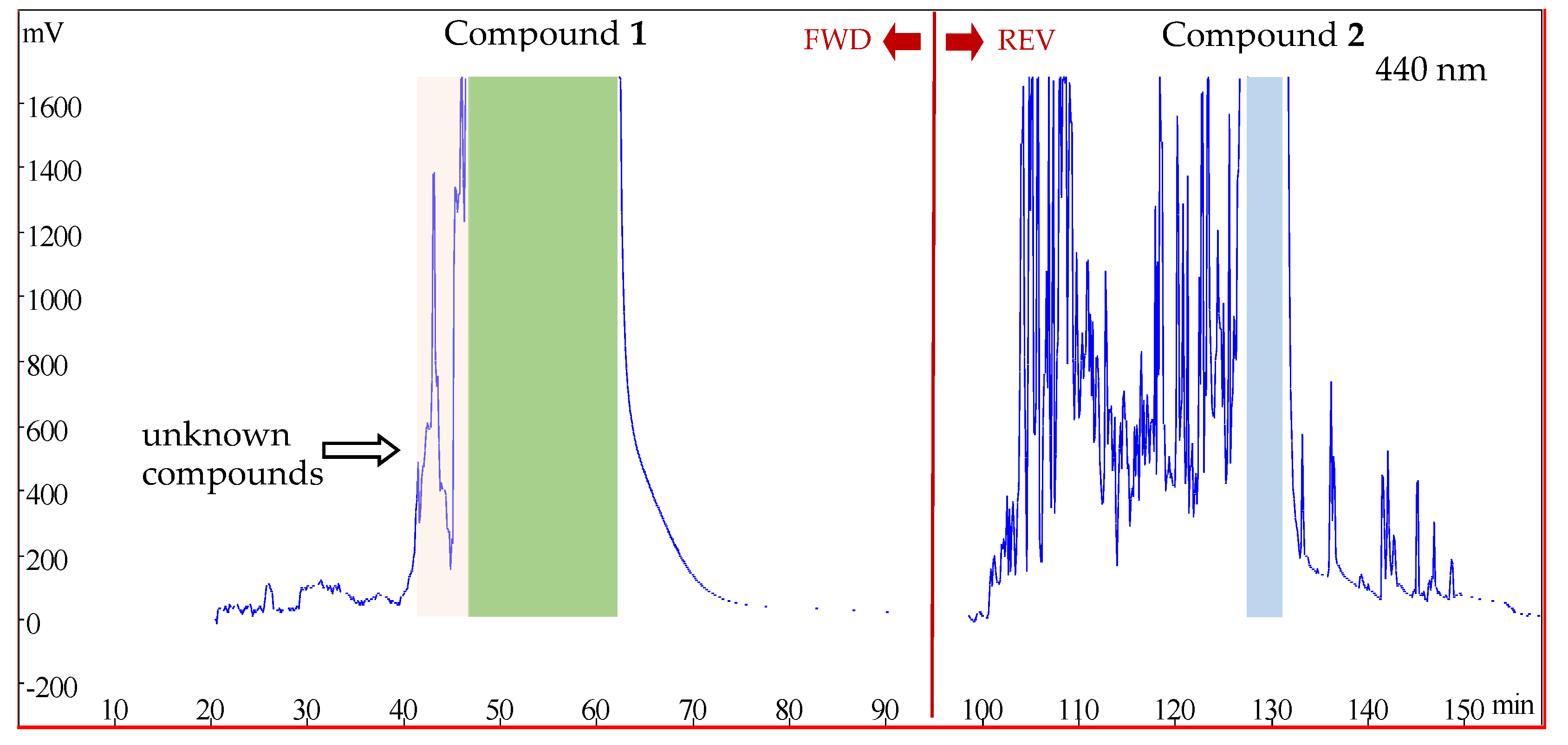
2.5. Forward and Reverse Rotations Successively Applied in the HSCCC Isolation Procedure
2.6. The Recoveries and the Purities of the Two Target Compounds
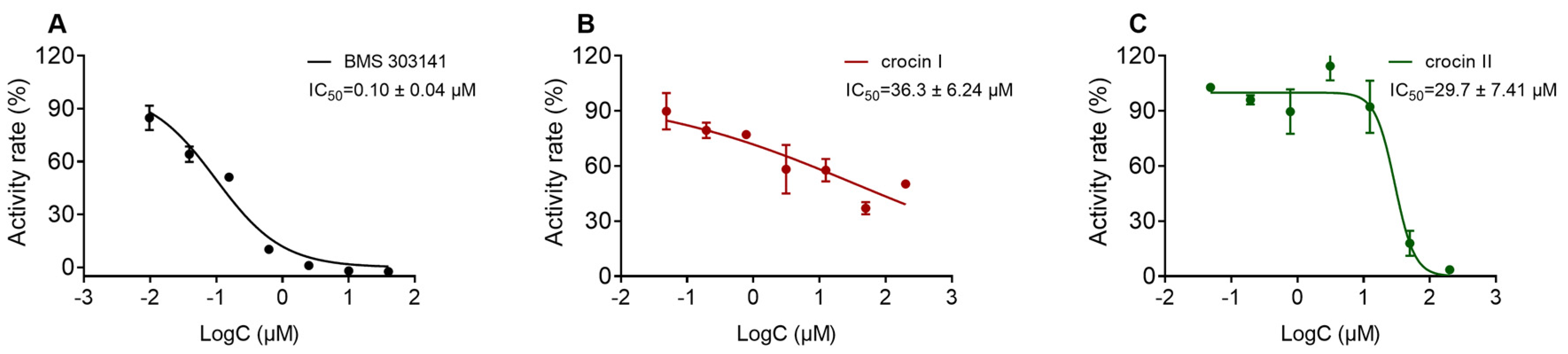
2.7. Inhibitory Activity of Compounds on ACLY
3. Materials and Methods
3.1. Reagents
3.2. Materials
3.3. Apparatus
3.4. Selection of the Concentration and the Amount of Loaded Sample on the Macroporous Resin Column Chromatography
3.5. Preparation of Crude Sample from the Fruits of G. jasminoides
3.6. Selection of HSCCC Solvent System
3.7. Selection of the Separation Parameter of HSCCC
3.8. Preparation of Two-Phase Solvent System and Sample Solution
3.9. The HSCCC Purification Procedure
3.10. HPLC Analysis
3.11. ACLY Enzymatic Assay Based on ADP-Coupled Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- Ghiasian, M.; Khamisabadi, F.; Kheiripour, N.; Karami, M.; Haddadi, R.; Ghaleiha, A.; Taghvaei, B.; Oliaie, S.S.; Salehi, M.; Samadi, P.; et al. Effects of crocin in reducing DNA damage, inflammation, and oxidative stress in multiple sclerosis patients: A double-blind, randomized, and placebo-controlled trial. J. Biochem. Mol. Toxicol. 2019, 33, e22410. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; He, Q.; Chen, H.; Lin, Z.; Xu, Y.; Yang, C. Crocin ameliorates chronic obstructive pulmonary disease-induced depression via PI3K/Akt mediated suppression of inflammation. Eur. J. Pharmacol. 2019, 862, 172640. [Google Scholar] [CrossRef] [PubMed]
- Talaei, A.; Hassanpour Moghadam, M.; Sajadi Tabassi, S.A.; Mohajeri, S.A. Crocin, the main active saffron constituent, as an adjunctive treatment in major depressive disorder: A randomized, double-blind, placebo-controlled, pilot clinical trial. J. Affect. Disord. 2015, 15, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Pashirzad, M.; Shafiee, M.; Avan, A.; Ryzhikov, M.; Fiuji, H.; Bahreyni, A.; Khazaei, M.; Soleimanpour, S.; Hassanian, S.M. Therapeutic potency of crocin in the treatment of inflammatory diseases: Current status and perspective. J. Cell. Physiol. 2019, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sepahi, S.; Mohajeri, S.A.; Hosseini, S.M.; Khodaverdi, E.; Shoeibi, N.; Namdari, M.; Tabassi, S.A.S. Effects of crocin on diabetic maculopathy: A placebo-controlled randomized clinical trial. Am. J. Ophthalmol. 2018, 190, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Colapietro, A.; Mancini, A.; D’Alessandro, A.M.; Festuccia, C. Crocetin and crocin from saffron in cancer chemotherapy and chemoprevention. Anticancer Agents Med. Chem. 2019, 19, 38–47. [Google Scholar] [CrossRef]
- Haeri, P.; Mohammadipour, A.; Heidari, Z.; Ebrahimzadeh-Bideskan, A. Neuroprotective effect of crocin on substantia nigra in MPTP-induced Parkinson’s disease model of mice. Anat Sci Int. 2019, 94, 119–127. [Google Scholar] [CrossRef]
- Li, L.; Zhang, H.; Jin, S.; Liu, C. Effects of crocin on inflammatory activities in human fibroblast-like synoviocytes and collagen- induced arthritis in mice. Immunol. Res. 2018, 66, 406–413. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Samarghandian, S.; Shaterzadeh Yazdi, H.; Samini, F. The protective effects of crocin in the management of neurodegenerative diseases: A review. Am. J. Neurodegener Dis. 2018, 5, 1–10. [Google Scholar]
- Yosri, H.; Elkashef, W.F.; Said, E.; Gameil, N.M. Crocin modulates IL-4/IL-13 signaling and ameliorates experimentally induced allergic airway asthma in a murine model. Int. Immunopharmacol. 2017, 50, 305–312. [Google Scholar] [CrossRef]
- Ghorbanzadeh, V.; Mohammadi, M.; Dariushnejad, H.; Abhari, A.; Chodari, L.; Mohaddes, G. Cardioprotective effect of crocin combined with voluntary exercise in rat: Role of mir-126 and mir-210 in heart angiogenesis. Arq. Bras. Cardiol. 2017, 109, 54–62. [Google Scholar] [CrossRef]
- Pitsikas, N.; Tarantilis, P.A. Crocins, the active constituents of Crocus sativus L., counteracted apomorphine-induced perfor- mance deficits in the novel object recognition task, but not novel object location task, in rats. Neurosci. Lett. 2017, 22, 37–42. [Google Scholar] [CrossRef]
- Finley, J.W.; Gao, S. A Perspective on Crocus sativus L. (Saffron) Constituent Crocin: A potent water-soluble antioxidant and potential therapy for Alzheimer’s disease. J. Agric. Food Chem. 2017, 8, 1005–1020. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Chen, T.; Xu, Z.; Huo, F.; Wei, Y.; Yang, X. Crocin protects retinal ganglion cells against H2O2-induced damage through the mitochondrial pathway and activation of NF-κB. Int. J. Mol. Med. 2016, 37, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Thushara, R.M.; Hemshekhar, M.; Santhosh, M.S.; Jnaneshwari, S.; Nayaka, S.C.; Naveen, S.; Kemparaju, K.; Girish, K.S. Crocin, a dietary additive protects platelets from oxidative stress-induced apoptosis and inhibits platelet aggregation. Mol. Cell Biochem. 2013, 373, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Deslauriers, A.M.; Afkhami-Goli, A.; Paul, A.M.; Bhat, R.K.; Acharjee, S.; Ellestad, K.K.; Noorbakhsh, F.; Michalak, M.; Power, C. Neuroinflammation and endoplasmic reticulum stress are coregulated by crocin to prevent demyelination and neurodegeneration. J. Immunol. 2011, 1, 4788–4799. [Google Scholar] [CrossRef]
- Pu, X.D.; He, C.N.; Yang, Y.; Wang, W.; Hu, K.; Xu, Z.C.; Song, J.Y. In vivo production of five crocins in the engineered Escherichia coli. Vet.Res. Forum. 2019, 10, 277–284. [Google Scholar] [CrossRef]
- Bathaie, S.Z.; Farajzade, A.; Hoshyar, R. A review of the chemistry and uses of crocins and crocetin, the carotenoid natural dyes in saffron, with particular emphasis on applications as colorants including their use as biological stains. Biotech. Histochem. 2014, 89, 401–411. [Google Scholar] [CrossRef]
- Liu, F.; Ding, F.; Shao, W.; He, B.; Wang, G. Regulated preparation of crocin-1 or crocin-2’ triggered by the cosolvent DMSO using Bs-GT/At-SuSy one-pot reaction. J. Agric. Food Chem. 2019, 13, 12496–12501. [Google Scholar] [CrossRef]
- Zhou, T.T.; Fan, G.R.; Hong, Z.Y.; Chai, Y.F.; Wu, Y.T. Large-scale isolation and purification of geniposide from the fruit of Gardenia jasminoides Ellis by high-speed counter-current chromatography. J. Chromatogr. A. 2005, 1100, 76–80. [Google Scholar] [CrossRef]
- Zhang, M.; Ignatova, S.; Hu, P.; Liang, Q.L.; Wang, Y.M.; Sutherland, I.; Jun, F.W.; Luo, G.A. Cost-efficient and process-efficient separation of geniposide from Gardenia jasminoides Ellis by high-performance counter-current chromatography. Sep. Purif. Technol. 2012, 89, 193–198. [Google Scholar] [CrossRef]
- Liang, Z.K.; Yang, M.; Xu, X.J.; Xie, Z.S.; Huang, J.Y.; Li, X.Y.; Yang, D.P. Isolation and purification of geniposide, crocin-1 and geniposidic acid from the fruit of Gardenia jasminoides Ellis by high-speed counter-current chromatography. Sep. Sci. Technol. 2014, 49, 1427–1433. [Google Scholar] [CrossRef]
- Wang, Y.R.; Chen, Y.; Deng, L.; Cai, S.N.; Liu, J.; Li, W.N.; Du, L.F.; Cui, G.Z.; Xu, X.; Lu, T.; et al. Systematic separation and purification of iridoid glycosides and crocetin derivatives from Gardenia jasminoides Ellis by high-speed counter-current chromatography. Phytochem. Anal. 2015, 26, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Mashmoul, M.; Azlan, A.; Yusof, B.N.M.; Khaza’ai, H.; Mohtarrudin, N.; Boroushaki, M.T. Effects of saffron extract and crocin on anthropometrical, nutritional and lipid profile parameters of rats fed a high fat diet. J. Funct. Foods 2014, 8, 180–187. [Google Scholar] [CrossRef]
- Li, J.; Lei, H.T.; Cao, L.; Mi, Y.N.; Li, S.; Cao, Y.X. Crocin alleviates coronary atherosclerosis via inhibiting lipid synthesis and inducing M2 macrophage polarization. Int. immunopharmacol. 2018, 55, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Xiao, Q.; Xiong, Z.; Yu, C.; Zhou, J.; Fu, Z. Crocin-I ameliorates the disruption of lipid metabolism and dysbiosis of the gut microbiota induced by chronic corticosterone in mice. Food Funct. 2019, 10, 6779–6791. [Google Scholar] [CrossRef] [PubMed]
- Pearce, N.J.; Yates, J.W.; Berkhout, T.A.; Tew, D.; Boyd, H.; Camilleri, P.; Sweeney, P.; Gribble, A.D.; Shaw, A.; Groot, P.H.E. Hypolipidaemic effects lactoneprodrug potent ATP citrate-lyase inhibitor SB-201076. Biochem. J. 1998, 334, 113–119. [Google Scholar] [CrossRef]
- Fernandez, S.; Viola, J.M.; Torres, A.; Wallace, M.; Trefely, S.; Zhao, S.; Affronti, H.C.; Gengatharan, J.M.; Guertin, D.A.; Snyder, N.W.; et al. Adipocyte ACLY facilitates dietary carbohydrate handling to maintain metabolic homeostasis in females. Cell Rep. 2019, 27, 2772–2784.e6. [Google Scholar] [CrossRef]
- Zhou, T.T. Studies on Chemome and Metabolome of Gardenia Jasminoides Ellis by Multiple-Dimensional Chromatography-Electrospray Ionization Mass Spectrometry. Second Military Medical University: Shanghai, China, 2007; pp. 41–60. [Google Scholar]
- Wu, H.; Feng, R.H.; Guan, S.H.; Yu, W.B.; Man, W.; Guo, J.L.; Liu, X.; Yang, M.; Jiang, B.H.; Wu, W.Y.; et al. Rapid preparative isolation of a new phenylpropanoid glycoside and four minor compounds Sparganium stoloniferum using high-speed counter-current chromatography as a fractionation tool. J. Sep. Sci. 2012, 35, 1160–1166. [Google Scholar] [CrossRef]
- Berthod, A.; Maryutina, T.; Spivakov, B.; Shpigun, O.; Sutherland, I.A. Countercurrent chromatography in analytical chemistry (IUPAC Technical Report). Pure Appl. Chem. 2009, 81, 355–387. [Google Scholar] [CrossRef]
- China Phamacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2015; pp. 129–130, 248–249. [Google Scholar]
- Yang, F.; Qi, Y.X.; Liu, W.; Li, J.; Wang, D.J.; Fang, L.; Zhang, Y.Q. Separation of five flavonoids from aerial parts of Salvia Miltiorrhiza Bunge using HSCCC and their antioxidant activities. Molecules. 2019, 24, 3448. [Google Scholar] [CrossRef] [PubMed]
| Concentration of Crude Extract (g/mL) | Loaded Volume (mL) | TLC Result | |
|---|---|---|---|
| 1 * | 1 g/30 mL | 50 | No Spot |
| 2 * | 1 g/40 mL | 80 | No Spot |
| 3 * | 1 g/50 mL | 160 | No Spot |
| 4 | 1 g/60 mL | 280 | Spot observed after the volume of loaded sample reached 250 mL |
| Final Choice | 1 g/60 mL | 240 | / |
| Solvent System (v/v) | Settling Time (sec) | KD | α | ||
|---|---|---|---|---|---|
| Crocin I | Crocin II | ||||
| 1 * | EtOAc-water (1:1) | 45 | / | / | / |
| 2 | EtOAc-n-BuOH-water (1:1:2) | 17 | 0.11 | 1.13 | 10.27 |
| 3 | EtOAc-n-BuOH-water (1:2:3) | 11 | 0.15 | 1.67 | 11.13 |
| 4 | EtOAc-n-BuOH-water (1:3:4) | 13 | 0.27 | 1.90 | 7.04 |
| 5 | n-BuOH-water (1:1) | 19 | 0.38 | 2.07 | 5.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, S.; Pu, Q.; Liu, Y.; Wu, H.; Yu, W.; Pi, Z.; Liu, S.; Song, F.; Li, J.; Guo, D.-A. Scale-Up Preparation of Crocins I and II from Gardeniajasminoides by a Two-Step Chromatographic Approach and Their Inhibitory Activity Against ATP Citrate Lyase. Molecules 2021, 26, 3137. https://doi.org/10.3390/molecules26113137
Guan S, Pu Q, Liu Y, Wu H, Yu W, Pi Z, Liu S, Song F, Li J, Guo D-A. Scale-Up Preparation of Crocins I and II from Gardeniajasminoides by a Two-Step Chromatographic Approach and Their Inhibitory Activity Against ATP Citrate Lyase. Molecules. 2021; 26(11):3137. https://doi.org/10.3390/molecules26113137
Chicago/Turabian StyleGuan, Shuguang, Qiaoli Pu, Yinan Liu, Honghong Wu, Wenbo Yu, Zifeng Pi, Shu Liu, Fengrui Song, Jingya Li, and De-An Guo. 2021. "Scale-Up Preparation of Crocins I and II from Gardeniajasminoides by a Two-Step Chromatographic Approach and Their Inhibitory Activity Against ATP Citrate Lyase" Molecules 26, no. 11: 3137. https://doi.org/10.3390/molecules26113137
APA StyleGuan, S., Pu, Q., Liu, Y., Wu, H., Yu, W., Pi, Z., Liu, S., Song, F., Li, J., & Guo, D.-A. (2021). Scale-Up Preparation of Crocins I and II from Gardeniajasminoides by a Two-Step Chromatographic Approach and Their Inhibitory Activity Against ATP Citrate Lyase. Molecules, 26(11), 3137. https://doi.org/10.3390/molecules26113137







