Abstract
The pH-dependent surface charging of tellurium (IV) oxide has been studied. The isoelectric point (IEP) of tellurium (IV) oxide was determined by microelectrophoresis in various 1-1 electrolytes over a concentration range of 0.001–0.1 M. In all electrolytes studied and irrespective of their concentration the zeta potential of TeO2 was negative over the pH range 3–12. In other words the IEP of TeO2 is at pH below 3 (if any). TeO2 specifically adsorbs ionic surfactants, and their presence strongly affects the zeta potential. In contrast the effect of multivalent inorganic ions on the zeta potential of TeO2 is rather insignificant (no shift in the IEP). In this respect TeO2 is very different from metal oxides.
1. Introduction
Tellurium dioxide is a white crystalline compound, chemically inert and practically insoluble in water. It occurs in Nature, and it can be easily obtained, for example, by synthesis from the constituent elements. TeO2 is used as an acousto-optic material and in optical fibers [1,2]. TeO2 is not particularly precious: a price for 1 kg of elementary tellurium is about $80.
Scientific knowledge on adsorption properties of TeO2 is practically absent. A search for adsorption AND (tellurium oxide OR tellurium dioxide) in scientific databases results in a dozen papers, which describe some kind of adsorption and they refer somehow to TeO2, but not to adsorption on TeO2 from solution. Very few papers describe adsorption of gases on TeO2 in the context of its possible application as a gas sensor [3].
A few papers report zeta potential of TeO2 which is directly related to adsorption of ions from solution. TeO2 nanoparticles had a zeta potential of about −50 mV at pH 5.3 (ionic strength not specified) [4]. Those particles were prepared in the presence of organic acids which might have induced negative zeta potentials. Properties of such particles are not relevant to electrokinetic properties of pure TeO2 in the presence of inert electrolyte. Another specimen of TeO2 had a zeta potential of +29 mV in deionized water [5]. The pH was not reported which makes this result useless in determination of the isoelectric point, IEP. Also, the level of purity of TeO2 used in electrophoretic measurements was not discussed.
The chemical analogs of TeO2, that is, SO2 and SeO2 (dioxides of the 16th group elements) undergo reactive dissolution in water, and PoO2 is seldom studied because of its high radioactivity, and high cost. Therefore, TeO2 is the only dioxide of the 16th group element which can be considered as adsorbent of ions from solution. We hypothesize that TeO2 behaves like the other sparingly soluble oxides [6,7], that is, it shows pH-dependent surface charging caused by reversible pH-dependent protonation and deprotonation of the surface oxygen atoms according to reactions (1) and (2):
where ≡Te is a tellurium atom on the surface.
≡TeOH + H+ = ≡TeOH2+ (below PZC, positively charged surface)
≡TeOH = ≡TeO- + H+ (above PZC, negatively charged surface)
We further hypothesize that positively charged surfaces of TeO2 adsorb anions by Coulombic attraction, and negatively charged surfaces of TeO2 adsorb cations by Coulombic attraction. The sign of the surface charge depends on pH, and more precisely on the relationship between the actual pH and the point of zero charge (PZC). Isoelectric point (IEP) and PZC are basically two different quantities, but for metal oxides in the presence of 1-1 salts (1st group metal halides, nitrates (V) and chlorates (VII)), they usually occur at the same pH value. We hypothesize that also with TeO2, the IEP determined by electrokinetic measurements in 1-1 salts coincides with the PZC. We further hypothesize that on top of the aforementioned electrostatic adsorption certain ions, e.g., ionic surfactants will specifically adsorb on TeO2, that is, these ions can adsorb even against electrostatic repulsion. In order to predict the sign of surface charge of we need to know the PZC. Unfortunately, there are no scientific papers reporting PZC/IEP of TeO2. We may estimate PZC of TeO2 using the correlations between points of zero charge and well-established physical properties, e.g., ionic radii and electronegativities [6,7] (Equations (3)–(10)). We cannot use a correlation between PZC and the 1st hydrolysis constant of metal ions, because the Te4+ species in aqueous media is non-existing:
PZC = 14.9 − 2.19·valence (not weighted)
PZC = 15 − 2.26·valence (weighted)
PZC = 17.74 − 1.36·electronegativity of oxide (not weighted)
PZC = 17.88 − 1.4·electronegativity of oxide (weighted)
PZC = 12.4 − 0.88·z/r (not weighted)
PZC = 11.66 − 0.75·z/r (weighted)
PZC = 15.03 − 7.73·z/R· (not weighted)
PZC = 15.28 − 8.08·z/R (weighted)
The PZC of TeO2 estimated from the Equations (3)–(10) [7] are summarized in Table 1.

Table 1.
PZC of TeO2 estimated from correlations reported in [7].
In Table 1 the electronegativity of an oxide is the electronegativity of metal plus 1/2 the metal valence times the electronegativity of oxygen, z is the valence of metal in the oxide, r is the radius of metal cation and R is r plus two radii of oxygen anion. “Weighted” is the PZC estimated from weighted PZC of other metal oxides, that is PZC of a certain oxide is taken with a weight proportional to the logarithm of the number of literature references reporting the PZC of this oxide. Particular correlations lead to very different PZC of TeO2 ranging from 5.3 to 8.5.
The PZC of oxides is obtained experimentally as a common intersection point CIP of at least three surface charging curves for different concentrations of a 1-1 electrolyte. This method requires a high specific surface area (>>100 m2/L), otherwise the potentiometric titration curves of the dispersions do not differ much from the titration curves of the electrolyte. The condition recalled above (>>100 m2/L) is a rule of thumb rather than a strict principle, and the applicability of salt titration depends also on other factors than the solid-to-liquid ratio. Even with relatively high specific surface area, many materials fail to produce a sharp CIP, but instead the titration curves obtained at various ionic strength merge over a broad pH range. This is (usually) the case for silica but merging titration curves have been also reported for other oxides [7]. A set of titration curves without CIP is useless in determination of the PZC. Inert electrolyte titration may be used to determine the PZC in the systems where the classical potentiometric titration fails [8]. PZC obtained by titration is affected by specific adsorption of ions, also by impurities in the studied material. CIP is often observed in impure materials, but such a CIP is different from the pristine PZC [7]. Discrepancies between PZC reported in different publications for the same oxide are chiefly due to impurities. Therefore, PZC obtained by titration has to be confirmed by electrokinetic measurements. Matching IEP and CIP are obtained in pure oxides while in impure materials, the CIP and IEP do not match. Electrokinetic measurements have also been used as a standalone method of determination of the PZC of oxides (which is assumed to match the IEP), but such a method is uncertain because of high sensitivity of IEP to impurities.
Production of materials with high specific surface area is not trivial, and commercially available specimens of high-purity TeO2 consist of coarse particles, and they are not suitable for determination of PZC by titration. Several recipes for TeO2 nanoparticles have been reported [9,10,11]. Due to a high specific surface area such nanomaterials may be more suitable for studies of the PZC by titration than coarse particles available commercially. The problem with nanoparticles is about their purity. The experience with other oxides shows that many nanomaterials contain surface-active impurities which shift the PZC. Therefore in order to test our hypothesis we used coarse particles of high-purity TeO2 rather than fine particles of unknown level of purity.
2. Results and Discussion
2.1. BET and XRD
The XRD pattern is shown in Figure 1a, and it represents tellurite, β-TeO2. Similar positions of peaks in XRD pattern of tellurite have been reported by others [4,12,13]. Narrow peaks indicate large crystallites in our specimen.
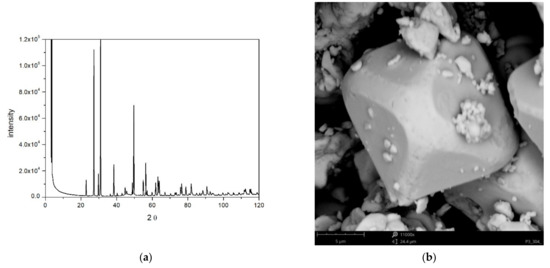
Figure 1.
(a) The XRD pattern. (b) Photograph of TeO2.
The BET specific surface area of TeO2 was 0.27 m2/g. This figure corresponds to monodispersed spherical particles 4 μm in diameter, which is the upper limit of particle size suitable for microelectrophoretic measurements. However, the actual particles are neither monodispersed nor spherical (Figure 1b), and on top of large particles (>10 µm) there is a substantial fraction of submicron particles in the sample. The size distribution is very broad and we find sufficient number of submicron particles to carry out an electrophoretic measurement. We also believe that the particle radii obtained by DLS (vide infra) which were 200–400 nm when the zeta potential was >50 mV in absolute value represent the actual sizes of particles whose zeta potentials were measured while the larger particles settled down, and they were not transferred into the zetameter cell. The smallest particles shown in Figure 1b roughly match the aforementioned sizes obtained by DLS.
2.2. Zeta Potential and Particle Size
The zeta potential of TeO2 in various 1-1 electrolytes is shown in Figure 2, Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7. These results can be summarized as follows. The absolute values of zeta potentials at constant pH decrease as the ionic strength increases. This behavior is commonplace in metal oxides [7], and it can be explained in terms of various models of the electric double layer. The maximum absolute value of the zeta potential of TeO2 is about 50 mV in 0.001 M electrolyte, 30 mV in 0.01 M electrolyte, and 15 mV in 0.1 M electrolyte. These absolute values are lower than typical results obtained with other oxides (in colloidal silica −100 mV in 0.001 M electrolyte, −60 mV in 0.01 M electrolyte, and −40 mV in 0.1 M electrolyte are commonplace), but a few publications report maximum absolute values of the zeta potential similar as the values obtained in this study [7]. The absolute value of the zeta potential at given pH decreases as the salt concentration increases according to a commonly observed trend. There was no specific salt effect (NaCl vs. KCl, etc.) at salt concentrations of 0.001 and 0.01 M.
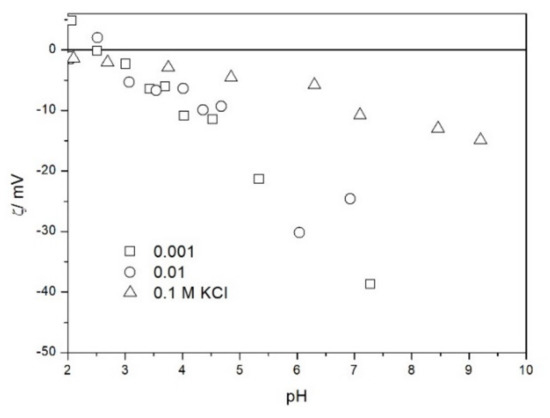
Figure 2.
The zeta potential of TeO2 in KCl.
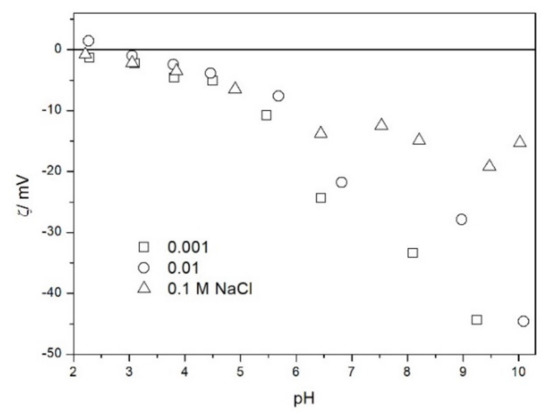
Figure 3.
The zeta potential of TeO2 in NaCl.
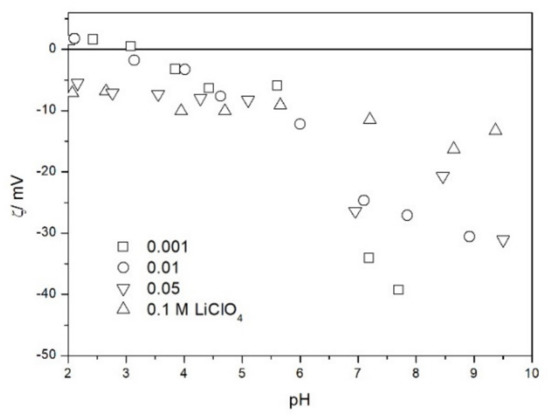
Figure 4.
The zeta potential of TeO2 in LiClO4.
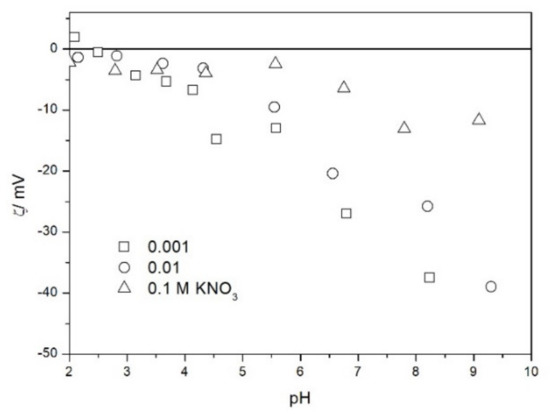
Figure 5.
The zeta potential of TeO2 in KNO3.
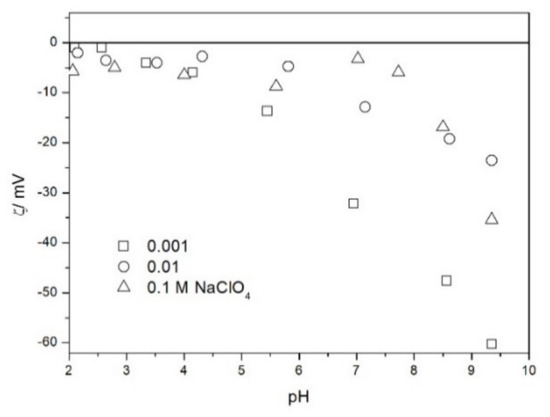
Figure 6.
The zeta potential of TeO2 in NaClO4.
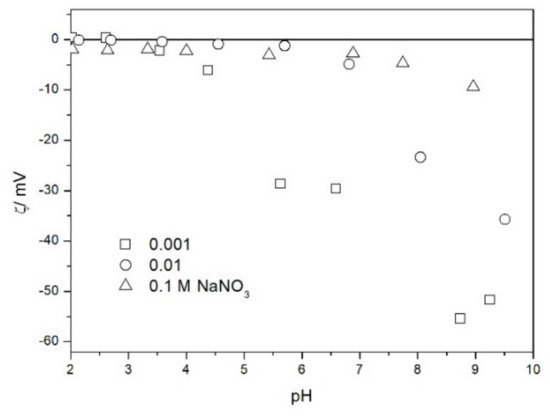
Figure 7.
The zeta potential of TeO2 in NaNO3.
The zeta potentials are negative at pH > 3. At pH 2–3 we have a mixture of small positive and small negative values. This result suggests an IEP between pH 2 and 3, that is, much lower than the predicted values in Table 1. Interestingly enough with 0.1 M electrolyte the zeta potentials assumed only negative values irrespective of the nature of the salt. The difficulties with exact determination of the IEP from electrokinetic curves are commonplace, e.g., in spite of large body of experimental data the position of IEP of silica is still under debate. The results presented in Figure 2, Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7 are in line with high negative zeta potentials at pH 5.3 reported in [4], although such a result could also be due to specific adsorption of acetate and gallate anions. There is no systematic effect of the nature or concentration of electrolyte on the apparent IEP: with Li, Na and K, and with chloride, perchlorate and nitrate we have a few electrokinetic curves with only negative zeta potentials and a few other electrokinetic curves with positive zeta potentials in the pH range 2–3. Apparently, chlorides, nitrates V and chlorates VII of alkali metals are inert electrolytes for TeO2. Inert character of these salts has been confirmed for many other oxides [7].
The electrokinetic properties of TeO2 in the presence of LiClO4 are different from other 1-1 salts, namely, in 0.05 and 0.1 M LiClO4 the zeta potential was clearly negative even at pH as low as 2, while in 0.1 M solutions of other 1-1 salts it was close to zero over the pH range 2–3. This effect may be due to specific adsorption of ions (in this case of anions) from concentrated solutions of electrolytes, which are indifferent at low concentrations [14]. The shifts in the IEP of metal oxides in the presence of 1-1 electrolytes were observed at electrolyte concentrations >1 M, but with silica such shifts were even observed at electrolyte concentrations below 0.1 M [15]. The other explanation of unusual behavior of TeO2 in the presence of LiClO4 is that both TeO2 and perchlorate anion are redox-active in acidic medium.
The results presented in Figure 2, Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7. are more scattered than most electrokinetic curves presented in the literature. The size of the TeO2 particles at the upper limit of particle size suitable for microelectrophoretic particles combined with high specific density explains the difficulties in the measurements. The specific density of our TeO2 is 5.67 g/cm3. We attempted to grind the material by means of a vibratory mill (mortar and ball), but our attempt was unsuccessful.
We also studied the zeta potentials of TeO2 in the presence of ionic surfactants (Figure 8). The zeta potential in the presence of sodium dodecylsulfate SDS was about −60 mV and it was pH-independent. Also the increase in SDS concentration from 10−4 to 10−3 M had rather insignificant effect on the zeta potential. This result is due to high affinity of SDS to the surface combined with low specific surface area of the powder. Thus, the uptake of SDS anions by TeO2 surface reaches a maximum value at relatively low SDS concentration, and the negative charge of pre-adsorbed SDS anions prevents from further uptake of these anions. The zeta potential in the presence of cetyltrimethlyammonium bromide CTMABr was about +50 mV and it was pH-independent at pH > 3. Also the increase in CTMABr concentration from 10−4 to 10−3 M had rather insignificant effect on the zeta potential. Lower absolute value of zeta potential in the presence of CTMABr as compared with SDS may be due to the fact that the negative charge of adsorbed SDS anions and the negative pristine surface charge of TeO2 add up, while the positive charge of adsorbed CTMA cations is partially balanced out by the negative pristine surface charge of TeO2. Unlike with SDS, the adsorption of CTMA cations on TeO2 is affected by the electrostatics. At pH about 2.5 TeO2 is not charged, and the effect of CTMABr on its zeta potential is insignificant. CTMABr adsorbs on the negatively charged surface, but not on electroneutral or positively charged surface. In this respect TeO2 is very different from metal oxides, which strongly adsorb CTMA cations even at pH below their pristine PZC [16].
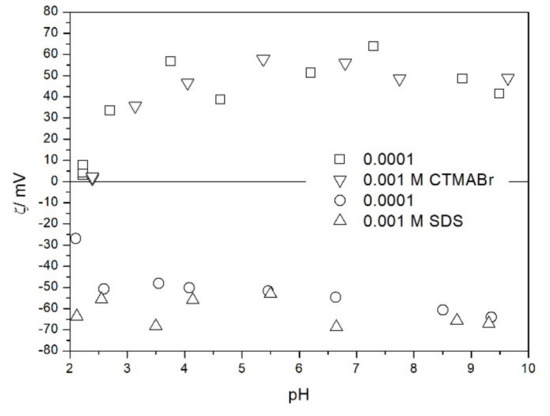
Figure 8.
The zeta potential of TeO2 in the presence of ionic surfactants and 0.001 M NaCl.
We also studied the zeta potentials of TeO2 in the presence of phosphate (Figure 9). The zeta potential was practically unaffected by the presence of phosphate (up to 10−3 M). In this respect TeO2 is very different from metal oxides, which strongly adsorb phosphate and other multivalent inorganic anions, and their IEP is shifted to low pH even at low concentrations of these anions [17]. In contrast with metal oxides, the effect of inorganic anions on the zeta potential of silica was seldom studied. A few results compiled in ref. [18] show that the uptake of inorganic anions by silica is low, and their effect on its zeta potential is rather insignificant.
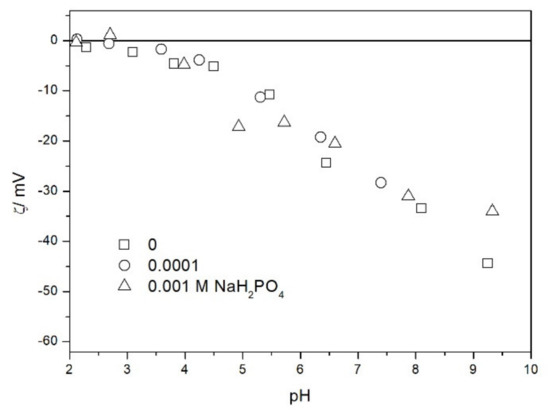
Figure 9.
The zeta potential of TeO2 in the presence of phosphate and 0.001 M NaCl.
We also studied the zeta potentials of TeO2 in the presence of barium (Figure 10). The IEP was practically unaffected by the presence of barium (up to 10−3 M). In this respect TeO2 is very different from metal oxides, which strongly adsorb barium and other multivalent inorganic cations, and their IEP is shifted to high pH even at low concentrations of these cations [19,20]. Depression of negative zeta potentials in 10−3 M Ba(NO3)2 at pH > 7 with respect to 10−3 M NaCl can be interpreted as the effect of increase in the ionic strength and it does not unequivocally indicate specific adsorption of Ba. In other words we do not need to invoke specific adsorption to explain the difference between Ba(NO3)2 (Figure 10) and NaH2PO4 (Figure 9): in view of divalent counterion (Figure 10) and monovalent counterion (Figure 9), the difference may be due to pure electrostatics. Moreover, NaH2PO4 is a weak electrolyte.
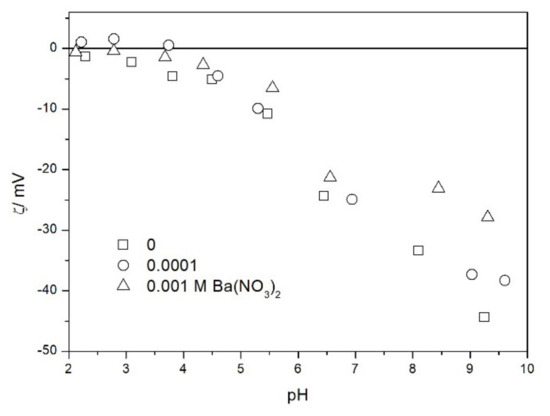
Figure 10.
The zeta potential of TeO2 in the presence of barium and 0.001 M NaCl.
The particle radii in the dispersions of TeO2 were very scattered, but they showed a common pattern. In 0.1 M 1-1 electrolyte the particle radius was >1000 nm. In 0.01 and 0.001 M 1-1 electrolyte the particle radius was >1000 nm at pH < 4, when the negative zeta potential was low in absolute value, and 200–1000 nm at pH > 4, when the negative zeta potential was high in absolute value. This result is in line with a typical behavior of colloidal particles which show high particle size when the zeta potential (negative or positive) is low in absolute value due to coagulation. In the presence of ionic surfactants the particle radius was 200–400 nm irrespective of the pH. Again, the particle size was low when the zeta potential was high in absolute value.
2.3. Salt Titration
The results of salt titration of TeO2 with KCl at 25 °C are presented in Figure 11. The point where KCl addition does not affect the pH falls at pH 6.4 which is halfway between the PZC predicted from a correlation with the valence and from the correlation with z/R (Table 1). Relatively high apparent PZC is also in line with positive zeta potential at (probably) nearly neutral pH reported in [5]. The apparent PZC obtained by salt titration (Figure 11) is much higher than the IEP determined by electrophoresis (Figure 2, Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7). Discrepancies between CIP and IEP even in apparently high-purity powders are commonplace in the literature [7]. They have been interpreted in terms of the presence of specifically adsorbing ions as an impurity in a high-purity reagent. Even a very small mass fraction of specifically adsorbing ions can induce a substantial shift in the IEP. Yet in the present case we believe that our IEP (pH < 3 if any) rather than the zero-point obtained by salt titration (pH 6.4) represents the actual pristine PZC. This is because our salt titration experiment was carried out beyond the normal application range of this method. We only have 54 m2/L of the surface area of solid in our experiment and the salt titration method requires >> 100 m2/L. The electrokinetic experiment directly indicates the sign of the particle charge while the salt titration method is based on several assumptions. The acid-base reactions in dispersions of metal oxides include surface protonation-deprotonation as well as protonation-deprotonation of solution monomers which are the products of dissolution of sparingly soluble metal oxides. The acid-base reactivity of solution monomers can be neglected when the solubility of oxide is very low and the surface area is very high, but apparently this not the case in our experiment. The apparent zero point obtained by salt titration decreased when the solid-to-liquid ratio increased, and this is a strong argument against using the apparent zero point from Figure 11 as a material constant. Perhaps some kind of extrapolation of apparent PZC obtained by salt titration to infinite solid-to-liquid ratio would result in the real PZC. Unfortunately, direct experiments at sufficiently high solid-to-liquid ratio cannot be conducted due to high viscosity of very concentrated dispersions. In our previous studies we applied the salt titration method to powders having much higher specific surface area (at least 10 m2/g), and such problems were not encountered. The results presented in Figure 11 are an example of an (expected) failure: the apparent PZC was dependent on the solid-to-liquid ratio. This is why we only performed such measurements with one salt (KCl, different solid-to-liquid ratios) and we have not attempted similar measurements with other salts. Moreover, the salt-specific surface-charging behavior is observed at high salt concentrations [14] while the salt titration is only efficient at low total salt concentration (addition of salt to dispersions, in which the salt concentration is already high has rather insignificant effect on pH, even far from the PZC).
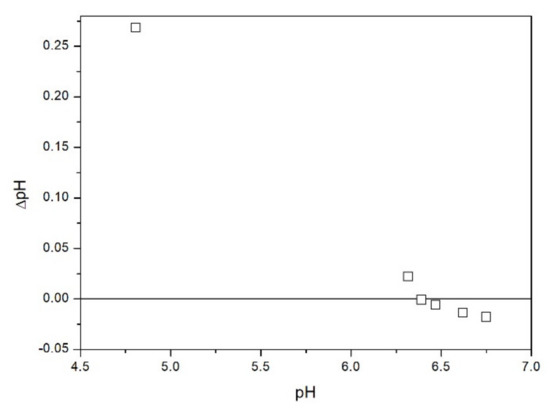
Figure 11.
Shifts in the pH of TeO2 dispersion induced by addition of KCl.
We also determined the natural pH of dispersion composed of 10 g of TeO2 and 50 mL of water, which is 4.81. Although the present authors are critical about using the natural pH of concentrated dispersion to determine the PZC, many other authors identified the natural pH of concentrated dispersion with the PZC [7].
2.4. Solubility
In the older literature (cf. [18] for details) we found allegations that the PZC of metal oxides matches the pH of minimum solubility. The present authors do not recommend pH of minimum solubility as a method of determination of PZC, but we can easily check how it works with TeO2, because the solubility data are available [21].
According to Figure 1 in [21] the minimum of solubility falls at pH 5.3, which coincides with the lowest predicted PZC in Table 1. The minimum solubility is 10−7 M (total concentration of soluble Te (IV) species), and it increases to 10−4 M at pH 2, and to 10−1 M at pH 10. Reference [21] presents also a Pourbaix-type plot for Te. Within the electrochemical window of water, elementary Te, Te(IV) and Te(VI) can be thermodynamically stable over various ranges of pH and redox potential.
3. Materials and Methods
TeO2 (99.995%) from Aldrich (St. Louis, MO, USA) was used as obtained. The other chemicals were of analytical grade from POCh (Lublin, Poland). Electrophoretic mobility and particle size were measured by means of a Zetasizer (Malvern, Malvern, UK). Zeta potential was estimated by the Smoluchowski equation. The measurements were carried out in fresh dispersions (a few hours after preparation) at 25 °C. The dispersions containing specifically adsorbing ions were also 0.001 M in NaCl. While in our previous electrokinetic study with Fe(OH)2 we designed a special procedure to exclude O2 and CO2 and in our previous electrokinetic study with BeO we designed a special procedure to exclude CO2 and no special efforts were made to exclude O2 or CO2 in the current electrokinetic study with TeO2. In acidic solution, the solubility of CO2 is low. Moreover, we are not aware of any special affinity of TeO2 to O2 or CO2. Exclusion of O2 or CO2 is not necessary in electrokinetic studies of most metal oxides, and the IEP obtained with and without exclusion of O2 or CO2 are often identical [6,7]. We also conducted a literature search on possible carbonate complexes of inorganic tellurium, but apparently such complexes do not exist or at least have not been detected yet. Negative charge of TeO2 particles is also an argument against hypothetical adsorption of carbonate anions and their hypothetical effect on the zeta potential.
An Empyrean system from PANalytical (currently Malvern, Malvern, UK) was used to obtain the XRD pattern. Gemini V from Micromeritics (Norcross, GA, USA) was used to measure the specific surface area. Adsorption of nitrogen at its boiling point was studied. In view of low specific surface area we used relatively large samples of powder (about 1 g) in specific surface area measurements, and we repeated the measurement 3 times. The samples were outgassed at 300 °C for 1 h. The data points for p/p0 < 0.3 were used to calculate the specific surface area from the linearized form of BET equation.
Salt titration of dispersion of 10 g of TeO2 in 50 mL of water was carried out at 25 °C in nitrogen atmosphere. 50 μL portions of 3 M KCl were added to pre-equilibrated dispersion, and the change in the pH induced by salt addition was recorded. When the pH increased, 0.1 or 0.005 M KOH was added to further increase the pH. When the pH decreased, 0.01 M HCl was added to further decrease the pH. After each addition of salt, acid or base the dispersion was equilibrated. The initial dispersion was stirred in a stream of nitrogen for about 1 h before the titration to remove any trace of CO2 and O2 from the dispersion, and an overpressure prevented the flow of external air into the reactor. The salt titration relies on very small changes in pH (cf. Figure 11) so any trace of CO2 in the reactor may affect the results, especially at pH about 7. In this respect the salt titration is very different from electrophoresis where 0.01 pH unit or so is immaterial (cf. the sizes of the symbols in Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9 and Figure 10 which are about 0.1 pH unit).
The pH measurements were carried out using a combined electrode calibrated against 3 commercial, fresh pH-buffers (pH 4, 7 and 10, POCh). The pH was measured just before the injection of the dispersion into the zetameter cell. The time of the contact between the electrode (glass!) and the dispersion was minimized to avoid silica contamination.
4. Conclusions
We showed that the adsorption properties of TeO2 are only partially similar to those of metal oxides. TeO2 shows a pH-dependent surface charging, and its zeta potential is strongly influenced by the adsorption of ionic surfactants. On the other hand, multivalent inorganic ions have rather insignificant effect on the zeta potential of TeO2. The MUSIC model [22] offers a possibility of prediction of PZC of oxides from the crystallographic data. So far it has been used for oxides whose PZC is already well-documented, but it can also be used for oxides whose PZC is less well-documented, and this may be a suggestion for further research.
TeO2 is one of numerous common materials whose adsorption properties are almost unknown. Obviously TeO2 will not compete with silica or with activated carbon as an adsorbent for practical applications, but perhaps adsorption studies are too focused on a small number of adsorbents (classes of adsorbents) whose adsorption properties are already well-documented while the adsorption properties of many other common materials remain unknown. Even if the studies of adsorption on materials like TeO2 have limited practical meaning, they may be important as fundamental research which will help to better understand the adsorption phenomenon.
Author Contributions
Conceptualization, writing—original draft preparation, writing—review and editing, supervision, M.K.; methodology, investigation, data curation, E.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Lublin University of Technology, grant numbers MK FD-EE-407, EM FD-EE-413.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available. All significant data related to this research are presented in the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stepien, R.; Buczynski, R.; Pysz, D.; Kujawa, I.; Mirkowska, M. Tellurite glasses for microstructured optical fibers manufacturing. Photonics Lett. Pol. 2010, 2, 16–18. [Google Scholar] [CrossRef]
- Jose, R.; Suzuki, T.; Ohishi, Y. Thermal and optical properties of TeO2–BaO–SrO–Nb2O5 based glasses: New broadband Raman gain media. J. Non-Crystall. Solids 2006, 352, 5564–5571. [Google Scholar] [CrossRef]
- Jiang, M.; Xu, K.; Liao, N.; Zhou, H. First principles investigation on selective hydrogen sensing properties of α-phase TeO2. Int. J. Hydrog. Energy 2021, 46, 4666–4672. [Google Scholar] [CrossRef]
- Qin, B.; Bai, Y.; Zhou, Y.; Liu, J.; Xie, X.; Zheng, W. Structure and characterization of TeO2 nanoparticles prepared in acid medium. Mater. Lett. 2009, 63, 1949–1951. [Google Scholar] [CrossRef]
- Gupta, P.K.; Sharma, P.P.; Sharma, A.; Khan, Z.H.; Solanki, P.R. Electrochemical and antimicrobial activity of tellurium oxide nanoparticles. Mater. Sci. Eng. B 2016, 211, 166–172. [Google Scholar] [CrossRef]
- Parks, G.A. The Isoelectric Points of Solid Oxides, Solid Hydroxides, and Aqueous Hydroxo Complex Systems. Chem. Rev. 1965, 65, 177–198. [Google Scholar] [CrossRef]
- Kosmulski, M. Isoelectric points and points of zero charge of metal (hydr)oxides: 50 years after Parks’ review. Adv. Colloid Interf. Sci. 2016, 238, 1–61. [Google Scholar] [CrossRef]
- Kosmulski, M.; Matysiak, J.; Szczypa, J. Solvent Effects on Standard Thermodynamic Functions of Surface Dissociation of Oxides. J. Colloid Interface Sci. 1994, 164, 280–284. [Google Scholar] [CrossRef]
- Cheon, S.; Yong, C.; Han, C.H.; Uhm, S. TeO2 nanoparticles synthesized by evaporation of tellurium in atmospheric microwave-plasma torch-flame. Chem. Phys. Lett. 2006, 429, 214–218. [Google Scholar] [CrossRef]
- Zhang, H.; Swihart, M.T. Synthesis of Tellurium Dioxide Nanoparticles by Spray Pyrolysis. Chem. Mater. 2007, 19, 1290–1301. [Google Scholar] [CrossRef]
- Amari, A.; Al Mesfer, M.K.; Alsaiari, N.S.; Danish, M.; Alshahrani, A.M.; Tahoon, M.A.; Rebah, F.B. Electrochemical and Optical Properties of Tellurium Dioxide (TeO2) Nanoparticles. Int. J. Electrochem. Sci. 2021, 16, 210235. [Google Scholar] [CrossRef]
- Grishechkin, M.; Islam, A.; Khomyakov, A.; Zykova, M.; Mozhevitina, E.; Avetisov, R.; Ermochenkov, I.; Avetissov, I. Extra pure tellurium oxide for the growth of high quality paratellurite crystals. IOP Conf. Ser. Mater. Sci. Eng. 2019, 613, 012021. [Google Scholar] [CrossRef]
- Arab, F.; Mousavi-Kamazani, M.; Salavati-Niasari, M. Synthesis, characterization, and optical properties of Te, Te/TeO2 and TeO2 nanostructures via a onepot hydrothermal method. RSC Adv. 2016, 6, 71472–71480. [Google Scholar] [CrossRef]
- Kosmulski, M.; Rosenholm, J.B. High Ionic Strength Electrokinetics. Adv. Colloid Interface Sci. 2004, 112, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Laven, J.; Stein, H.N. The Electroviscous Behavior of Aqueous Dispersions of Amorphous Silica (Ludox). J. Colloid Interface Sci. 2001, 238, 8–15. [Google Scholar] [CrossRef]
- Grządka, E. Adsorption and electrokinetic properties in the system: Beta-cyclodextrin/alumina in the presence of ionic and non-ionic surfactants. Colloids Surf. A 2015, 481, 261–268. [Google Scholar] [CrossRef]
- Del Nero, M.; Galindo, C.; Barillon, R.; Halter, E.; Madé, B. Surface reactivity of α-Al2O3 and mechanisms of phosphate sorption: In situ ATR-FTIR spectroscopy and ζ potential studies. J. Colloid Interface Sci. 2010, 342, 437–444. [Google Scholar] [CrossRef]
- Kosmulski, M. Chemical Properties of Materials Surfaces; Taylor and Francis: Boca Raton, FL, USA, 2001. [Google Scholar] [CrossRef]
- Escudey, M.; Gil-Llambias, F. Effect of cation and anion adsorption on the electrophoretic behavior of MoO3/γ-Al2O3 catalysts. J. Colloid Interface Sci. 1985, 107, 272–275. [Google Scholar] [CrossRef]
- Huang, C.P.; Stumm, W. Specific adsorption of cations on hydrous γ-Al2O3. J. Colloid Interface Sci. 1973, 43, 409–420. [Google Scholar] [CrossRef]
- Murase, K.; Suzuki, T.; Umenaka, Y.; Hirato, T.; Awakura, Y. Thermodynamics of Cathodic ZnTe Electrodeposition Using Basic Ammoniacal Electrolytes: Why CdTe Can Deposit While ZnTe Cannot. High Temp. Mater. Proc. 2011, 30, 451–458. [Google Scholar] [CrossRef]
- Hiemstra, T.; van Riemsdijk, W.H.; Bolt, G.H. Multisite proton adsorption modeling at the solid/solution interface of (hydr)oxides: A new approach: I. Model description and evaluation of intrinsic reaction constants. J. Colloid Interface Sci. 1989, 133, 91–104. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).