Combined Ultrahigh Pressure Extraction and High-Speed Counter-Current Chromatography for Separation and Purification of Three Glycoside Compounds from Dendrobium officinale Protocorm
Abstract
:1. Introduction
2. Results and Discussion
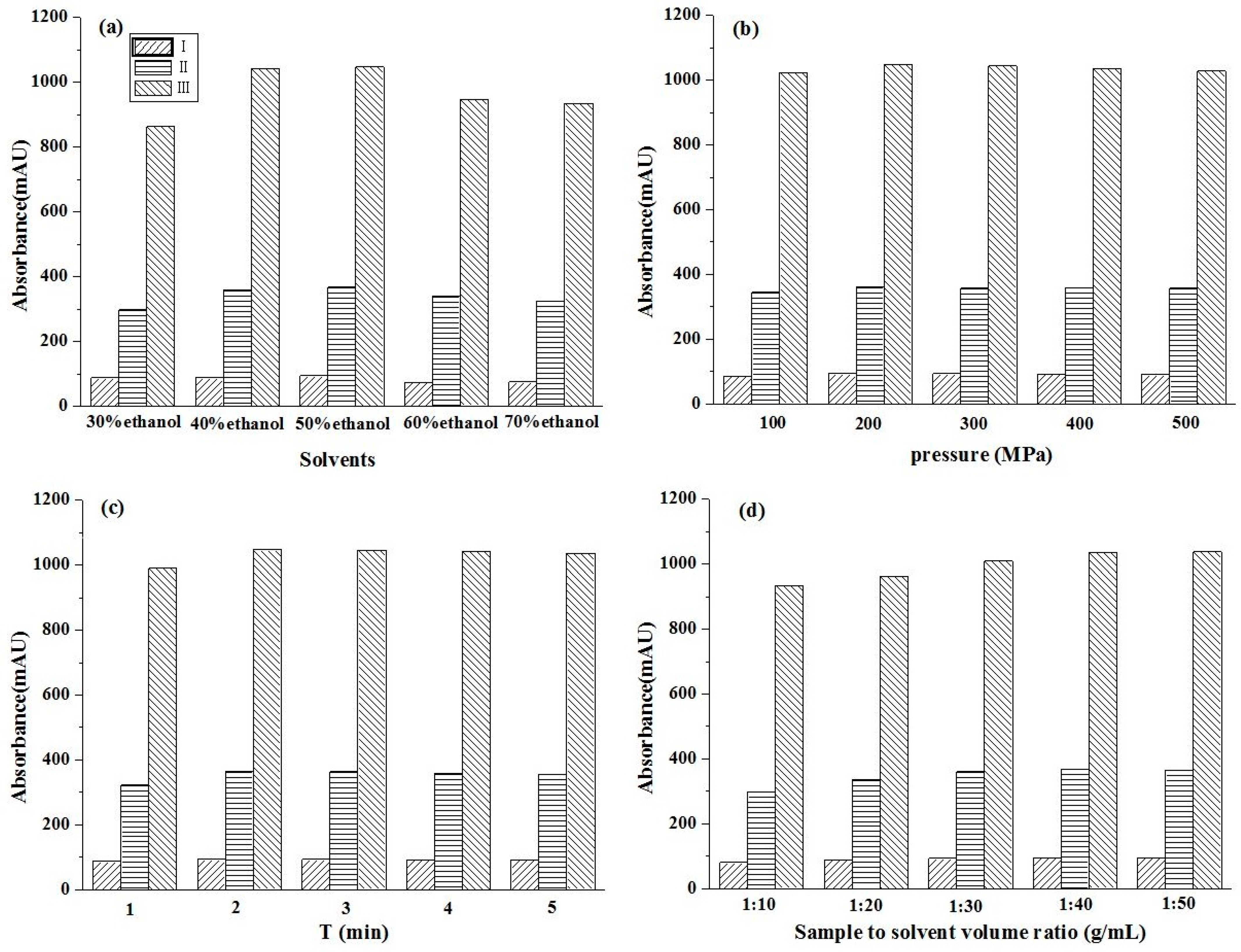
2.1. Ultrahigh Pressure Extraction Parameters
2.1.1. The Effect of Ethanol Concentration
2.1.2. The Effect of Pressure
2.1.3. The Effect of Extraction Time
2.1.4. The Effect of Solid-Liquid Ratio
2.2. Comparison of UPE and Hot Reflux Extraction
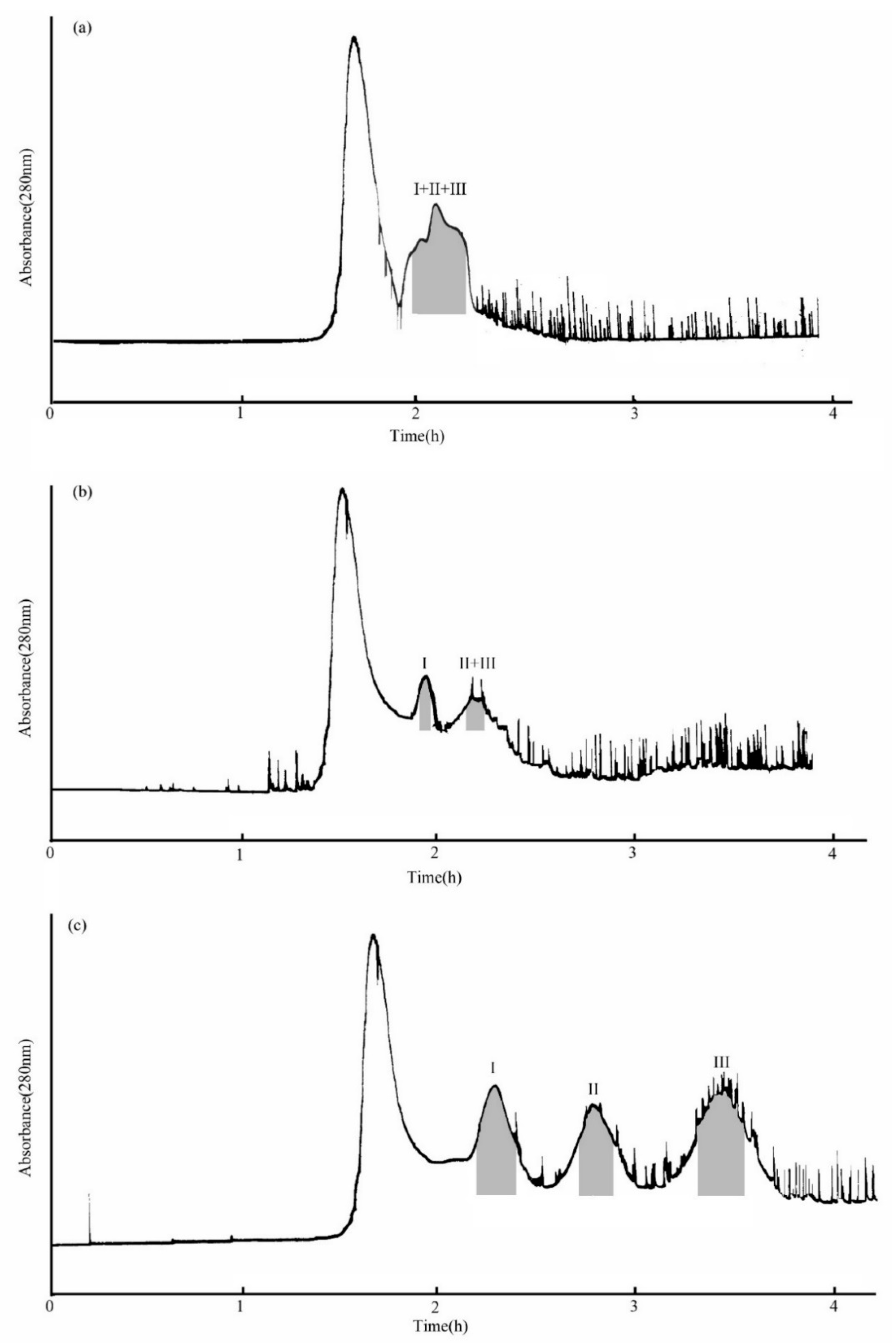
2.3. Selection of the Optimized Two-Phase Solvent System
2.4. Purification of Three Compounds by HSCCC
2.5. Identification of Purified Compounds
2.6. DPPH Radical Scavenging Effect
3. Experimental Section
3.1. Reagents
3.2. Apparatus
3.3. UPE Conditions
3.4. Heat Reflux Extraction
3.5. Silica Gel Column Chromatography Separation
3.6. Selection of Two-Phase Solvent System and Preparation of Sample for HSCCC
3.7. HSCCC Separation Procedures
3.8. HPLC Analysis and Identification of HSCCC Fractions
3.9. DPPH Radical Scavenging Effect
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Yang, F.; Wei, N.-N.; Gao, R.; Piao, X.-C.; Lian, M.-L. Effect of several medium factors on polysaccharide and alkaloid accumulation in protocorm-like bodies of Dendrobium candidum during bioreactor culture. Acta Physiol. Plant. 2015, 37. [Google Scholar] [CrossRef]
- Chin, C.K.; Stanly, C.; Muniandy, A.; Ramanathan, S.; Murugaiyah, V.; Chew, B.L.; Subramaniam, S. Protocorm-like bodies (PLBs) of Dendrobium Sabin Blue: A novel source for in vitro production of dendrobine and anthocyanin. In Vitro Cell. Dev. Biol. Plant. 2021. [Google Scholar] [CrossRef]
- Wei, M.; Yang, C.Y.; Wei, S.H. Enhancement of the differentiation of protocorm-like bodies of Dendrobium officinale to shoots by ultrasound treatment. J. Plant. Physiol. 2012, 169, 770–774. [Google Scholar] [CrossRef]
- Tang, H.; Zhao, T.; Sheng, Y.; Zheng, T.; Fu, L.; Zhang, Y. Dendrobium officinale Kimura et Migo: A Review on Its Ethnopharmacology, Phytochemistry, Pharmacology, and Industrialization. Evid Based Complement. Alternat Med. 2017, 2017, 7436259. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.-Y.; Murthy, H.N.; Moh, S.H.; Cui, Y.-Y.; Lee, E.-J.; Paek, K.-Y. Production of biomass and bioactive compounds in protocorm cultures of Dendrobium candidum Wall ex Lindl. using balloon type bubble bioreactors. Ind. Crops Prod. 2014, 53, 28–33. [Google Scholar] [CrossRef]
- Jiao, C.; Song, C.; Zheng, S.; Zhu, Y.; Jin, Q.; Cai, Y.; Lin, Y. Metabolic Profiling of Dendrobium officinale in Response to Precursors and Methyl Jasmonate. Int. J. Mol. Sci. 2018, 19, 728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, H.; Ji, Y.; Li, S.; Lu, L.; Tian, M.; Yang, W.; Li, H. Extensive Metabolic Profiles of Leaves and Stems from the Medicinal Plant Dendrobium officinale Kimura et Migo. Metabolites 2019, 9, 215. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.-Q.; Jin, M.-Y.; Paek, K.-Y.; Piao, X.-C.; Lian, M.-L. An efficient strategy for enhancement of bioactive compounds by protocorm-like body culture of Dendrobium candidum. Ind. Crops Prod. 2016, 84, 121–130. [Google Scholar] [CrossRef]
- Mai, Y.; Niu, Z.; He, W.; Lai, X.; Huang, S.; Zheng, X. The Reparative Effect of Dendrobium officinale Protocorms against Photodamage Caused by UV-Irradiation in Hairless Mice. J. Pharm. Soc. Jpn. 2019, 42, 728–735. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.-Y.; Murthy, H.N.; Moh, S.H.; Cui, Y.; Lee, E.-J.; Paek, K.-Y. Protocorm culture of Dendrobium candidum in balloon type bubble bioreactors. Biochem. Eng. J. 2014, 88, 26–29. [Google Scholar] [CrossRef]
- Cardoso, J.C.; Zanello, C.A.; Chen, J.-T. An Overview of Orchid Protocorm-Like Bodies: Mass Propagation, Biotechnology, Molecular Aspects, and Breeding. Int. J. Mol. Sci. 2020, 21, 985. [Google Scholar] [CrossRef] [Green Version]
- James Antony, J.J.; Zakaria, S.; Zakaria, R.; Anak Ujang, J.; Othman, N.; Subramaniam, S. Biochemical analyses of Dendrobium Sabin Blue PLBs during cryopreservation by vitrification. Physiol. Mol. Biol. Plants 2019, 25, 1457–1467. [Google Scholar] [CrossRef]
- Cao, Y.; Meng, D.; Han, Y.; Chen, T.; Jiao, C.; Chen, Y.; Jin, Q.; Cai, Y. Comparative analysis of B-BOX genes and their expression pattern analysis under various treatments in Dendrobium officinale. BMC Plant. Biol. 2019, 19, 245. [Google Scholar] [CrossRef]
- An, H.; Zhu, Q.; Pei, W.; Fan, J.; Liang, Y.; Cui, Y.; Lv, N.; Wang, W. Whole-Transcriptome Selection and Evaluation of Internal Reference Genes for Expression Analysis in Protocorm Development of Dendrobium officinale Kimura et Migo. PLoS ONE 2016, 11, e0163478. [Google Scholar] [CrossRef]
- Zeng, Q.; Ko, C.H.; Siu, W.S.; Li, L.F.; Han, X.Q.; Yang, L.; Bik-San Lau, C.; Hu, J.M.; Leung, P.C. Polysaccharides of Dendrobium officinale Kimura & Migo protect gastric mucosal cell against oxidative damage-induced apoptosis in vitro and in vivo. J. Ethnopharmacol. 2017, 208, 214–224. [Google Scholar] [CrossRef]
- Youssef, F.S.; Ashour, M.L.; Sobeh, M.; El-Beshbishy, H.A.; Singab, A.N.; Wink, M. Eremophila maculata-Isolation of a rare naturally-occurring lignan glycoside and the hepatoprotective activity of the leaf extract. Phytomedicine 2016, 23, 1484–1493. [Google Scholar] [CrossRef] [PubMed]
- Palasz, A.; Ciez, D.; Trzewik, B.; Miszczak, K.; Tynor, G.; Bazan, B. In the Search of Glycoside-Based Molecules as Antidiabetic Agents. Top. Curr. Chem. 2019, 377, 19. [Google Scholar] [CrossRef] [Green Version]
- Du, K.; Yang, X.; Li, J.; Meng, D. Antiproliferative diterpenoids and acetophenone glycoside from the roots of Euphorbia fischeriana. Phytochemistry 2020, 177, 112437. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.-Z.; Yong, Y.; Dong, Y.-H.; Wang, R.-J.; Li, H.-X.; Zhang, H.; Guo, D.-L.; Zhang, S.-J.; Dong, X.-P.; Xie, X.-F. A new secoiridoid glycoside and a new sesquiterpenoid glycoside from Valeriana jatamansi with neuroprotective activity. Phytochem. Lett. 2016, 17, 177–180. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-G.; Li, X.; Xiong, D.-C.; Yu, B.; Pu, X.; Ye, X.-S. Synthetic phenylethanoid glycoside derivatives as potent neuroprotective agents. Eur. J. Med. Chem. 2015, 95, 313–323. [Google Scholar] [CrossRef]
- Bai, L.-J.; Luo, J.-G.; Chen, C.; Kong, L.-Y. Pharesinosides A-G, acylated glycosidic acid methyl esters derivatized by NH2 silica gel on-column catalyzation from the crude resin glycosides of Pharbitis Semen. Tetrahedron 2017, 73, 2863–2871. [Google Scholar] [CrossRef]
- Jin, H.G.; Kim, A.R.; Ko, H.J.; Woo, E.R. A new megastigmane glycoside from Akebia quinata. Arch. Pharm. Res. 2015, 38, 591–597. [Google Scholar] [CrossRef]
- Chen, T.; Li, H.; Zou, D.; Liu, Y.; Chen, C.; Zhou, G.; Li, Y. Separation of three anthraquinone glycosides including two isomers by preparative high-performance liquid chromatography and high-speed countercurrent chromatography from Rheum tanguticum Maxim. ex Balf. J. Sep. Sci. 2016, 39, 3105–3112. [Google Scholar] [CrossRef]
- Zheng, L.; Chen, L.; Li, J.; Liang, L.; Fan, Y.; Qiu, L.; Deng, Z. Two Kaempferol Glycosides Separated from Camellia Oleifera Meal by High-Speed Countercurrent Chromatography and Their Possible Application for Antioxidation. J. Food Sci. 2019, 84, 2805–2811. [Google Scholar] [CrossRef]
- Ali, I.; Li, J.; Cui, L.; Zhao, H.; He, Q.; Wang, D. Efficient extraction and purification of benzo[c]phenanthridine alkaloids from Macleaya cordata (Willd) R. Br. by combination of ultrahigh pressure extraction and pH-zone-refining counter-current chromatography with anti-breast cancer activity in vitro. Phytochem. Anal. 2021, 32, 423–432. [Google Scholar] [CrossRef]
- He, F.; Chen, L.; Liu, Q.; Wang, X.; Li, J.; Yu, J. Preparative Separation of Phenylethanoid and Secoiridoid Glycosides from Ligustri Lucidi Fructus by High-Speed Counter-Current Chromatography Coupled with Ultrahigh Pressure Extraction. Molecules 2018, 23, 3353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdin, M.; Hamed, Y.S.; Akhtar, H.M.S.; Chen, D.; Chen, G.; Wan, P.; Zeng, X. Antioxidant and anti-inflammatory activities of target anthocyanins di-glucosides isolated from Syzygium cumini pulp by high speed counter-current chromatography. J. Food Biochem. 2020, 44, 1050–1062. [Google Scholar] [CrossRef] [PubMed]
- Kan, X.; Yan, Y.; Ran, L.; Lu, L.; Mi, J.; Zhang, Z.; Li, X.; Zeng, X.; Cao, Y. Ultrasonic-assisted extraction and high-speed counter-current chromatography purification of zeaxanthin dipalmitate from the fruits of Lycium barbarum L. Food Chem. 2020, 310, 125854. [Google Scholar] [CrossRef]
- Ito, Y. High-speed countercurrent chromatography. Nature 1987, 326, 419–420. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Huang, X.Y.; Pei, D.; Duan, W.D.; Zhang, X.; Sun, X.; Di, D.L. The applicability of high-speed counter current chromatography to the separation of natural antioxidants. J. Chromatogr. A 2020, 1623, 461150. [Google Scholar] [CrossRef]
- Wang, C.-X.; Wang, L.-X.; Li, C.-Y.; Hu, C.; Zhao, S.-h. Anti-proliferation activities of three bioactive components purified by high-speed counter-current chromatography in essential oil from ginger. Eur. Food Res. Technol. 2020, 246, 795–805. [Google Scholar] [CrossRef]
- Wang, X.; Liu, C.; Ma, Q.Y.; Tian, Z.H.; Jiang, H.Q.; Lv, Q.T.; Rong, R. A rapid and practical prediction method for the Arizona solvent system family used in high speed countercurrent chromatography. J. Chromatogr. A 2020, 1629, 461426. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Che, F.; Lu, Y.; Li, A.; Li, H.; Liu, J.; Wei, Y. Isolation and purification of alkaloids from the fruits of Macleaya cordata by ionic-liquid-modified high-speed counter-current chromatography. J. Sep. Sci. 2020, 43, 2459–2466. [Google Scholar] [CrossRef] [PubMed]
- Xi, J. Ultrahigh pressure extraction of bioactive compounds from plants-a review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Liu, F.; Xu, M.; Lin, X.; Wang, X. Ultrahigh pressure extraction of lignan compounds from Dysosma versipellis and purification by high-speed counter-current chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2012, 905, 145–149. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Q.; Li, P.; Yang, H. High-Speed Countercurrent Chromatography-Based Method for Simultaneous Recovery and Separation of Natural Products from Deep Eutectic Solvent Extracts. ACS Sustain. Chem. Eng. 2020, 8, 2073–2080. [Google Scholar] [CrossRef]
- Wu, X.; Gao, X.; Liu, X.; Zhang, S.; Yang, H.; Zhu, X.; Song, H.; Li, F.; Chen, Q. Quality Control of Psoralea corylifolia L. Based on High-Speed Countercurrent Chromatographic Fingerprinting. Molecules 2020, 25, 279. [Google Scholar] [CrossRef] [Green Version]
- Oka, F.; Oka, H.; Ito, Y. Systematic search for suitable two-phase solvent systems for high-speed counter-current chromatography. J. Chromatogr. 1991, 538, 99–108. [Google Scholar] [CrossRef]
- Ina, H.; Komakid, K.; Iida, H. Hydroxycinnamylglucoes from Spiraea thunbergii. Planta Med. 1987, 53, 502. [Google Scholar] [CrossRef]
- JKim, J.E.; Jung, M.; Jung, H.A.; Woo, J.J.; Cheigh, H.S.; Chung, H.Y.; Choi, H.S. A new Kaempferol 7-O-Triglucoside from the leaves of Brassical juncea L. Arch. Pham. Res. 2002, 25, 621–624. [Google Scholar] [CrossRef]
- Fan, S.; Yang, G.; Zhang, J.; Li, J.; Bai, B. Optimization of Ultrasound-Assisted Extraction Using Response Surface Methodology for Simultaneous Quantitation of Six Flavonoids in Flos Sophorae Immaturus and Antioxidant Activity. Molecules 2020, 25, 1767. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Solvent System | Volume Ratio (v/v) | KD Values | ||
|---|---|---|---|---|
| Compound I | Compound II | Compound III | ||
| MTBE-n-butyl alcohol-acetonitrile-water | 4:2:3:8 | 0.39 | 0.79 | 0.95 |
| 5:1:2:6 | 0.98 | 1.24 | 1.45 | |
| 2:0:2:3 | 0.23 | 0.36 | 0.39 | |
| 6:0:3:8 | 0.21 | 0.38 | 0.43 | |
| chloroform-methanol-water | 4:3:3 | 2.56 | 2.98 | 3.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Zhang, Y.; Wang, J.; Duan, W.; Liu, F. Combined Ultrahigh Pressure Extraction and High-Speed Counter-Current Chromatography for Separation and Purification of Three Glycoside Compounds from Dendrobium officinale Protocorm. Molecules 2021, 26, 3934. https://doi.org/10.3390/molecules26133934
Zhang W, Zhang Y, Wang J, Duan W, Liu F. Combined Ultrahigh Pressure Extraction and High-Speed Counter-Current Chromatography for Separation and Purification of Three Glycoside Compounds from Dendrobium officinale Protocorm. Molecules. 2021; 26(13):3934. https://doi.org/10.3390/molecules26133934
Chicago/Turabian StyleZhang, Wei, Yingjie Zhang, Jinying Wang, Wenjuan Duan, and Feng Liu. 2021. "Combined Ultrahigh Pressure Extraction and High-Speed Counter-Current Chromatography for Separation and Purification of Three Glycoside Compounds from Dendrobium officinale Protocorm" Molecules 26, no. 13: 3934. https://doi.org/10.3390/molecules26133934
APA StyleZhang, W., Zhang, Y., Wang, J., Duan, W., & Liu, F. (2021). Combined Ultrahigh Pressure Extraction and High-Speed Counter-Current Chromatography for Separation and Purification of Three Glycoside Compounds from Dendrobium officinale Protocorm. Molecules, 26(13), 3934. https://doi.org/10.3390/molecules26133934





