Combined Acute Ozone and Water Stress Alters the Quantitative Relationships between O3 Uptake, Photosynthetic Characteristics and Volatile Emissions in Brassica nigra
Abstract
1. Introduction
2. Results
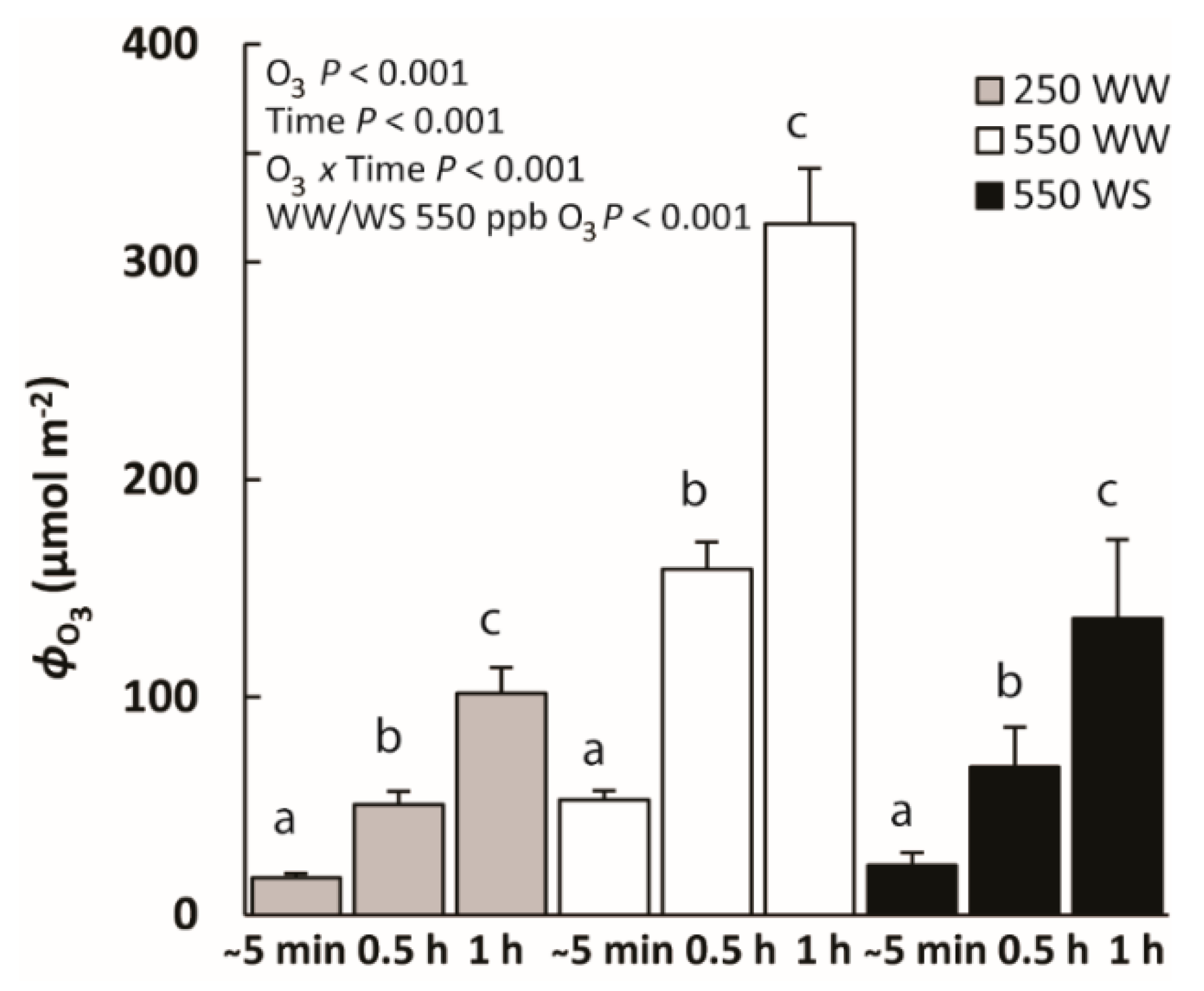
2.1. Impacts of Water Stress on Photosynthetic Characteristics and O3 Uptake by Leaf Surface and Stomata
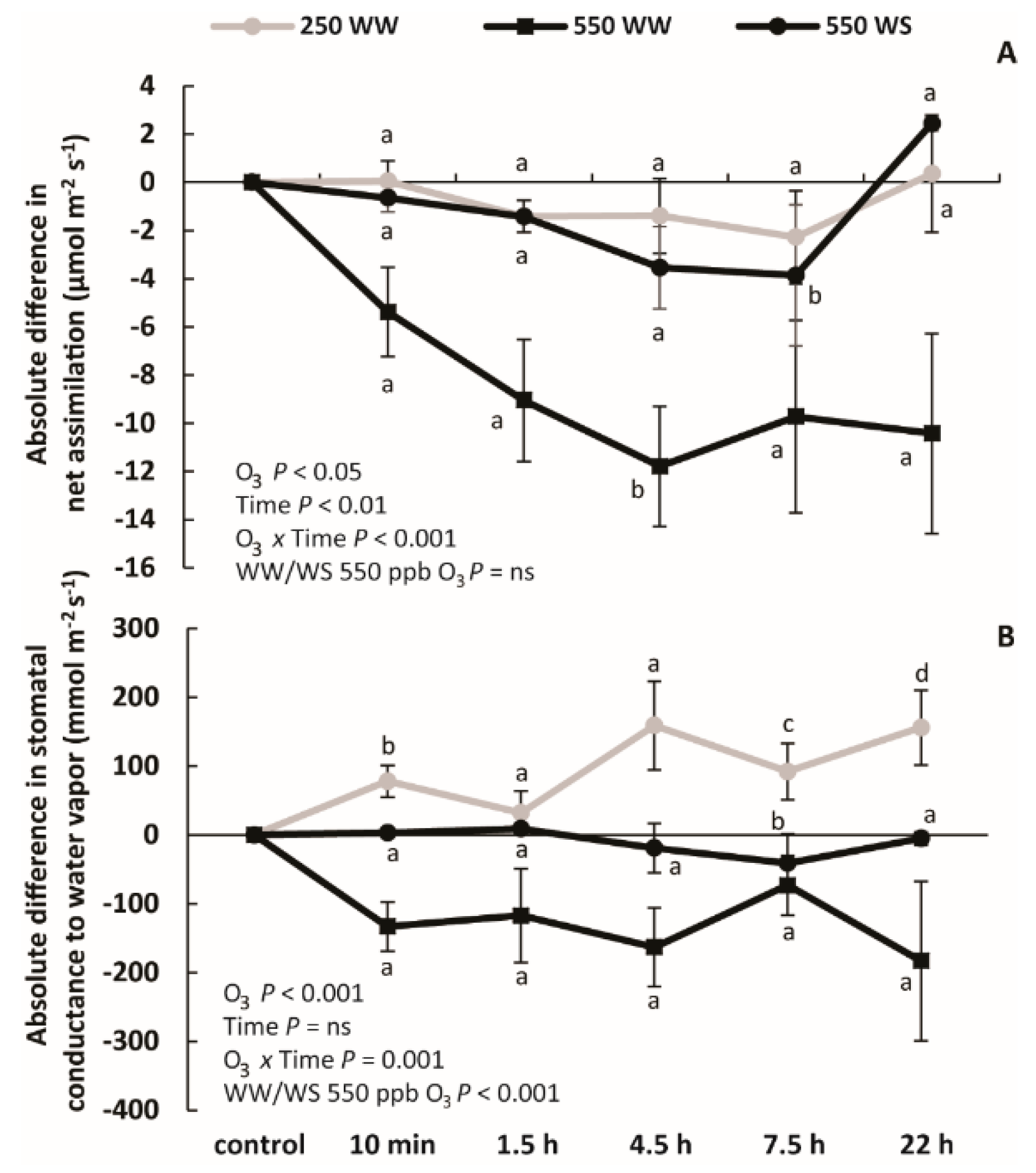
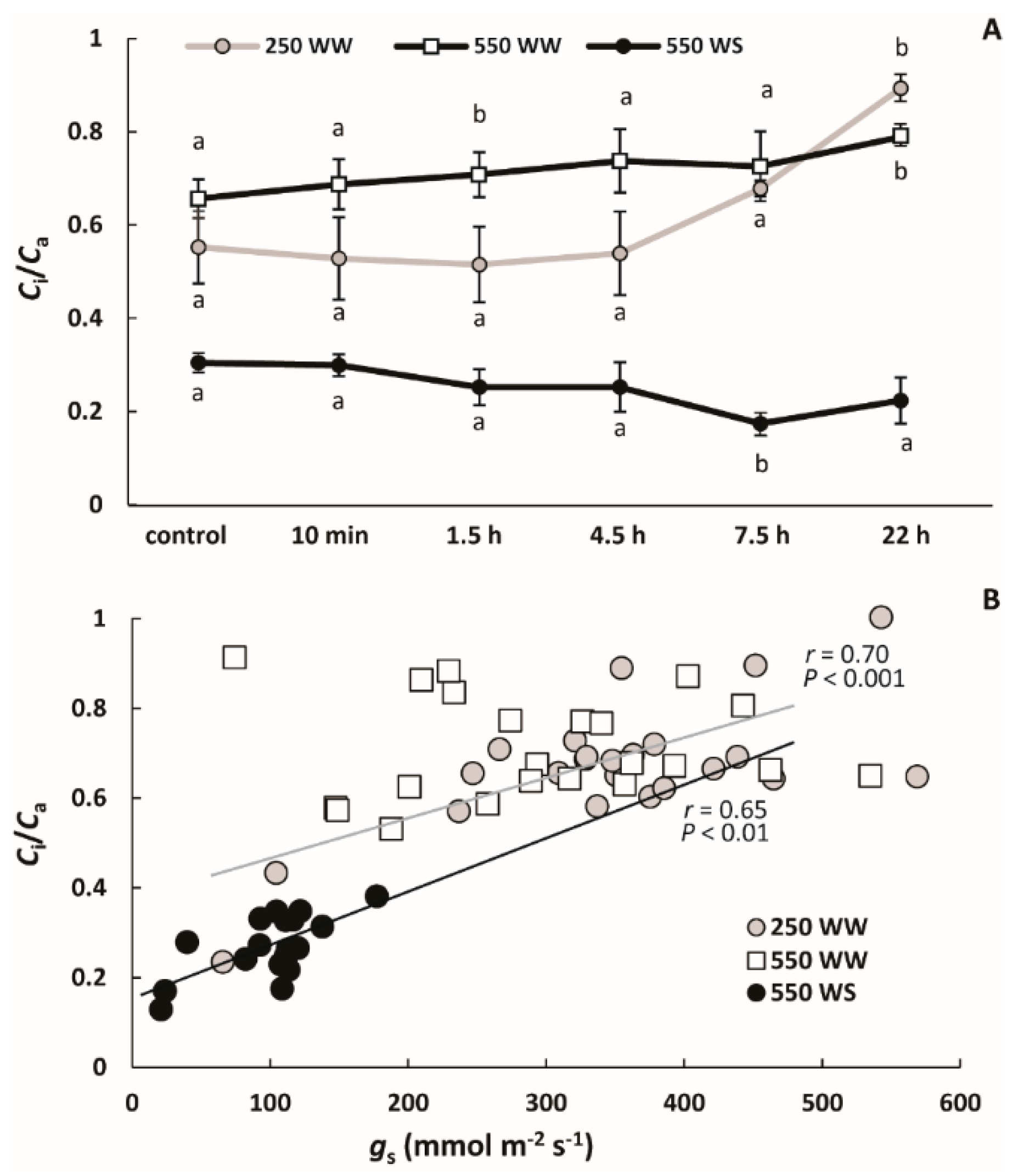
2.2. Changes in Photosynthetic Characteristics Upon Ozone Exposure
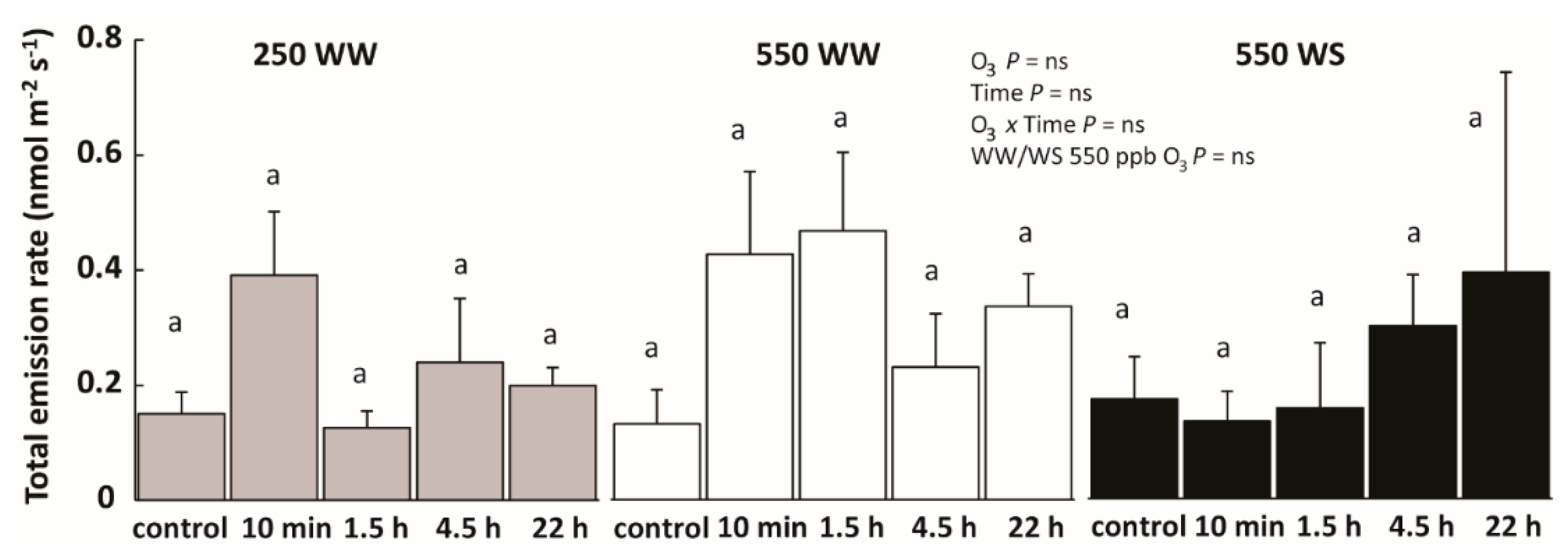
2.3. Drought and O3 Impacts on Total Volatile Emissions and Emissions of Different Volatile Groups
2.4. Elicitation of Emissions of Individual LOX Pathway Compounds by O3 Exposure
2.5. Impact of Glucosinolate Breakdown Products on the O3-Induced Smell Bouquet
2.6. O3 Exposure Effects on Other Volatiles
3. Discussion
3.1. How Drought and Different O3 Levels Affect Leaf Photosynthetic Characteristics in B. nigra
3.2. Water Stress Effects on Non-Ozonated Plant Volatile Emissions
3.3. O3 Effects on Total VOC Emissions in Well-Watered and Water-Stressed Plants
3.4. Effects of O3 and Water Stress Treatments on Emissions of Specific LOX Pathway Compounds
3.5. O3 Effects on Species—Specific Glucosinolate Degradation Products
3.6. O3 Effects on Other Volatile Groups
4. Materials and Methods
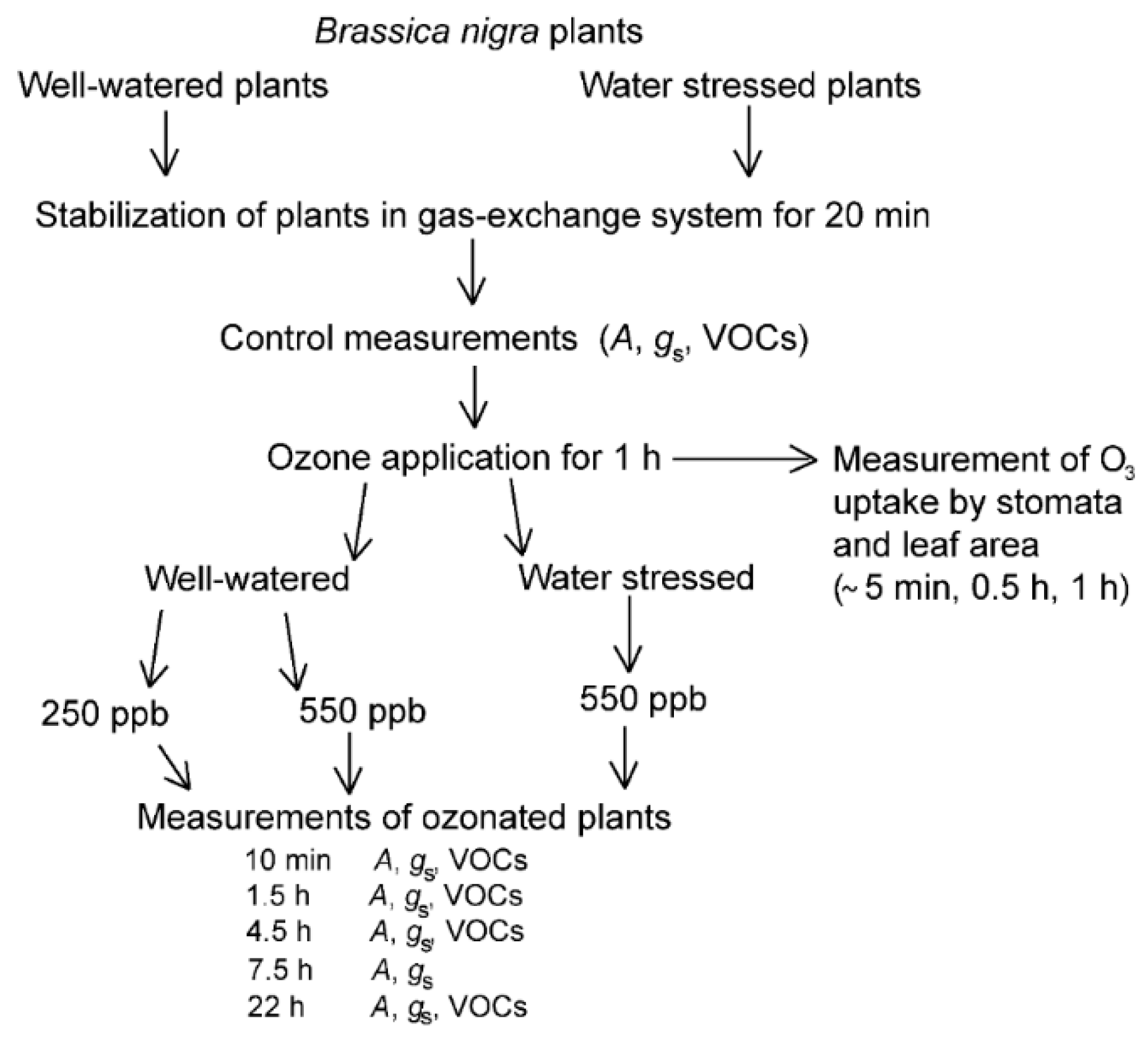
4.1. Plant Material
4.2. Experimental Set-Up and Gas Exchange Measurements
4.3. Ozone Stress Application
4.4. Volatile Sampling and GC-MS Analysis
4.5. Statistical Analyses
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lacour, S.A.; de Monte, M.; Diot, P.; Brocca, J.; Veron, N.; Colin, P.; Leblond, V. Relationship between ozone and temperature during the 2003 heat wave in France: Consequences for health data analysis. BMC Public Health 2006, 6. [Google Scholar] [CrossRef]
- Langematz, U. Stratospheric ozone: Down and up through the anthropocene. Chemtexts 2019, 5, 12. [Google Scholar] [CrossRef]
- Ainsworth, E.A. Understanding and improving global crop response to ozone pollution. Plant J. 2017, 90, 886–897. [Google Scholar] [CrossRef]
- Bais, A.F.; Bernhard, G.; McKenzie, R.L.; Aucamp, P.J.; Young, P.J.; Ilyas, M.; Jockel, P.; Deushi, M. Ozone-climate interactions and effects on solar ultraviolet radiation. Photochem. Photobiol. Sci. 2019, 18, 602–640. [Google Scholar] [CrossRef] [PubMed]
- Chipperfield, M.P.; Bekki, S.; Dhomse, S.; Harris, N.R.P.; Hassler, B.; Hossaini, R.; Steinbrecht, W.; Thieblemont, R.; Weber, M. Detecting recovery of the stratospheric ozone layer. Nature 2017, 549, 211–218. [Google Scholar] [CrossRef]
- Dhomse, S.S.; Feng, W.; Montzka, S.A.; Hossaini, R.; Keeble, J.; Pyle, J.A.; Daniel, J.S.; Chipperfield, M.P. Delay in recovery of the Antarctic ozone hole from unexpected CFC-11 emissions. Nat. Commun. 2019, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.Z.; Calatayud, V.; Zhu, J.G.; Kobayashi, K. Ozone exposure- and flux-based response relationships with photosynthesis of winter wheat under fully open air condition. Sci. Total Environ. 2018, 619, 1538–1544. [Google Scholar] [CrossRef]
- Masutomi, Y.; Kinose, Y.; Takimoto, T.; Yonekura, T.; Oue, H.; Kobayashi, K. Ozone changes the linear relationship between photosynthesis and stomatal conductance and decreases water use efficiency in rice. Sci. Total Environ. 2019, 655, 1009–1016. [Google Scholar] [CrossRef]
- Chaudhary, N.; Agrawal, S.B. The role of elevated ozone on growth, yield and seed quality amongst six cultivars of mung bean. Ecotoxicol. Environ. Saf. 2015, 111, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Schaub, M.; Haeni, M.; Calatayud, V.; Ferretti, M.; Gottardini, E. Forests Ozone Concentrations Are Decreasing but Exposure Remains High in European Forests; ICP forests brief; Thünen Institute of Forest Ecosystems: Eberswalde, Germany, 2018; 4p. [Google Scholar] [CrossRef]
- Querol, X.; Alastuey, A.; Gangoiti, G.; Perez, N.; Lee, H.K.; Eun, H.R.; Park, Y.; Mantilla, E.; Escudero, M.; Titos, G.; et al. Phenomenology of summer ozone episodes over the Madrid Metropolitan Area, central Spain. Atmos. Chem. Phys. 2018, 18, 6511–6533. [Google Scholar] [CrossRef]
- Archibald, A.T.; Turnock, S.T.; Griffiths, P.T.; Cox, T.; Derwent, R.G.; Knote, C.; Shin, M. On the changes in surface ozone over the twenty-first century: Sensitivity to changes in surface temperature and chemical mechanisms. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2020, 378, 16. [Google Scholar] [CrossRef]
- Chen, C.P.; Frank, T.D.; Long, S.P. Is a short, sharp shock equivalent to long-term punishment? Contrasting the spatial pattern of acute and chronic ozone damage to soybean leaves via chlorophyll fluorescence imaging. Plant Cell Environ. 2009, 32, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Schraudner, M.; Moeder, W.; Wiese, C.; Van Camp, W.; Inze, D.; Langebartels, C.; Sandermann, H. Ozone-induced oxidative burst in the ozone biomonitor plant, tobacco Bel W3. Plant J. 1998, 16, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Vollenweider, P.; Ottiger, M.; Gunthardt-Goerg, M.S. Validation of leaf ozone symptoms in natural vegetation using microscopical methods. Environ. Pollut. 2003, 124, 101–118. [Google Scholar] [CrossRef]
- Brosché, M.; Merilo, E.; Mayer, F.; Pechter, P.; Puzõrjova, I.; Brader, G.; Kangasjärvi, J.; Kollist, H. Natural variation in ozone sensitivity among Arabidopsis thaliana accessions and its relation to stomatal conductance. Plant Cell Environ. 2010, 33, 914–925. [Google Scholar] [CrossRef]
- Guidi, L.; Degl’Innocenti, E.; Genovesi, S.; Soldatini, G.F. Photosynthetic process and activities of enzymes involved in the phenylpropanoid pathway in resistant and sensitive genotypes of Lycopersicon esculentum L. exposed to ozone. Plant Sci. 2005, 168, 153–160. [Google Scholar] [CrossRef]
- Kangasjärvi, J.; Jaspers, P.; Kollist, H. Signalling and cell death in ozone-exposed plants. Plant Cell Environ. 2005, 28, 1021–1036. [Google Scholar] [CrossRef]
- Dickinson, B.C.; Chang, C.J. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat. Chem. Biol. 2011, 7, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, J.; Wisthaler, A.; Hansel, A.; Kleist, E.; Miebach, M.; Niinemets, Ü.; Schurr, U.; Wildt, J. Ozone induced emissions of biogenic VOC from tobacco: Relationships between ozone uptake and emission of LOX products. Plant Cell Environ. 2005, 28, 1334–1343. [Google Scholar] [CrossRef]
- Rozpądek, P.; Nosek, M.; Ślesak, I.; Kunicki, E.; Dziurka, M.; Miszalski, Z. Ozone fumigation increases the abundance of nutrients in Brassica vegetables: Broccoli (Brassica oleracea var. italica) and Chinese cabbage (Brassica pekinensis). Eur. Food Res. Technol. 2015, 240, 459–462. [Google Scholar] [CrossRef]
- Rozpądek, P.; Ślesak, I.; Cebula, S.; Waligórski, P.; Dziurka, M.; Skoczowski, A.; Miszalski, Z. Ozone fumigation results in accelerated growth and persistent changes in the antioxidant system of Brassica oleracea L. var. capitata f. alba. J. Plant Physiol. 2013, 170, 1259–1266. [Google Scholar] [CrossRef]
- Li, S.; Harley, P.C.; Niinemets, Ü. Ozone-induced foliar damage and release of stress volatiles is highly dependent on stomatal openness and priming by low-level ozone exposure in Phaseolus vulgaris. Plant Cell Environ. 2017, 40, 1984–2003. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ballesta, M.D.; Moreno, D.A.; Carvajal, M. The physiological importance of glucosinolates on plant response to abiotic stress in Brassica. Int. J. Mol. Sci. 2013, 14, 11607–11625. [Google Scholar] [CrossRef] [PubMed]
- Dorokhov, Y.L.; Sheshukova, E.V.; Komarova, T.V. Methanol in plant life. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Peron, A.; Kaser, L.; Fitzky, A.C.; Graus, M.; Halbwirth, H.; Greiner, J.; Wohlfahrt, G.; Rewald, B.; Sanden, H.; Karl, T. Combined effects of ozone and drought stress on the emission of biogenic volatile organic compounds from Quercus robur L. Biogeosciences 2021, 18, 535–556. [Google Scholar] [CrossRef]
- Pokhrel, Y.; Felfelani, F.; Satoh, Y.; Boulange, J.; Burek, P.; Gadeke, A.; Gerten, D.; Gosling, S.N.; Grillakis, M.; Gudmundsson, L.; et al. Global terrestrial water storage and drought severity under climate change. Nat. Clim. Chang. 2021, 11, 226–233. [Google Scholar] [CrossRef]
- Taylor, R.G.; Scanlon, B.; Doll, P.; Rodell, M.; van Beek, R.; Wada, Y.; Longuevergne, L.; Leblanc, M.; Famiglietti, J.S.; Edmunds, M.; et al. Ground water and climate change. Nat. Clim. Chang. 2013, 3, 322–329. [Google Scholar] [CrossRef]
- Catola, S.; Centritto, M.; Cascone, P.; Ranieri, A.; Loreto, F.; Calamai, L.; Balestrini, R.; Guerrieri, E. Effects of single or combined water deficit and aphid attack on tomato volatile organic compound (VOC) emission and plant-plant communication. Environ. Exp. Bot. 2018, 153, 54–62. [Google Scholar] [CrossRef]
- Liu, Y.L.; Guo, X.S.; Ma, M.S.; Yu, X.F. Maize seedlings response to drought stress and re-watering: Abscisic acid, a key regulator of physio-biochemical traits and gas exchange parameters. Pak. J. Bot. 2018, 50, 2131–2139. [Google Scholar]
- Saunier, A.; Ormeno, E.; Wortham, H.; Temime-Roussel, B.; Lecareux, C.; Boissard, C.; Fernandez, C. Chronic drought decreases anabolic and catabolic BVOC emissions of Quercus pubescens in a Mediterranean forest. Front. Plant Sci. 2017, 8, 11. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Keenan, T. Photosynthetic responses to stress in Mediterranean evergreens: Mechanisms and models. Environ. Exp. Bot. 2014, 103, 24–41. [Google Scholar] [CrossRef]
- Jud, W.; Fischer, L.; Canaval, E.; Wohlfahrt, G.; Tissier, A.; Hansel, A. Plant surface reactions: An opportunistic ozone defence mechanism impacting atmospheric chemistry. Atmos. Chem. Phys. 2016, 16, 277–292. [Google Scholar] [CrossRef]
- Khan, M.A.M.; Ulrichs, C.; Mewis, I. Influence of water stress on the glucosinolate profile of Brassica oleracea var. italica and the performance of Brevicoryne brassicae and Myzus persicae. Entomol. Exp. Appl. 2010, 137, 229–236. [Google Scholar] [CrossRef]
- Salerno, G.; Frati, F.; Marino, G.; Ederli, L.; Pasqualini, S.; Loreto, F.; Colazza, S.; Centritto, M. Effects of water stress on emission of volatile organic compounds by Vicia faba, and consequences for attraction of the egg parasitoid Trissolcus basalis. J. Pest Sci. 2017, 90, 635–647. [Google Scholar] [CrossRef]
- Shang, B.; Yuan, X.Y.; Li, P.; Xu, Y.S.; Feng, Z.Z. Effects of elevated ozone and water deficit on poplar saplings: Changes in carbon and nitrogen stocks and their allocation to different organs. For. Ecol. Manag. 2019, 441, 89–98. [Google Scholar] [CrossRef]
- Kigathi, R.N.; Weisser, W.W.; Reichelt, M.; Gershenzon, J.; Unsicker, S.B. Plant volatile emission depends on the species composition of the neighboring plant community. BMC Plant Biol. 2019, 19, 17. [Google Scholar] [CrossRef]
- Kanagendran, A.; Pazouki, L.; Niinemets, Ü. Differential regulation of volatile emission from Eucalyptus globulus leaves upon single and combined ozone and wounding treatments through recovery and relationships with ozone uptake. Environ. Exp. Bot. 2018, 145, 21–38. [Google Scholar] [CrossRef]
- Niederbacher, B.; Winkler, J.B.; Schnitzler, J.P. Volatile organic compounds as non-invasive markers for plant phenotyping. J. Exp. Bot. 2015, 66, 5403–5416. [Google Scholar] [CrossRef]
- Niinemets, Ü. Mild versus severe stress and BVOCs: Thresholds, priming and consequences. Trends Plant Sci. 2010, 15, 145–153. [Google Scholar] [CrossRef]
- Jud, W.; Vanzo, E.; Li, Z.R.; Ghirardo, A.; Zimmer, I.; Sharkey, T.D.; Hansel, A.; Schnitzler, J.P. Effects of heat and drought stress on post-illumination bursts of volatile organic compounds in isoprene-emitting and non-emitting poplar. Plant Cell Environ. 2016, 39, 1204–1215. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, F.; Jia, H.X.; Hu, J.J.; Feng, Z.Z. Molecular response of poplar to single and combined ozone and drought. Sci. Total Environ. 2019, 655, 1364–1375. [Google Scholar] [CrossRef] [PubMed]
- Buckley, T.N. How do stomata respond to water status? New Phytol. 2019, 224, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Hoshika, Y.; Omasa, K.; Paoletti, E. Both ozone exposure and soil water stress are able to induce stomatal sluggishness. Environ. Exp. Bot. 2013, 88, 19–23. [Google Scholar] [CrossRef]
- Otu-Larbi, F.; Conte, A.; Fares, S.; Wild, O.; Ashworth, K. Current and future impacts of drought and ozone stress on Northern Hemisphere forests. Glob. Chang. Biol. 2020, 26, 6218–6234. [Google Scholar] [CrossRef]
- Pääkkönen, E.; Vahala, J.; Pohjolai, M.; Holopainen, T.; Kärenlampi, L. Physiological, stomatal and ultrastructural ozone responses in birch (Betula pendula Roth.) are modified by water stress. Plant Cell Environ. 1998, 21, 671–684. [Google Scholar] [CrossRef]
- Kask, K.; Kännaste, A.; Talts, E.; Copolovici, L.; Niinemets, Ü. How specialized volatiles respond to chronic and short-term physiological and shock heat stress in Brassica nigra. Plant Cell Environ. 2016, 39, 2027–2042. [Google Scholar] [CrossRef]
- Veromann, E.; Toome, M.; Kännaste, A.; Kaasik, R.; Copolovici, L.; Flink, J.; Kovacs, G.; Narits, L.; Luik, A.; Niinemets, Ü. Effects of nitrogen fertilization on insect pests, their parasitoids, plant diseases and volatile organic compounds in Brassica napus. Crop Prot. 2013, 43, 79–88. [Google Scholar] [CrossRef]
- Kissen, R.; Rossiter, J.T.; Bones, A.M. The ‘mustard oil bomb’: Not so easy to assemble?! Localization, expression and distribution of the components of the myrosinase enzyme system. Phytochem. Rev. 2009, 8, 69–86. [Google Scholar] [CrossRef]
- Pang, Q.Y.; Chen, S.X.; Li, L.X.; Yan, X.F. Characterization of glucosinolate-myrosinase system in developing salt cress Thellungiella halophila. Physiol. Plant. 2009, 136, 1–9. [Google Scholar] [CrossRef]
- Mithen, R.F. Glucosinolates and their degradation products. Adv. Bot. Res. 2001, 35, 213–262. [Google Scholar] [CrossRef]
- Ponzio, C.; Papazian, S.; Albrectsen, B.R.; Dicke, M.; Gols, R. Dual herbivore attack and herbivore density affect metabolic profiles of Brassica nigra leaves. Plant Cell Environ. 2017, 40, 1356–1367. [Google Scholar] [CrossRef] [PubMed]
- Stam, J.M.; Chretien, L.; Dicke, M.; Poelman, E.H. Response of Brassica oleracea to temporal variation in attack by two herbivores affects preference and performance of a third herbivore. Ecol. Entomol. 2017, 42, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.J.; Gharibeshghi, A.; Mewis, I.; Forster, N.; Beck, W.; Ulrichs, C. Effect of different durations of moderate ozone exposure on secondary metabolites of Brassica campestris L. ssp. chinensis. J. Hortic. Sci. Biotechnol. 2021, 96, 110–120. [Google Scholar] [CrossRef]
- Himanen, S.J.; Nissinen, A.; Auriola, S.; Poppy, G.M.; Stewart, C.N., Jr.; Holopainen, J.K.; Nerg, A.-M. Constitutive and herbivore-inducible glucosinolate concentrations in oilseed rape (Brassica napus) leaves are not affected by Bt Cry1Ac insertion but change under elevated atmospheric CO2 and O3. Planta 2008, 227, 427–437. [Google Scholar] [CrossRef]
- Khaling, E.; Li, T.; Holopainen, J.K.; Blande, J.D. Elevated ozone modulates herbivore-induced volatile emissions of Brassica nigra and alters a tritrophic interaction. J. Chem. Ecol. 2016, 42, 368–381. [Google Scholar] [CrossRef]
- Saunier, A.; Blande, J.D. The effect of elevated ozone on floral chemistry of Brassicaceae species. Environ. Pollut. 2019, 255, 10. [Google Scholar] [CrossRef]
- Brosset, A.; Saunier, A.; Kivimäenpää, M.; Blande, J.D. Does ozone exposure affect herbivore-induced plant volatile emissions differently in wild and cultivated plants? Environ. Sci. Pollut. Res. 2020, 27, 30448–30459. [Google Scholar] [CrossRef]
- Dicke, M.; Baldwin, I.T. The evolutionary context for herbivore-induced plant volatiles: Beyond the ‘cry for help’. Trends Plant Sci. 2010, 15, 167–175. [Google Scholar] [CrossRef]
- Kessler, A.; Halitschke, R.; Poveda, K. Herbivory-mediated pollinator limitation: Negative impacts of induced volatiles on plant-pollinator interactions. Ecology 2011, 92, 1769–1780. [Google Scholar] [CrossRef]
- Fatouros, N.E.; Lucas-Barbosa, D.; Weldegergis, B.T.; Pashalidou, F.G.; van Loon, J.J.A.; Dicke, M.; Harvey, J.A.; Gols, R.; Huigens, M.E. Plant volatiles induced by herbivore egg deposition affect insects of different trophic levels. PLoS ONE 2012, 7, e43607. [Google Scholar] [CrossRef]
- Jeschke, V.; Kearney, E.E.; Schramm, K.; Kunert, G.; Shekhov, A.; Gershenzon, J.; Vassao, D.G. How glucosinolates affect generalist lepidopteran larvae: Growth, development and glucosinolate metabolism. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Giron-Calva, P.S.; Li, T.; Blande, J.D. Plant-plant interactions affect the susceptibility of plants to oviposition by pests but are disrupted by ozone pollution. Agric. Ecosyst. Environ. 2016, 233, 352–360. [Google Scholar] [CrossRef]
- Augustine, R.; Bisht, N.C. Regulation of glucosinolate metabolism: From model plant Arabidopsis thaliana to Brassica crops. Glucosinolates 2017, 163–199. [Google Scholar] [CrossRef]
- Bonnet, C.; Lassueur, S.; Ponzio, C.; Gols, R.; Dicke, M.; Reymond, P. Combined biotic stresses trigger similar transcriptomic responses but contrasting resistance against a chewing herbivore in Brassica nigra. BMC Plant Biol. 2017, 17. [Google Scholar] [CrossRef]
- Pineda, A.; Soler, R.; Pastor, V.; Li, Y.H.; Dicke, M. Plant-mediated species networks: The modulating role of herbivore density. Ecol. Entomol. 2017, 42, 449–457. [Google Scholar] [CrossRef]
- Douma, J.C.; Vermeulen, P.J.; Poelman, E.H.; Dicke, M.; Anten, N.P.R. When does it pay off to prime for defense? A modeling analysis. New Phytol. 2017, 216, 782–797. [Google Scholar] [CrossRef]
- Papazian, S.; Khaling, E.; Bonnet, C.; Lassueur, S.; Reymond, P.; Moritz, T.; Blande, J.D.; Albrectsen, B.R. Central metabolic responses to ozone and herbivory affect photosynthesis and stomatal closure. Plant Physiol. 2016, 172, 2057–2078. [Google Scholar] [CrossRef]
- Hetherington, A.M.; Woodward, F.I. The role of stomata in sensing and driving environmental change. Nature 2003, 424, 901–908. [Google Scholar] [CrossRef]
- Vahisalu, T.; Puzõrjova, I.; Brosché, M.; Valk, E.; Lepiku, M.; Moldau, H.; Pechter, P.; Wang, Y.S.; Lindgren, O.; Salojärvi, J.; et al. Ozone-triggered rapid stomatal response involves the production of reactive oxygen species, and is controlled by SLAC1 and OST1. Plant J. 2010, 62, 442–453. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Reichstein, M. Controls on the emission of plant volatiles through stomata: Differential sensitivity of emission rates to stomatal closure explained. J. Geophys. Res. Atmos. 2003, 108. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Escalona, J.M.; Sampol, B.; Medrano, H. Effects of drought on photosynthesis in grapevines under field conditions: An evaluation of stomatal and mesophyll limitations. Funct. Plant Biol. 2002, 29, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Correia, B.; Hancock, R.D.; Amaral, J.; Gomez-Cadenas, A.; Valledor, L.; Pinto, G. Combined drought and heat activates protective responses in Eucalyptus globulus that are not activated when subjected to drought or heat stress alone. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Moldau, H.; Sõber, J.; Sõber, A. Impact of acute ozone exposure on CO2 uptake by two cultivars of Phaseolus vulgaris. Photosynthetica 1993, 28, 133–141. [Google Scholar]
- Kull, O.; Moldau, H. Absorption of ozone on Betula pendula Roth leaf surface. Water Air Soil Pollut. 1994, 75, 79–86. [Google Scholar] [CrossRef]
- Moldau, H.; Bichele, I. Plasmalemma protection by the apoplast as assessed from above-zero ozone concentrations in leaf intercellular air spaces. Planta 2002, 214, 484–487. [Google Scholar] [CrossRef]
- Hassan, I.A.; Ashmore, M.R.; Bell, J.N.B. Effects of O3 on the stomatal behaviour of Egyptian varieties of radish (Raphanus sativus L. cv. Baladey) and turnip (Brassica rapa L. cv. Sultani). New Phytol. 1994, 128, 243–249. [Google Scholar] [CrossRef]
- Bergmann, E.; Bender, J.; Weigel, H.J. Impact of tropospheric ozone on terrestrial biodiversity: A literature analysis to identify ozone sensitive taxa. J. Appl. Bot. Food Qual. 2017, 90. [Google Scholar] [CrossRef]
- Tardieu, F.; Parent, B.; Simonneau, T. Control of leaf growth by abscisic acid: Hydraulic or non-hydraulic processes? Plant Cell Environ. 2010, 33, 636–647. [Google Scholar] [CrossRef]
- Wilkinson, S.; Davies, W.J. Ozone suppresses soil drying- and abscisic acid (ABA)-induced stomatal closure via an ethylene-dependent mechanism. Plant Cell Environ. 2009, 32, 949–959. [Google Scholar] [CrossRef]
- Hoshika, Y.; Katata, G.; Deushi, M.; Watanabe, M.; Koike, T.; Paoletti, E. Ozone-induced stomatal sluggishness changes carbon and water balance of temperate deciduous forests. Sci. Rep. 2015, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Alonso, R.; Elvira, S.; González-Fernández, I.; Calvete, H.; García-Gómez, H.; Bermejo, V. Drought stress does not protect Quercus ilex L. from ozone effects: Results from a comparative study of two subspecies differing in ozone sensitivity. Plant Biol. 2014, 16, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Tingey, D.T.; Hogsett, W.E. Water stress reduces ozone injury via a stomatal mechanism. Plant Physiol. 1985, 77, 944–947. [Google Scholar] [CrossRef]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Sconiers, W.B.; Rowland, D.L.; Eubanks, M.D. Pulsed drought: The effects of varying water stress on plant physiology and predicting herbivore response. Crop Sci. 2020, 60, 2543–2561. [Google Scholar] [CrossRef]
- Guidi, L.; Tonini, M.; Soldatini, G.F. Effects of high light and ozone fumigation on photosynthesis in Phaseolus vulgaris. Plant Physiol. Biochem. 2000, 38, 717–725. [Google Scholar] [CrossRef]
- Lombardozzi, D.; Sparks, J.P.; Bonan, G.; Levis, S. Ozone exposure causes a decoupling of conductance and photosynthesis: Implications for the Ball-Berry stomatal conductance model. Oecologia 2012, 169, 651–659. [Google Scholar] [CrossRef]
- Kanagendran, A.; Pazouki, L.; Li, S.; Liu, B.; Kännaste, A.; Niinemets, Ü. Ozone-triggered surface uptake and stress volatile emissions in Nicotiana tabacum ‘Wisconsin’. J. Exp. Bot. 2018, 69, 681–697. [Google Scholar] [CrossRef]
- Zhang, W.W.; Feng, Z.Z.; Wang, X.K.; Niu, J.F. Impacts of elevated ozone on growth and photosynthesis of Metasequoia glyptostroboides Hu et Cheng. Plant Sci. 2014, 226, 182–188. [Google Scholar] [CrossRef]
- Singh, E.; Tiwari, S.; Agrawal, M. Effects of elevated ozone on photosynthesis and stomatal conductance of two soybean varieties: A case study to assess impacts of one component of predicted global climate change. Plant Biol. 2009, 11, 101–108. [Google Scholar] [CrossRef]
- Paoletti, E.; Grulke, N.E. Does living in elevated CO2 ameliorate tree response to ozone? A review on stomatal responses. Environ. Pollut. 2005, 137, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.S.P. Response of plants to water stress. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.H.; Shen, Y.; Niinemets, Ü. Responses of isoprene emission and photochemical efficiency to severe drought combined with prolonged hot weather in hybrid Populus. J. Exp. Bot. 2020, 71, 7364–7381. [Google Scholar] [CrossRef]
- Mewis, I.; Khan, M.A.M.; Glawischnig, E.; Schreiner, M.; Ulrichs, C. Water stress and aphid feeding differentially influence metabolite composition in Arabidopsis thaliana (L.). PLoS ONE 2012, 7, e0048661. [Google Scholar] [CrossRef] [PubMed]
- Weldegergis, B.T.; Zhu, F.; Poelman, E.H.; Dicke, M. Drought stress affects plant metabolites and herbivore preference but not host location by its parasitoids. Oecologia 2018, 187, 873–874. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, Ü.; Arneth, A.; Kuhn, U.; Monson, R.K.; Peñuelas, J.; Staudt, M. The emission factor of volatile isoprenoids: Stress, acclimation, and developmental responses. Biogeosciences 2010, 7, 2203–2223. [Google Scholar] [CrossRef]
- Khaling, E.; Papazian, S.; Poelman, E.H.; Holopainen, J.K.; Albrectsen, B.R.; Blande, J.D. Ozone affects growth and development of Pieris brassicae on the wild host plant Brassica nigra. Environ. Pollut. 2015, 199, 119–129. [Google Scholar] [CrossRef]
- Bailey, A.; Burkey, K.; Taggart, M.; Rufty, T. Leaf traits that contribute to differential ozone response in ozone-tolerant and sensitive soybean genotypes. Plants 2019, 8, 235. [Google Scholar] [CrossRef]
- Li, S.; Tosens, T.; Harley, P.C.; Jiang, Y.F.; Kanagendran, A.; Grosberg, M.; Jaamets, K.; Niinemets, Ü. Glandular trichomes as a barrier against atmospheric oxidative stress: Relationships with ozone uptake, leaf damage, and emission of LOX products across a diverse set of species. Plant Cell Environ. 2018, 41, 1263–1277. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Loughrin, J.H.; McCall, P.J.; Rose, U.S.R.; Lewis, W.J.; Tumlinson, J.H. How caterpillar-damaged plants protect themselves by attracting parasitic wasps. Proc. Natl. Acad. Sci. USA 1995, 92, 4169–4174. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.; Beck, J.J. Duration of emission of volatile organic compounds from mechanically damaged plant leaves. J. Plant Physiol. 2015, 188, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Turan, S.; Kask, K.; Kanagendran, A.; Li, S.; Anni, R.; Talts, E.; Rasulov, B.; Kännaste, A.; Niinemets, Ü. Lethal heat stress-dependent volatile emissions from tobacco leaves: What happens beyond the thermal edge? J. Exp. Bot. 2019, 70, 5017–5030. [Google Scholar] [CrossRef]
- Portillo-Estrada, M.; Kazantsev, T.; Talts, E.; Tosens, T.; Niinemets, Ü. Emission timetable and quantitative patterns of wound-induced volatiles across different leaf damage treatments in aspen (Populus tremula). J. Chem. Ecol. 2015, 41, 1105–1117. [Google Scholar] [CrossRef] [PubMed]
- Brilli, F.; Ruuskanen, T.M.; Schnitzhofer, R.; Muller, M.; Breitenlechner, M.; Bittner, V.; Wohlfahrt, G.; Loreto, F.; Hansel, A. Detection of plant volatiles after leaf wounding and darkening by proton transfer reaction “time-of-flight” mass spectrometry (PTR-TOF). PLoS ONE 2011, 6. [Google Scholar] [CrossRef]
- Jiang, Y.F.; Ye, J.Y.; Li, S.; Niinemets, Ü. Methyl jasmonate-induced emission of biogenic volatiles is biphasic in cucumber: A high-resolution analysis of dose dependence. J. Exp. Bot. 2017, 68, 4679–4694. [Google Scholar] [CrossRef]
- Hu, Z.-H.; Leng, P.-S.; Shen, Y.-B.; Wang, W.-H. Emissions of saturated C6-C10 aldehydes from poplar (Populus simonii × P. pyramidalis ‘Opera 8277’) cuttings at different levels of light intensity. J. For. Res. 2011, 22, 233–238. [Google Scholar] [CrossRef]
- Shen, J.; Tieman, D.; Jones, J.B.; Taylor, M.G.; Schmelz, E.; Huffaker, A.; Bies, D.; Chen, K.; Klee, H.J. A 13-lipoxygenase, TomloxC, is essential for synthesis of C5 flavour volatiles in tomato. J. Exp. Bot. 2014, 65, 419–428. [Google Scholar] [CrossRef]
- Heiden, A.C.; Kobel, K.; Langebartels, C.; Schuh-Thomas, G.; Wildt, J. Emissions of oxygenated volatile organic compounds from plants—Part I: Emissions from lipoxygenase activity. J. Atmos. Chem. 2003, 45, 143–172. [Google Scholar] [CrossRef]
- Adams, A.; Bouckaert, C.; Van Lancker, F.; De Meulenaer, B.; De Kimpe, N. Amino acid catalysis of 2-alkylfuran formation from lipid oxidation-derived α,β-unsaturated aldehydes. J. Agric. Food Chem. 2011, 59, 11058–11062. [Google Scholar] [CrossRef]
- Redovniković, I.R.; Glivetić, T.; Delonga, K.; Vorkapić-Furač, J. Glucosinolates and their potential role in plant. Period. Biol. 2008, 110, 297–309. [Google Scholar]
- Miękus, N.; Marszałek, K.; Podlacha, M.; Iqbal, A.; Puchalski, C.; Świergiel, A.H. Health benefits of plant-derived sulfur compounds, glucosinolates, and organosulfur compounds. Molecules 2020, 25, 3804. [Google Scholar] [CrossRef] [PubMed]
- Tulio, A.Z.; Yamanaka, H.; Ueda, Y.; Imahori, Y. Formation of methanethiol and dimethyl disulfide in crushed tissues of broccoli florets and their inhibition by freeze-thawing. J. Agric. Food Chem. 2002, 50, 1502–1507. [Google Scholar] [CrossRef]
- Dan, K.; Nagata, M.; Yamashita, I. Mechanism of off-flavor production in Brassica vegetables under anaerobic conditions. JARQ-Jpn. Agric. Res. Q. 1999, 33, 109–114. [Google Scholar]
- Ratzka, A.; Vogel, H.; Kliebenstein, D.J.; Mitchell-Olds, T.; Kroymann, J. Disarming the mustard oil bomb. Proc. Natl. Acad. Sci. USA 2002, 99, 11223–11228. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E. Sites of synthesis, biochemistry and functional role of plant volatiles. S. Afr. J. Bot. 2010, 76, 612–631. [Google Scholar] [CrossRef]
- Staudt, M.; Lhoutellier, L. Monoterpene and sesquiterpene emissions from Quercus coccifera exhibit interacting responses to light and temperature. Biogeosciences 2011, 8, 2757–2771. [Google Scholar] [CrossRef]
- Loreto, F.; Pinelli, P.; Manes, F.; Kollist, H. Impact of ozone on monoterpene emissions and evidence for an isoprene-like antioxidant action of monoterpenes emitted by Quercus ilex leaves. Tree Physiol. 2004, 24, 361–367. [Google Scholar] [CrossRef]
- Loreto, F.; Förster, A.; Durr, M.; Csiky, O.; Seufert, G. On the monoterpene emission under heat stress and on the increased thermotolerance of leaves of Quercus ilex L. fumigated with selected monoterpenes. Plant Cell Environ. 1998, 21, 101–107. [Google Scholar] [CrossRef]
- Acton, W.J.F.; Jud, W.; Ghirardo, A.; Wohlfahrt, G.; Hewitt, C.N.; Taylor, J.E.; Hansel, A. The effect of ozone fumigation on the biogenic volatile organic compounds (BVOCs) emitted from Brassica napus above- and below-ground. PLoS ONE 2018, 13, 19. [Google Scholar] [CrossRef]
- Cui, H.Y.; Wei, J.I.; Su, J.W.; Li, C.Y.; Ge, F. Elevated O3 increases volatile organic compounds via jasmonic acid pathway that promote the preference of parasitoid Encarsia formosa for tomato plants. Plant Sci. 2016, 253, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Nogués, I.; Brilli, F.; Loreto, F. Dimethylallyl diphosphate and geranyl diphosphate pools of plant species characterized by different isoprenoid emissions. Plant Physiol. 2006, 141, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Fruekilde, P.; Hjorth, J.; Jensen, N.R.; Kotzias, D.; Larsen, B. Ozonolysis at vegetation surfaces: A source of acetone, 4-oxopentanal, 6-methyl-5-hepten-2-one, and geranyl acetone in the troposphere. Atmos. Environ. 1998, 32, 1893–1902. [Google Scholar] [CrossRef]
- Pellegrini, E.; Cioni, P.L.; Francini, A.; Lorenzini, G.; Nali, C.; Flamini, G. Volatiles emission patterns in Poplar clones clones varying in response to ozone. J. Chem. Ecol. 2012, 38, 924–932. [Google Scholar] [CrossRef]
- Simkin, A.J.; Schwartz, S.H.; Auldridge, M.; Taylor, M.G.; Klee, H.J. The tomato carotenoid cleavage dioxygenase 1 genes contribute to the formation of the flavor volatiles beta-ionone, pseudoionone, and geranylacetone. Plant J. 2004, 40, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.H.; Strack, D. Carotenoids and their cleavage products: Biosynthesis and functions. Nat. Prod. Rep. 2011, 28, 663–692. [Google Scholar] [CrossRef] [PubMed]
- Beisel, K.G.; Jahnke, S.; Hofmann, D.; Koppchen, S.; Schurr, U.; Matsubara, S. Continuous turnover of carotenes and chlorophyll a in mature leaves of Arabidopsis revealed by 14CO2 pulse-chase labeling. Plant Physiol. 2010, 152, 2188–2199. [Google Scholar] [CrossRef] [PubMed]
- Tieman, D.M.; Zeigler, M.; Schmelz, E.A.; Taylor, M.G.; Bliss, P.; Kirst, M.; Klee, H.J. Identification of loci affecting flavour volatile emissions in tomato fruits. J. Exp. Bot. 2006, 57, 887–896. [Google Scholar] [CrossRef]
- García-Plazaola, J.I.; Portillo-Estrada, M.; Fernandez-Marin, B.; Kännaste, A.; Niinemets, Ü. Emissions of carotenoid cleavage products upon heat shock and mechanical wounding from a foliose lichen. Environ. Exp. Bot. 2017, 133, 87–97. [Google Scholar] [CrossRef]
- Desikan, R.; Mackerness, S.A.H.; Hancock, J.T.; Neill, S.J. Regulation of the Arabidopsis transcriptome by oxidative stress. Plant Physiol. 2001, 127, 159–172. [Google Scholar] [CrossRef]
- Kaiser, H. The relation between stomatal aperture and gas exchange under consideration of pore geometry and diffusional resistance in the mesophyll. Plant Cell Environ. 2009, 32, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Mano, J.; Biswas, M.S.; Sugimoto, K. Reactive carbonyl species: A missing link in ROS signaling. Plants 2019, 8, 391. [Google Scholar] [CrossRef] [PubMed]
- Daszkowska-Golec, A.; Szarejko, I. Open or close the gate—Stomata action under the control of phytohormones in drought stress conditions. Front. Plant Sci. 2013, 4, 16. [Google Scholar] [CrossRef]
- Bruinsma, M.; Lucas-Barbosa, D.; ten Broeke, C.J.M.; van Dam, N.M.; van Beek, T.A.; Dicke, M.; van Loon, J.J.A. Folivory affects composition of nectar, floral odor and modifies pollinator behavior. J. Chem. Ecol. 2014, 40, 39–49. [Google Scholar] [CrossRef]
- Wildt, J.; Kobel, K.; Schuh-Thomas, G.; Heiden, A.C. Emissions of oxygenated volatile organic compounds from plants—Part II: Emissions of saturated aldehydes. J. Atmos. Chem. 2003, 45, 173–196. [Google Scholar] [CrossRef]
- Giron-Calva, P.S.; Li, T.; Blande, J.D. Volatile-mediated interactions between cabbage plants in the field and the impact of ozone pollution. J. Chem. Ecol. 2017, 43, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Copolovici, L.; Niinemets, Ü. Flooding induced emissions of volatile signalling compounds in three tree species with differing waterlogging tolerance. Plant Cell Environ. 2010, 33, 1582–1594. [Google Scholar] [CrossRef] [PubMed]
- Von Caemmerer, S.; Farquhar, G.D. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 1981, 153, 376–387. [Google Scholar] [CrossRef]
- Jardine, K.J.; Monson, R.K.; Abrell, L.; Saleska, S.R.; Arneth, A.; Jardine, A.; Ishida, F.Y.; Yanez Serrano, A.M.; Artaxo, P.; Karl, T.; et al. Within-plant isoprene oxidation confirmed by direct emissions of oxidation products methyl vinyl ketone and methacrolein. Glob. Chang. Biol. 2012, 18, 973–984. [Google Scholar] [CrossRef]
- Kännaste, A.; Copolovici, L.; Niinemets, Ü. Gas chromatography-mass spectrometry method for determination of biogenic volatile organic compounds emitted by plants. In Plant Isoprenoids: Methods and Protocols; Rodriguez Concepcion, M., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2014; Volume 1153, pp. 161–169. [Google Scholar]
- Niinemets, Ü.; Kuhn, U.; Harley, P.C.; Staudt, M.; Arneth, A.; Cescatti, A.; Ciccioli, P.; Copolovici, L.; Geron, C.; Guenther, A.; et al. Estimations of isoprenoid emission capacity from enclosure studies: Measurements, data processing, quality and standardized measurement protocols. Biogeosciences 2011, 8, 2209–2246. [Google Scholar] [CrossRef]
- Wold, S.; Esbensen, K.; Geladi, P. Principal component analysis. Chemom. Intell. Lab. Syst. 1987, 2, 37–52. [Google Scholar] [CrossRef]
- Niinemets, Ü. Leaf trait plasticity and evolution in different plant functional types. Annu. Plant Rev. 2020, 3, 473–522. [Google Scholar] [CrossRef]
- Guidi, L.; Nali, C.; Lorenzini, G.; Filippi, F.; Soldatini, G.F. Effect of chronic ozone fumigation on the photosynthetic process of poplar clones showing different sensitivity. Environ. Pollut. 2001, 113, 245–254. [Google Scholar] [CrossRef]
- Mills, G.; Hayes, F.; Wilkinson, S.; Davies, W.J. Chronic exposure to increasing background ozone impairs stomatal functioning in grassland species. Glob. Chang. Biol. 2009, 15, 1522–1533. [Google Scholar] [CrossRef]


| Uptake Rate | WW/250 ppb O3 | WW/550 ppb O3 | WS/550 ppb O3 | p Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | Time | Time | ||||||||||
| ~5 min | 0.5 h | 1 h | ~5 min | 0.5 h | 1 h | ~5 min | 0.5 h | 1 h | O3 | Time | O3 x Time | |
| Surface O3 uptake rate (nmol m−2 s−1) | 15.6 ± 2.4 a | 12.1 ± 2.0 b | 11.5 ± 2.2 b | 43.1 ± 4.0 a | 35.8 ± 4.1 a | 44 ± 8 a | 26 ± 9 a | 25 ± 9 a | 25 ± 9 a | <0.01 | <0.05 | ns |
| Stomatal O3 uptake rate (nmol m−2 s−1) | 13.1 ± 2.8 a | 16.1 ± 3.1 b | 16.8 ± 3.5 b | 54 ± 11 a | 60 ± 12 a | 44 ± 5 a | 12 ± 2 a | 13.3 ± 1.7 a | 12.4 ± 1.4 a | 0.001 | ns | <0.05 |
| Surface O3 uptake rate/Whole leaf uptake rate (%) | 56 ± 8 a | 45 ± 8 b | 43 ± 10 b | 50.5 ± 2.8 a | 43 ± 6 a | 50 ± 7 a | 66 ± 5 a | 61 ± 7 a | 63 ± 8 a | ns | 0.01 | ns |
| Stomatal O3 uptake rate/Whole leaf uptake rate (%) | 44 ± 8 a | 55 ± 8 b | 57 ± 10 b | 49.5 ± 2.8 a | 57 ± 6 a | 50 ± 7 a | 34 ± 5 a | 39 ± 7 a | 37 ± 8 a | ns | <0.05 | <0.05 |
| WW/250 ppb O3 | WW/550 ppb O3 | WS/550 ppb O3 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | Control | 10 min | 1.5 h | 4.5 h | 22 h | Control | 10 min | 1.5 h | 4.5 h | 22 h | Control | 10 min | 1.5 h | 4.5 h | 22 h | |
| LOX pathway | ||||||||||||||||
| 1 | 2-Ethylfuran | 0.015 ± 0.013 | 0.018 * | 0.008 ± 0.007 a | 0.02 ± 0.01 a | |||||||||||
| 2 | (E, E)-2,4-Hexadienal | 0.024 | ||||||||||||||
| 3 | Hexanal | 0.0090 ± 0.0028 a | 0.018 ± 0.005 a | 0.0070 ± 0.0018 a | 0.0098 ± 0.0030 a | 0.015 ± 0.005 a | 0.0030 ± 0.0012 a | 0.010 ± 0.004 b | 0.018 ± 0.007 c | 0.0064 ± 0.0020 a | 0.0128 ± 0.0007 a | 0.011 ± 0.008 a | 0.0028 ± 0.0005 a | 0.0017 ± 0.0004 a | 0.0086 ± 0.0034 a | 0.00070 ± 0.00050 a |
| 4 | 1-Hexanol | 0.0014 | 0.018 | 0.0022 ± 0.0003 | 0.0015 | 0.0036 ± 0.0030 | ||||||||||
| 5 | (E)-3-hexen-1-ol | 0.180 | ||||||||||||||
| 6 | (Z)-3-hexen-1-ol | 0.010 | 0.03 ± 0.01 | 0.002 | 0.0197 | 0.027 ± 0.025 | ||||||||||
| 7 | 2-Methyl-2-cyclopenten-1-one | 0.012 | ||||||||||||||
| 8 | Pentanal | 0.0068 ± 0.0014 a | 0.010 ± 0.002 a | 0.0039 ± 0.0013 a | 0.0070 ± 0.0024 a | 0.0103 ± 0.0017 a | 0.0062 ± 0.0015 a | 0.0063 ± 0.0013 a | 0.0042± 0.002 a | 0.0026 ± 0.0010 a | 0.0070 ± 0.0026 a | 0.0048 ± 0.0026 a | 0.0023 ± 0.0005 a | 0.0026 ± 0.0007 a | 0.0018 ± 0.0010 a | 0.00119 ± 0.00003 a |
| 9 | 1-Penten-3-ol | 0.034 | 0.022 | |||||||||||||
| 10 | (Z)-2-Penten-1-ol | 0.053 | ||||||||||||||
| 11 | 1-Penten-3-one | 0.016 | ||||||||||||||
| Glucosinolate degradation products | ||||||||||||||||
| 12 | Cyclohexyl isocyanate | 0.0087 ± 0.0029 a | 0.053 | 0.0008 | 0.070 ± 0.066 a | 0.0176 ± 0.0032 a | 0.0073 ± 0.0040 a | 0.030 ± 0.020 a | 0.034 ± 0.030 a | 0.0083 ± 0.0050 a | 0.0097 ± 0.0010 a | 0.012 ± 0.007 a | 0.0015 ± 0.0004 a | 0.0011 ± 0.0003 a | 0.0050 ± 0.0027 a | 0.16 |
| 13 | Cyclohexyl isothiocyanate | 0.0017 ± 0.0011 a | 0.005 ± 0.004 a | 0.00005 | 0.006 ± 0.005 a | 0.00121± 0.00040 a | 0.003 | 0.0062 ± 0.0040 a | 0.0031± 0.0029 a | 0.009 | 0.00305 ± 0.00006 a | 0.0017 ± 0.0006 a | 0.00111 ± 0.00020 a | 0.0019 ± 0.0005 a | 0.0015 ± 0.0005 a | 0.09 |
| 14 | Dimethyl disulfide | 0.004 | 0.0031 ± 0.0010 a | 0.0017 ± 0.0012 a | 0.0007 | 0.003 | 0.011 | 0.0004 | 0.00023 ± 0.00007 a | |||||||
| 15 | Methanethiol | 0.0056 ± 0.0034 a | 0.003 | 0.016 ± 0.013 a | 0.0080 | 0.013 | 0.0011 | 0.0003 | 0.003 | |||||||
| 16 | 2-Propenenitrile | 0.09 ± 0.08 a | 0.036 ± 0.018 a | 0.18 ± 0.16 a | 0.043 ± 0.020 a | 0.04 | 0.24 ± 0.17 a | 0.18 ± 0.15 a | 0.084 ± 0.040 a | 0.09 ± 0.05 a | 0.053 ± 0.020 a | 0.08 ± 0.06 a | 0.22 ± 0.21 a | 0.14 ± 0.09 a | 0.22 ± 0.21 a | |
| 17 | Methyl isothiocyanate | 0.0039 | 0.006 | |||||||||||||
| 18 | Tetramethylthiourea | 0.0007 | 0.008 ± 0.007 | 0.0005 | 0.0041 | 0.031 ± 0.030 a | 0.011 | 0.0036 ± 0.0030 a | 0.0019 ± 0.0007 a | 0.0014 ± 0.0009 a | 0.009 | |||||
| 19 | Tetramethylurea | 0.0020 ± 0.0016 a | 0.042 ± 0.035 a | 0.0035 ± 0.0020 a | 0.0046 ± 0.0040 a | 0.004 | 0.0057 ± 0.0050 a | 0.002 ± 0.001 a | 0.027 | 0.014 ± 0.007 a | 0.0032 ± 0.0020 a | 0.002 ± 0.001 a | 0.0031 ± 0.0004 a | 0.0040 ± 0.0022 a | 0.025 | |
| GDP pathway | ||||||||||||||||
| 20 | Camphene | 0.0017 ± 0.0008 a | 0.0006± 0.0002 a | 0.0007 ± 0.0002 a | 0.0005 ± 0.0001 a | 0.0015 ± 0.0008 a | 0.0013 ± 0.0006 a | 0.0017 ± 0.0009 a | 0.00067 ± 0.00018 a | 0.014 ± 0.013 a | 0.00141± 0.00015 a | 0.00044 ± 0.00009 a | 0.00041 ± 0.00013 a | 0.00020 ± 0.00002 a | 0.00032 ± 0.00015 a | 0.00057 ± 0.00031 a |
| 21 | 3-Carene | 0.013 ± 0.006 a | 0.014 ± 0.003 a | 0.012 ± 0.002 a | 0.009 ± 0.003 a | 0.019 ± 0.003 a | 0.005 ± 0.003 a | 0.014 ± 0.006 a | 0.014 ± 0.006 a | 0.0147 ± 0.0027 a | 0.025 ± 0.00 9 b | 0.004 ± 0.002 a | 0.0017 ± 0.0009 a | 0.0025 ± 0.0010 a | 0.0062 ± 0.0027 a | 0.0049 ± 0.0032 a |
| 22 | Limonene | 0.013 ± 0.008 a | 0.005 ± 0.001 a | 0.005 ± 0.001 a | 0.0019 ± 0.0006 a | 0.0040 ± 0.0005 a | 0.003 | 0.003 ± 0.002 a | 0.004 ± 0.002 a | 0.0021 ± 0.0004 a | 0.004 ± 0.002 a | 0.0023 ± 0.0016 a | 0.0006 ± 0.0001 a | 0.0005 ± 0.0002 a | 0.002 ± 0.001 a | 0.0013 |
| 23 | α-Pinene | 0.025 ± 0.006 a | 0.019 ± 0.004 a | 0.017 ± 0.003 a | 0.015 ± 0.003 b | 0.022 ± 0.004 a | 0.0080 ± 0.0001 a | 0.014 ± 0.006 a | 0.012 ± 0.007 a | 0.016 ± 0.005 a | 0.04 ± 0.01 a | 0.009 ± 0.004 a | 0.005 ± 0.001 a | 0.005 ± 0.003 a | 0.012 ± 0.002 a | 0.012 ± 0.007 a |
| 24 | β-Pinene | 0.0012 ± 0.0002 a | 0.0012± 0.0004 a | 0.0007 ± 0.0003 a | 0.0005 ± 0.0002 a | 0.0008 ± 0.0001 a | 0.0004 | 0.0005 | 0.002 | 0.00076 ± 0.00003 a | 0.0021 ± 0.0007 a | 0.0021 ± 0.0013 a | 0.00028 ± 0.00002 a | 0.00049 ± 0.00001 a | 0.0006 ± 0.0001 a | 0.0006 ± 0.0003 a |
| GGDP pathway | ||||||||||||||||
| 25 | Geranyl acetone | 0.0108 ± 0.0039 a | 0.036 ± 0.018 a | 0.0090 ± 0.0034 a | 0.013 ± 0.005 a | 0.015 ± 0.006 a | 0.030 ± 0.006 a | 0.042 ± 0.027 a | 0.049 ± 0.020 a | 0.058 ± 0.050 a | 0.049 ± 0.008 a | 0.010 ± 0.007 a | 0.0071 ± 0.0048 a | 0.0029 ± 0.0016 a | 0.013 ± 0.003 a | 0.0063 ± 0.0056 a |
| 26 | 6-Methyl-5-hepten-2-one | 0.0034 ± 0.0015 a | 0.062 ± 0.047 a | 0.010 ± 0.005 a | 0.0077 ± 0.0031 a | 0.014 ± 0.008 a | 0.0031 ± 0.0015 a | 0.013 ± 0.010 a | 0.055 ± 0.027 a | 0.0077 ± 0.0050 a | 0.0057 ± 0.0014 a | 0.0087 ± 0.0081 a | 0.0042 ± 0.0033 a | 0.0037 ± 0.0022 a | 0.020 ± 0.008 a | 0.00033 ± 0.00011 a |
| Saturated aldehydes | ||||||||||||||||
| 27 | Decanal | 0.0132 ± 0.0030 a | 0.077 ± 0.030 b | 0.026 ± 0.007 a | 0.018 ± 0.006 a | 0.030 ± 0.006 a | 0.060 ± 0.044 a | 0.030 ± 0.016 a | 0.071 ± 0.012 a | 0.029 ± 0.016 a | 0.046 ± 0.017 a | 0.035 ± 0.016 a | 0.0190 ± 0.0070 a | 0.013 ± 0.002 a | 0.050 ± 0.027 a | 0.0029 ± 0.0008 a |
| 28 | Heptanal | 0.0072 ± 0.0011 a | 0.010 ± 0.003 b | 0.0042 ± 0.0010 a | 0.0053 ± 0.0011 a | 0.0071 ± 0.0020 a | 0.0055 ± 0.0020 a | 0.0040 ± 0.0003 a | 0.014 ± 0.005 a | 0.0072 ± 0.0020 a | 0.0078 ± 0.0002 a | 0.004 ± 0.001 a | 0.003 ± 0.001 a | 0.0019 ± 0.0003 a | 0.0083 ± 0.0006 b | 0.0014 ± 0.0001 a |
| 29 | Nonanal | 0.0182 ± 0.0043 a | 0.063 ± 0.020 b | 0.025 ± 0.007 a | 0.014 ± 0.005 a | 0.022 ± 0.007 a | 0.035 ± 0.020 a | 0.026 ± 0.008 a | 0.074 ± 0.020 a | 0.026 ± 0.009 a | 0.033 ± 0.018 a | 0.019 ± 0.007 a | 0.011 ± 0.004 a | 0.010 ± 0.002 a | 0.050 ± 0.017 a | 0.0026 ± 0.0003 a |
| 30 | Octanal | 0.0086 ± 0.0010 a | 0.022 ± 0.007 b | 0.011 ± 0.003 a | 0.006 ± 0.002 a | 0.012 ± 0.002 a | 0.013 ± 0.006 a | 0.0076 ± 0.0020 a | 0.033 ± 0.012 a | 0.0068 ± 0.0022 a | 0.0090 ± 0.0020 a | 0.012 ± 0.006 a | 0.004 ± 0.001 a | 0.0040 ± 0.0012 a | 0.021 ± 0.005 a | 0.0013 ± 0.0003 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kask, K.; Kaurilind, E.; Talts, E.; Kännaste, A.; Niinemets, Ü. Combined Acute Ozone and Water Stress Alters the Quantitative Relationships between O3 Uptake, Photosynthetic Characteristics and Volatile Emissions in Brassica nigra. Molecules 2021, 26, 3114. https://doi.org/10.3390/molecules26113114
Kask K, Kaurilind E, Talts E, Kännaste A, Niinemets Ü. Combined Acute Ozone and Water Stress Alters the Quantitative Relationships between O3 Uptake, Photosynthetic Characteristics and Volatile Emissions in Brassica nigra. Molecules. 2021; 26(11):3114. https://doi.org/10.3390/molecules26113114
Chicago/Turabian StyleKask, Kaia, Eve Kaurilind, Eero Talts, Astrid Kännaste, and Ülo Niinemets. 2021. "Combined Acute Ozone and Water Stress Alters the Quantitative Relationships between O3 Uptake, Photosynthetic Characteristics and Volatile Emissions in Brassica nigra" Molecules 26, no. 11: 3114. https://doi.org/10.3390/molecules26113114
APA StyleKask, K., Kaurilind, E., Talts, E., Kännaste, A., & Niinemets, Ü. (2021). Combined Acute Ozone and Water Stress Alters the Quantitative Relationships between O3 Uptake, Photosynthetic Characteristics and Volatile Emissions in Brassica nigra. Molecules, 26(11), 3114. https://doi.org/10.3390/molecules26113114







