Chemical Profile, Cytotoxic Activity and Oxidative Stress Reduction of Different Syringa vulgaris L. Extracts
Abstract
1. Introduction
2. Results and Discussion
2.1. HPLC-DAD-ESI+ Analysis
2.2. Vibrational IR Spectra of Syringa vulgaris L., Bark and Fruit Extracts
2.3. Quantification of Total Polyphenolic (TPC), Flavonoids (TFC) and Phenolic Acids Content (TPA)
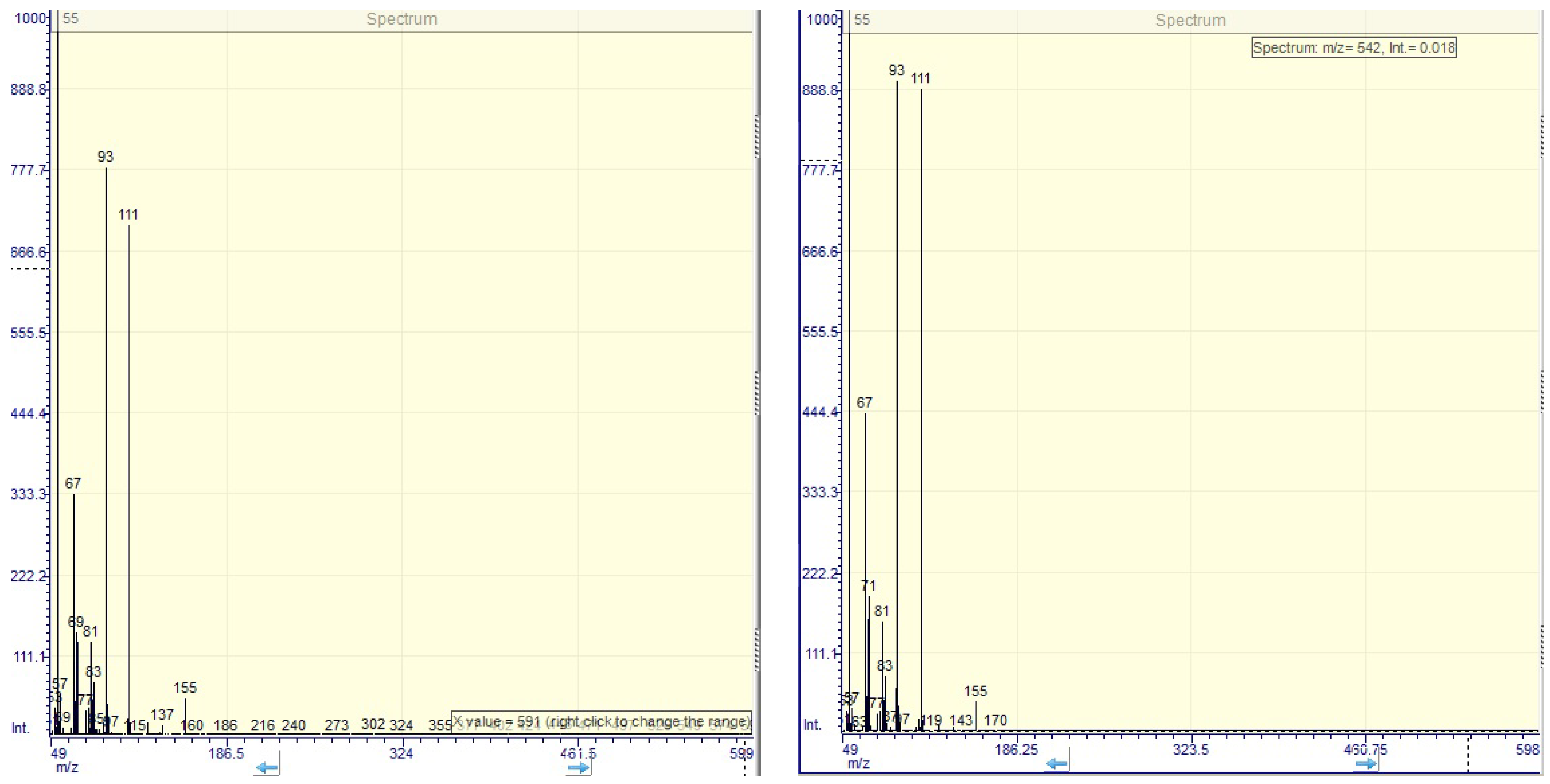
2.4. GC-MS Analysis
2.5. Antioxidant Activity Assays
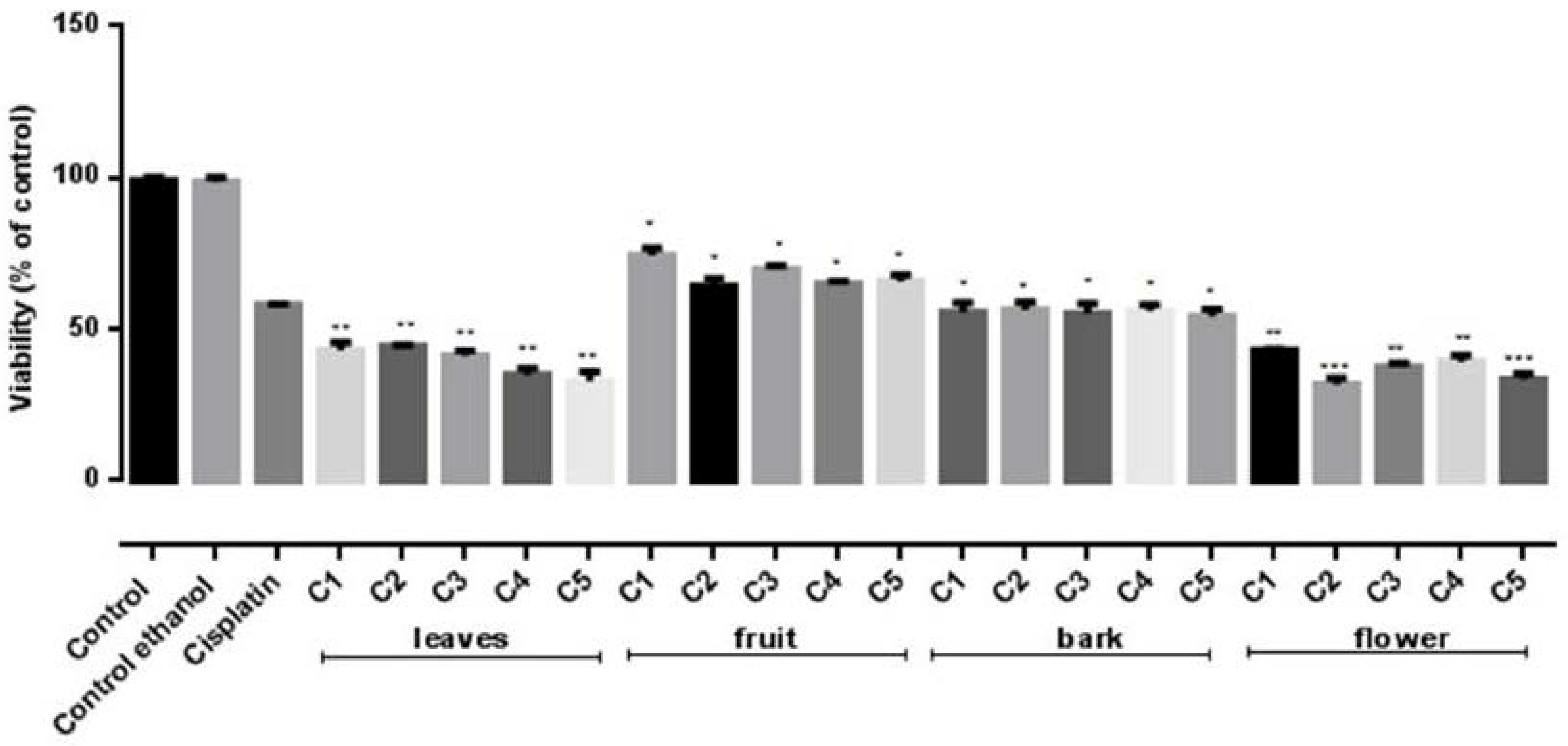
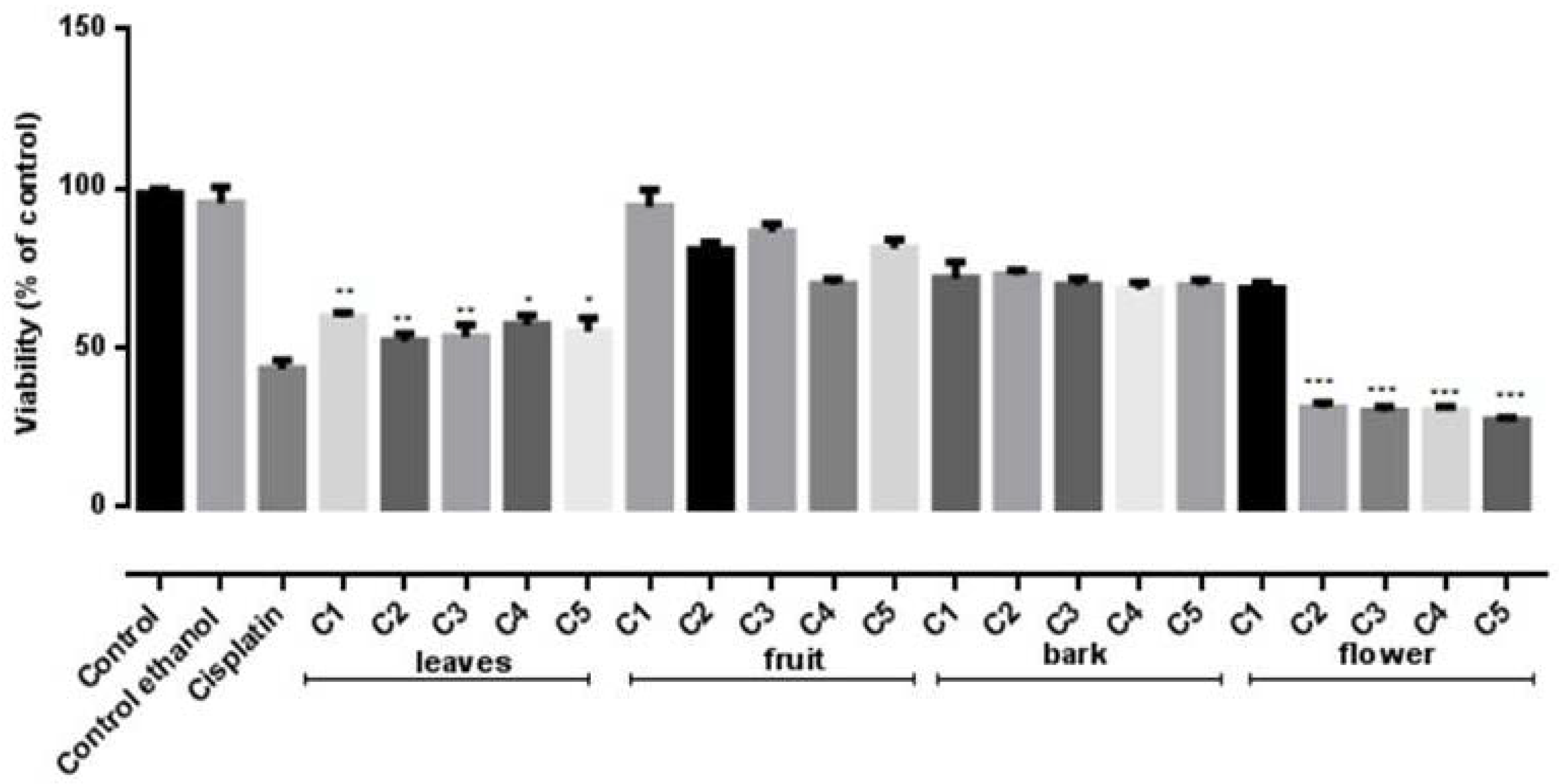
2.6. Cytotoxiciy Assays
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material and Preparation of Extracts
3.3. HPLC-DAD-ESI+ Analysis of Polyphenolic Compounds
3.4. FT-IR Spectroscopy
3.5. Quantification of Total Polyphenols, Flavonoids and Phenolic Acids Content
3.6. GC-MS Analysis
3.7. Antioxidant Activity Assays
3.7.1. DPPH Radical Scavenging Activity
3.7.2. Ferric-Reducing Antioxidant Power Assay (FRAP)
3.7.3. Cupric Ion Reducing Antioxidant Capacity (CUPRAC)
3.8. Cytotoxicity Assays
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wozniak, M.; Michalak, B.; Wyszomierska, J.; Dudek, M.K.; Kiss, A.K. Effects of phytochemically characterized extracts from Syringa vulgaris and isolated secoiridoids on mediators of inflammation in a human neutrophil model. Front. Pharm. 2018, 9, 349. [Google Scholar] [CrossRef] [PubMed]
- Simpson, M.G. Diversity and Classification of Flowering Plants: Eudicots. In Plant Systematics; Elsevier: Amsterdam, The Netherlands, 2010; Volume 315, pp. 275–448. [Google Scholar]
- Lendvay, B.; Kadereit, J.W.; Westberg, E.; Cornejo, C.; Pedryc, A.; Höhn, M. Phylogeography of Syringa josikaea (Oleaceae): Early Pleistocene divergence from East Asian relatives and survival in small populations in the Carpathians. Biol. J. Linn. Soc. 2016, 119, 689–703. [Google Scholar] [CrossRef]
- Su, G.; Cao, Y.; Li, C.; Yu, X.; Gao, X.; Tu, P.; Chai, X. Phytochemical and pharmacological progress on the genus Syringa. Chem. Cent. J. 2015, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Wang, Z.; Sun, Y.; Yang, B.; Wang, Q.; Kuang, H. Traditional uses, phytochemistry and pharmacology of genus Syringa: A comprehensive review. J. Ethnopharmacol. 2021, 26, 113465. [Google Scholar] [CrossRef] [PubMed]
- Hanlidou, E.; Karousou, R.; Kleftoyanni, V.; Kokkini, S. The herbal market of Thessaloniki (N Greece) and its relation to the ethnobotanical tradition. J. Ethnopharmacol. 2004, 91, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Jarić, S.; MačUkanović-Jocić, M.; Djurdjević, L.; Mitrović, M.; Kostić, O.; Karadžić, B.; Pavlović, P. An ethnobotanical survey of traditionally used plants on Suva planina mountain (south-eastern Serbia). J. Ethnopharmacol. 2015, 175, 93–108. [Google Scholar] [CrossRef]
- Leporatti, M.L.; Ivancheva, S. Preliminary comparative analysis of medicinal plants used in the traditional medicine of Bulgaria and Italy. J. Ethnopharmacol. 2003, 87, 123–142. [Google Scholar] [CrossRef]
- Papp, N.; Birkás-Frendl, K.; Bencsik, T.; Stranczinger, S.; Czégényi, D. Survey of traditional beliefs in the Hungarian Csángó and Székely ethnomedicine in Transylvania, Romania. Braz. J. Pharm. 2014, 24, 141–152. [Google Scholar] [CrossRef]
- Tóth, G.; Barabás, C.; Tóth, A.; Kéry, Á.; Béni, S.; Boldizsár, I.; Varga, E.; Noszál, B. Characterization of antioxidant phenolics in Syringa vulgaris L. flowers and fruits by HPLC-DAD-ESI-MS. Biomed. Chromatogr. 2016, 30, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Varga, E.; Barabás, C.; Tóth, A.; Boldizsár, I.; Noszál, B.; Tóth, G. Phenolic composition, antioxidant and antinociceptive activities of Syringa vulgaris L. bark and leaf extracts. Nat. Prod. Res. 2019, 33, 1664–1669. [Google Scholar] [CrossRef]
- Ao, W.L.J.; Wang, Q.H.; Si-Qin; Mu-Dan; Sa-Ren-Tu-Ya; Dai, N.Y.T.; Du-Ri-Si-Ha-La-Tu. The structural elucidation and antimicrobial activities of two new sesquiterpenes from Syringa pinnatifolia Hemsl. Chin. J. Nat. Med. 2012, 10, 477–480. [Google Scholar] [CrossRef]
- Dudek, M.K.; Michalak, B.; Woźniak, M.; Czerwińska, M.E.; Filipek, A.; Granica, S.; Kiss, A.K. Hydroxycinnamoyl derivatives and secoiridoid glycoside derivatives from Syringa vulgaris flowers and their effects on the pro-inflammatory responses of human neutrophils. Fitoterapia 2017, 121, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Filipek, A.; Wyszomierska, J.; Michalak, B.; Kiss, A.K. Syringa vulgaris bark as a source of compounds affecting the release of inflammatory mediators from human neutrophils and monocytes/macrophages. Phytochem. Lett. 2019, 30, 309–313. [Google Scholar] [CrossRef]
- Oku, H.; Maeda, M.; Kitagawa, F.; Ishiguro, K. Effect of polyphenols from Syringa vulgaris on blood stasis syndrome. J. Clin. Biochem. Nutr. 2020, 67, 84–88. [Google Scholar] [CrossRef]
- Su, G.; Zhang, R.; Yang, X.; Bai, R.; Yin, X.; Gao, X.; Li, L.; Tu, P.; Chai, X. Lignans from the stem bark of Syringa pinnatifolia. Fitoterapia 2016, 114, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.S.C.; Subramanian, I.P.; Subramanian, S.P. Isolation, characterization of syringin, phenylpropanoid glycoside from Musa paradisiaca tepal extract and evaluation of its antidiabetic effect in streptozotocin-induced diabetic rats. Biomed. Prev. Nutr. 2014, 4, 105–111. [Google Scholar] [CrossRef]
- Kikuchi, M.; Yaoita, Y.; Mano, N.; Kikuchi, M. Glycosides from the Leaves of Syringa vulgaris and Their Growth Inhibitory Activity against Human Cancer Cell Lines. Jpn. Soc. Pharm. 2010, 64, 104–105. [Google Scholar]
- Qi, L.M.; Zhang, J.; Zhao, Y.L.; Zuo, Z.T.; Jin, H.; Wang, Y.Z. Quantitative and qualitative characterization of Gentiana rigescens Franch (Gentianaceae) on different parts and cultivations years by HPLC and FTIR spectroscopy. J. Anal. Methods Chem. 2017, 2017. [Google Scholar] [CrossRef]
- Mosoarca, G.; Vancea, C.; Popa, S.; Gheju, M.; Boran, S. Syringa vulgaris leaves powder a novel low-cost adsorbent for methylene blue removal: Isotherms, kinetics, thermodynamic and optimization by Taguchi method. Sci. Rep. 2020, 10, 17676. [Google Scholar] [CrossRef]
- Kenđel, A.; Zimmermann, B. Chemical Analysis of Pollen by FT-Raman and FTIR Spectroscopies. Front. Plant Sci. 2020, 11, 352. [Google Scholar] [CrossRef]
- Schulz, H.; Pfeffer, S.; Quilitzsch, R.; Steuer, B.; Reif, K. Rapid and non-destructive determination of the echinacoside content in Echinacea roots by ATR-IR and NIR spectroscopy. Planta Med. 2002, 68, 926–929. [Google Scholar] [CrossRef] [PubMed]
- Agatonovic-Kustrin, S.; Ristivojevic, P.; Gegechkori, V.; Litvinova, T.M.; Morton, D.W. Essential oil quality and purity evaluation via ft-ir spectroscopy and pattern recognition techniques. Appl. Sci. 2020, 10, 7294. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Y.; Zuo, Z.; Zhang, J.; Shi, Y.; Wang, Y. Investigation of a medical plant for hepatic diseases with secoiridoids using HPLC and FT-IR spectroscopy for a case of gentiana rigescens. Molecules 2020, 25, 1219. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.M.; Zhang, J.; Zuo, Z.T.; Zhao, Y.L.; Wang, Y.Z.; Hang, J. Determination of Iridoids in Gentiana rigescens by Infrared Spectroscopy and Multivariate Analysis. Anal. Lett. 2017, 50, 389–401. [Google Scholar] [CrossRef]
- Min, X.; Dong, W.; Jun, Z.Y.; Chong-Ren, Y. A New Secoiridoidal Secoiridoidal Glucoside from Gentiana rigescens (Gentianaceae). Acta Bot. Yunnanica 2006, 28, 669–672. [Google Scholar]
- Selvaraj, K.; Chowdhury, R.; Bhattacharjee, C. Isolation and structural elucidation of flavonoids from aquatic fern Azolla microphylla and evaluation of free radical scavenging activity. Int. J. Pharm. Pharm. Sci. 2013, 5 (Suppl. 3), 743–749. [Google Scholar]
- Bakkialakshmi, S.; Roy, J. Infrared Spectrum Analysis of Some Flavonoids with Hemoglobin. Int. J. Appl. Adv. Sci. Res. 2017, 2, 107–110. [Google Scholar]
- Crişan, I.; Vidican, R.; Olar, L.; Stoian, V.; Morea, A.; Ştefan, R. Screening for Changes on Iris germanica L. Rhizomes following inoculation with arbuscular mycorrhiza using Fourier transform infrared spectroscopy. Agronomy 2019, 9, 815. [Google Scholar] [CrossRef]
- Kowczyk-Sadowy, M.; Wisłocka, R.; Lewandowska, H.; Piekut, J.; Lewandowski, W. Spectroscopic (FT-IR, FT-Raman, 1H- And13C-NMR), theoretical and microbiological study of trans o-coumaric acid and alkali metal o-coumarates. Molecules 2015, 20, 3146–3369. [Google Scholar] [CrossRef]
- Ielciu, I.; Sevastre, B.; Olah, N.-K.; Turdean, A.; Chişe, E.; Marica, R.; Oniga, I.; Uifălean, A.; Sevastre-Berghian, A.C.; Niculae, M.; et al. Evaluation of Hepatoprotective Activity and Oxidative Stress Reduction of Rosmarinus officinalis L. Shoots Tincture in Rats with Experimentally Induced Hepatotoxicity. Molecules 2021, 26, 1737. [Google Scholar] [CrossRef] [PubMed]
- Ielciu, I.; Hanganu, D.; Păltinean, R.; Vlase, L.; Frédérich, M.; Gheldiu, A.-M.; Benedec, D.; Crişan, G. Antioxidant capacity and polyphenolic content of the Echinocystis lobata (Michx.) Torr. et A.Gray flowers. Pak. J. Pharm. Sci. 2018, 31 (Suppl. 2), 677–683. [Google Scholar]
- Ielciu, I.; Frederich, M.; Hanganu, D.; Angenot, L.; Olah, N.-K.; Ledoux, A.; Crişan, G.; Păltinean, R. Flavonoid Analysis and Antioxidant Activities of the Bryonia alba L. Aerial Parts. Antioxidants 2019, 8, 108. [Google Scholar] [CrossRef]
- Castejón, M.L.; Montoya, T.; Alarcón-de-la-Lastra, C.; Sánchez-Hidalgo, M. Potential protective role exerted by secoiridoids from Olea europaea L. in cancer, cardiovascular, neurodegenerative, aging-related, and immunoinflammatory diseases. Antioxidants 2020, 9, 149. [Google Scholar] [CrossRef]
- Taleghani, A.; Tayarani-Najaran, Z. Potent Cytotoxic Natural Flavonoids: The Limits of Perspective. Curr. Pharm. Des. 2019, 24, 5555–5579. [Google Scholar] [CrossRef] [PubMed]
- Neelam, A.K.; Sharma, K.K. Phenylpropanoids and its derivatives: Biological activities and its role in food, pharmaceutical and cosmetic industries. Crit. Rev. Food Sci. Nutr. 2020, 60, 2655–2675. [Google Scholar] [CrossRef]
- Karihtala, P.; Soini, Y. Reactive oxygen species and antioxidant mechanisms in human tissues and their relation to malignancies. Apmis 2007, 115, 81–103. [Google Scholar] [CrossRef]
- Aung, T.N.; Qu, Z.; Kortschak, R.D.; Adelson, D.L. Understanding the effectiveness of natural compound mixtures in cancer through their molecular mode of action. Int. J. Mol. Sci. 2017, 18, 656. [Google Scholar] [CrossRef]
- Huang, C.Y.; Ju, D.T.; Chang, C.F.; Muralidhar, R.P.; Velmurugan, B.K. A review on the effects of current chemotherapy drugs and natural agents in treating non-small cell lung cancer. Biomededicine 2017, 7, 12–23. [Google Scholar] [CrossRef]
- Kikuchi, M.; Yaoita, Y.; Mano, N.; Kikuchi, M. Structure and Cytotoxic Activity of Enzymatic Hydrolysis Products of Secoiridoid Glucosides, Isoligustroside and Isooleuropein. Chem. Biodivers. 2011, 8, 651–657. [Google Scholar] [CrossRef]
- Ielciu, I.; Mouithys-Mickalad, A.; Franck, T.; Angenot, L.; Ledoux, A.; Păltinean, R.; Cieckiewicz, E.; Etienne, D.; Tits, M.; Crişan, G.; et al. Flavonoid composition, cellular antioxidant activity and (myelo)peroxidase inhibition of a Bryonia alba L. (Cucurbitaceae) leaves extract. J. Pharm. Pharm. 2019, 71, 230–239. [Google Scholar] [CrossRef]
- Bădălica-Petrescu, M.; Drăgan, S.; Ranga, F.; Fetea, F.; Socaciu, C. Comparative HPLC-DAD-ESI(+)MS fingerprint and quantification of phenolic and flavonoid composition of aqueous leaf extracts of Cornus mas and Crataegus monogyna, in relation to their cardiotonic potential. Not. Bot. Horti Agrobot. 2014, 42, 9–18. [Google Scholar] [CrossRef]
- Hanganu, D.; Benedec, D.; Olah, N.-K.; Ranga, F.; Mirel, S.; Tiperciuc, B.; Oniga, I. Research on enzyme inhibition potential and phenolic compounds from Origanum vulgare ssp. vulgare. Farmacia 2020, 68, 1075–1080. [Google Scholar] [CrossRef]
- Sevastre-Berghian, A.C.; Ielciu, I.; Mitre, A.O.; Filip, G.A.; Oniga, I.; Vlase, L.; Benedec, D.; Gheldiu, A.-M.; Toma, V.A.; Miharţ, B.; et al. Targeting Oxidative Stress Reduction and Inhibition of HDAC1, MECP2, and NF-kB Pathways in Rats with Experimentally Induced Hyperglycemia by Administration of Thymus marshallianus Willd. Extracts. Front. Pharm. 2020, 11, 581470. [Google Scholar] [CrossRef]
- Stan, M.S.; Voicu, S.N.; Căruntu, S.; Nica, I.C.; Olah, N.-K.; Burtescu, R.; Balta, C.; Rosu, M.; Herman, H.; Hermenean, A.; et al. Antioxidant and anti-inflammatory properties of a Thuja occidentalis mother tincture for the treatment of ulcerative colitis. Antioxidants 2019, 8, 416. [Google Scholar] [CrossRef] [PubMed]
- Moţ, A.C.; Damian, G.; Sârbu, C.; Silaghi-Dumitrescu, R. Redox reactivity in propolis: Direct detection of free radicals in basic medium and interaction with hemoglobin. Redox Rep. 2009, 14, 267–274. [Google Scholar] [CrossRef]
- Benzie, I.; Strain, J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Oniga, I.; Puşcaş, C.; Silaghi-Dumitrescu, R.; Olah, N.-K.; Sevastre, B.; Marica, R.; Marcus, I.; Sevastre-Berghian, A.C.; Benedec, D.; Pop, C.E.; et al. Origanum vulgare ssp. vulgare: Chemical composition and biological studies. Molecules 2018, 23, 2077. [Google Scholar]
- Özyürek, M.; Güngör, N.; Baki, S.; Güçlü, K.; Apak, R. Development of a silver nanoparticle-based method for the antioxidant capacity measurement of polyphenols. Anal. Chem. 2012, 84, 8052–8059. [Google Scholar] [CrossRef]
- Olah, N.-K.; Osser, G.; Câmpean, R.F.; Furtuna, F.R.; Benedec, D.; Filip, L.; Raita, O.; Hanganu, D. The study of polyphenolic compounds profile of some Rosmarinus officinalis L. extracts. Pak. J. Pharm. Sci. 2016, 29 (Suppl. 6), 2355–2361. [Google Scholar]
- Iacoviţă, C.; Florea, A.; Scoruş, L.; Pall, E.; Dudric, R.; Moldovan, A.I.; Ştiufiuc, R.; Tetean, R.; Lucaciu, C.M. Hyperthermia, cytotoxicity, and cellular uptake properties of manganese and zinc ferrite magnetic nanoparticles synthesized by a polyol-mediated process. Nanomaterials 2019, 9, 1489. [Google Scholar] [CrossRef]
- Marian, E.; Duteanu, N.; Vicas, L.; Rusu, G.; Jurca, T.; Muresan, M.; Micle, O.; Hangan, A.C.; Stan, R.L.; Ionescu, C.; et al. Synthesis, characterization of inclusion compounds of amygdalin with β-cyclodextrin and sod-like activity and cytotoxicity on hela tumor cells. Arab. J. Chem. 2020, 13, 6828–6837. [Google Scholar] [CrossRef]

| Peak No. | Compound | Rt (min) | UV λmax (nm) | [M + H]+ (m/z) | Syringa vulgaris Leaves | Syringa vulgaris Bark | Syringa vulgaris Flowers | Syringa vulgaris Fruit |
|---|---|---|---|---|---|---|---|---|
| 1 | p-Coumaroyl-glycolic acid | 3.16 | 332 | 223 | 3814.80 ± 0.96 | 788.46 ± 0.58 | 6748.16 ± 2.03 | 1094.85 ± 1.05 |
| 2 | Secologanoside | 9.71 | 233 | 391 | 17,539.42 ± 0.5 | 3020.98 ± 1.65 | 27,663.00 ± 0.6 | 467.04 ± 0.36 |
| 3 | Syringin | 12.27 | 265 | 373 | 9245.97 ± 0.54 | 74,535.30 ± 2.3 | 14,653.98 ± 0.5 | 1214.39 ± 0.89 |
| 4 | 10-Hydroxy-oleuropein | 13.19 | 235 | 556 | 2153.98 ± 0.89 | 8943.89 ± 1.44 | 3405.45 ± 0.88 | 300.31 ± 1.25 |
| 5 | Acteoside | 13.95 | 324 | 625 | 5813.26 ± 1.02 | 2658.10 ± 0.88 | 9408.78 ± 0.59 | - |
| 6 | Echinacoside | 15.13 | 328 | 787 | 4537.26 ± 0.45 | 38,299.52 ± 0.1 | 7417.81 ± 1.23 | 2151.03 ± 0.71 |
| 7 | Quercetin-rutinoside (Rutin) | 15.78 | 256, 355 | 611 | 4779.29 ± 0.35 | 1604.54 ± 1.23 | 7642.07 ± 0.56 | 72.32 ± 0.99 |
| 8 | Ligstroside | 16.10 | 275 | 525 | 6921.53 ± 1.25 | 24,820.71 ± 2.0 | 11,019.22 ± 0.21 | 565.12 ± 3.01 |
| 9 | Demethyl oleuropein | 16.41 | 231 | 527 | 28,607.49 ± 0.71 | 6396.82 ± 0.55 | 35,729.89 ± 0.2 | 5345.42 ± 0.21 |
| 10 | Oleuropein | 17.12 | 280 | 540 | 4224.40 ± 0.89 | 9139.07 ± 0.78 | 6744.01 ± 0.58 | - |
| 11 | Kaempferol-glucoside | 17.42 | 265, 341 | 449 | 2377.10 ± 0.21 | 1690.59 ± 0.25 | 3814.93 ± 1.02 | 462.39 ± 0.66 |
| 12 | Syringalactone A | 17.96 | 223 | 511 | 8457.43 ± 0.65 | 17,161.82 ± 1.3 | 13,652.60 ± 0.6 | 1380.14 ± 1.22 |
| 13 | Nuzhenide | 18.33 | 275 | 687 | 9928.59 ± 1.02 | 129.65 ± 1.03 | 15,893.68 ± 0.3 | 1365.43 ± 0.5 |
| 14 | Oleuropein-aglycone | 18.85 | 280 | 379 | 7541.38 ± 1.56 | 42,796.39 ± 2.0 | 12,078.46 ± 0.7 | 1195.76 ± 0.25 |
| S. vulgaris Leaves | S. vulgaris Bark | S. vulgaris Fruit | S. vulgaris Flowers | Tentative Assignement | References |
|---|---|---|---|---|---|
| ~605 | ~605 | ~593 | ~598 | β(CH) | [20,30] |
| ~704 | ~704 | ~704 | ~705 | γ(C=O) | [30] |
| ~766 | ~766 | ~766 | ~774 | C-O-C ring vibration | [29] |
| ~816 | ~816 | ~816 | ~814 | Phenyl-propanoid-associated signals of echinacoside | [21,22] |
| - | ~853 | ~853 | - | γ(CH)ar | [30] |
| ~885 | ~890 | ~890 | ~884 | -CH2 out-of-plane deformation (Terpene) | [23] |
| ~930 | ~925 | ~925 | ~924 | C-O-C ring vibration Symmetric stretching Rutin | [27,28,29] |
| ~1026 | ~1033 | ~1032 | ~1039 | -C-O, C-O-H, C-O-C, C-C | [20] |
| ~1076 | ~1076 | ~1076 | ~1079 | -C-OH stretching (iridoids skeletal vibration) | [24,25,26] |
| - | ~1106 | - | - | C-O stretch | [23,28] |
| 1162 | 1162 | 1162 | 1159 | C=C Stretching Rutin | [27,28] |
| ~1268 | ~1261 | ~1256 | ~1263 | C-O stretching (due to to terpenes, phenols or carbohydrates from iridoids) | [20,24,25] |
| ~1404 | ~1385 | ~1394 | ~1401 | symmetrical CH3 deformation Rutin | [20,23,27,28] |
| ~1454 | ~1454 | ~1443 | ~1454 | C-H asymmetric bending (esters, carbohydrate) | [25] |
| 1515 | 1515 | 1515 | ~1516 | Phenyl-propanoid-associated signals of echinacoside | [21,22] |
| 1608 | 1608 | 1608 | ~1596 | C=C stretching Rutin (iridoids skeletal vibration) | [24,26,27,28] |
| ~1702 | ~1702 | ~1696 | ~1694 | C=O stretch (terpenoids) | [23] |
| S. vulgaris Leaves | S. vulgaris Bark | S. vulgaris Fruit | S. vulgaris Flowers | Tentative Assignement | References |
|---|---|---|---|---|---|
| 2901 | 2901 | 2909 | C-H stretch (terpenoid) | [23] | |
| 2933 | 2925 | 2933 | -CH2 asymmetric stretching (from iridoids) | [20,24,25] | |
| ~3384 | ~3376 | ~3376 | -OH stretching vibration of phenols, carboxylic acids and alcohols | [20,27,28] |
| Sample | TPC (mg GAE/g) | TFC (mg RE/g) | TPA (mg CAE/g) |
|---|---|---|---|
| bark | 3.36 ± 0.42 | 0.19 ± 0.24 | 3.99 ± 0.84 |
| leaves | 3.86 ± 0.31 | 0.71 ± 0.41 | 4.90 ± 0.42 |
| flowers | 3.98 ± 0.28 | 1.21 ± 0.12 | 2.36 ± 0.06 |
| fruit | 2.25 ± 0.02 | 0.75 ± 0.01 | 1.22 ± 0.01 |
| Identified Compound | Retention Time (min) | Area | Content% |
|---|---|---|---|
| Benzyl alcohol | 6.52 | 472,069 | 27.36 ± 0.19 |
| Diethyl malonate | 6.80 | 26,382 | 1.53 ± 1.29 |
| 5-ethoxydihydro-2-furanone | 6.88 | 72,759 | 4.22 ± 0.95 |
| 3,4-dihydroxytetrahydro-2-furanone | 7.43 | 86,244 | 5.00 ± 0.31 |
| Lilac alcohol C | 8.86 | 132,859 | 7.70 ± 1.02 |
| Lilac alcohol D | 8.98 | 569,808 | 33.02 ± 0.88 |
| 5-oxotetrahydrofuran-2 carboxylic acod, ethyl ester | 9.11 | 33,140 | 1.92 ± 0.89 |
| 2-methyl-propanoic acid, propyl ester | 9.45 | 34,760 | 2.01± 0.10 |
| 3-Phenylpropanal | 10.27 | 23,867 | 1.83 ± 0.09 |
| 4-hydroxy-2-methylacetophenone | 10.41 | 40,576 | 2.35 ± 0.92 |
| Ethyl 3,3-diethoxypropionate | 10.44 | 64,276 | 3.72 ± 0.37 |
| 2-hydroxy-3-methylsuccinic acid | 10.51 | 16,343 | 0.95 ± 0.41 |
| Tetrahydro [2,2] bifuranyl-5-one | 10.59 | 10,896 | 0.63 ± 0.56 |
| 4-hydroxy-benzaldehyde | 10.81 | 23,664 | 1.50 ± 1.16 |
| Methyleugenol | 11.50 | 14,156 | 0.82 ± 0.72 |
| p-hydroxycinnamic acid, ethyl ester | 15.39 | 15,797 | 0.92 ± 0.19 |
| Tetradecanoic acid, ethyl ester | 16.33 | 31,256 | 1.81 ± 1.02 |
| n-hexadecanoic acid | 18.66 | 38,934 | 2.26 ± 0.94 |
| 11,14-eicosadienoic acid, methyl ester | 21.66 | 7722 | 0.45 ± 0.01 |
| Identified Compound | Retention Time (min) | Area | Content% |
|---|---|---|---|
| á-Psi-Carotene | 6.50 | 663.200 | 0.61 ± 0.22 |
| Benzoic acid, 4 formyl, methyl ester | 11.14 | 13,207.20 | 12.20 ± 0.79 |
| 2-metoxyphenol | 15.81 | 5445.60 | 5.03 ± 0.64 |
| n-Hexadecanoic acid | 18.66 | 8976.40 | 8.35 ± 0.09 |
| Astaxanthin | 19.31 | 1347.60 | 1.24 ± 0.12 |
| trans-Sinapyl alcohol | 19.42 | 15,466.00 | 14.28 ± 0.90 |
| Oleic acid | 21.77 | 17,973.60 | 16.06 ± 0.02 |
| Identified Compound | Retention Time (min) | Area | Content% |
|---|---|---|---|
| Benzyl alcohol | 6.54 | 4403.200 | 3.26 ± 0.75 |
| Benzofuran,2,3-dihydro | 8.92 | 5456.000 | 4.05 ± 0.95 |
| Benzoic acid, 4-formyl, methyl ester | 11.15 | 10,302.400 | 7.64 ± 0.01 |
| Benzaldehyde, 2-hydroxy-6-methyl | 11.94 | 12,287.600 | 9.12 ± 0.56 |
| á-Phellandrene | 13.15 | 1151.200 | 0.85 ± 0.22 |
| 4-{(1E)-3-Hydroxy-1-propenyl)-2-metoxyphenol | 15.82 | 5185.200 | 3.85 ± 0.33 |
| n-Hexadecanoic acid | 16.67 | 5146.400 | 3.83 ± 0.57 |
| Identified Compound | Retention Time (min) | Area | Content% |
|---|---|---|---|
| n-Hexadecanoic acid | 18.67 | 17,880.400 | 11.83 ± 0.06 |
| trans-13-octadecenoic acid | 21.60 | 6479.200 | 4.28 ± 0.09 |
| Trans-13-octadecanoic acid | 21.77 | 32,274.00 | 21.36 ± 0.22 |
| Oleic acid | 21.85 | 8079.60 | 5.35 ± 0.25 |
| Octadecanoic acid | 22.18 | 12,766.80 | 8.45 ± 0.36 |
| Sample | DPPH (IC50 µg/mL) | FRAP (µM TE/g) | CUPRAC (µM TE/g) |
|---|---|---|---|
| bark | 956 ± 1.71 | 157.92 ± 1.74 | 279.4 ± 1.18 |
| leaves | 865 ± 1.10 | 178.92 ± 0.62 | 169.7 ± 0.73 |
| flowers | 36.83 ± 0.47 * | 182.52 ± 0.99 * | 329.3 ± 0.15 * |
| fruit | 103.19 ± 1.02 | 116.85 ± 0.42 | 110.5 ± 1.04 |
| IC50 | B16F10 | HeLa |
|---|---|---|
| Flower | 5.74 ± 0.20 | 5.13 ± 0.12 |
| Leaves | 3.08 ± 0.23 | 4.91 ± 0.25 |
| Fruit | 2.28 ± 0.6 | 2.74 ± 0.4 |
| Bark | 2.62 ± 0.09 | 3.32 ± 0.09 |
| Standard: Cisplatin 0.2 μM/mL | ||
| Time | Temperature | Rate |
|---|---|---|
| 0 min | 80 °C | 0 °C/min |
| 7 min | 220 °C | 20 °C/min |
| 11 min | 240 °C | 5 °C/min |
| 24 min | 240 °C | 0 °C/min |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanganu, D.; Niculae, M.; Ielciu, I.; Olah, N.-K.; Munteanu, M.; Burtescu, R.; Ștefan, R.; Olar, L.; Pall, E.; Andrei, S.; et al. Chemical Profile, Cytotoxic Activity and Oxidative Stress Reduction of Different Syringa vulgaris L. Extracts. Molecules 2021, 26, 3104. https://doi.org/10.3390/molecules26113104
Hanganu D, Niculae M, Ielciu I, Olah N-K, Munteanu M, Burtescu R, Ștefan R, Olar L, Pall E, Andrei S, et al. Chemical Profile, Cytotoxic Activity and Oxidative Stress Reduction of Different Syringa vulgaris L. Extracts. Molecules. 2021; 26(11):3104. https://doi.org/10.3390/molecules26113104
Chicago/Turabian StyleHanganu, Daniela, Mihaela Niculae, Irina Ielciu, Neli-Kinga Olah, Melania Munteanu, Ramona Burtescu, Răzvan Ștefan, Loredana Olar, Emoke Pall, Sanda Andrei, and et al. 2021. "Chemical Profile, Cytotoxic Activity and Oxidative Stress Reduction of Different Syringa vulgaris L. Extracts" Molecules 26, no. 11: 3104. https://doi.org/10.3390/molecules26113104
APA StyleHanganu, D., Niculae, M., Ielciu, I., Olah, N.-K., Munteanu, M., Burtescu, R., Ștefan, R., Olar, L., Pall, E., Andrei, S., Vodnar, D. C., Benedec, D., & Oniga, I. (2021). Chemical Profile, Cytotoxic Activity and Oxidative Stress Reduction of Different Syringa vulgaris L. Extracts. Molecules, 26(11), 3104. https://doi.org/10.3390/molecules26113104













