Unexpected Radical Telomerisation of Vinylidene Fluoride with 2-Mercaptoethanol
Abstract
1. Introduction
2. Results
2.1. Radical Telomerisation of VDF with 2-Mercaptoethanol: Synthesis and Characterisation
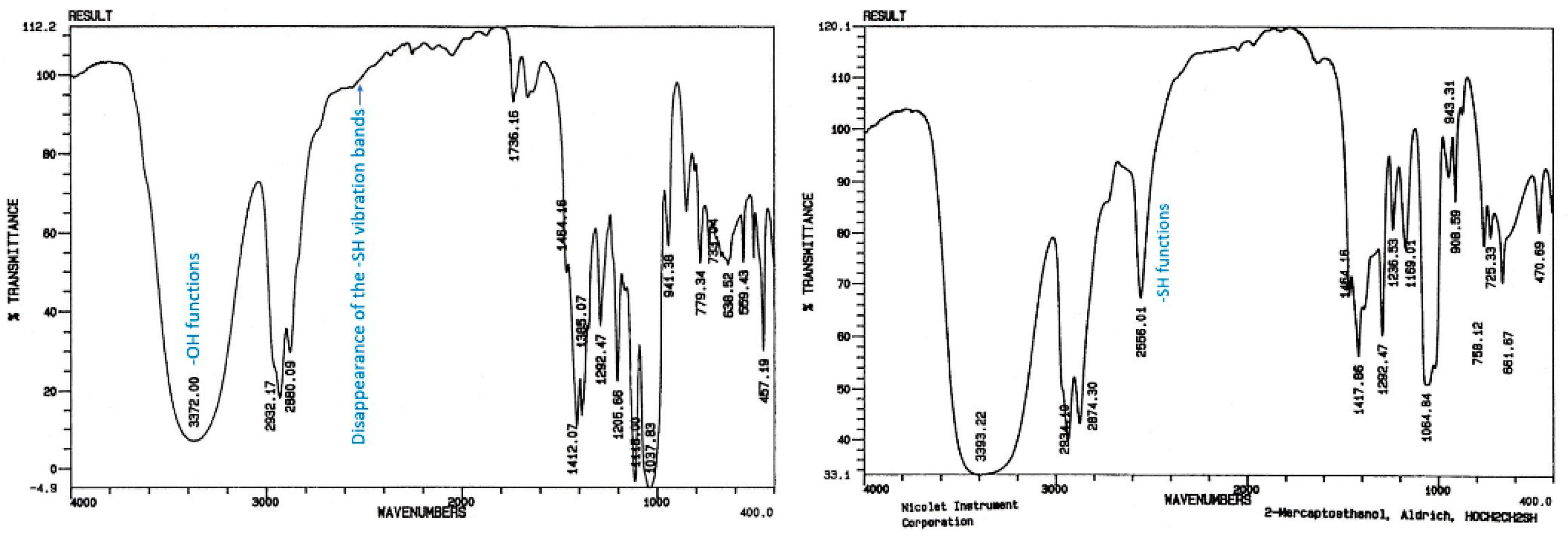
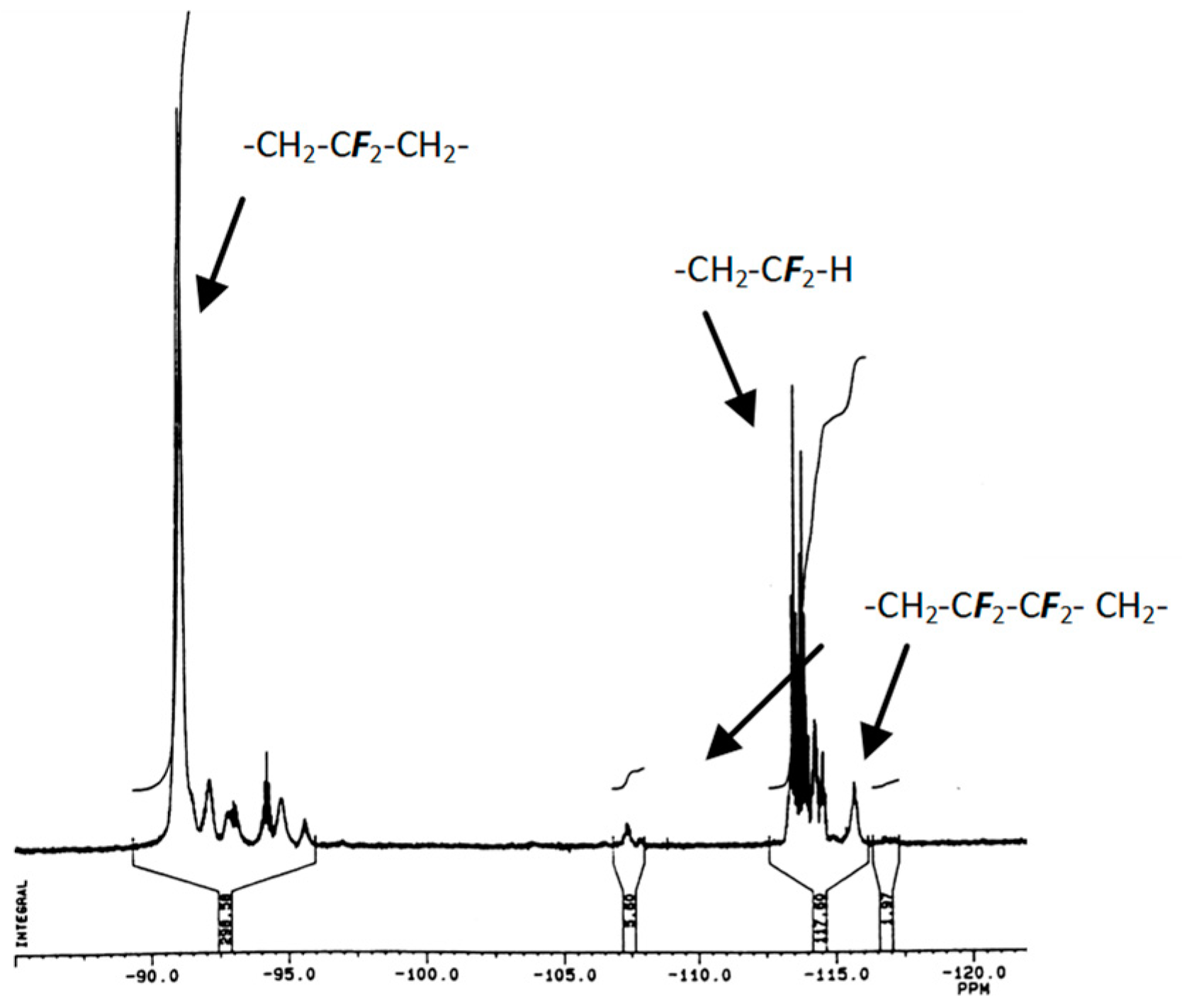
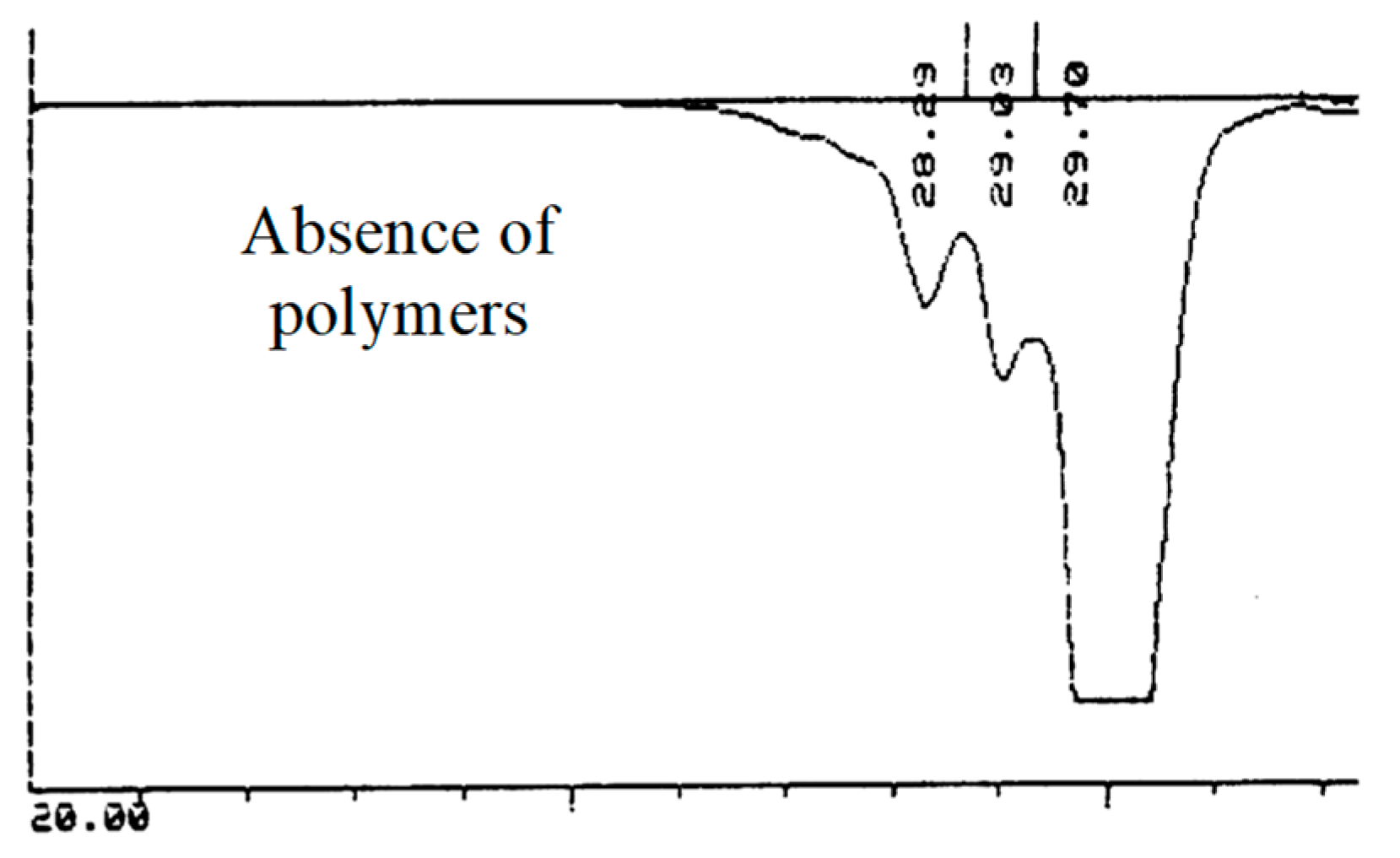
2.1.1. First Adducts
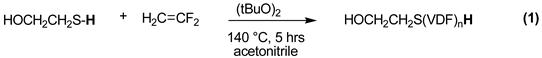
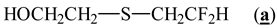
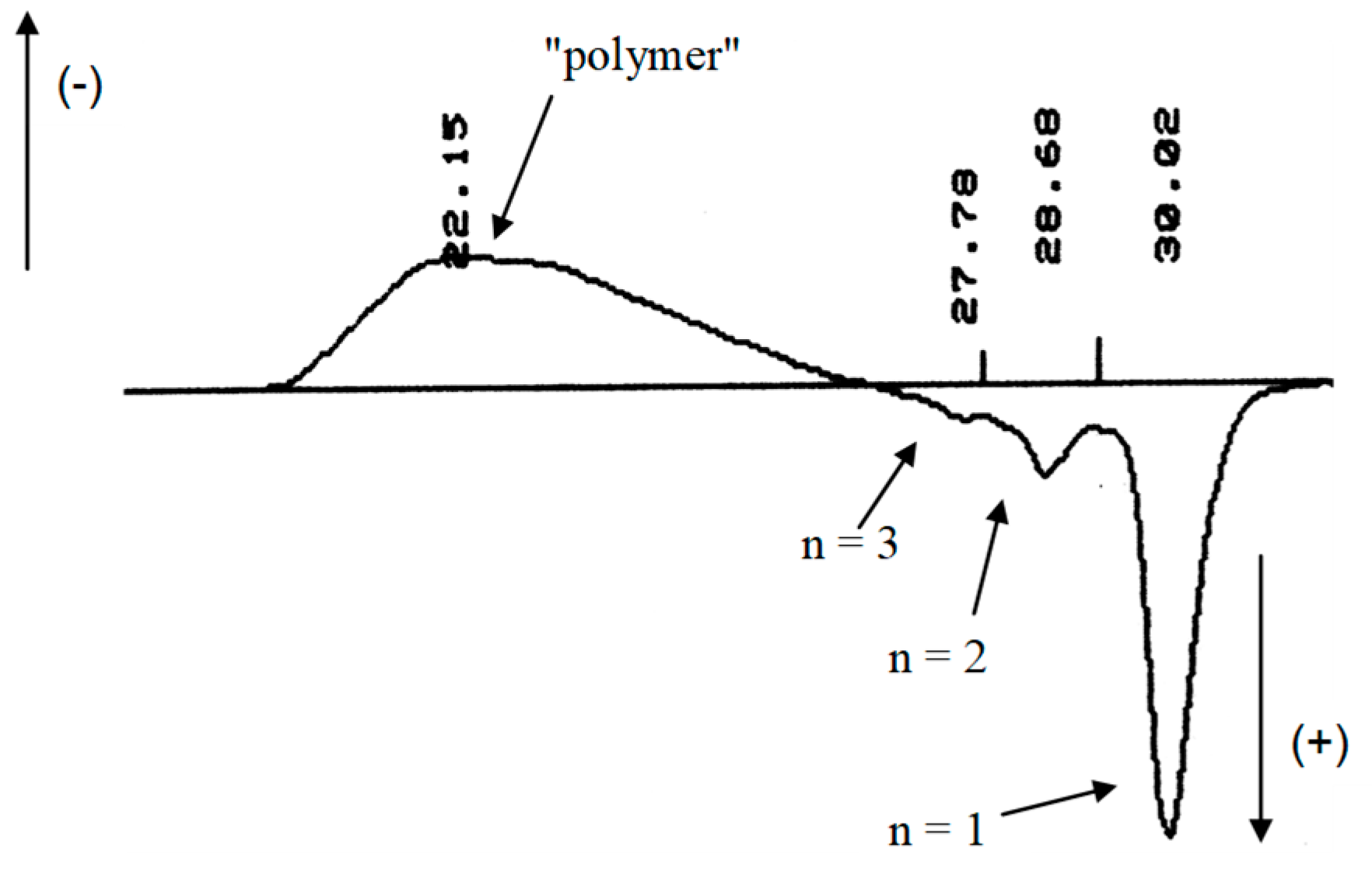
2.1.2. Higher Molar Mass-Telomers
2.2. Kinetics of Radical Telomerisation of VDF with 2-Mercaptoethanol

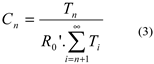
3. Materials and Methods
3.1. Materials
3.2. Reactions
3.3. Apparatus and Analysis of Products
3.4. Typical Procedure for the Radical Telomerisation of VDF with 2-Mercaptoethanol: From an Initial Molar Ratio R0 = [CTA]0/[VDF]0 = 1
3.5. Photochemical Telomerisation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Scheirs, J. Modern Fluoropolymers; John Wiley and Sons Ltd.: New York, NY, USA, 1997. [Google Scholar]
- Fluoropolymers: Synthesis and Applications; Hougham, G., Cassidy, P.E., Johns, K., Davidson, T., Eds.; Plenum Press: New York, NY, USA, 1999; Volume 1–2. [Google Scholar]
- Ameduri, B.; Boutevin, B. Well-Architectured Fluoropolymers: Synthesis, Properties and Applications; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Ameduri, B. From Vinylidene Fluoride (VDF) to the Applications of VDF-Containing Polymers and Copolymers: Recent Developments and Future Trends. Chem. Rev. 2009, 109, 6632–6686. [Google Scholar] [CrossRef]
- Smith, D.W.; Iacono, S.T.; Iyer, S.S. Handbook of Fluoropolymer Science and Technology; Wiley: New York, NY, USA, 2014. [Google Scholar]
- Ameduri, B.; Sawada, H. Fluoropolymers: Volume 1: Synthesis, Properties, Processing and Simulations; Royal Society Chemistry: Oxford, UK, 2016. [Google Scholar]
- Ameduri, B.; Fomin, S. Progress in Fluorine Science; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Boutevin, B. From Telomerization to Living Radical Polymerization. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 3235–3243. [Google Scholar] [CrossRef]
- Long, K.F.; Bongiardina, N.J.; Mayordomo, P.; Olin, M.J.; Ortega, A.D.; Bowman, C.N. Effects of 1°, 2°, and 3° Thiols on Thiol–Ene Reactions: Polymerization Kinetics and Mechanical Behavior. Macromolecules 2020, 53, 5805–5815. [Google Scholar] [CrossRef]
- Duc, M.; Ameduri, B.; Boutevin, B.; Kharroubi, M.; Sage, J.M. Telomerization of Vinylidene Fluoride with Methanol. Elucidation of the Reaction Process and Mechanism by a Structural Analysis of the Telomers. Chem. Phys. 1998, 199, 1271–1289. [Google Scholar] [CrossRef]
- Duc, M.; Boutevin, B.; Ameduri, B. Radical Telomerisation of Vinylidene Fluoride with Diethyl Hydrogenphosphonate. J. Fluor. Chem. 2001, 112, 3–12. [Google Scholar] [CrossRef]
- Duc, M.; Ameduri, B.; David, G.; Boutevin, B. Kinetics of Radical Telomerization of Vinylidene Fluoride in the Presence of CCl3Z Chain Transfer Agent. J. Fluor. Chem. 2007, 128, 144–149. [Google Scholar] [CrossRef]
- Laflamme, P.; Porzio, P.; Ameduri, B.; Soldera, A. Characterization of the Telomerization Reaction Path for Vinylidene Fluoride with ĊCl3 Radicals. Polym. Chem. 2012, 3, 2222–2230. [Google Scholar] [CrossRef]
- Ameduri, B.; Boutevin, B.; Kostov, K. Synthesis and Copolymerization of Vinylidene Fluoride (VDF) with Trifluorovinyl Monomers, 11. Macromol. Chem. Phys. 2002, 203, 1763–1771. [Google Scholar] [CrossRef]
- Ameduri, B.; Ladavière, C.; Delolme, F.; Boutevin, B. First MALDI-TOF Mass Spectrometry of Vinylidene Fluoride Telomers Endowed with Low Defect Chaining. Macromolecules 2004, 37, 7602–7609. [Google Scholar] [CrossRef]
- Boyer, C.; Valade, D.; Lacroix-Desmazes, P.; Ameduri, B.; Boutevin, B. Kinetics of the Iodine Transfer Polymerization of Vinylidene Fluoride. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 5763–5777. [Google Scholar] [CrossRef]
- Guerre, M.; Wadihur Rahaman, R.M.; Ameduri, B.; Poli, R.; Ladmiral, V. Limitations of the MADIX Polymerization of VDF. Macromolecules 2016, 49, 5386–5396. [Google Scholar] [CrossRef]
- Kharroubi, M.; Manséri, A.; Améduri, B.; Boutevin, B. Radical Addition of Iodine Monochloride to Vinylidene Fluoride. J. Fluor. Chem. 2000, 103, 145–153. [Google Scholar] [CrossRef]
- Monteiro, I.S.; Mendez Ecoscia, A.C.; McKenna, T.F.L. Investigation of the Chain Transfer Agent Effect on the Polymerization of Vinylidene Fluoride. Indust. Eng. Chem. Res. 2019, 58, 20976–20986. [Google Scholar] [CrossRef]
- Beuermann, S.; Imran-ul-haq, M. Homogeneous Phase Polymerization of Vinylidene Fluoride in Supercritical CO2: Surfactant Free Synthesis and Kinetics. Macromol. Symp. 2007, 259, 210–217. [Google Scholar] [CrossRef]
- Asandei, A.D.; Adebolu, O.I.; Simpson, C.P. Mild-Temperature Mn2 (CO)10-Photomediated Controlled Radical Polymerization of Vinylidene Fluoride and Synthesis of Well-Defined Poly(Vinylidene Fluoride) Block Copolymers. J. Am. Chem. Soc. 2012, 134, 6080–6083. [Google Scholar] [CrossRef]
- Asandei, A.D. Photomediated Controlled Radical Polymerization and Block Copolymerization of Vinylidene Fluoride. Chem. Rev. 2016, 116, 2244–2274. [Google Scholar] [CrossRef]
- Harris, J.; Stacey, F. The Free Radical Addition of Trifluoromethanethiol to Fluoroölefins. J. Am. Chem. Soc. 1961, 83, 840–845. [Google Scholar] [CrossRef]
- Harris, J.; Stacey, F. The Free Radical Addition of Hydrogen Sulfide to Fluoroethylenes. J. Am. Chem. Soc. 1963, 85, 749–754. [Google Scholar] [CrossRef]
- Sharp, D.W.A.; Miguel, H.T. Fluorine-Containing Dithioethers. Isr. J. Chem. 1978, 17, 144–147. [Google Scholar] [CrossRef]
- Haran, G.; Sharp, D.W.A. Photochemically Initiated Reactions of Bistrifluoromethyl Disulphide with Olefins. J. Chem. Soc. Perkin Trans. 1972, 1, 34–38. [Google Scholar] [CrossRef]
- Dear, R.; Gilbert, G. Telomerization of Bis (Trifluoromethyl) Disulfide with Polyfluoro-Olefins. J. Fluor. Chem. 1974, 4, 107–110. [Google Scholar] [CrossRef]
- Ameduri, B.; Billard, T.; Langlois, B. Telomerization of Vinylidene Fluoride with Alkyl (or Aryl) Trifluoromethanethiosulfonates. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 4538–4549. [Google Scholar] [CrossRef]
- Russo, S.; Behari, K.; Chengji, S.; Pianca, S.; Barchiesi, E.; Moggi, G. Synthesis and Microstructural Characterization of Low-Molar-Mass Poly (Vinylidene Fluoride). Polymer 1993, 34, 4777–4785. [Google Scholar] [CrossRef]
- Boutevin, B.; Furet, Y.; Hervaud, Y.; Rigal, G. Etude de la Télomérisation et de la Cotélomérisation du Fluorure de Vinylidène (VF2). Part, I. Cotélomérisation du VF2 Avec L’acétate de Vinyle et le 2-Hydroxyéthylmercaptan. J. Fluor. Chem. 1994, 69, 11–18. [Google Scholar] [CrossRef]
- Duc, M. Télomérisation du Fluorure de Vinylidène-Applications à la Synthèse d’Oligomères Fluorés Fonctionnels. Ph.D. Dissertation, University of Montpellier, Montpellier, France, 1997. [Google Scholar]
- Golzari, N.; Adams, J.; Beuermann, S. Inducing β Phase Crystallinity in Block Copolymers of Vinylidene Fluoride with Methyl Methacrylate or Styrene. Polymers 2017, 9, 306. [Google Scholar] [CrossRef]
- David, C.; Gosselain, P.A. Etude de la Réaction de Télomérisation de L’éthylène et du Tétrachlorure de Carbone Initiée par Rayonnement Gamma. Tetrahedron 1962, 70, 639. [Google Scholar] [CrossRef]
- Bauduin, G.; Boutevin, B.; Bertocchio, R.; Lantz, A.; Verge, C. Etude de la Télomérisation de C2F4 Avec les Iodures de Perfluoroalkyle Partie, I. Télomérisation Radicalaire. J. Fluor. Chem. 1998, 90, 107–116. [Google Scholar] [CrossRef]
- Boyer, C.; Valade, D.; Sauguet, L.; Ameduri, B.; Boutevin, B. Iodine Transfer Polymerization (ITP) of Vinylidene Fluoride (VDF). Influence of the Defect of VDF Chaining on the Control of ITP. Macromolecules 2005, 38, 10353–10362. [Google Scholar] [CrossRef]
- Kostov, G.; Boschet, F.; Brandstadter, S.; Ameduri, B. Random and Sequential Radical Cotelomerizations of 3,3,3-Trifluoropropene (H2C=CHCF3) with Vinylidene Fluoride (F2C=CH2). J. Polym. Sci. Part A Polym. Chem. Wiley 2009, 47, 3964–3981. [Google Scholar] [CrossRef]
- Luo, Y.R. Comprehensive Handbook of Chemical Bond Energies; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Taguet, A.; Ameduri, B.; Boutevin, B. Synthesis of Original Para-Sulfonic Acid Aminoethylthioethylbenzenesulfonic by Telomerization, and Its Grafting onto Poly(VDF-co-HFP) Copolymers for Proton Exchange Membrane for Fuel Cell. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 121–136. [Google Scholar] [CrossRef]
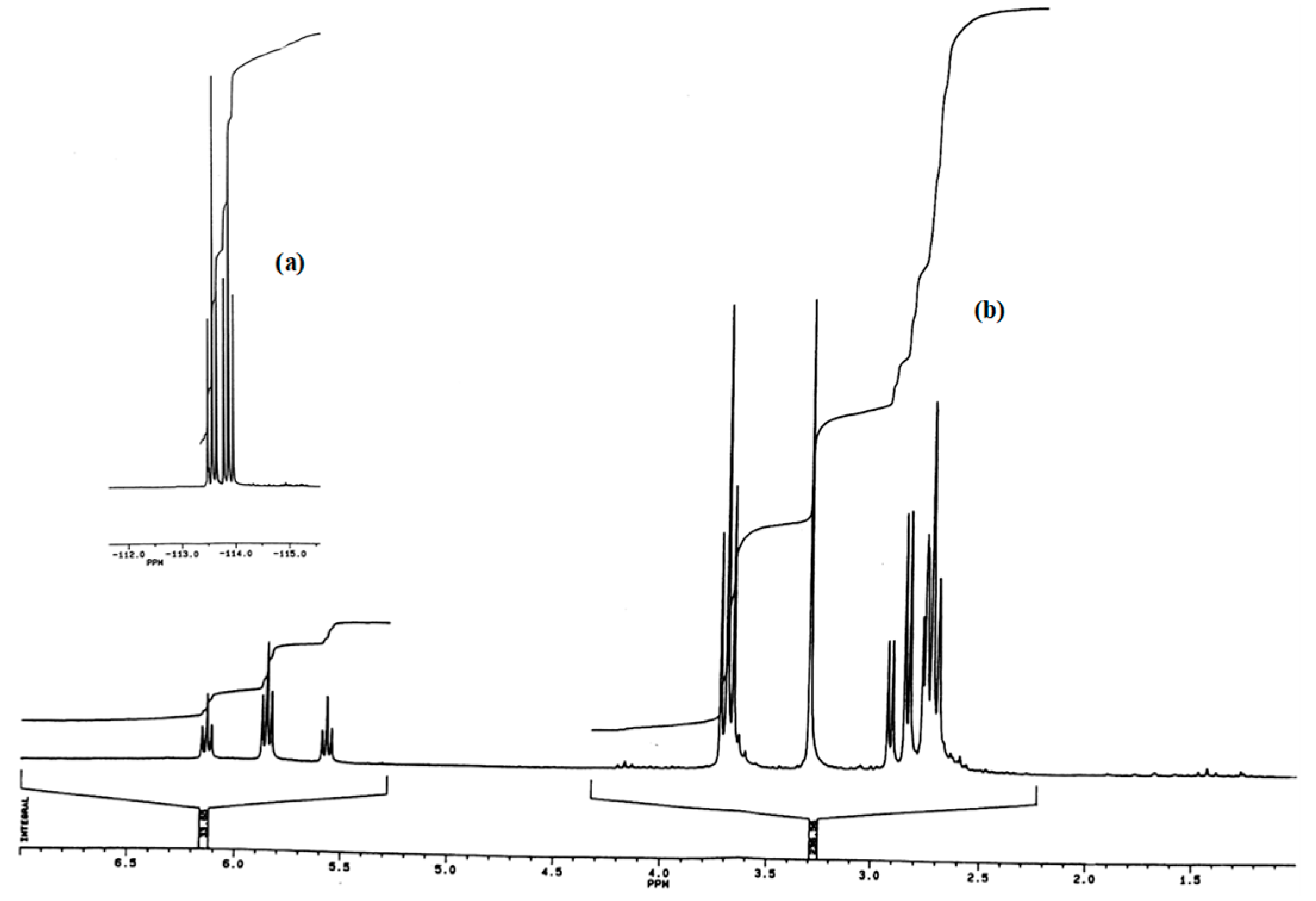

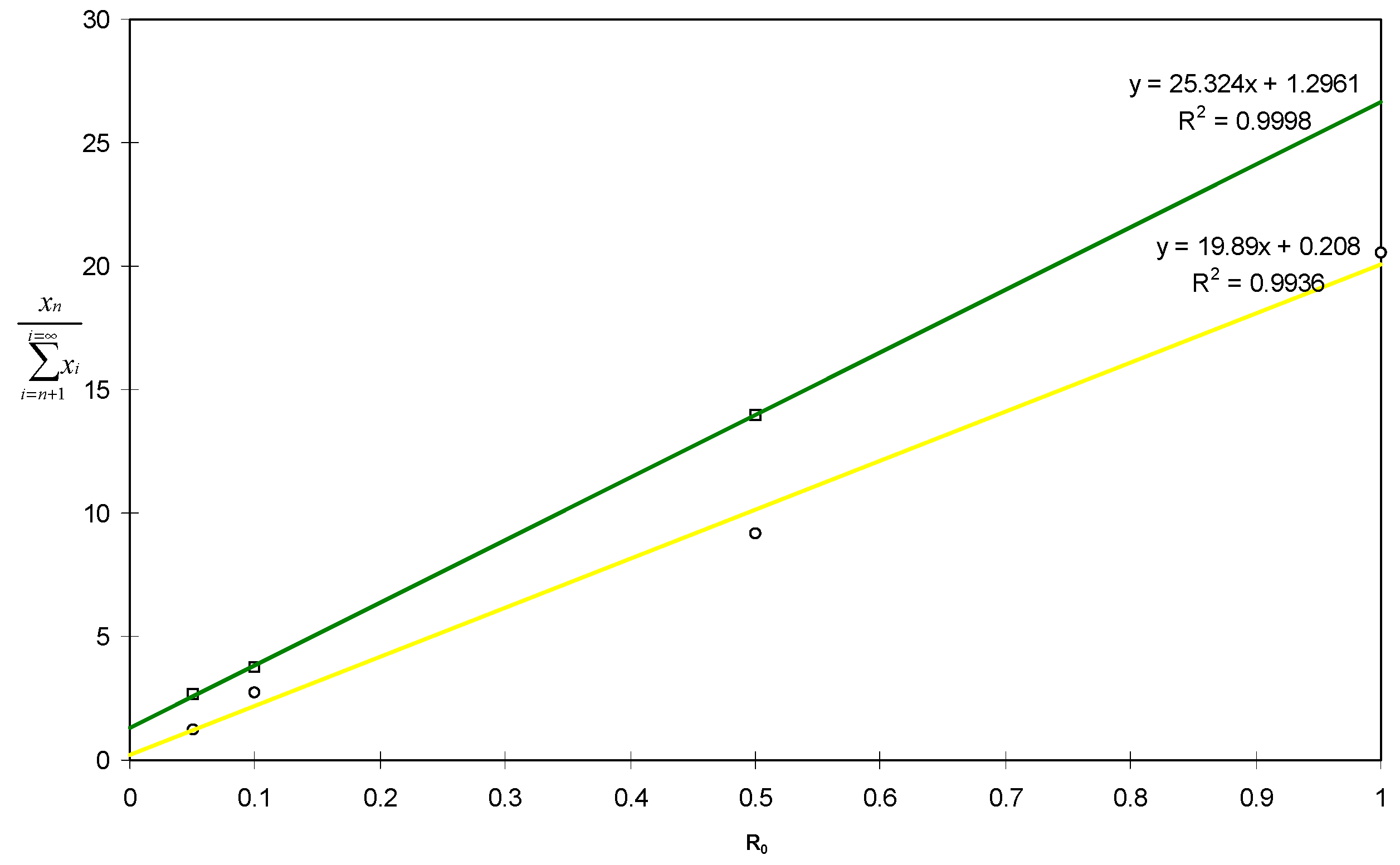
| Chain Transfer Agent | Transfer Constant (T/°C) | References |
|---|---|---|
| HOCH3 | 8 × 10−3 (140) | [10] |
| (EtO)2P(O)-H | 0.34 (140) | [11] |
| Cl3C-H | 0.06 (141) | [12,13] |
| Cl3C-Cl | 0.25 (141) | [12,13] |
| BrCF2CFCl-Br | 1.30 (75) | [14] |
| C6F13-I | 7.4 (75) | [16] |
| HCF2CF2CH2I | 0.3 (75) | [16] |
| Xanthate | 49 (73) | [17] |
| Cl3C-Br | 34 (141) | [12,13] |
| ICl | >40 (130) | [18] |
| RSH | 40 (140) | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duc, M.; Boutevin, B.; Ameduri, B. Unexpected Radical Telomerisation of Vinylidene Fluoride with 2-Mercaptoethanol. Molecules 2021, 26, 3082. https://doi.org/10.3390/molecules26113082
Duc M, Boutevin B, Ameduri B. Unexpected Radical Telomerisation of Vinylidene Fluoride with 2-Mercaptoethanol. Molecules. 2021; 26(11):3082. https://doi.org/10.3390/molecules26113082
Chicago/Turabian StyleDuc, Michel, Bernard Boutevin, and Bruno Ameduri. 2021. "Unexpected Radical Telomerisation of Vinylidene Fluoride with 2-Mercaptoethanol" Molecules 26, no. 11: 3082. https://doi.org/10.3390/molecules26113082
APA StyleDuc, M., Boutevin, B., & Ameduri, B. (2021). Unexpected Radical Telomerisation of Vinylidene Fluoride with 2-Mercaptoethanol. Molecules, 26(11), 3082. https://doi.org/10.3390/molecules26113082







