The Effects of Medicinal Plants and Bioactive Natural Compounds on Homocysteine
Abstract
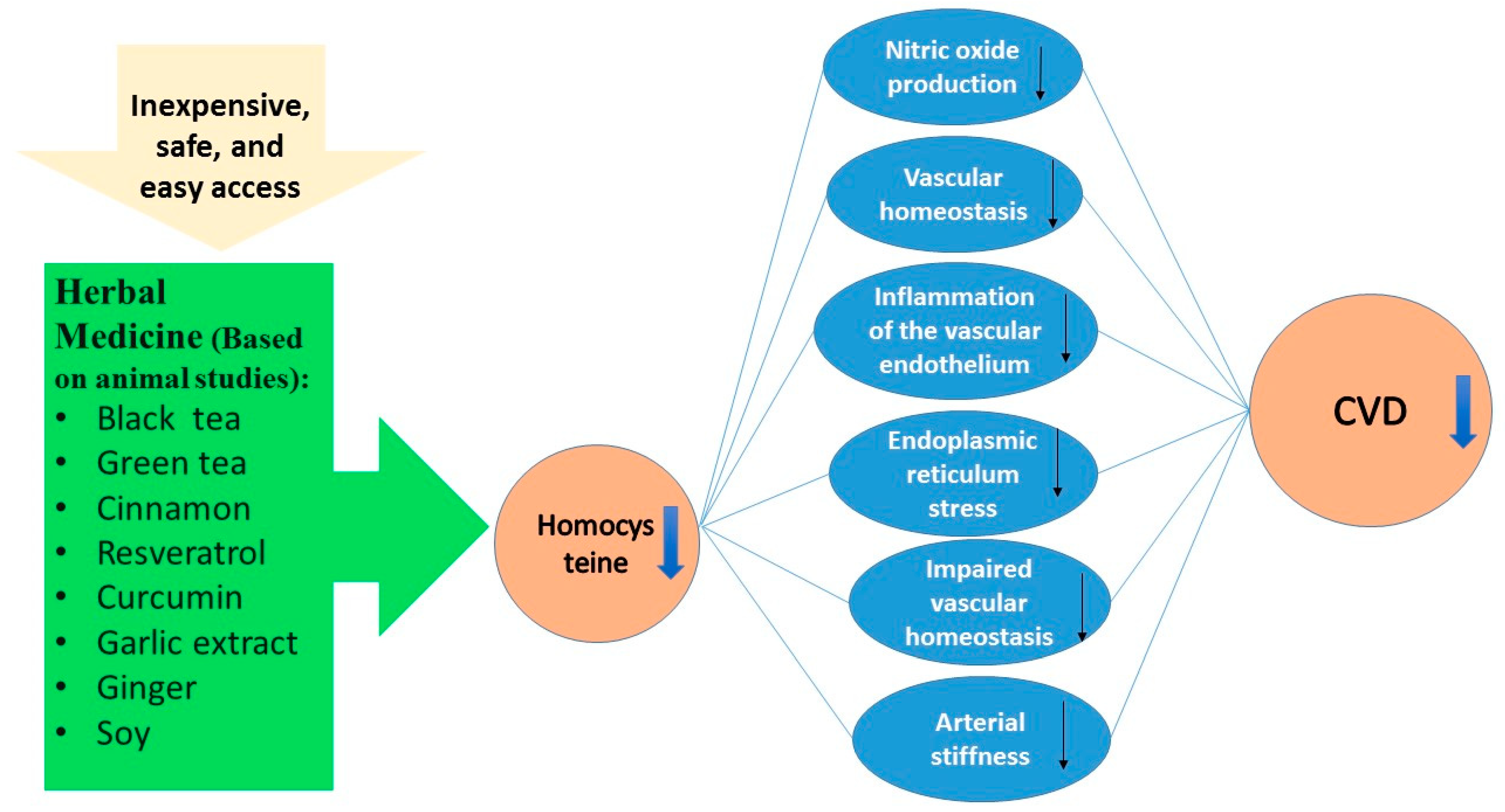
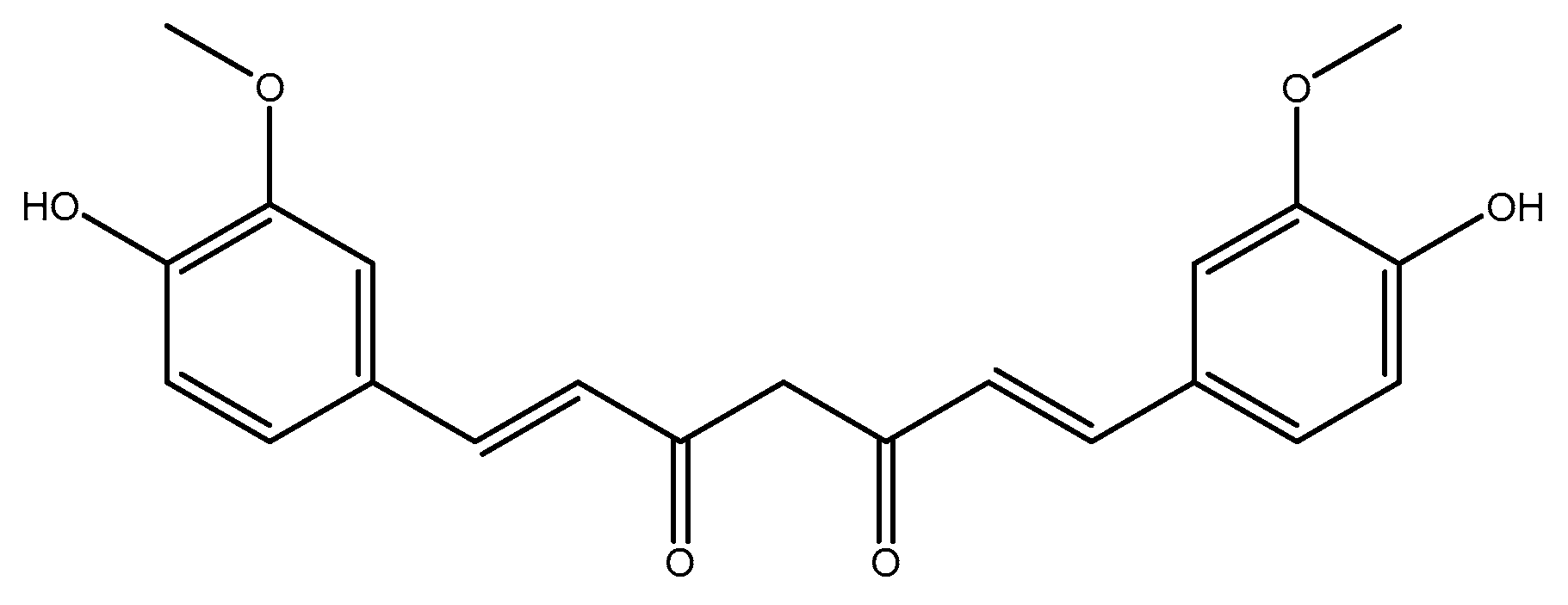
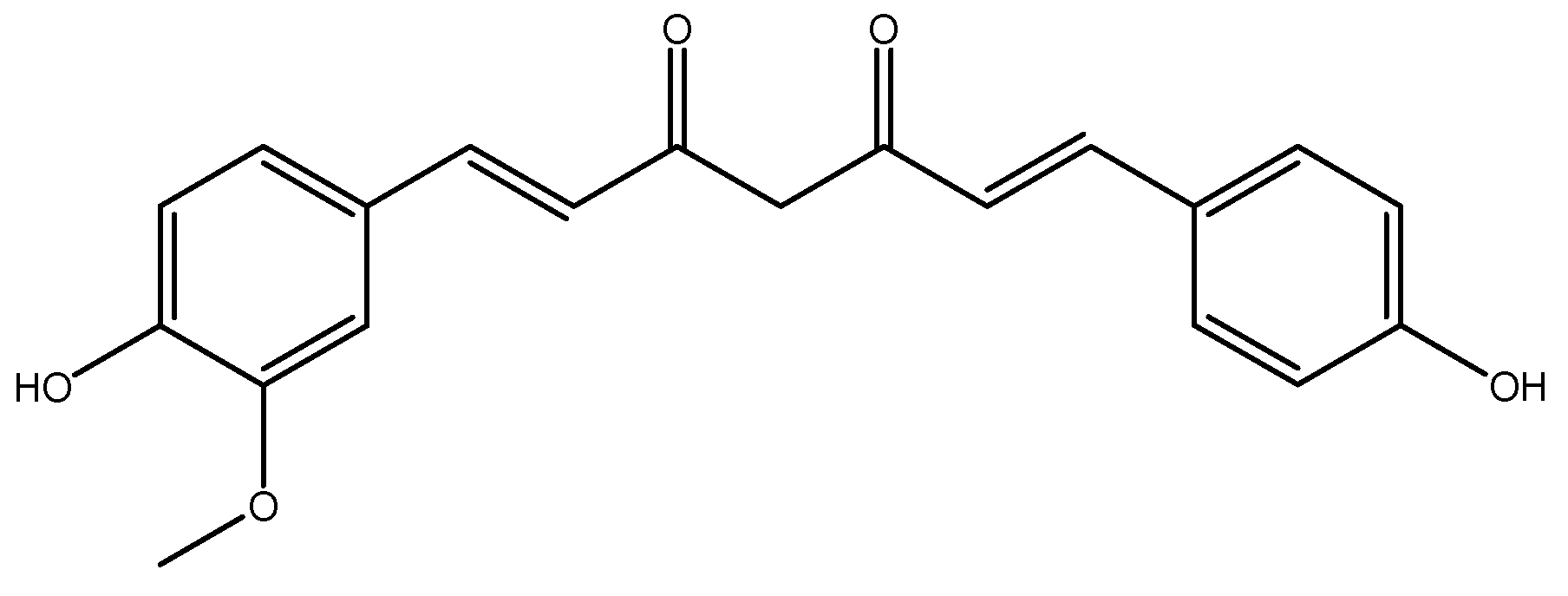
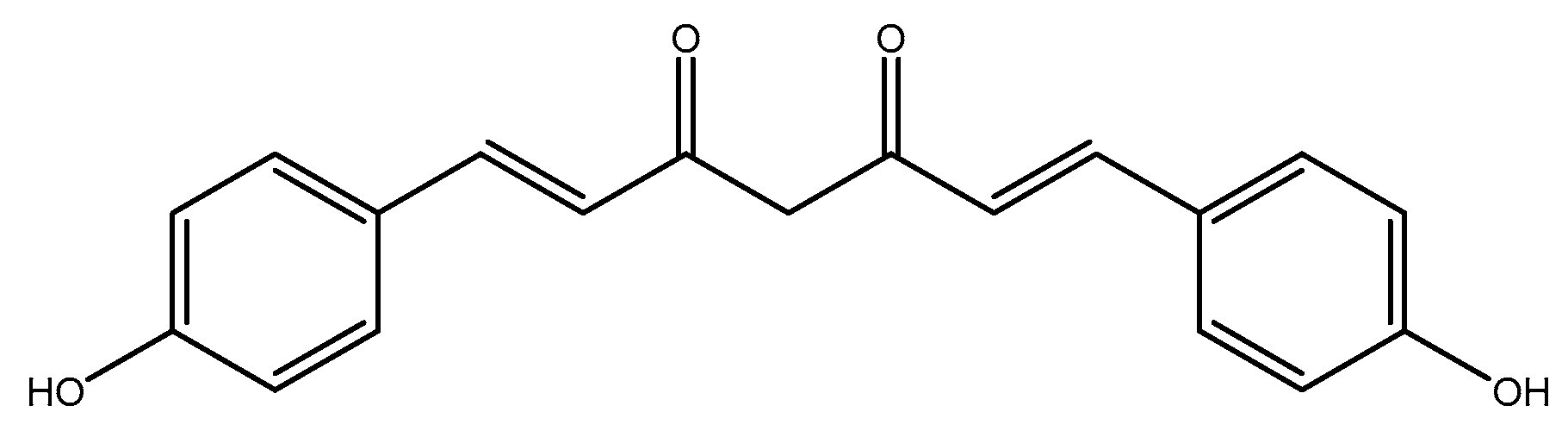
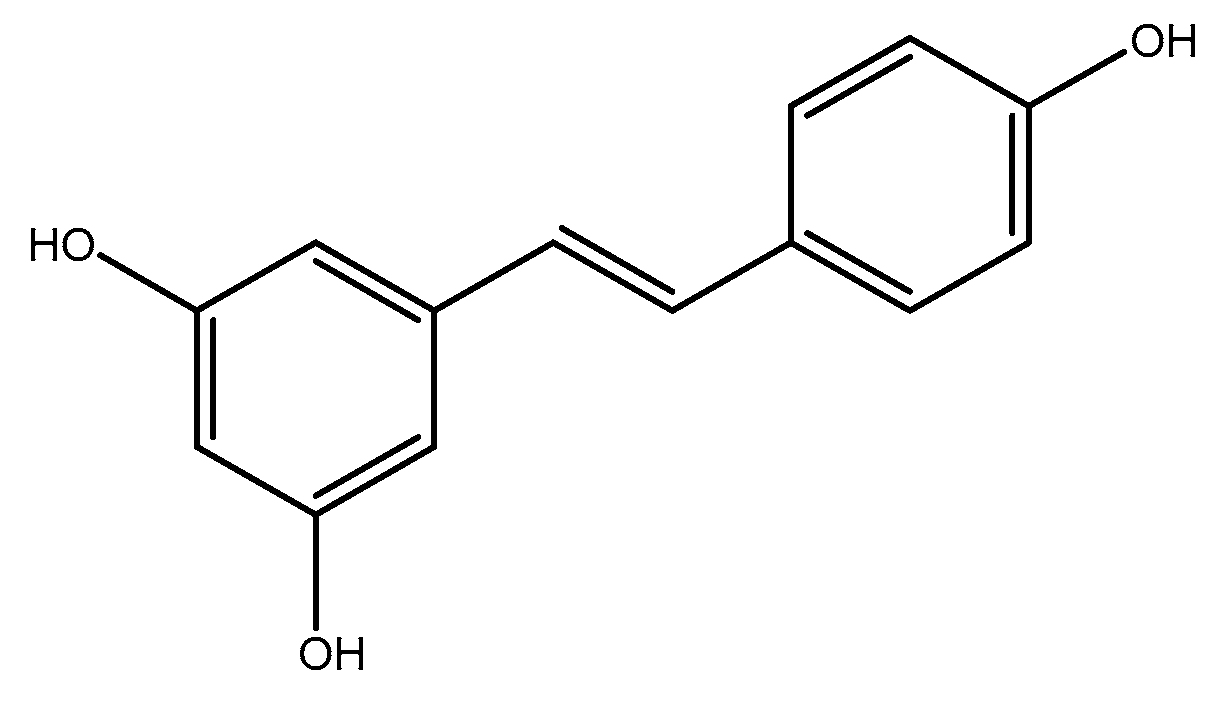
1. Introduction
2. Results
2.1. Plants
2.1.1. Black Tea
2.1.2. Green Tea
2.1.3. Cinnamon
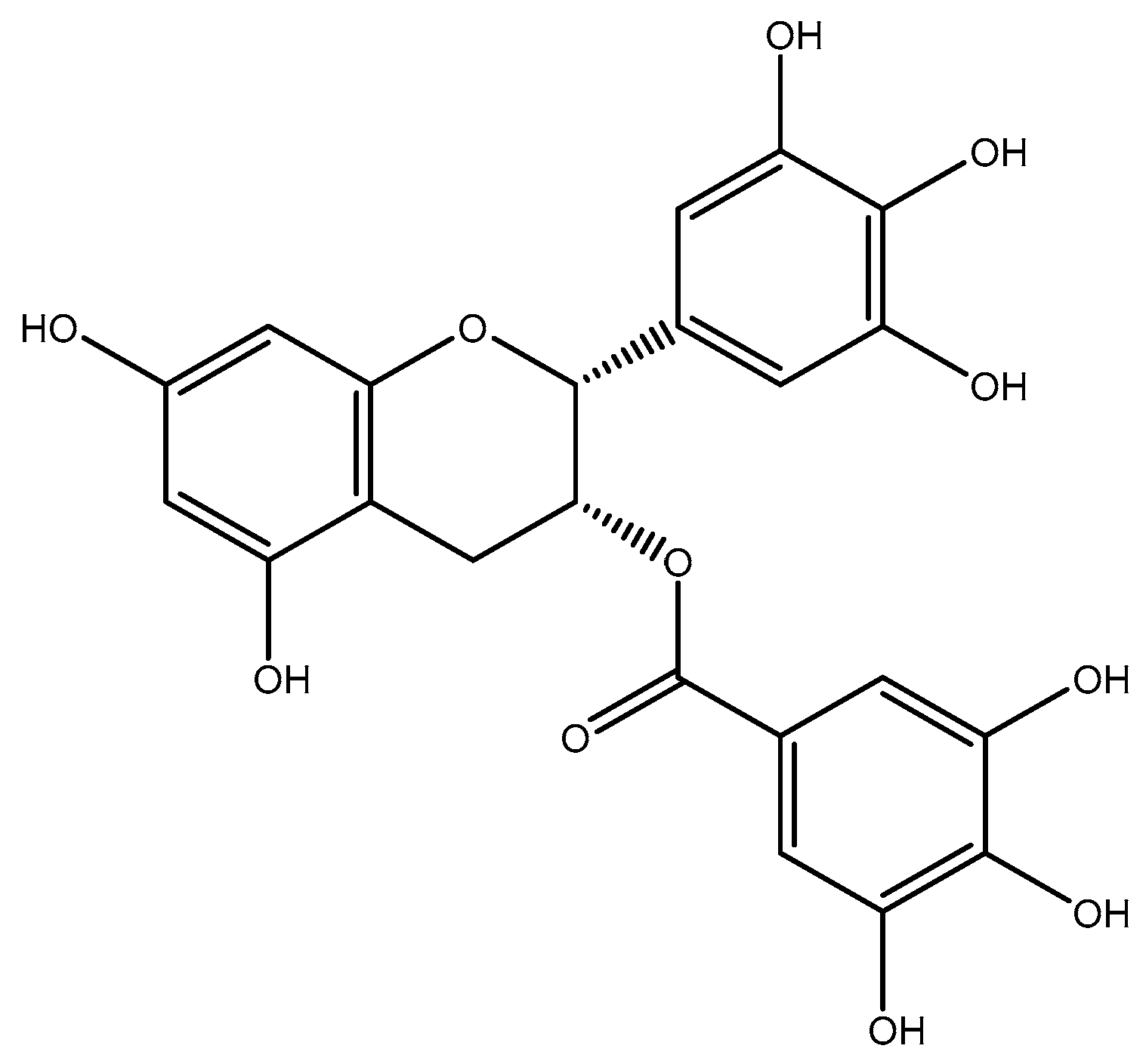
2.1.4. Anthocyanin
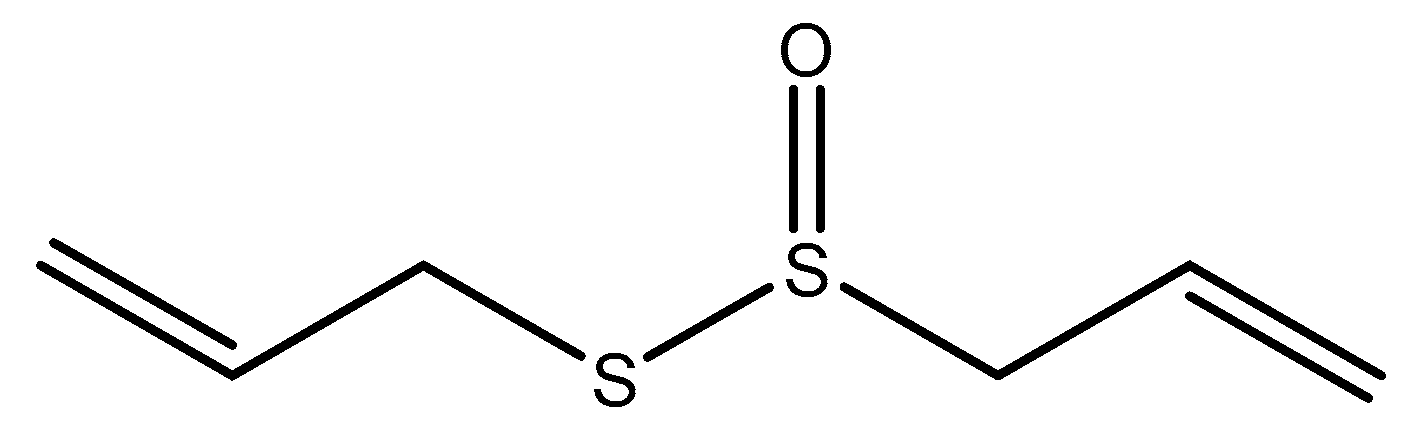
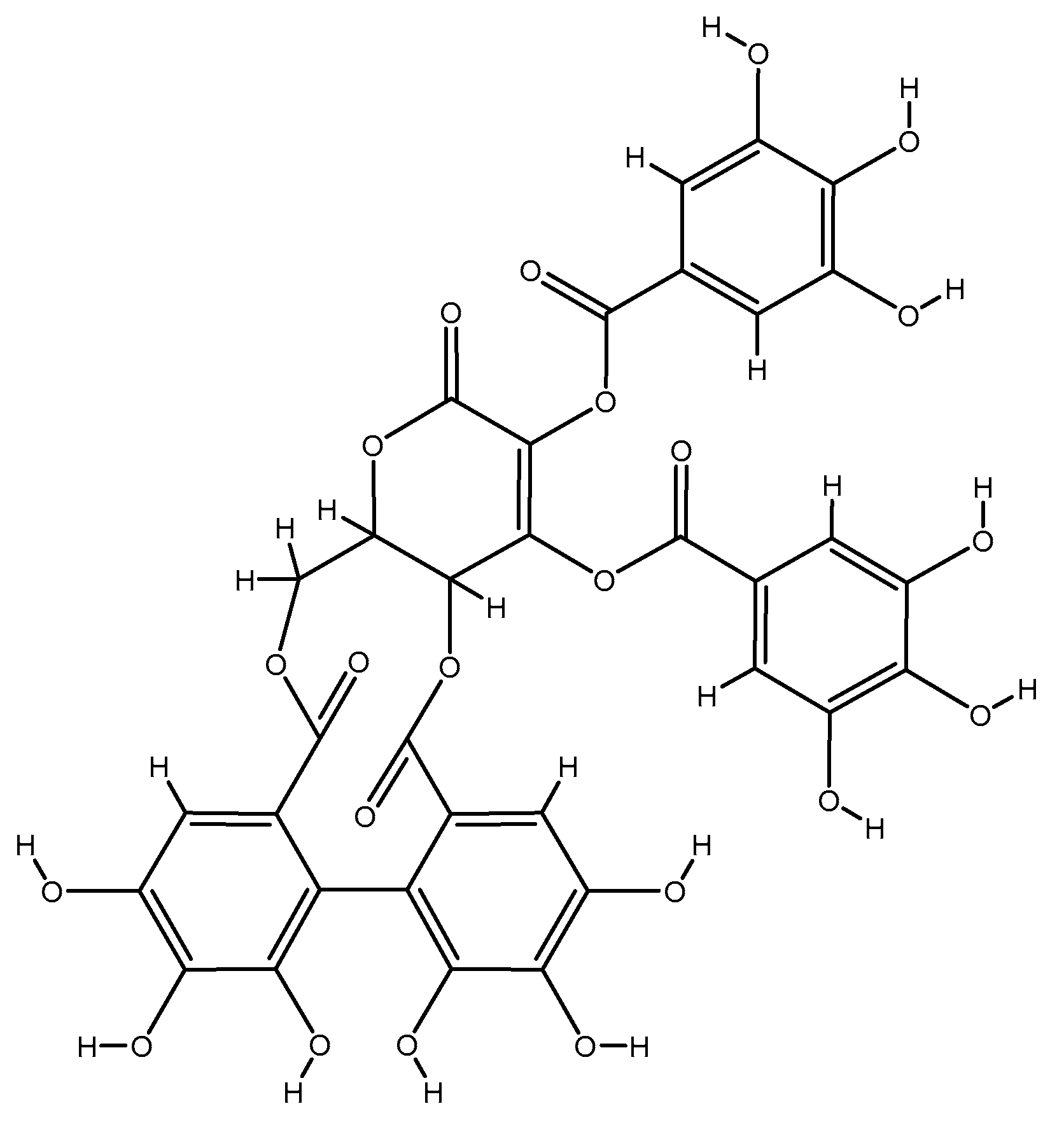
2.1.5. Garlic Extract
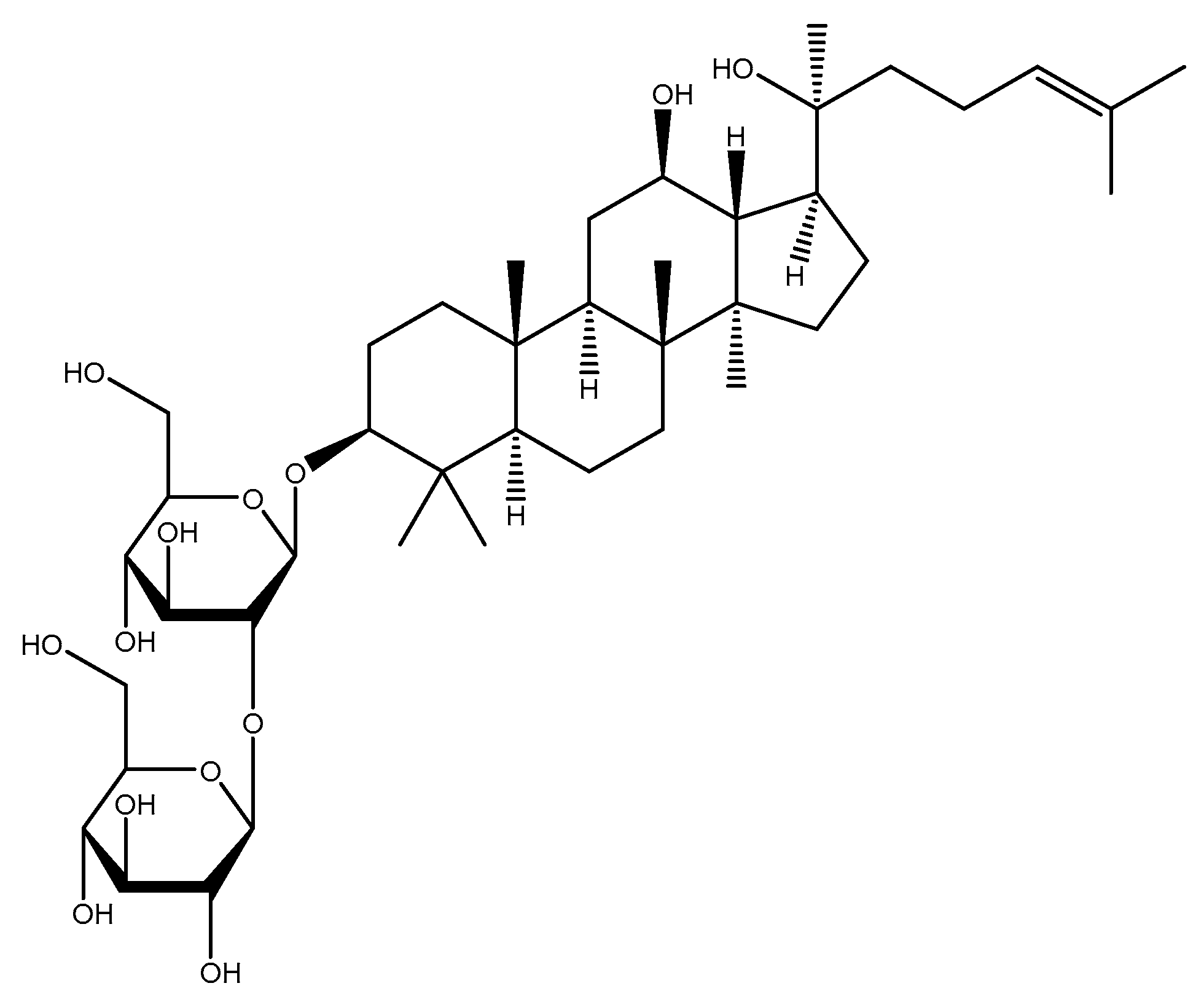
2.1.6. Ginseng
2.1.7. Chlorella
2.1.8. Ginger
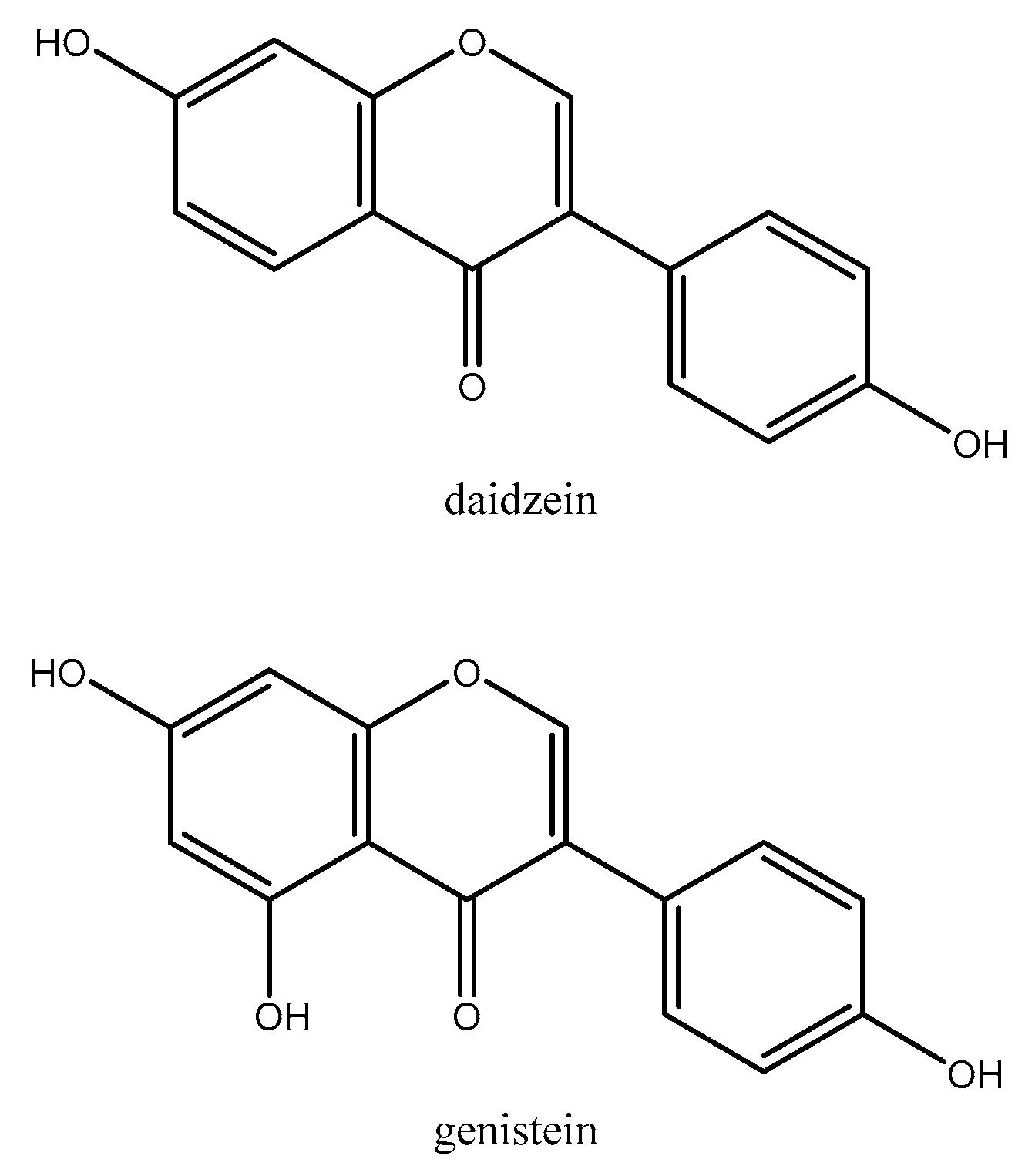
2.1.9. Soy
2.1.10. Emblica Officinals (Amla)
2.1.11. Nuts
2.1.12. Olive Oil
2.2. Phytochemicals
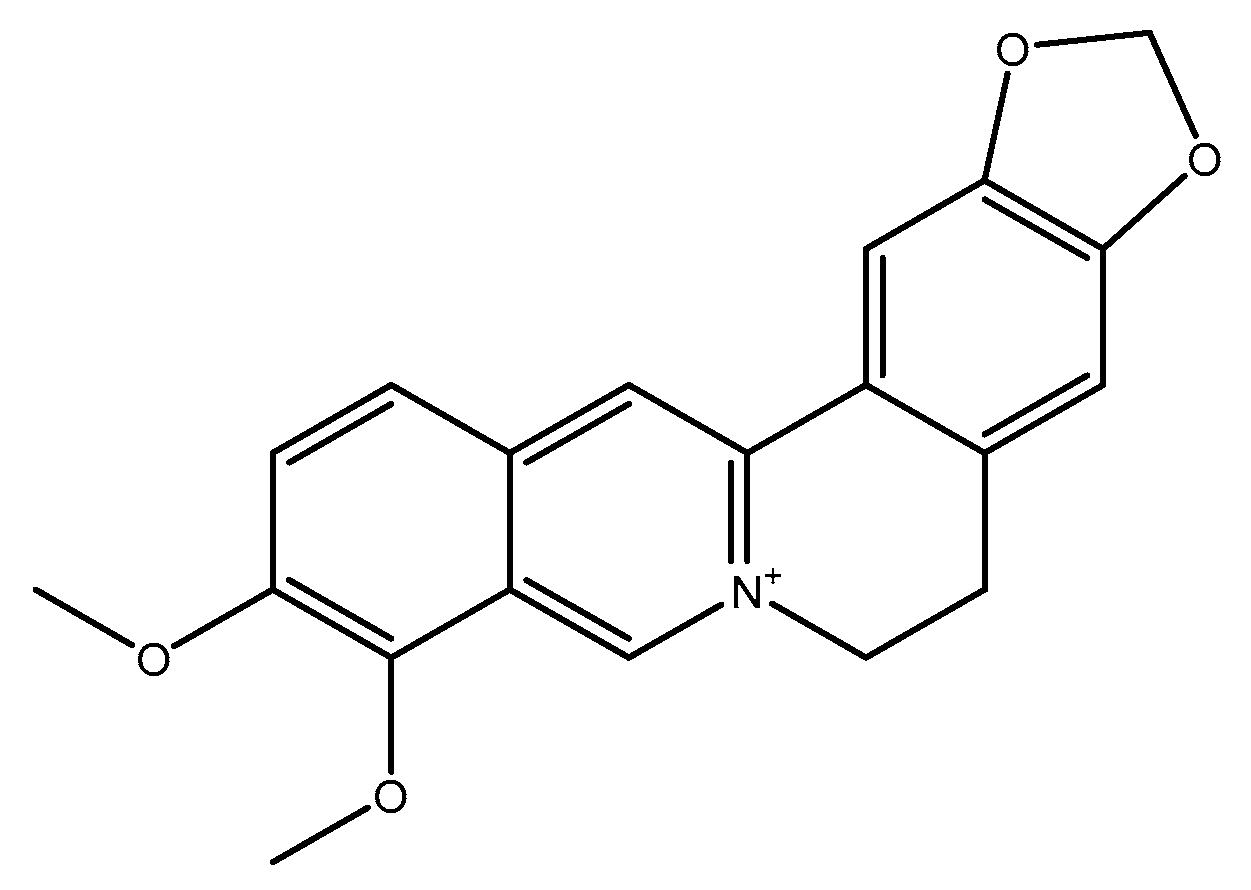
2.2.1. Berberine
2.2.2. Curcumin
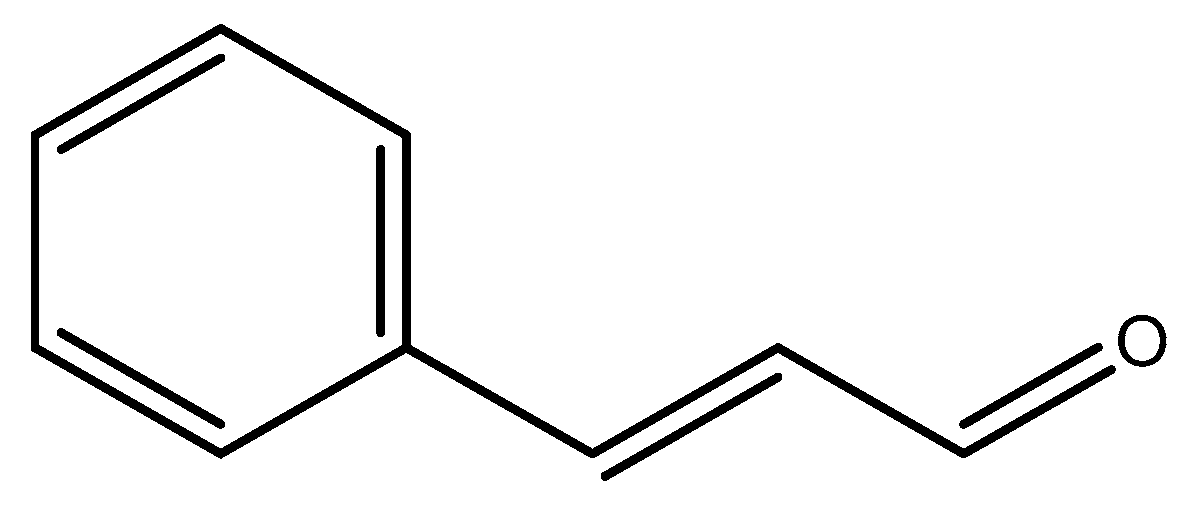
2.2.3. Resveratrol
2.3. Other
Soluble Fiber
3. Conclusions and Future Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kavey, R.-E.W.; Daniels, S.R.; Lauer, R.M.; Atkins, D.L.; Hayman, L.L.; Taubert, K. American Heart Association Guidelines for Primary Prevention of Atherosclerotic Cardiovascular Disease Beginning in Childhood. Circulation 2003, 107, 1562–1566. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.L.; Hayman, L.L.; Daniels, S.R.; Robinson, T.N.; Steinberger, J.; Paridon, S.; Bazzarre, T.J.C. Cardiovascular health in childhood: A statement for health professionals from the Committee on Atherosclerosis, Hypertension, and Obesity in the Young (AHOY) of the Council on Cardiovascular Disease in the Young, American Heart Association. Circulation 2002, 106, 143–160. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Lopez, A.D.; W.H.O. The Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability from Diseases, Injuries, and Risk Factors in 1990 and Projected to 2020: Summary; World Health Organization: Geneva, Switzerland, 1996. [Google Scholar]
- Abubakar, I.; Tillmann, T.; Banerjee, A.J.L. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar]
- Yusuf, S.; Wood, D.; Ralston, J.; Reddy, K.S.J.T.L. The World Heart Federation’s vision for worldwide cardiovascular disease prevention. Lancet 2015, 386, 399–402. [Google Scholar] [CrossRef]
- Levenson, J.W.; Ms, P.J.S.; Gaziano, J.M. Reducing the Global Burden of Cardiovascular Disease: The Role of Risk Factors. Prev. Cardiol. 2002, 5, 188–199. [Google Scholar] [CrossRef]
- Okwuosa, I.S.; Lewsey, S.C.; Adesiyun, T.; Blumenthal, R.S.; Yancy, C.W. Worldwide disparities in cardiovascular disease: Challenges and solutions. Int. J. Cardiol. 2016, 202, 433–440. [Google Scholar] [CrossRef]
- W.H.O.; World Economic Forum. From Burden to “Best Buys”: Reducing the Economic Impact of Non-Communicable Diseases in Low-and Middle-Income Countries; World Health Organization: Geneva, Switzerland; World Economic Forum: Cologny, Switzerland, 2011. [Google Scholar]
- G.H.S.I. Shifting Paradigm: How BRICS are Reshaping Global Health and Development; Global Health Strategies Initiatives: New York, NY, USA, 2012. [Google Scholar]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.J.C. Heart disease and stroke statistics—2018 update: A report from the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef]
- Laslett, L.J.; Alagona, P.; Clark, B.A.; Drozda, J.P.; Saldivar, F.; Wilson, S.R.; Poe, C.; Hart, M. The worldwide environment of cardiovascular disease: Prevalence, diagnosis, therapy, and policy issues: A report from the American College of Cardiology. J. Am. Coll. Cardiol. 2012, 60, S1–S49. [Google Scholar] [CrossRef]
- Finkelstein, J.D.; Martin, J.J. Homocysteine. Int. J. Biochem. Cell Biol. 2000, 32, 385–389. [Google Scholar] [CrossRef]
- Mudd, S.; Ebert, M.H.; Scriver, C.R. Labile methyl group balances in the human: The role of sarcosine. Metabolism 1980, 29, 707–720. [Google Scholar] [CrossRef]
- Mudd, S.; Poole, J.R. Labile methyl balances for normal humans on various dietary regimens. Metabolism 1975, 24, 721–735. [Google Scholar] [CrossRef]
- Finkelstein, J.D.J. Methionine metabolism in mammals. J. Nutr. Biochem. 1990, 1, 228–237. [Google Scholar] [CrossRef]
- McKeever, M.P.; Weir, D.G.; Molloy, A.; Scott, J.M. Betaine-homocysteine methyltransferase: Organ distribution in man, pig and rat and subcellular distribution in the rat. Clin. Sci. 1991, 81, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Selhub, J. Homocysteine metabolism. Annu. Rev. Nutr. 1999, 19, 217–246. [Google Scholar] [CrossRef] [PubMed]
- Christensen, B.; Refsum, H.; Vintermyr, O.; Ueland, P.M. Homocysteine export from cells cultured in the presence of physiological or superfluous levels of methionine: Methionine loading of non-transformed, transformed, proliferating, and quiescent cells in culture. J. Cell. Physiol. 1991, 146, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Ueland, P.M.; Refsum, H.; Male, R.; Lillehaug, J.R. Disposition of endogenous homocysteine by mouse fibroblast C3H/10T1/2 CI 8 and the chemically transformed C3H/10T1/2 MCA CI 16 cells following methotrexate exposure. J. Natl. Cancer Inst. 1986, 77, 283–289. [Google Scholar] [PubMed]
- Refsum, H.; Ueland, P.M. Recent data are not in conflict with homocysteine as a cardiovascular risk factor. Curr. Opin. Lipidol. 1998, 9, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-S.; Wong, P.W.; Malinow, M.R. Hyperhomocyst (e) inemia as a risk factor for occlusive vascular disease. Annu. Rev. Nutr. 1992, 12, 279–298. [Google Scholar] [CrossRef]
- Maron, B.A.; Loscalzo, J. The treatment of hyperhomocysteinemia. Annu. Rev. Med. 2009, 60, 39–54. [Google Scholar] [CrossRef]
- Martí-Carvajal, A.J.; Solà, I.; Lathyris, D.; Dayer, M. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst. Rev. 2017, 8, CD006612. [Google Scholar] [CrossRef]
- Lindqvist, M.; Hellström, A.; Henriksson, A.E. Abdominal aortic aneurysm and the association with serum levels of Homocysteine, vitamins B6, B12 and Folate. Am. J. Cardiovasc. Dis. 2012, 2, 318. [Google Scholar] [PubMed]
- Warsi, A.; Davies, B.; Morris-Stiff, G.; Hullin, D.; Lewis, M. Abdominal Aortic Aneurysm and its Correlation to Plasma Homocysteine, and Vitamins. Eur. J. Vasc. Endovasc. Surg. 2004, 27, 75–79. [Google Scholar] [CrossRef]
- Wald, D.S.; Law, M.; Morris, J. Homocysteine and cardiovascular disease: Evidence on causality from a meta-analysis. BMJ 2002, 325, 1202–1206. [Google Scholar] [CrossRef]
- Humphrey, L.L.; Fu, R.; Rogers, K.; Freeman, M.; Helfand, M. Homocysteine Level and Coronary Heart Disease Incidence: A Systematic Review and Meta-analysis. Mayo Clin. Proc. 2008, 83, 1203–1212. [Google Scholar] [CrossRef]
- Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke. JAMA 2002, 288, 2015–2022. [Google Scholar] [CrossRef]
- Maciel, F.R.; Punaro, G.R.; Rodrigues, A.M.; Bogsan, C.S.; Rogero, M.M.; Oliveira, M.N.; Mouro, M.G.; Higa, E.M. Immunomodulation and nitric oxide restoration by a probiotic and its activity in gut and peritoneal macrophages in diabetic rats. Clin. Nutr. 2016, 35, 1066–1072. [Google Scholar] [CrossRef]
- Klerk, M.; Verhoef, P.; Clarke, R.; Blom, H.J.; Kok, F.J.; Schouten, E.G. MTHFR 677C→ T polymorphism and risk of coronary heart disease: A meta-analysis. JAMA 2002, 288, 2023–2031. [Google Scholar] [CrossRef]
- Azad, A.K.; Huang, P.; Liu, G.; Ren, W.; Teklebrh, T.; Yan, W.; Zhou, X.; Yin, Y. Hyperhomocysteinemia and cardiovascular disease in animal model. Amino Acids 2018, 50, 3–9. [Google Scholar] [CrossRef]
- Balint, B.; Jepchumba, V.K.; Guéant, J.-L.; Guéant-Rodriguez, R.-M. Mechanisms of homocysteine-induced damage to the endothelial, medial and adventitial layers of the arterial wall. Biochimie 2020, 173, 100–106. [Google Scholar] [CrossRef]
- Dayal, S.; Bottiglieri, T.; Arning, E.; Maeda, N.; Malinow, M.R.; Sigmund, C.D.; Heistad, D.D.; Faraci, F.M.; Lentz, S.R. Endothelial Dysfunction and Elevation of S-Adenosylhomocysteine in Cystathionine β-Synthase–Deficient Mice. Circ. Res. 2001, 88, 1203–1209. [Google Scholar] [CrossRef]
- Eberhardt, R.T.; Forgione, M.A.; Cap, A.; Leopold, J.A.; Rudd, M.A.; Trolliet, M.; Heydrick, S.; Stark, R.; Klings, E.S.; Moldovan, N.I.; et al. Endothelial dysfunction in a murine model of mild hyperhomocyst(e)inemia. J. Clin. Investig. 2000, 106, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yang, F.; Tan, H.; Liao, D.; Bryan, R.M., Jr.; Randhawa, J.K.; Rumbaut, R.E.; Durante, W.; Schafer, A.I.; Yang, X.J. Hyperhomocystinemia impairs endothelial function and eNOS activity via PKC activation. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2515–2521. [Google Scholar] [CrossRef] [PubMed]
- Lentz, S.R.; Erger, R.A.; Dayal, S.; Maeda, N.; Malinow, M.R.; Heistad, D.D.; Faraci, F.M. Folate dependence of hyperhomocysteinemia and vascular dysfunction in cystathionine β-synthase-deficient mice. Am. J. Physiol. Circ. Physiol. 2000, 279, H970–H975. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hasegawa, H.; Inaba, N.; Yoshioka, W.; Chang, D.; Liu, J.; Ichida, K. Diet-induced hyperhomocysteinemia impairs vasodilation in 5/6-nephrectomized rats. Amino Acids 2018, 50, 1485–1494. [Google Scholar] [CrossRef]
- Liu, L.-H.; Guo, Z.; Feng, M.; Wu, Z.-Z.; He, Z.-M.; Xiong, Y. Protection of DDAH2 Overexpression Against Homocysteine-Induced Impairments of DDAH/ADMA/NOS/NO Pathway in Endothelial Cells. Cell. Physiol. Biochem. 2012, 30, 1413–1422. [Google Scholar] [CrossRef]
- Szabo, C.J. Hydrogen sulfide, an enhancer of vascular nitric oxide signaling: Mechanisms and implications. Am. J. Physiol. Cell Physiol. 2017, 312, C3–C15. [Google Scholar] [CrossRef]
- Han, S.; Wu, H.; Li, W.; Gao, P. Protective effects of genistein in homocysteine-induced endothelial cell inflammatory injury. Mol. Cell. Biochem. 2015, 403, 43–49. [Google Scholar] [CrossRef]
- Kamat, P.; Kalani, A.; Givvimani, S.; Sathnur, P.; Tyagi, S.; Tyagi, N. Hydrogen sulfide attenuates neurodegeneration and neurovascular dysfunction induced by intracerebral-administered homocysteine in mice. Neuroscience 2013, 252, 302–319. [Google Scholar] [CrossRef]
- Li, J.; Luo, M.; Xie, N.; Wang, J.; Chen, L. Curcumin protects endothelial cells against homocysteine induced injury through inhibiting inflammation. Am. J. Transl. Res. 2016, 8, 4598–4604. [Google Scholar]
- Wu, X.; Zhang, L.; Miao, Y.; Yang, J.; Wang, X.; Wang, C.-C.; Feng, J.; Wang, L. Homocysteine causes vascular endothelial dysfunction by disrupting endoplasmic reticulum redox homeostasis. Redox Biol. 2019, 20, 46–59. [Google Scholar] [CrossRef]
- Zulli, A.; Widdop, R.E.; Hare, D.L.; Buxton, B.F.; Black, M.J. High Methionine and Cholesterol Diet Abolishes Endothelial Relaxation. Arter. Thromb. Vasc. Biol. 2003, 23, 1358–1363. [Google Scholar] [CrossRef]
- Sipkens, J.A.; Hahn, N.; Brand, C.S.V.D.; Meischl, C.; Cillessen, S.A.G.M.; Smith, D.E.C.; Juffermans, L.J.M.; Musters, R.J.P.; Roos, D.; Jakobs, C.; et al. Homocysteine-Induced Apoptosis in Endothelial Cells Coincides with Nuclear NOX2 and Peri-nuclear NOX4 Activity. Cell Biophys. 2011, 67, 341–352. [Google Scholar] [CrossRef]
- Gurda, D.; Handschuh, L.; Kotkowiak, W.; Jakubowski, H. Homocysteine thiolactone and N-homocysteinylated protein induce pro-atherogenic changes in gene expression in human vascular endothelial cells. Amino Acids 2015, 47, 1319–1339. [Google Scholar] [CrossRef]
- Buemi, M.; Marino, D.; Di Pasquale, G.; Floccari, F.; Ruello, A.; Aloisi, C.; Corica, F.; Senatore, M.; Romeo, A.; Frisina, N. Effects of Homocysteine on Proliferation, Necrosis, and Apoptosis of Vascular Smooth Muscle Cells in Culture and Influence of Folic Acid. Thromb. Res. 2001, 104, 207–213. [Google Scholar] [CrossRef]
- Tsai, J.C.; Wang, H.; Perrella, M.A.; Yoshizumi, M.; Sibinga, N.E.; Tan, L.C.; Haber, E.; Chang, T.H.; Schlegel, R.; Lee, M.E. Induction of cyclin A gene expression by homocysteine in vascular smooth muscle cells. J. Clin. Investig. 1996, 97, 146–153. [Google Scholar] [CrossRef]
- Küskü-Kiraz, Z.; Genc, S.; Bekpınar, S.; Ünlücerci, Y.; Çevik, A.; Olgaç, V.; Gürdöl, F.; Uysal, M. Effects of betaine supplementation on nitric oxide metabolism, atherosclerotic parameters, and fatty liver in guinea pigs fed a high cholesterol plus methionine diet. Nutrients 2018, 45, 41–48. [Google Scholar] [CrossRef]
- Majesky, M.W. Developmental basis of vascular smooth muscle diversity. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1248–1258. [Google Scholar] [CrossRef]
- Hofmann, M.A.; Lalla, E.; Lu, Y.; Gleason, M.R.; Wolf, B.M.; Tanji, N.; Ferran, L.J.; Kohl, B.; Rao, V.; Kisiel, W.; et al. Hyperhomocysteinemia enhances vascular inflammation and accelerates atherosclerosis in a murine model. J. Clin. Investig. 2001, 107, 675–683. [Google Scholar] [CrossRef]
- Liu, X.; Luo, F.; Li, J.; Wu, W.; Li, L.; Chen, H. Homocysteine induces connective tissue growth factor expression in vascular smooth muscle cells. J. Thromb. Haemost. 2007, 6, 184–192. [Google Scholar] [CrossRef]
- Ovechkin, A.V.; Tyagi, N.; Sen, U.; Lominadze, D.; Steed, M.M.; Moshal, K.S.; Tyagi, S.C. 3-Deazaadenosine mitigates arterial remodeling and hypertension in hyperhomocysteinemic mice. Am. J. Physiol. Cell. Mol. Physiol. 2006, 291, L905–L911. [Google Scholar] [CrossRef]
- Wilson, K.; Lindholt, J.; Hoskins, P.; Heickendorff, L.; Vammen, S.; Bradbury, A. The Relationship Between Abdominal Aortic Aneurysm Distensibility and Serum Markers of Elastin and Collagen Metabolism. Eur. J. Vasc. Endovasc. Surg. 2001, 21, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Leon, A.S.; Bronas, U.G. Dyslipidemia and risk of coronary heart disease: Role of lifestyle approaches for its management. Am. J. Lifestyle Med. 2009, 3, 257–273. [Google Scholar] [CrossRef]
- Bagherniya, M.; Nobili, V.; Blesso, C.N.; Sahebkar, A. Medicinal plants and bioactive natural compounds in the treatment of non-alcoholic fatty liver disease: A clinical review. Pharmacol. Res. 2018, 130, 213–240. [Google Scholar] [CrossRef] [PubMed]
- Gurib-Fakim, A. Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol. Asp. Med. 2006, 27, 1–93. [Google Scholar] [CrossRef]
- Moss, J.W.; Ramji, D.P. Nutraceutical therapies for atherosclerosis. Nat. Rev. Cardiol. 2016, 13, 513–532. [Google Scholar] [CrossRef]
- Sosnowska, B.; Penson, P.; Banach, M. The role of nutraceuticals in the prevention of cardiovascular disease. Cardiovasc. Diagn. Ther. 2017, 67, S21–S31. [Google Scholar] [CrossRef]
- DeFelice, S.L. The nutraceutical revolution: Its impact on food industry R&D. Trends Food Sci. Technol. 1995, 6, 59–61. [Google Scholar]
- Santini, A.; Novellino, E. Nutraceuticals: Beyond the diet before the drugs. Curr. Bioact. Compd. 2014, 10, 1–12. [Google Scholar] [CrossRef]
- Santini, A.; Novellino, E. Nutraceuticals in hypercholesterolaemia: An overview. Br. J. Pharmacol. 2017, 174, 1450–1463. [Google Scholar] [CrossRef]
- Alissa, E.M.; Ferns, G.A. Functional Foods and Nutraceuticals in the Primary Prevention of Cardiovascular Diseases. J. Nutr. Metab. 2012, 2012, 1–16. [Google Scholar] [CrossRef]
- Ramaa, C.S.; Shirode, A.R.; Mundada, A.S.; Kadam, V.J. Nutraceuticals—An Emerging Era in the Treatment and Prevention of Cardiovascular Diseases. Curr. Pharm. Biotechnol. 2006, 7, 15–23. [Google Scholar] [CrossRef]
- Zuchi, C.; Ambrosio, G.; Lüscher, T.F.; Landmesser, U. Nutraceuticals in Cardiovascular Prevention: Lessons from Studies on Endothelial Function. Cardiovasc. Ther. 2010, 28, 187–201. [Google Scholar] [CrossRef]
- Badimon, L.; Vilahur, G.; Padro, T. Nutraceuticals and Atherosclerosis: Human Trials. Cardiovasc. Ther. 2010, 28, 202–215. [Google Scholar] [CrossRef]
- McCarty, M.F. Nutraceutical resources for diabetes prevention—An update. Med. Hypotheses 2005, 64, 151–158. [Google Scholar] [CrossRef]
- Davì, G.; Santilli, F.; Patrono, C. Nutraceuticals in Diabetes and Metabolic Syndrome. Cardiovasc. Ther. 2010, 28, 216–226. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Azizi, F. Dietary polyphenols as potential nutraceuticals in management of diabetes: A review. J. Diabetes Metab. Disord. 2013, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Houston, M. The role of nutrition and nutraceutical supplements in the treatment of hypertension. World J. Cardiol. 2014, 6, 38–66. [Google Scholar] [CrossRef]
- Houston, M.C. Nutraceuticals, Vitamins, Antioxidants, and Minerals in the Prevention and Treatment of Hypertension. Prog. Cardiovasc. Dis. 2005, 47, 396–449. [Google Scholar] [CrossRef]
- Houston, M.C. Nutrition and nutraceutical supplements in the treatment of hypertension. Expert Rev. Cardiovasc. Ther. 2010, 8, 821–833. [Google Scholar] [CrossRef]
- Mahdavi, A.; Bagherniya, M.; Fakheran, O.; Reiner, Ž.; Xu, S.; Sahebkar, A. Medicinal plants and bioactive natural compounds as inhibitors of HMG-CoA reductase: A literature review. BioFactors 2020, 46, 906–926. [Google Scholar] [CrossRef]
- Talebi, S.; Bagherniya, M.; Atkin, S.L.; Askari, G.; Orafai, H.M.; Sahebkar, A. The beneficial effects of nutraceuticals and natural products on small dense LDL levels, LDL particle number and LDL particle size: A clinical review. Lipids Health Dis. 2020, 19, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Izzo, R.; de Simone, G.; Giudice, R.; Chinali, M.; Trimarco, V.; De Luca, N.; Trimarco, B. Effects of nutraceuticals on prevalence of metabolic syndrome and on calculated Framingham Risk Score in individuals with dyslipidemia. J. Hypertens. 2010, 28, 1482–1487. [Google Scholar] [CrossRef]
- Houston, M. The Role of Nutraceutical Supplements in the Treatment of Dyslipidemia. J. Clin. Hypertens. 2012, 14, 121–132. [Google Scholar] [CrossRef]
- Sirtori, C.R.; Galli, C.; Anderson, J.W.; Arnoldi, A. Nutritional and nutraceutical approaches to dyslipidemia and atherosclerosis prevention: Focus on dietary proteins. Atherosclerosis 2009, 203, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Mannarino, M.R.; Ministrini, S.; Pirro, M. Nutraceuticals for the treatment of hypercholesterolemia. Eur. J. Intern. Med. 2014, 25, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Scicchitano, P.; Cameli, M.; Maiello, M.; Modesti, P.A.; Muiesan, M.L.; Novo, S.; Palmiero, P.; Saba, P.S.; Pedrinelli, R.; Ciccone, M.M. Nutraceuticals and dyslipidaemia: Beyond the common therapeutics. J. Funct. Foods 2014, 6, 11–32. [Google Scholar] [CrossRef]
- Arab, L.; Liu, W.; Elashoff, D. Green and black tea consumption and risk of stroke: A meta-analysis. Stroke 2009, 40, 1786–1792. [Google Scholar] [CrossRef] [PubMed]
- Bahorun, T.; Luximon-Ramma, A.; Gunness, T.K.; Sookar, D.; Bhoyroo, S.; Jugessur, R.; Reebye, D.; Googoolye, K.; Crozier, A.; Aruoma, O.I. Black tea reduces uric acid and C-reactive protein levels in humans susceptible to cardiovascular diseases. Toxicology 2010, 278, 68–74. [Google Scholar] [CrossRef]
- Vasisht, K. Study to Promote the Industrial Exploitation of Green Tea Polyphenols in India; UNIDO: Vienna, Austria, 2003. [Google Scholar]
- Hayat, K.; Iqbal, H.; Malik, U.; Bilal, U.; Mushtaq, S. Tea and Its Consumption: Benefits and Risks. Crit. Rev. Food Sci. Nutr. 2015, 55, 939–954. [Google Scholar] [CrossRef]
- Cheang, W.S.; Ngai, C.Y.; Tam, Y.Y.; Tian, X.Y.; Wong, W.T.; Zhang, Y.; Lau, C.W.; Chen, Z.Y.; Bian, Z.-X.; Huang, Y.; et al. Black tea protects against hypertension-associated endothelial dysfunction through alleviation of endoplasmic reticulum stress. Sci. Rep. 2015, 5, 10340. [Google Scholar] [CrossRef]
- Hodgson, J.M.; Puddey, I.B.; Van Bockxmeer, F.M.; Burke, V. Acute effects of tea on fasting and non-fasting plasma total homocysteine concentrations in human subjects. Br. J. Nutr. 2007, 97, 842–846. [Google Scholar] [CrossRef][Green Version]
- Hodgson, J.M.; Burke, V.; Beilin, L.J.; Croft, K.D.; Puddey, I.B. Can black tea influence plasma total homocysteine concentrations? Am. J. Clin. Nutr. 2003, 77, 907–911. [Google Scholar] [CrossRef]
- Olthof, M.R.; Hollman, P.C.; Zock, P.L.; Katan, M.B. Consumption of high doses of chlorogenic acid, present in coffee, or of black tea increases plasma total homocysteine concentrations in humans. Am. J. Clin. Nutr. 2001, 73, 532–538. [Google Scholar] [CrossRef]
- Urgert, R.; Van Vliet, T.; Zock, P.L.; Katan, M.B. Heavy coffee consumption and plasma homocysteine: A randomized controlled trial in healthy volunteers. Am. J. Clin. Nutr. 2000, 72, 1107–1110. [Google Scholar] [CrossRef]
- Verhoef, P.; Pasman, W.J.; Van Vliet, T.; Urgert, R.; Katan, M.B. Contribution of caffeine to the homocysteine-raising effect of coffee: A randomized controlled trial in humans. Am. J. Clin. Nutr. 2002, 76, 1244–1248. [Google Scholar] [CrossRef]
- Hartley, L.; Flowers, N.; Holmes, J.; Clarke, A.; Stranges, S.; Hooper, L.; Rees, K. Green and black tea for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef]
- Mineharu, Y.; Koizumi, A.; Wada, Y.; Iso, H.; Watanabe, Y.; Date, C.; Yamamoto, A.; Kikuchi, S.; Inaba, Y.; Toyoshima, H.; et al. Coffee, green tea, black tea and oolong tea consumption and risk of mortality from cardiovascular disease in Japanese men and women. J. Epidemiol. Community Health 2009, 65, 230–240. [Google Scholar] [CrossRef]
- Tang, J.; Zheng, J.-S.; Fang, L.; Jin, Y.; Cai, W.; Li, D. Tea consumption and mortality of all cancers, CVD and all causes: A meta-analysis of eighteen prospective cohort studies. Br. J. Nutr. 2015, 114, 673–683. [Google Scholar] [CrossRef]
- Cabrera, C.; Artacho, R.; Giménez, R. Beneficial effects of green tea—A review. J. Am. Coll. Nutr. 2006, 25, 79–99. [Google Scholar] [CrossRef]
- Goszcz, K.; Duthie, G.G.; Stewart, D.; Leslie, S.J.; Megson, I.L. Bioactive polyphenols and cardiovascular disease: Chemical antagonists, pharmacological agents or xenobiotics that drive an adaptive response? Br. J. Pharmacol. 2017, 174, 1209–1225. [Google Scholar] [CrossRef]
- Šilarová, P.; Česlová, L.; Meloun, M. Fast gradient HPLC/MS separation of phenolics in green tea to monitor their degradation. Food Chem. 2017, 237, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Assi, M. The Impact of Physical Activity and Antioxidants on Tumor-Skeletal Muscle Crosstalk during Cancer: Deciphering Signaling Pathways Involved in Tumor Growth and Muscle Wasting. Ph.D. Thesis, University Rennes 2, Rennes, France, 2016. [Google Scholar]
- Lee, S.-R.; Suh, S.-I.; Kim, S.-P. Protective effects of the green tea polyphenol (−)-epigallocatechin gallate against hippocampal neuronal damage after transient global ischemia in gerbils. Neurosci. Lett. 2000, 287, 191–194. [Google Scholar] [CrossRef]
- Ortiz-López, L.; Márquez-Valadez, B.; Gómez-Sánchez, A.; Silva-Lucero, M.; Torres-Pérez, M.; Téllez-Ballesteros, R.; Ichwan, M.; Meraz-Ríos, M.; Kempermann, G.; Ramírez-Rodríguez, G. Green tea compound epigallo-catechin-3-gallate (EGCG) increases neuronal survival in adult hippocampal neurogenesis in vivo and in vitro. Neuroscience 2016, 322, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Bagherniya, M.; Khedmatgozar, H.; Fakheran, O.; Xu, S.; Johnston, T.P.; Sahebkar, A. Medicinal plants and bioactive natural products as inhibitors of NLRP3 inflammasome. Phytother. Res. 2021. [Google Scholar] [CrossRef]
- Wang, L.; Tian, X. Epigallocatechin-3-Gallate Protects against Homocysteine-Induced Brain Damage in Rats. Planta Med. 2017, 84, 34–41. [Google Scholar] [CrossRef]
- El-Missiry, M.A.; Othman, A.I.; El-Sawy, M.R.; Lebede, M.F. Neuroprotective effect of epigallocatechin-3-gallate (EGCG) on radiation-induced damage and apoptosis in the rat hippocampus. Int. J. Radiat. Biol. 2018, 94, 798–808. [Google Scholar] [CrossRef]
- Ranasinghe, P.; Pigera, S.; Premakumara, G.A.S.; Galappaththy, P.; Constantine, G.R.; Katulanda, P. Medicinal properties of ‘true’ cinnamon (Cinnamomum zeylanicum): A systematic review. BMC Complement. Altern. Med. 2013, 13, 275. [Google Scholar] [CrossRef]
- Bandara, T.; Uluwaduge, I.; Jansz, E.R. Bioactivity of cinnamon with special emphasis on diabetes mellitus: A review. Int. J. Food Sci. Nutr. 2011, 63, 380–386. [Google Scholar] [CrossRef]
- Deng, R. A review of the hypoglycemic effects of five commonly used herbal food supplements. Recent Pat. Foodnutrition Agric. 2012, 4, 50–60. [Google Scholar]
- Mousavi, S.M.; Rahmani, J.; Kord-Varkaneh, H.; Sheikhi, A.; Larijani, B.; Esmaillzadeh, A. Cinnamon supplementation positively affects obesity: A systematic review and dose-response meta-analysis of randomized controlled trials. Clin. Nutr. 2020, 39, 123–133. [Google Scholar] [CrossRef]
- Mahdavi, A.; Bagherniya, M.; Mirenayat, M.S.; Atkin, S.L.; Sahebkar, A. Medicinal Plants and Phytochemicals Regulating Insulin Resistance and Glucose Homeostasis in Type 2 Diabetic Patients: A Clinical Review. Adv. Exp. Med. Biol. 2021, 1308, 161–183. [Google Scholar] [CrossRef]
- Barceloux, D.G. Cinnamon (Cinnamomum Species). Dis. Mon. 2009, 55, 327–335. [Google Scholar] [CrossRef]
- Amin, K.A.; El-Twab, T.M.A. Oxidative markers, nitric oxide and homocysteine alteration in hypercholesterolimic rats: Role of atorvastatine and cinnamon. Int. J. Clin. Exp. Med. 2009, 2, 254–265. [Google Scholar]
- Waterhouse, A.L. Determination of total phenolics. Curr. Protoc. Food Anal. Chem. 2002, 6. [Google Scholar] [CrossRef]
- Buchert, J.; Koponen, J.M.; Suutarinen, M.; Mustranta, A.; Lille, M.; Törrönen, R.; Poutanen, K. Effect of enzyme-aided pressing on anthocyanin yield and profiles in bilberry and blackcurrant juices. J. Sci. Food Agric. 2005, 85, 2548–2556. [Google Scholar] [CrossRef]
- Valenti, L.; Riso, P.; Mazzocchi, A.; Porrini, M.; Fargion, S.; Agostoni, C. Dietary Anthocyanins as Nutritional Therapy for Nonalcoholic Fatty Liver Disease. Oxidative Med. Cell. Longev. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the pH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Chandrasekhar, J.; Madhusudhan, M.; Raghavarao, K. Extraction of anthocyanins from red cabbage and purification using adsorption. Food Bioprod. Process. 2012, 90, 615–623. [Google Scholar] [CrossRef]
- Ho, S.-C.; Hwang, L.S.; Shen, Y.-J.; Lin, C.-C. Suppressive Effect of a Proanthocyanidin-rich Extract from Longan (Dimocarpus longan Lour.) Flowers on Nitric Oxide Production in LPS-Stimulated Macrophage Cells. J. Agric. Food Chem. 2007, 55, 10664–10670. [Google Scholar] [CrossRef]
- Duthie, S.J.; Jenkinson, A.M.E.; Crozier, A.; Mullen, W.; Pirie, L.; Kyle, J.; Yap, L.S.; Christen, P.; Duthie, G.G. The effects of cranberry juice consumption on antioxidant status and biomarkers relating to heart disease and cancer in healthy human volunteers. Eur. J. Nutr. 2006, 45, 113–122. [Google Scholar] [CrossRef]
- Mikaili, P.; Maadirad, S.; Moloudizargari, M.; Aghajanshakeri, S.; Sarahroodi, S. Therapeutic Uses and Pharmacological Properties of Garlic, Shallot, and Their Biologically Active Compounds. Iran J. Basic Med. Sci. 2013, 16, 1031–1048. [Google Scholar]
- Ankri, S.; Mirelman, D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999, 1, 125–129. [Google Scholar] [CrossRef]
- Josling, P. Preventing the common cold with a garlic supplement: A double-blind, placebo-controlled survey. Adv. Ther. 2001, 18, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Khodavandi, A.; Alizadeh, F.; Aala, F.; Sekawi, Z.; Chong, P.P. In Vitro Investigation of Antifungal Activity of Allicin Alone and in Combination with Azoles Against Candida Species. Mycopathology 2009, 169, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.M.B.L.; Freitas, F.I.D.S.; Morais, L.C.S.L.D.; Cavalcanti, M.G.D.S.; Silva, L.F.D.; Padilha, R.J.R.; Barbosa, C.G.S.; Santos, F.A.B.D.; Alves, L.C.; Diniz, M.D.F.F.M. Ultrastructural study on the morphological changes to male worms of Schistosoma mansoni after in vitro exposure to allicin. Revista Sociedade Brasileira Medicina Tropical 2011, 44, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Younis, F.; Mirelman, D.; Rabinkov, A.; Rosenthal, T. S-Allyl-Mercapto-Captopril: A Novel Compound in the Treatment of Cohen-Rosenthal Diabetic Hypertensive Rats. J. Clin. Hypertens. 2010, 12, 451–455. [Google Scholar] [CrossRef]
- Krishna, A.; Yadav, A. Lead compound design for TPR/COX dual inhibition. J. Mol. Model. 2012, 18, 4397–4408. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, G.-Y.; Choi, I.-W.; Kim, N.D.; Hwang, H.J.; Choi, Y.-W.; Choi, Y.H. Inhibition of Matrix Metalloproteinase Activities and Tightening of Tight Junctions by Diallyl Disulfide in AGS Human Gastric Carcinoma Cells. J. Food Sci. 2011, 76, T105–T111. [Google Scholar] [CrossRef]
- Tattelman, E. Health effects of garlic. Am. Fam. Physician 2005, 72, 103–106. [Google Scholar]
- Ahmadi, N.; Nabavi, V.; Hajsadeghi, F.; Zeb, I.; Flores, F.; Ebrahimi, R.; Budoff, M. Aged garlic extract with supplement is associated with increase in brown adipose, decrease in white adipose tissue and predict lack of progression in coronary atherosclerosis. Int. J. Cardiol. 2013, 168, 2310–2314. [Google Scholar] [CrossRef]
- Budoff, M.J.; Ahmadi, N.; Gul, K.M.; Liu, S.T.; Flores, F.R.; Tiano, J.; Takasu, J.; Miller, E.; Tsimikas, S. Aged garlic extract supplemented with B vitamins, folic acid and l-arginine retards the progression of subclinical atherosclerosis: A randomized clinical trial. Prev. Med. 2009, 49, 101–107. [Google Scholar] [CrossRef]
- Yeh, Y.-Y.; Yeh, S.-M. Homocysteine-Lowering Action Is Another Potential Cardiovascular Protective Factor of Aged Garlic Extract. J. Nutr. 2006, 136, 745S–749S. [Google Scholar] [CrossRef]
- Budoff, M.J.; Takasu, J.; Flores, F.R.; Niihara, Y.; Lu, B.; Lau, B.H.; Rosen, R.T.; Amagase, H. Inhibiting progression of coronary calcification using Aged Garlic Extract in patients receiving statin therapy: A preliminary study. Prev. Med. 2004, 39, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.Y.; Lee, S.R.; Kim, H.K.; Baek, Y.H.; Kwak, Y.S.; Ko, T.H.; Kim, N.; Rhee, B.D.; Ko, K.S.; Park, B.J.; et al. Independent beneficial effects of aged garlic extract intake with regular exercise on cardiovascular risk in postmenopausal women. Nutr. Res. Pr. 2012, 6, 226–231. [Google Scholar] [CrossRef]
- McCrindle, B.W.; Helden, E.; Conner, W.T. Garlic Extract Therapy in Children with Hypercholesterolemia. Arch. Pediatr. Adolesc. Med. 1998, 152, 1089–1094. [Google Scholar] [CrossRef]
- Ried, K.; Travica, N.; Sali, A. The effect of aged garlic extract on blood pressure and other cardiovascular risk factors in uncontrolled hypertensives: The AGE at Heart trial. Integr. Blood Press. Control. 2016, 9, 9–21. [Google Scholar] [CrossRef]
- Wee, J.J.; Park, K.M.; Chung, A.-S. Biological activities of ginseng and its application to human health. Herb. Med. Biomol. Clin. Asp. 2011, 2, 157–174. [Google Scholar]
- Park, S.E.; Park, C.; Kim, S.H.; Hossain, M.A.; Kim, M.Y.; Chung, H.Y.; Son, W.S.; Kim, G.-Y.; Choi, Y.H.; Kim, N.D. Korean red ginseng extract induces apoptosis and decreases telomerase activity in human leukemia cells. J. Ethnopharmacol. 2009, 121, 304–312. [Google Scholar] [CrossRef]
- Vuksan, V.; Sung, M.-K.; Sievenpiper, J.L.; Stavro, P.M.; Jenkins, A.L.; Di Buono, M.; Lee, K.-S.; Leiter, L.A.; Nam, K.Y.; Arnason, J.T.; et al. Korean red ginseng (Panax ginseng) improves glucose and insulin regulation in well-controlled, type 2 diabetes: Results of a randomized, double-blind, placebo-controlled study of efficacy and safety. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 46–56. [Google Scholar] [CrossRef]
- Kim, J.-H.; Cho, S.Y.; Lee, J.-H.; Jeong, S.M.; Yoon, I.-S.; Lee, B.-H.; Lee, J.-H.; Pyo, M.K.; Lee, S.-M.; Chung, J.-M.; et al. Neuroprotective effects of ginsenoside Rg3 against homocysteine-induced excitotoxicity in rat hippocampus. Brain Res. 2007, 1136, 190–199. [Google Scholar] [CrossRef]
- Kim, J.-H. Ginseng Total Saponin Attenuate Cardiac Hypertrophy Induced by Homocysteine in Rats. J. Ginseng Res. 2009, 33, 260–267. [Google Scholar] [CrossRef][Green Version]
- Belasco, W. Algae Burgers for a Hungry World? The Rise and Fall of Chlorella Cuisine. Technol. Cult. 1997, 38, 608. [Google Scholar] [CrossRef]
- Komiyama, K.; Hirokawa, Y.; Mocota, T. Chlorella in cancer therapy. Chemotherapy 1986, 34, 302–307. [Google Scholar]
- Konishi, F.; Tanaka, K.; Himeno, K.; Taniguchi, K.; Nomoto, K. Antitumor effect induced by a hot water extract of Chlorella vulgaris (CE): Resistance to meth-A tumor growth mediated by CE-induced polymorphonuclear leukocytes. Cancer Immunol. Immunother. 1985, 19, 73–78. [Google Scholar] [CrossRef]
- Merchant, R.E.; Rice, C.D.; Young, H.F. Dietary Chlorella pyrenoidosa for patients with malignant glioma: Effects on immunocompetence, quality of life, and survival. Phytother. Res. 1990, 4, 220–231. [Google Scholar] [CrossRef]
- Mitsuda, H.; Nishikawa, Y.; Higuchi, M.; Nakajima, K.; Kawai, F. Effect of the Breaking of Chlorella Cells on the Digestibility of Chlorella Protein. Eiyo Shokuryo 1977, 30, 93–98. [Google Scholar] [CrossRef]
- Miyazawa, Y.; Murayama, T.; Ooya, N.; Wang, L.; Tung, Y.; Yamaguchi, N. Immunomodulation by a unicellular green algae (Chlorella pyrenoidosa) in tumor-bearing mice. J. Ethnopharmacol. 1988, 24, 135–146. [Google Scholar] [CrossRef]
- Tanaka, K.; Koga, T.; Konishi, F.; Nakamura, M.; Mitsuyama, M.; Himeno, K.; Nomoto, K. Augmentation of host defense by a unicellular green alga, Chlorella vulgaris, to Escherichia coli infection. Infect. Immun. 1986, 53, 267–271. [Google Scholar] [CrossRef]
- Merchant, R.E.; Phillips, T.W.; Udani, J. Nutritional Supplementation with Chlorella pyrenoidosa Lowers Serum Methylmalonic Acid in Vegans and Vegetarians with a Suspected Vitamin B12 Deficiency. J. Med. Food 2015, 18, 1357–1362. [Google Scholar] [CrossRef]
- White, B. Ginger: An overview. Am. Fam. Physician 2007, 75, 1689–1691. [Google Scholar]
- Borrelli, F.; Capasso, R.; Aviello, G.; Pittler, M.H.; Izzo, A. Effectiveness and Safety of Ginger in the Treatment of Pregnancy-Induced Nausea and Vomiting. Obstet. Gynecol. 2005, 105, 849–856. [Google Scholar] [CrossRef]
- Chaiyakunapruk, N.; Kitikannakorn, N.; Nathisuwan, S.; Leeprakobboon, K.; Leelasettagool, C. The efficacy of ginger for the prevention of postoperative nausea and vomiting: A meta-analysis. Am. J. Obstet. Gynecol. 2006, 194, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Ernst, E.; Pittler, M.H. Efficacy of ginger for nausea and vomiting: A systematic review of randomized clinical trials. Br. J. Anaesth. 2000, 84, 367–371. [Google Scholar] [CrossRef]
- Jewell, D.; Young, G. Interventions for nausea and vomiting in early pregnancy. Cochrane Database Syst. Rev. 2010, CD000145. [Google Scholar] [CrossRef]
- Manusirivithaya, S.; Sripramote, M.; Tangjitgamol, S.; Sheanakul, C.; Leelahakorn, S.; Thavaramara, T.; Tangcharoenpanich, K. Antiemetic effect of ginger in gynecologic oncology patients receiving cisplatin. Int. J. Gynecol. Cancer 2004, 14, 1063–1069. [Google Scholar] [CrossRef]
- Altman, R.D.; Marcussen, K.C. Effects of a ginger extract on knee pain in patients with osteoarthritis. Arthritis Rheum. 2001, 44, 2531–2538. [Google Scholar] [CrossRef]
- Bliddal, H.; Rosetzsky, A.; Schlichting, P.; Weidner, M.; Andersen, L.; Ibfelt, H.-H.; Christensen, K.; Jensen, O.; Barslev, J. A randomized, placebo-controlled, cross-over study of ginger extracts and Ibuprofen in osteoarthritis. Osteoarthr. Cartil. 2000, 8, 9–12. [Google Scholar] [CrossRef]
- Srivastava, K.; Mustafa, T. Ginger (Zingiber officinale) in rheumatism and musculoskeletal disorders. Med. Hypotheses 1992, 39, 342–348. [Google Scholar] [CrossRef]
- Ilkhanizadeh, B.; Shirpoor, A.; Ansari, M.H.K.; Nemati, S.; Rasmi, Y. Protective Effects of Ginger (Zingiber officinale) Extract against Diabetes-Induced Heart Abnormality in Rats. Diabetes Metab. J. 2016, 40, 46–53. [Google Scholar] [CrossRef]
- Akbari, A.; Nasiri, K.; Heydari, M.; Mosavat, S.H.; Iraji, A. The Protective Effect of Hydroalcoholic Extract of Zingiber officinale Roscoe (Ginger) on Ethanol-Induced Reproductive Toxicity in Male Rats. J. Evid. Based Integr. Med. 2017, 22, 609–617. [Google Scholar] [CrossRef]
- Golbitz, P. Traditional soyfoods: Processing and products. J. Nutr. 1995, 125, 570–572. [Google Scholar]
- Lee, G.-A.; Crawford, G.W.; Liu, L.; Sasaki, Y.; Chen, X. Archaeological Soybean (Glycine max) in East Asia: Does Size Matter? PLoS ONE 2011, 6, e26720. [Google Scholar] [CrossRef] [PubMed]
- Abuajah, C.I.; Ogbonna, A.C.; Osuji, C.M. Functional components and medicinal properties of food: A review. J. Food Sci. Technol. 2015, 52, 2522–2529. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.; Ryder, J.; Kurzer, M.; Lampe, J.; Messina, M.; Phipps, W.; Cassidy, A. Effects of soy protein and isoflavones on circulating hormone concentrations in pre- and post-menopausal women: A systematic review and meta-analysis. Hum. Reprod. Updat. 2009, 15, 423–440. [Google Scholar] [CrossRef]
- Kohama, T.; Kobayashi, H.; Inoue, M. The Effect of Soybeans on the Anovulatory Cycle. J. Med. Food 2005, 8, 550–551. [Google Scholar] [CrossRef]
- Tokede, O.A.; Onabanjo, T.A.; Yansane, A.; Gaziano, J.M.; Djoussé, L. Soya products and serum lipids: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2015, 114, 831–843. [Google Scholar] [CrossRef]
- Song, X.; Zeng, R.; Ni, L.; Liu, C. The effect of soy or isoflavones on homocysteine levels: A meta-analysis of randomised controlled trials. J. Hum. Nutr. Diet. 2016, 29, 797–804. [Google Scholar] [CrossRef]
- Llaneza, P.; González, C.; Fernandez-Iñarrea, J.; Alonso, A.; Diaz, F.; Arnott, I.; Ferrer-Barriendos, J. Soy isoflavones, diet and physical exercise modify serum cytokines in healthy obese postmenopausal women. Phytomedicine 2011, 18, 245–250. [Google Scholar] [CrossRef]
- Høie, L.H.; Graubaum, H.-J.; Harde, A.; Gruenwald, J.; Wernecke, K.-D. Lipid-lowering effect of 2 dosages of a soy protein supplement in Hypercholesterolemia. Adv. Ther. 2005, 22, 175–186. [Google Scholar] [CrossRef]
- Greany, K.A.; Nettleton, J.A.; Wangen, K.E.; Thomas, W.; Kurzer, M.S. Consumption of isoflavone-rich soy protein does not alter homocysteine or markers of inflammation in postmenopausal women. Eur. J. Clin. Nutr. 2007, 62, 1419–1425. [Google Scholar] [CrossRef]
- Imani, H.; Tabibi, H.; Atabak, S.; Rahmani, L.; Ahmadinejad, M.; Hedayati, M. Effects of Soy Consumption on Oxidative Stress, Blood Homocysteine, Coagulation Factors, and Phosphorus in Peritoneal Dialysis Patients. J. Ren. Nutr. 2009, 19, 389–395. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Kendall, C.W.C.; Jackson, C.-J.C.; Connelly, P.W.; Parker, T.; Faulkner, D.; Vidgen, E.; Cunnane, S.C.; Leiter, L.A.; Josse, R.G. Effects of high- and low-isoflavone soyfoods on blood lipids, oxidized LDL, homocysteine, and blood pressure in hyperlipidemic men and women. Am. J. Clin. Nutr. 2002, 76, 365–372. [Google Scholar] [CrossRef]
- Hanson, L.N.; Engelman, H.M.; Alekel, D.L.; Schalinske, K.L.; Kohut, M.L.; Reddy, M.B. Effects of soy isoflavones and phytate on homocysteine, C-reactive protein, and iron status in postmenopausal women. Am. J. Clin. Nutr. 2006, 84, 774–780. [Google Scholar] [CrossRef]
- Reimann, M.; Dierkes, J.; Carlsohn, A.; Talbot, D.; Ferrari, M.; Hallund, J.; Hall, W.L.; Vafeiadou, K.; Huebner, U.; Branca, F.; et al. Consumption of Soy Isoflavones Does Not Affect Plasma Total Homocysteine or Asymmetric Dimethylarginine Concentrations in Healthy Postmenopausal Women. J. Nutr. 2006, 136, 100–105. [Google Scholar] [CrossRef]
- Brandão, L.C.; Hachul, H.; Bittencourt, L.R.; Baracat, E.C.; Tufik, S.; D’Almeida, V. Effects of isoflavone on oxidative stress parameters and homocysteine in postmenopausal women complaining of insomnia. Biol. Res. 2009, 42, 281–287. [Google Scholar] [CrossRef]
- Snelson, M.; Mamo, J.C.L.; Lam, V.; Giles, C.; Takechi, R. Differential Effects of High-Protein Diets Derived from Soy and Casein on Blood–Brain Barrier Integrity in Wild-type Mice. Front. Nutr. 2017, 4, 35. [Google Scholar] [CrossRef]
- Adam, S.K.; Das, S.; Soelaiman, I.N.; Umar, N.A.; Jaarin, K. Consumption of Repeatedly Heated Soy Oil Increases the Serum Parameters Related to Atherosclerosis in Ovariectomized Rats. Tohoku J. Exp. Med. 2008, 215, 219–226. [Google Scholar] [CrossRef]
- Kumar, K.B.H.; Sabu, M.C.; Lima, P.S.; Kuttan, R. Modulation of Haematopoetic System and Antioxidant Enzymes by Emblica Officinalis Gaertn and its Protective Role Against γ-radiation Induced Damages in Mice. J. Radiat. Res. 2004, 45, 549–555. [Google Scholar] [CrossRef]
- Singh, I.; Sharma, A.; Nunia, V.; Goyal, P.K. Radioprotection of Swiss albino mice by Emblica officinalis. Phytother. Res. 2005, 19, 444–446. [Google Scholar] [CrossRef]
- Singh, I.; Soyal, D.; Goyal, P.K. Emblica officinalis (Linn.) Fruit Extract Provides Protection against Radiation-Induced Hematological and Biochemical Alterations in Mice. J. Environ. Pathol. Toxicol. Oncol. 2006, 25, 643–654. [Google Scholar] [CrossRef]
- Kim, H.Y.; Yokozawa, T.; Tohda, C.; Rao, T.P.; Juneja, L.R. Influence of Amla (Emblica officinalis Gaertn.) on Hypercholesterolemia and Lipid Peroxidation in Cholesterol-Fed Rats. J. Nutr. Sci. Vitaminol. 2005, 51, 413–418. [Google Scholar] [CrossRef]
- Panda, S.; Kar, A. Fruit extract of Emblica officinalis ameliorates hyperthyroidism and hepatic lipid peroxidation in mice. Die Pharmazie 2003, 58, 753–755. [Google Scholar] [PubMed]
- Rajak, S.; Banerjee, S.; Sood, S.; Dinda, A.; Gupta, Y.; Gupta, S.; Maulik, S. Emblica officinalis causes myocardial adaptation and protects against oxidative stress in ischemic-reperfusion injury in rats. Phytother. Res. 2004, 18, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Rao, T.; Sakaguchi, N.; Juneja, L.; Wada, E.; Yokozawa, T. Amla (Emblica officinalis Gaertn.) Extracts Reduce Oxidative Stress in Streptozotocin-Induced Diabetic Rats. J. Med. Food 2005, 8, 362–368. [Google Scholar] [CrossRef]
- Yokozawa, T.; Kim, H.Y.; Okubo, T.; Chu, D.-C.; Juneja, L.R. Amla (Emblica officinalis Gaertn.) prevents dyslipidaemia and oxidative stress in the ageing process. Br. J. Nutr. 2007, 97, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Anilakumar, K.R.; Nagaraj, N.S.; Santhanam, K. Reduction of hexachlorocyclohexane-induced oxidative stress and cytotoxicity in rat liver by Emblica officinalis gaertn. Indian J. Exp. Biol. 2007, 45, 450–454. [Google Scholar] [PubMed]
- Sultana, S.; Ahmad, S.; Khan, N.; Jahangir, T. Effect of Emblica officinalis (Gaertn) on CCl4 induced hepatic toxicity and DNA synthesis in Wistar rats. Indian J. Exp. Biol. 2005, 43, 430–436. [Google Scholar]
- Sultana, S.; Ahmed, S.; Sharma, S.; Jahangir, T. Emblica officinalis reverses thioacetamide-induced oxidative stress and early promotional events of primary hepatocarcinogenesis. J. Pharm. Pharmacol. 2010, 56, 1573–1579. [Google Scholar] [CrossRef]
- Tasduq, S.A.; Kaisar, P.; Gupta, D.K.; Kapahi, B.K.; Jyotsna, S.; Maheshwari, H.S.; Johri, R.K. Protective effect of a 50% hydroalcoholic fruit extract of Emblica officinalis against anti-tuberculosis drugs induced liver toxicity. Phytother. Res. 2005, 19, 193–197. [Google Scholar] [CrossRef]
- Ram, M.S.; Neetu, D.; Yogesh, B.; Anju, B.; Dipti, P.; Pauline, T.; Sharma, S.; Sarada, S.; Ilavazhagan, G.; Kumar, D.; et al. Cytoprotective and immunomodulating properties of Amla (Emblica officinalis) on lymphocytes: An in-vitro study. J. Ethnopharmacol. 2002, 81, 5–10. [Google Scholar] [CrossRef]
- Sai Ram, M.; Neetu, D.; Deepti, P.; Vandana, M.; Ilavazhagan, G.; Kumar, D.; Selvamurthy, W. Cytoprotective activity of Amla (Emblica officinalis) against chromium (VI) induced oxidative injury in murine macrophages. Phytother. Res. 2003, 17, 430–433. [Google Scholar] [CrossRef]
- Mathur, R.; Sharma, A.; Dixit, V.; Varma, M. Hypolipidaemic effect of fruit juice of Emblica officinalis in cholesterol-fed rabbits. J. Ethnopharmacol. 1996, 50, 61–68. [Google Scholar] [CrossRef]
- Brown, R.P.; Gerbarg, P.L.; Ramazanov, Z. Rhodiola rosea: A phytomedicinal overview. HerbalGram 2002, 56, 40–52. [Google Scholar]
- Winston, D. Harmony Remedies: An Overview of Adaptogens; Herbal Therapeutics Research Library: Washington, NJ, USA, 2004. [Google Scholar]
- Upadya, H.; Prabhu, S.; Prasad, A.; Subramanian, D.; Gupta, S.; Goel, A. A randomized, double blind, placebo controlled, multicenter clinical trial to assess the efficacy and safety of Emblica officinalis extract in patients with dyslipidemia. BMC Complement. Altern. Med. 2019, 19, 27. [Google Scholar] [CrossRef]
- Chen, T.-S.; Liou, S.-Y.; Chang, Y.-L. Supplementation of Emblica Officinalis (Amla) Extract Reduces Oxidative Stress in Uremic Patients. Am. J. Chin. Med. 2009, 37, 19–25. [Google Scholar] [CrossRef]
- Rainey, C.; Nyquist, L. Nuts—Nutrition and Health Benefits of Daily Use. Nutr. Today 1997, 32, 157–163. [Google Scholar] [CrossRef]
- Segura, R.; Javierre, C.; Lizarraga, M.A.; Ros, E. Other relevant components of nuts: Phytosterols, folate and minerals. Br. J. Nutr. 2006, 96, S36–S44. [Google Scholar] [CrossRef]
- Yang, J.; Liu, R.H.; Halim, L. Antioxidant and antiproliferative activities of common edible nut seeds. LWT 2009, 42, 1–8. [Google Scholar] [CrossRef]
- Mercanlıgil, S.M.; Arslan, P.; Alasalvar, C.; Okut, E.; Akgül, E.; Pınar, A.; Geyik, P.Ö.; Tokgözoğlu, L.; Shahidi, F. Effects of hazelnut-enriched diet on plasma cholesterol and lipoprotein profiles in hypercholesterolemic adult men. Eur. J. Clin. Nutr. 2006, 61, 212–220. [Google Scholar] [CrossRef]
- Morgan, J.M.; Horton, K.; Reese, D.; Carey, C.; Walker, K.; Capuzzi, D.M. Effects of Walnut Consumption as Part of a Low-Fat, Low-Cholesterol Diet on Serum Cardiovascular Risk Factors. Int. J. Vitam. Nutr. Res. 2002, 72, 341–347. [Google Scholar] [CrossRef]
- Caramia, G. Virgin olive oil. From legend to scientific knowledge of the nutraceutical aspects. Med Surg. Pediatrics 2006, 28, 9. [Google Scholar]
- Covas, M.-I.; Konstantinidou, V.; Fitó, M. Olive Oil and Cardiovascular Health. J. Cardiovasc. Pharmacol. 2009, 54, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Kipnis, V.; Freedman, L.S.; Brown, C.C.; Hartman, A.; Schatzkin, A.; Wacholder, S. Interpretation of Energy Adjustment Models for Nutritional Epidemiology. Am. J. Epidemiol. 1993, 137, 1376–1380. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Dilis, V. Olive oil and longevity. Mol. Nutr. Food Res. 2007, 51, 1275–1278. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Lagiou, P. Healthy Traditional Mediterranean Diet: An Expression of Culture, History, and Lifestyle. Nutr. Rev. 1997, 55, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Braga, C.; Vecchia, C.L.; Franceschi, S.; Negri, E.; Parpinel, M.; Decarli, A.; Giacosa, A.; Trichopoulos, D. Olive oil, other seasoning fats, and the risk of colorectal carcinoma. Cancer 1998, 82, 448–453. [Google Scholar] [CrossRef]
- Buckland, G.; Gonzalez, C.A. The role of olive oil in disease prevention: A focus on the recent epidemiological evidence from cohort studies and dietary intervention trials. Br. J. Nutr. 2015, 113, S94–S101. [Google Scholar] [CrossRef] [PubMed]
- Martin-Moreno, J.M.; Willett, W.C.; Gorgojo, L.; Banegas, J.R.; Rodriguez-Artalejo, F.; Fernandez-Rodriguez, J.C.; Maisonneuve, P.; Boyle, P. Dietary fat, olive oil intake and breast cancer risk. Int. J. Cancer 1994, 58, 774–780. [Google Scholar] [CrossRef]
- Tzonou, A.; Kalandidi, A.; Trichopoulou, A.; Hsieh, C.C.; Toupadaki, N.; Willett, W.; Trichopoulos, D. Diet and coronary heart disease: A case-control study in Athens, Greece. Epidemiology 1993, 4, 511–516. [Google Scholar] [CrossRef]
- Rodrigues, A.P.D.S.; Rosa, L.P.D.S.; Noll, M.; Silveira, E.A. Traditional Brazilian Diet and Olive Oil Reduce Cardiometabolic Risk Factors in Severely Obese Individuals: A Randomized Trial. Nutrients 2020, 12, 1413. [Google Scholar] [CrossRef]
- Battu, S.K.; Repka, M.A.; Maddineni, S.; Chittiboyina, A.G.; Avery, M.A.; Majumdar, S. Physicochemical Characterization of Berberine Chloride: A Perspective in the Development of a Solution Dosage Form for Oral Delivery. AAPS PharmSciTech 2010, 11, 1466–1475. [Google Scholar] [CrossRef]
- Bhutada, P.; Mundhada, Y.; Bansod, K.; Dixit, P.; Umathe, S.; Mundhada, D. Anticonvulsant activity of berberine, an isoquinoline alkaloid in mice. Epilepsy Behav. 2010, 18, 207–210. [Google Scholar] [CrossRef]
- Domitrović, R.; Cvijanović, O.; Pernjak-Pugel, E.; Škoda, M.; Mikelić, L.; Crnčević-Orlić, Ž. Berberine exerts nephroprotective effect against cisplatin-induced kidney damage through inhibition of oxidative/nitrosative stress, inflammation, autophagy and apoptosis. Food Chem. Toxicol. 2013, 62, 397–406. [Google Scholar] [CrossRef]
- Kulkarni, S.K.; Dhir, A. Berberine: A plant alkaloid with therapeutic potential for central nervous system disorders. Phytother. Res. 2009, 24, 317–324. [Google Scholar] [CrossRef]
- Kumar, A.; Ekavali; Chopra, K.; Mukherjee, M.; Pottabathini, R.; Dhull, D.K. Current knowledge and pharmacological profile of berberine: An update. Eur. J. Pharmacol. 2015, 761, 288–297. [Google Scholar] [CrossRef]
- Li, M.-H.; Zhang, Y.-J.; Yu, Y.-H.; Yang, S.-H.; Iqbal, J.; Mi, Q.-Y.; Li, B.; Wang, Z.-M.; Mao, W.-X.; Xie, H.-G.; et al. Berberine improves pressure overload-induced cardiac hypertrophy and dysfunction through enhanced autophagy. Eur. J. Pharmacol. 2014, 728, 67–76. [Google Scholar] [CrossRef]
- Chang, X.-X.; Yan, H.-M.; Xu, Q.; Xia, M.-F.; Bian, H.; Zhu, T.-F.; Gao, X. The effects of berberine on hyperhomocysteinemia and hyperlipidemia in rats fed with a long-term high-fat diet. Lipids Health Dis. 2012, 11, 86. [Google Scholar] [CrossRef]
- Shidfar, F.; Ebrahimi, S.S.; Hosseini, S.; Heydari, I.; Shidfar, S.; Hajhassani, G. The Effects of Berberis vulgaris Fruit Extract on Serum Lipoproteins, apoB, apoA-I, Homocysteine, Glycemic Control and Total Antioxidant Capacity in Type 2 Diabetic Patients. Iran. J. Pharm. Res. 2012, 11, 643–652. [Google Scholar]
- Alikiaii, B.; Bagherniya, M.; Askari, G.; Johnston, T.P.; Sahebkar, A. The role of phytochemicals in sepsis: A mechanistic and therapeutic perspective. BioFactors 2021, 47, 19–40. [Google Scholar] [CrossRef]
- Alikiaii, B.; Bagherniya, M.; Askari, G.; Sathyapalan, T.; Sahebkar, A. Evaluation of the effect of curcumin on pneumonia: A systematic review of preclinical studies. Phytother. Res. 2020. [Google Scholar] [CrossRef]
- Ghandadi, M.; Sahebkar, A. Curcumin: An Effective Inhibitor of Interleukin-6. Curr. Pharm. Des. 2017, 23, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Momtazi, A.A.; Derosa, G.; Maffioli, P.; Banach, M.; Sahebkar, A. Role of microRNAs in the Therapeutic Effects of Curcumin in Non-Cancer Diseases. Mol. Diagn. Ther. 2016, 20, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Ahmadi, Y.; Teymouri, M.; Johnston, T.P.; Sahebkar, A. Curcumin as a potential candidate for treating hyperlipidemia: A review of cellular and metabolic mechanisms. J. Cell. Physiol. 2018, 233, 141–152. [Google Scholar] [CrossRef]
- Teymouri, M.; Pirro, M.; Johnston, T.P.; Sahebkar, A. Curcumin as a multifaceted compound against human papilloma virus infection and cervical cancers: A review of chemistry, cellular, molecular, and preclinical features. BioFactors 2017, 43, 331–346. [Google Scholar] [CrossRef]
- Iranshahi, M.; Sahebkar, A.; Hosseini, S.; Takasaki, M.; Konoshima, T.; Tokuda, H. Cancer chemopreventive activity of diversin from Ferula diversivittata in vitro and in vivo. Phytomedicine 2010, 17, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Simental-Mendía, L.E.; Majeed, M.; Sahebkar, A.; Simental-Mendía, L. Effects of Curcuminoids Plus Piperine on Glycemic, Hepatic and Inflammatory Biomarkers in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blind Placebo-Controlled Trial. Drug Res. 2018, 68, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Bianconi, V.; Sahebkar, A.; Atkin, S.L.; Pirro, M. The regulation and importance of monocyte chemoattractant protein-1. Curr. Opin. Hematol. 2018, 25, 44–51. [Google Scholar] [CrossRef]
- Ghasemi, F.; Shafiee, M.; Banikazemi, Z.; Pourhanifeh, M.H.; Khanbabaei, H.; Shamshirian, A.; Moghadam, S.A.; ArefNezhad, R.; Sahebkar, A.; Avan, A.; et al. Curcumin inhibits NF-kB and Wnt/β-catenin pathways in cervical cancer cells. Pathol. Res. Pr. 2019, 215, 152556. [Google Scholar] [CrossRef]
- Martin, R.C.; Aiyer, H.S.; Malik, D.J.; Li, Y. Effect on pro-inflammatory and antioxidant genes and bioavailable distribution of whole turmeric vs curcumin: Similar root but different effects. Food Chem. Toxicol. 2012, 50, 227–231. [Google Scholar] [CrossRef]
- Sahebkar, A. Why it is necessary to translate curcumin into clinical practice for the prevention and treatment of metabolic syndrome? BioFactors 2013, 39, 197–208. [Google Scholar] [CrossRef]
- Campbell, M.S.; Ouyang, A.; Krishnakumar, I.M.; Charnigo, R.J.; Westgate, P.M.; Fleenor, B.S. Influence of enhanced bioavailable curcumin on obesity-associated cardiovascular disease risk factors and arterial function: A double-blinded, randomized, controlled trial. Nutrients 2019, 62, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Varatharajalu, R.; Garige, M.; Leckey, L.C.; Reyes-Gordillo, K.; Shah, R.; Lakshman, M.R. Protective Role of Dietary Curcumin in the Prevention of the Oxidative Stress Induced by Chronic Alcohol with respect to Hepatic Injury and Antiatherogenic Markers. Oxidative Med. Cell. Longev. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Madaric, A.; Kadrabova, J.; Krajcovicova-Kudlackova, M.; Valachovicova, M.; Spustova, V.; Mislanova, C.; Kajaba, I.; Blazicek, P. The effect of bioactive complex of quercetin, selenium, catechins and curcumin on cardiovascular risk markers in healthy population after a two month consumption. Bratislavske Lekarske Listy 2013, 114, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, Z.; Sabetkasaei, M.; Moradi, F.; Masoudnia, F.; Ataie, A. Curcumin has Neuroprotection Effect on Homocysteine Rat Model of Parkinson. J. Mol. Neurosci. 2012, 47, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Jelodar, G.; Azimifar, A. Evaluation of serum cancer antigen 125, resistin, leptin, homocysteine, and total antioxidant capacity in rat model of endometriosis treated with Curcumin. Physiol. Rep. 2019, 7, e14016. [Google Scholar] [CrossRef]
- Sahebkar, A. Effects of resveratrol supplementation on plasma lipids: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2013, 71, 822–835. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Serban, C.; Ursoniu, S.; Wong, N.D.; Muntner, P.; Graham, I.M.; Mikhailidis, D.P.; Rizzo, M.; Rysz, J.; Sperling, L.S.; et al. Lack of efficacy of resveratrol on C-reactive protein and selected cardiovascular risk factors—Results from a systematic review and meta-analysis of randomized controlled trials. Int. J. Cardiol. 2015, 189, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Abu-Amero, K.K.; Kondkar, A.A.; Chalam, K.V. Resveratrol and Ophthalmic Diseases. Nutrients 2016, 8, 200. [Google Scholar] [CrossRef]
- Limmongkon, A.; Janhom, P.; Amthong, A.; Kawpanuk, M.; Nopprang, P.; Poohadsuan, J.; Somboon, T.; Saijeen, S.; Surangkul, D.; Srikummool, M.; et al. Antioxidant activity, total phenolic, and resveratrol content in five cultivars of peanut sprouts. Asian Pac. J. Trop. Biomed. 2017, 7, 332–338. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Suleria, H.A.R.; Ahmad, B.; Peters, D.G.; Mubarak, M.S. A comprehensive review of the health perspectives of resveratrol. Food Funct. 2017, 8, 4284–4305. [Google Scholar] [CrossRef]
- Koz, S.T.; Etem, E.O.; Baydas, G.; Yuce, H.; Ozercan, H.I.; Kuloğlu, T.; Koz, S.; Etem, A.; Demir, N. Effects of resveratrol on blood homocysteine level, on homocysteine induced oxidative stress, apoptosis and cognitive dysfunctions in rats. Brain Res. 2012, 1484, 29–38. [Google Scholar] [CrossRef]
- Noll, C.; Hamelet, J.; Ducros, V.; Belin, N.; Paul, J.-L.; Delabar, J.-M.; Janel, N. Resveratrol supplementation worsen the dysregulation of genes involved in hepatic lipid homeostasis observed in hyperhomocysteinemic mice. Food Chem. Toxicol. 2009, 47, 230–236. [Google Scholar] [CrossRef]
- Yilmaz, O.; Keser, S.; Tuzcu, M.; Çetintaş, B. Resveratrol (trans-3,4′,5-trihydroxystilbene) decreases lipid peroxidation level and protects antioxidant capacity in sera and erythrocytes of old female Wistar rats induced by the kidney carcinogen potassium bromate. Environ. Toxicol. Pharmacol. 2007, 24, 79–85. [Google Scholar] [CrossRef]
- Schroecksnadel, K.; Winkler, C.; Wirleitner, B.; Schennach, H.; Weiss, G.; Fuchs, D.; Weiss, G. Anti-inflammatory compound resveratrol suppresses homocysteine formation in stimulated human peripheral blood mononuclear cells in vitro. Clin. Chem. Lab. Med. 2005, 43, 1084–1088. [Google Scholar] [CrossRef]
- Andreson, J. Dietary fiber: Hyperlipidemia, hypertension, and coronary heart disease. Am. J. Gastroenterol. 1986, 81, 907–909. [Google Scholar]
- Theuwissen, E.; Mensink, R.P. Water-soluble dietary fibers and cardiovascular disease. Physiol. Behav. 2008, 94, 285–292. [Google Scholar] [CrossRef]
- Anderson, J.W.; Baird, P.; Davis, R.H.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health benefits of dietary fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef]
- Wood, R.J.; Volek, J.S.; Davis, S.R.; Dell’Ova, C.; Fernandez, M.L. Effects of a carbohydrate-restricted diet on emerging plasma markers for cardiovascular disease. Nutr. Metab. 2006, 3, 19. [Google Scholar] [CrossRef]
- Sprecher, D.L.; Pearce, G.L. Fiber-multivitamin combination therapy: A beneficial influence on low-density lipoprotein and homocysteine. Metabolism 2002, 51, 1166–1170. [Google Scholar] [CrossRef]

| Number | Author, Year | Agent | Dose per Day | Treatment Duration | Subjects | Main Outcome(s) | Final Effects of Nutraceuticals on Homocysteine Level |
|---|---|---|---|---|---|---|---|
| 1 | Hodgson et al., 2007 [85] | Black Tea | 2.2 g tea leaves | 3.5 h | Adults with CAD | Black tea significantly increased plasma homocysteine levels | ↑ |
| 2 | Hodgson JM et al., 2003 [86] | Black Tea | 2 g tea leave/250 mL boiled water | 12 weeks | Healthy subjects | Black tea did not significantly alter mean homocysteine concentrations | No effect |
| 3 | Olthof MR et al., 2001 [87] | Black tea | 4 g black tea | 4 weeks | Healthy subjects | Black tea raised total plasma homocysteine concentrations | ↑ |
| 4 | Duthie et al., 2006 [115] | Anthocyanins | 750 mL/day cranberry juice (2.80 mg/L anthocyanins) | 2 weeks | Healthy volunteer females | Cranberry juice had no effect on plasma homocysteine levels | No effect |
| 5 | Ahmadi N et al., 2013 [125] | Garlic extract | Aged garlic extract (AGE) 250 mg + B12 100 μg + B9 300 μg + B6 12.5 mg + L-arginine 100 mg | 12 months | Healthy subjects | Garlic extract plus other supplements reduced homocysteine level | ↓ |
| 6 | Budoff MJ et al., 2009 [126] | Garlic extract | AGE 250 mg + B12 100 μg + B9 300 μg + B6 12.5 mg + L-arginine 100 mg | 1 year | Healthy subjects | Garlic extract plus other supplements reduced homocysteine level | ↓ |
| 7 | Budoff MJ et al., 2004 [128] | Garlic extract | 4 mL | 1 year | Patients with CAD | Garlic extract did not significantly improve homocysteine level | No effect |
| 8 | Seo DY et al., 2012 [129] | Garlic extract | 5 × 65 mg per week | 12 weeks | 30 postmenopausal women | Homocysteine was significantly decreased | ↓ |
| 9 | McCrindle BW et al., 1998 [130] | Garlic extract | 3 × 300 mg per day | 8weeks | 30 pediatric (8 to 18 years old) | No significant difference between the groups | No effect |
| 10 | Ried K et al., 2016 [131] | Garlic extract | 1.2 g powder per day + 1.2 mg S-allylcysteine | 12 weeks | 88 uncontrolled hypertensive patients | No significant differences between the groups | No effect |
| 11 | Merchant RE et al., 2015 [144] | Chorella | 9 g per day | 60 days | 17 vegetarian or vegan | Homocysteine level decreased by an average of 10% | ↑ |
| 12 | Llaneza P et al., 2011 [163] | Soy | 80 mg of soy isoflavone | 6 months | 87 healthy postmenopausal women | No change in both groups | No effect |
| 13 | Høie LH et al., 2005 [164] | Soy | 15 or 25 g of soy protein | 8 weeks | 117 Hypercholesterolemic patients | No change in all groups | No effect |
| 14 | Greany K et al., 2008 [165] | Soy | 26 ± 5 g/day of soy isolated protein | 2 × 4 weeks + 2 weeks washout | 34 postmenopausal women | No change between both groups | No effect |
| 15 | Imani H et al., 2009 [166] | Soy | 28 g/day of soy textured soy flour | 8 weeks | 40 peritoneal dialysis patients | No change between groups | No effect |
| 16 | Jenkins DJ et al., 2002 [167] | Soy | Low fat dairy food control diet, with low or high isoflavone soy food diets | 3 × 1 months + 2 weeks washout | 41 hyperlipidemic men and postmenopausal women | Homocysteine was lower in both isoflavone groups | ↓ |
| 17 | Hanson LN et al., 2006 [168] | Soy | 40 g/day soy protein isolate | 6 weeks | 55 postmenopausal women | homocysteine was significantly reduced in soy protein normal phytate group, while no significant change was detected in soy protein normal isoflavone group | soy protein normal phytat: ↓ soy protein normal isoflavone: no effect |
| 18 | Reimann M et al., 2006 [169] | Soy | 50 mg soy isoflavone | 8 weeks | 89 postmenopausal women | Homocysteine level did not change in both groups | No effect |
| 19 | Brandao LC et al., 2009 [170] | Soy | 80 mg soy isoflavone | 4 months | 38 postmenopausal women | Homocysteine level did not change in both groups | No effect |
| 20 | Upadya H et al., 2019 [190] | Amla | 500 mg capsule of Amla extract | 12 weeks | 98 patients with dyslipidemia | Homocysteine level did not change significantly between the groups | No effect |
| 21 | Chen T-S et al., 2009 [191] | Amla | amla extract tablets (300 mg, 50% dextrin + 50% amla extract) four times a day | 4 months | 17 uremic patients | Homocysteine level did not change in subjects | No effect |
| 22 | Mercanlıgil S et al., 2007 [195] | Nut | 40 g/day hazeinut | 8 weeks | 15 hypercholesterolemic patients | Homocysteine level did not change between the groups | No effect |
| 23 | Morgan J et al., 2002 [196] | Nut | 64 g/day walnut | 18 weeks | 67 patients (serum total cholesterol > 5.2 mmol/L) | No statically significant effects were observed | No effect |
| 24 | Rodrigues APdS et al., 2020 [206] | Olive oil | 52 mL/d EVOO | 12 weeks | 121 obese adult subjects | Homocysteine levels did not change significantly between the groups | No effect |
| 25 | Shidfar F et al., 2012 [214] | Berberis vulgaris Fruit Extract | 3 g/d | 3 months | Diabetic patients | Berberine did not significantly alter mean serum homocysteine concentration | No effect |
| 26 | Campbell MS et al., 2019 [227] | Curcumin | 500 mg | 12 weeks | Obese men | Homocysteine was significantly reduced in the curcumin group | ↓ |
| 27 | Madaric A et al., 2013 [229] | Curcumin | 100 g of biscuits per day with 1.3 g curcuma | 2 months | Healthy men | Curcumin significantly decreased homocysteine level | ↓ |
| 28 | Schroecksnadel K et al., 2005 [240] | Resveratrol | 1–100 µg | 72 h | Healthy voluntary blood donors | Pretreatment of unstimulated cells with 10–100 mM resveratrol only slightly decreased homocysteine production in the resting cells | ↓ |
| 29 | Wood RJ et al., 2006 [244] | soluble fiber | 3 g soluble fiber | 12 weeks | 29 overweight men | Homocysteine level did not significantly increase in fiber group compared to the placebo group | No effect |
| 30 | Sprecher DL et al., 2002 [245] | soluble fiber | 4 g soluble fiber | 8 weeks | 119 subjects | Homocysteine levels significantly reduced in fiber blend group compared to the placebo group | ↓ |
| Number | Author, Year | Agent | Dose per Day | Treatment Duration | Animals | Main Outcome(s) | Final Effects of Nutraceuticals on Homocysteine Level |
|---|---|---|---|---|---|---|---|
| 1 | San Cheang et al., 2015 [84] | Black Tea extract | 15 mg/kg/day | 2 weeks | Rats | Black tea extract significantly reduced plasma homocysteine levels | ↓ |
| 2 | El-Missiry MA et al., 2018 [101] | Green tea | 2.5 or 5 mg/kg body weight EGCG | 3 days | Adult male Wister rats | EGCG at a dose of 2.5 and 5 mg/kg significantly decreased plasma homocysteine | ↓ |
| 3 | Amin KA et al., 2009 [108] | Cinnamon extract | 20 mg/day/rat | 5–8 weeks | Male rats | Cinnamon extract reduced homocysteine levels | ↓ |
| 4 | Yeh YY et al., 2006 [127] | Garlic extract | 4% of diet | 6 weeks | Rats | Garlic extract significantly reduced homocysteine level | ↓ |
| 5 | Kim JH, 2019 [136] | ginsenoside | 50 mg/kg every 12 h | 60 days | 40 wistar male rats | Met reduced plasma Homocysteine level, whereas GTS did not affect basal plasma levels | GTS alone: No effect Met: ↓ |
| 6 | Ilkhanizadeh B et al., 2016 [154] | ginger | 50 mg/kg body weight daily | 6 weeks | 24 male Wistar rats | Significant decrease in homocystein level was found in the ginger extract-treated diabetic group | ↓ |
| 7 | Akbari A et al., 2017 [155] | ginger | 1 g/kg body weight daily | 28 days | 28 adult male Sprague-Dawley | In ginger-ethanol group, ginger improved antioxidant enzymes’ activity and reduced tHcy and MDA compared to the ethanol group | ↓ |
| 8 | Snelson M et al., 2017 [171] | Soy | 55% total energy | 12 weeks | 30 female wild-type mice | homocysteine level was the same in soy and control group | No effect |
| 9 | Adam SK et al., 2008 [172] | Soy | Fresh soy oil One-heated soy oil Five-time-heated soy oil | 4 months | 24 adult female Sprague Dawley rats | fresh soy oil significantly reduced homocysteine level compared to the other groups | ↓ |
| 10 | Chang X-x et al., 2012 [213] | Berberine | 200 mg/kg/day | 24 weeks | Healthy male rats | Serum homocysteine level was significantly decreased after berberine consumption in rats fed with a high-fat diet | ↓ |
| 11 | Varatharajalu R et al., 2016 [228] | Curcumin | 150 mg/kg body weight/day | 8 weeks | Female Wistar-Furth rats | Curcumin significantly increased homocysteine thiolactonase activity | ↓ |
| 12 | Mansouri Z et al., 2012 [230] | Curcumin | 50 mg/kg | 10 days | Adult male Wister rats | Investigated the neuroprotective effects of curcumin against homocysteine neurotoxicity | ↓ |
| 13 | Jelodar G et al., 2019 [231] | Curcumin | 48 mg/kg | 4 weeks | female Sprague-Dawley rats | no significant difference was observed between all groups | No effect |
| 14 | Koz ST et al.2012 [237] | Resveratrol | 20 mg/kg/day | 30 days | Rats | Resveratrol significantly reduced plasma Homocysteine levels | ↓ |
| 15 | Noll C et al., 2009 [238] | Resveratrol | 50 µg/day | 3 months | Mice | Resveratrol significantly increased homocysteine levels compared to the control group | ↑ |
| 16 | Yilmaz Ö et al., 2007 [239] | Resveratrol | 33mg/kg four times per week | 5 weeks | Old female rats | Resveratrol significantly decreased homocysteine levels | ↓ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atazadegan, M.A.; Bagherniya, M.; Askari, G.; Tasbandi, A.; Sahebkar, A. The Effects of Medicinal Plants and Bioactive Natural Compounds on Homocysteine. Molecules 2021, 26, 3081. https://doi.org/10.3390/molecules26113081
Atazadegan MA, Bagherniya M, Askari G, Tasbandi A, Sahebkar A. The Effects of Medicinal Plants and Bioactive Natural Compounds on Homocysteine. Molecules. 2021; 26(11):3081. https://doi.org/10.3390/molecules26113081
Chicago/Turabian StyleAtazadegan, Mohammad Amin, Mohammad Bagherniya, Gholamreza Askari, Aida Tasbandi, and Amirhossein Sahebkar. 2021. "The Effects of Medicinal Plants and Bioactive Natural Compounds on Homocysteine" Molecules 26, no. 11: 3081. https://doi.org/10.3390/molecules26113081
APA StyleAtazadegan, M. A., Bagherniya, M., Askari, G., Tasbandi, A., & Sahebkar, A. (2021). The Effects of Medicinal Plants and Bioactive Natural Compounds on Homocysteine. Molecules, 26(11), 3081. https://doi.org/10.3390/molecules26113081






