Electrochemical Assessment of Indigo Carmine Dye in Lithium Metal Polymer Technology
Abstract
1. Introduction
2. Results and Discussion
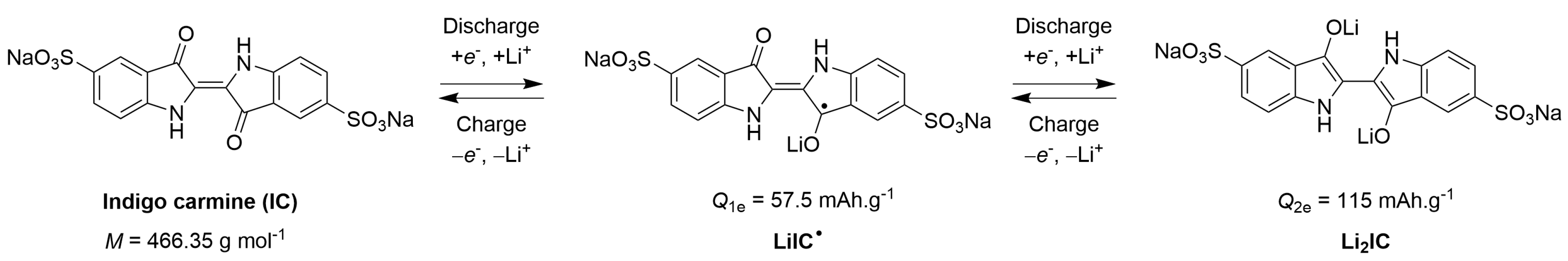
2.1. Recap of the Electrochemical Behavior Concerning Li||IC Cells Measured in Carbonate-Based Liquid Electrolytes at Room Temperature
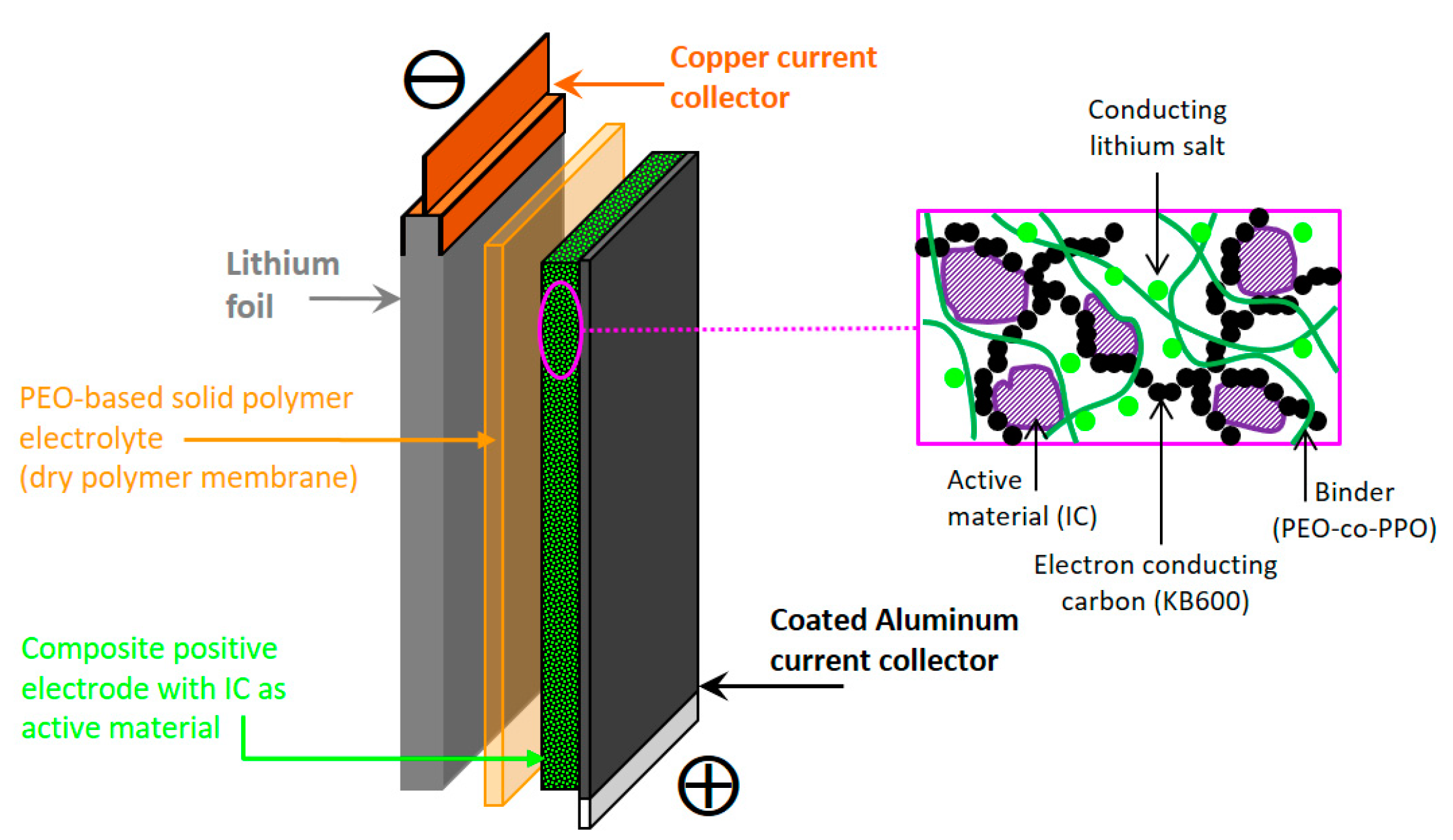
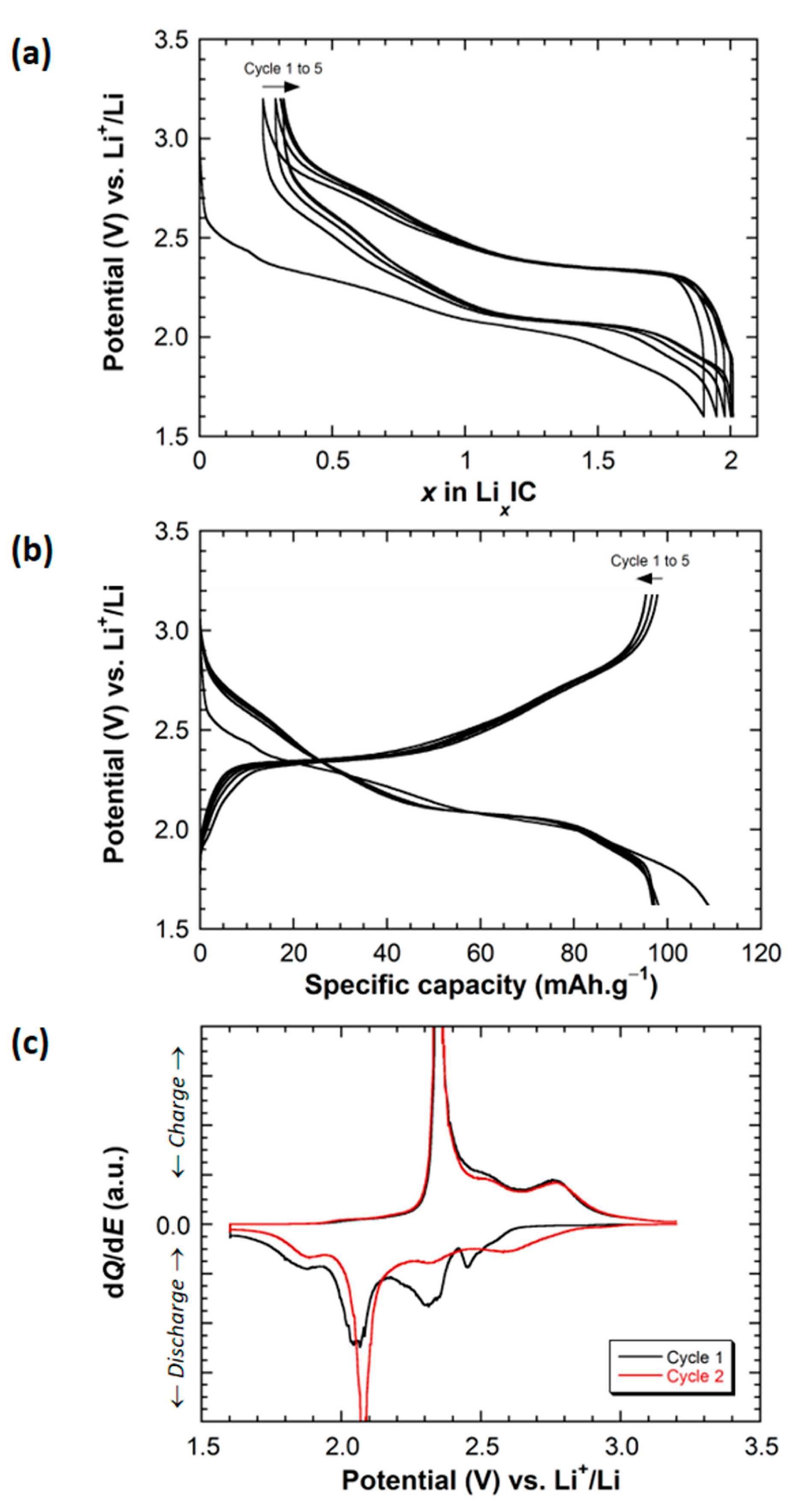
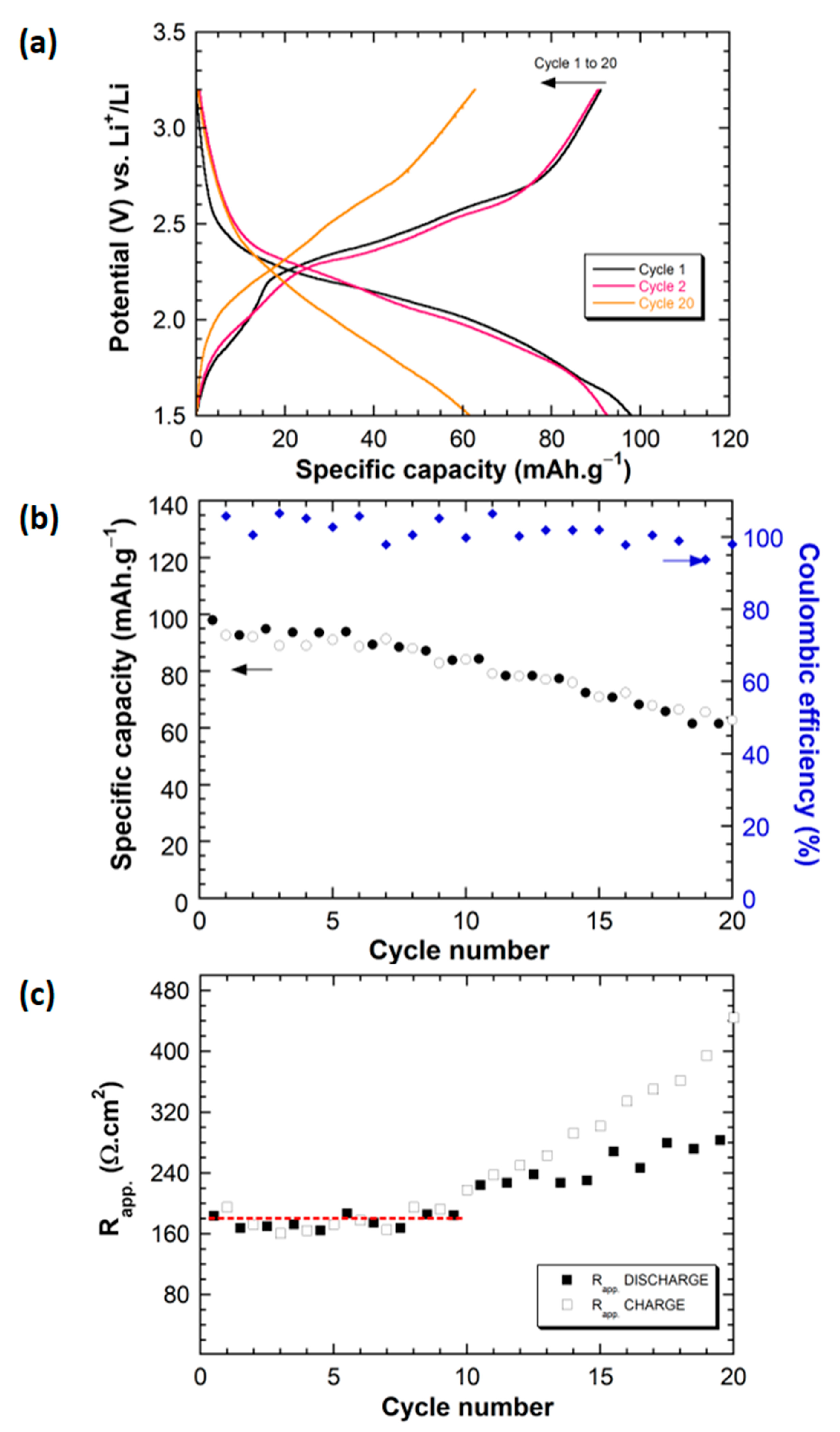
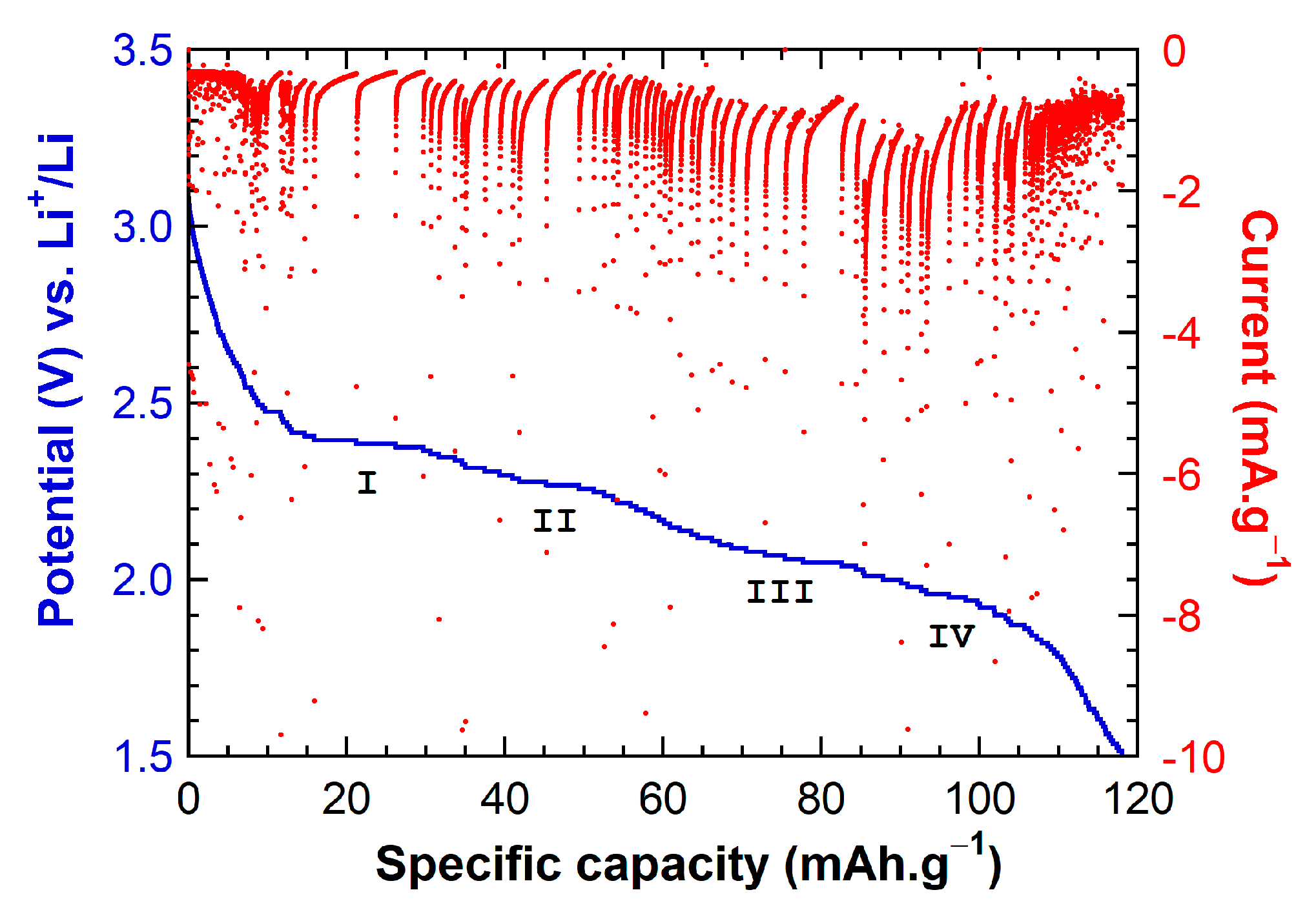
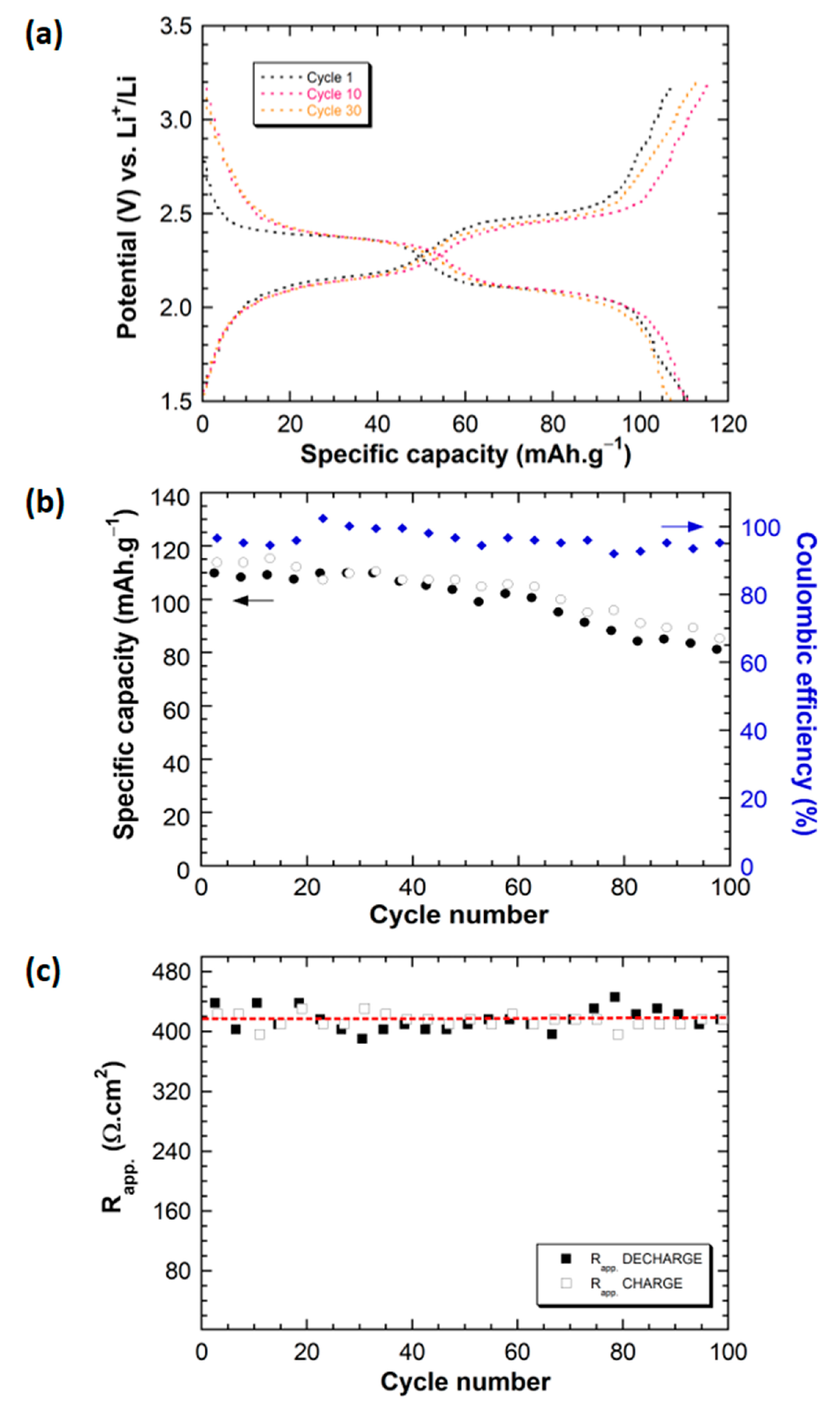
2.2. Electrochemical Behavior of Li||IC Cells Using the LMP® Technology
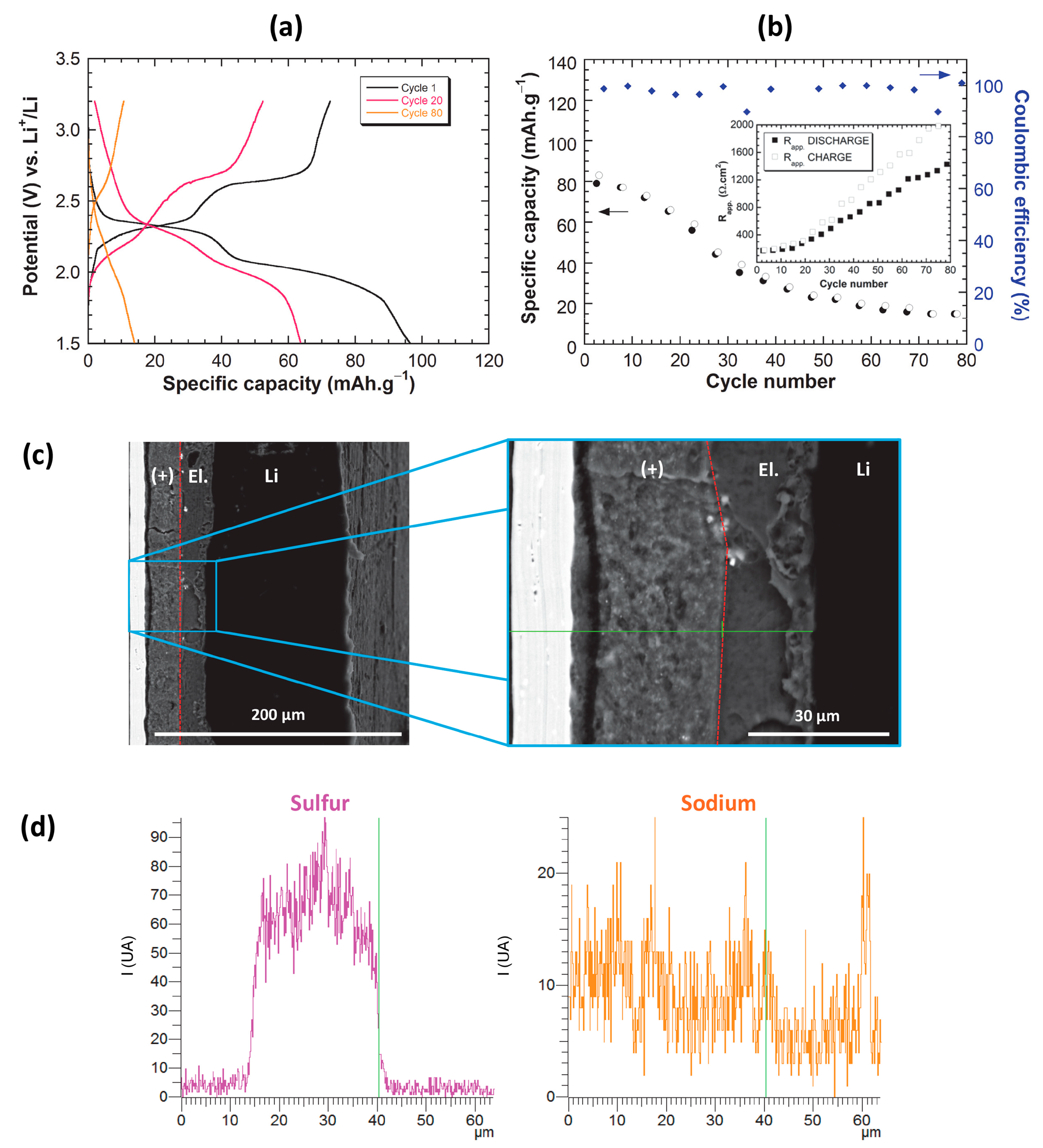
2.3. Post-Mortem Analyses and Failure Identification
3. Materials and Methods
3.1. Reagents, Electrode Preparation, and LMP® Cell Assembly
3.2. Electrochemical Measurements and Characterization Techniques
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ziegler, M.S.; Trancik, J.E. Re-Examining Rates of Lithium-Ion Battery Technology Improvement and Cost Decline. Energy Environ. Sci. 2021, 14, 1635–1651. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C.; Eshetu, G.G.; Laruelle, S.; Grugeon, S.; Zaghib, K.; Julien, C.; Mauger, A.; Guyomard, D.; Rojo, T.; et al. From Solid-Solution Electrodes and the Rocking-Chair Concept to Today’s Batteries. Angew. Chem. Int. Ed. 2020, 59, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Brandt, K. Historical Development of Secondary Lithium Batteries. Solid State Ion. 1994, 69, 173–183. [Google Scholar] [CrossRef]
- Yoshino, A. The Birth of the Lithium-Ion Battery. Angew. Chem. Int. Ed. 2012, 51, 5798–5800. [Google Scholar] [CrossRef]
- Blomgren, G.E. The Development and Future of Lithium Ion Batteries. J. Electrochem. Soc. 2017, 164, A5019–A5025. [Google Scholar] [CrossRef]
- Goodenough, J.B. How We Made the Li-Ion Rechargeable Battery. Nat. Electron. 2018, 1, 204. [Google Scholar] [CrossRef]
- Mauger, A.; Julien, C.M.; Goodenough, J.B.; Zaghib, K. Tribute to Michel Armand: From Rocking Chair—Li-Ion to Solid-State Lithium Batteries. J. Electrochem. Soc. 2020, 167, 070507. [Google Scholar] [CrossRef]
- Shanmukaraj, D.; Ranque, P.; Ben Youcef, H.; Rojo, T.; Poizot, P.; Grugeon, S.; Laruelle, S.; Guyomard, D. Review—Towards Efficient Energy Storage Materials: Lithium Intercalation/Organic Electrodes to Polymer Electrolytes—A Road Map (Tribute to Michel Armand). J. Electrochem. Soc. 2020, 167, 070530. [Google Scholar] [CrossRef]
- Hérold, A. Insertion Compounds of Graphite with Bromine and the Alkali Metals. Bull. Soc. Chim. Fr. 1955, 187, 999–1012. [Google Scholar]
- Rüdorff, W. Über Die Einlagerung von Unedlen Metallen in Graphit Sowie in Metallchalkogenide Vom Typ MeX2. Chimia 1965, 19, 489–499. [Google Scholar]
- Danot, M.; Le Blanc, A.; Rouxel, J. Les Composés Intercalaires KxTiS2. Bull. Soc. Chim. Fr. 1969, 8, 2670–2675. [Google Scholar]
- Rouxel, J.; Danot, M.; Bichon, J. Les Composés Intercalaires NaxTiS2. Etude Structurale Générale des Phases NaxTiS2 et KxTiS2. Bull. Soc. Chim. Fr. 1971, 11, 3930–3935. [Google Scholar]
- Bichon, J.; Danot, M.; Rouxel, J. Systématique Structurale pour les Séries d’Intercalaires MxTiS2 (M = Li, Na, K, Rb, Cs). C. R. Acad. Sci. 1973, 276, 1283–1286. [Google Scholar]
- van Gool, W. Fast Ion Transport in Solids: Solid State Balteries and Devices. In Proceedings of the NATO Sponsored Advanced Study Institute on Fast Ion Transport in Solids, Solid State Balteries and Devices, Belgirate, Italy, 5–16 September 1972. [Google Scholar]
- Winn, D.A.; Shemilt, J.M.; Steele, B.C.H. Titanium Disulphide: A Solid Solution Electrode for Sodium and Lithium. Mater. Res. Bull. 1976, 11, 559–566. [Google Scholar] [CrossRef]
- Whittingham, M.S. Electrical Energy Storage and Intercalation Chemistry. Science 1976, 192, 1126–1127. [Google Scholar] [CrossRef]
- Rouxel, J. Recent Progress in Intercalation Chemistry: Alkali Metals in Chacogenide Host Structures. Rev. Inorg. Chem. 1979, 1, 245–279. [Google Scholar]
- Brec, R.; Schleich, D.M.; Ouvrard, G.; Louisy, A.; Rouxel, J. Physical Properties of Lithium Intercalation Compounds of the Layered Transition-Metal Chalcogenophosphites. Inorg. Chem. 1979, 18, 1814–1818. [Google Scholar] [CrossRef]
- Brec, R.; Ouvrard, G.; Louisy, A.; Rouxel, J.; Lemehaute, A. The Influence, on Lithium Electrochemical Intercalation, of Bond Ionicity in Layered Chalcogenophosphates of Transition Metals. Solid State Ion. 1982, 6, 185–190. [Google Scholar] [CrossRef]
- Lazzari, M.; Scrosati, B. A Cyclable Lithium Organic Electrolyte Cell Based on Two Intercalation Electrodes. J. Electrochem. Soc. 1980, 127, 773–774. [Google Scholar] [CrossRef]
- Guyomard, D.; Tarascon, J.M. Rechargeable Li1+xMn2O4/Carbon Cells with a New Electrolyte Composition: Potentiostatic Studies and Application to Practical Cells. J. Electrochem. Soc. 1993, 140, 3071–3081. [Google Scholar] [CrossRef]
- Armand, M.B.; Chabagno, J.M.; Duclot, M. Extended Abstracts. In Proceedings of the Second International Meeting on Solid Electrolytes, St Andrews, Scotland, 20–22 September 1978. [Google Scholar]
- Armand, M.B.; Chabagno, J.M.; Duclot, M. Fast Ion Transport in Solids; Vashishta, P., Mundy, J.N., Shenoy, J.K., Eds.; North Holland Publishers: Amsterdam, The Netherlands, 1979; p. 131. [Google Scholar]
- Fenton, D.E.; Parker, J.M.; Wright, P.V. Complexes of Alkali Metal Ions with Poly(Ethylene Oxide). Polymer 1973, 14, 589. [Google Scholar] [CrossRef]
- Wright, P.V. Electrical Conductivity in Ionic Complexes of Poly(Ethylene Oxide). Brit. Poly. J. 1975, 7, 319–327. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, J.-G.; Xu, W. Advancing Lithium Metal Batteries. Joule 2018, 2, 833–845. [Google Scholar] [CrossRef]
- Albertus, P.; Anandan, V.; Ban, C.; Balsara, N.; Belharouak, I.; Buettner-Garrett, J.; Chen, Z.; Daniel, C.; Doeff, M.; Dudney, N.J.; et al. Challenges for and Pathways toward Li-Metal-Based All-Solid-State Batteries. ACS Energy Lett. 2021, 1399–1404. [Google Scholar] [CrossRef]
- Vandepaer, L.; Cloutier, J.; Amor, B. Environmental Impacts of Lithium Metal Polymer and Lithium-Ion Stationary Batteries. Renew. Sustain. Energy Rev. 2017, 78, 46–60. [Google Scholar] [CrossRef]
- Esser, B.; Dolhem, F.; Becuwe, M.; Poizot, P.; Vlad, A.; Brandell, D. A Perspective on Organic Electrode Materials and Technologies for next Generation Batteries. J. Power Sources 2021, 482, 228814. [Google Scholar] [CrossRef]
- Judez, X.; Qiao, L.; Armand, M.; Zhang, H. Energy Density Assessment of Organic Batteries. ACS Appl. Energy Mater. 2019, 2, 4008–4015. [Google Scholar] [CrossRef]
- Lécuyer, M.; Gaubicher, J.; Barrès, A.-L.; Dolhem, F.; Deschamps, M.; Guyomard, D.; Poizot, P. A Rechargeable Lithium/Quinone Battery Using a Commercial Polymer Electrolyte. Electrochem. Commun. 2015, 55, 22–25. [Google Scholar] [CrossRef]
- Li, W.; Chen, L.; Sun, Y.; Wang, C.; Wang, Y.; Xia, Y. All-Solid-State Secondary Lithium Battery Using Solid Polymer Electrolyte and Anthraquinone Cathode. Solid State Ion. 2017, 300, 114–119. [Google Scholar] [CrossRef]
- Poizot, P.; Gaubicher, J.; Renault, S.; Dubois, L.; Liang, Y.; Yao, Y. Opportunities and Challenges for Organic Electrodes in Electrochemical Energy Storage. Chem. Rev. 2020, 120, 6490–6557. [Google Scholar] [CrossRef]
- Bouridah, A.; Dalard, F.; Armand, M.B. Electrochemical Properties of Poly(Decaviologen) in Polymer Media. J. Appl. Electrochem. 1990, 20, 1040–1044. [Google Scholar] [CrossRef]
- Chen, H.; Armand, M.; Demailly, G.; Dolhem, F.; Poizot, P.; Tarascon, J.-M. From Biomass to a Renewable LixC6O6 Organic Electrode for Sustainable Li-Ion Batteries. ChemSusChem 2008, 1, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Armand, M.; Courty, M.; Jiang, M.; Grey, C.P.; Dolhem, F.; Tarascon, J.-M.; Poizot, P. Lithium Salt of Tetrahydroxybenzoquinone: Toward the Development of a Sustainable Li-Ion Battery. J. Am. Chem. Soc. 2009, 131, 8984–8988. [Google Scholar] [CrossRef] [PubMed]
- Renault, S.; Geng, J.; Dolhem, F.; Poizot, P. Evaluation of Polyketones with N-Cyclic Structure as Electrode Material for Electrochemical Energy Storage: Case of Pyromellitic Diimide Dilithium Salt. Chem. Commun. 2011, 47, 2414–2416. [Google Scholar] [CrossRef]
- Yao, M.; Araki, M.; Senoh, H.; Yamazaki, S.; Sakai, T.; Yasuda, K. Indigo Dye as a Positive-Electrode Material for Rechargeable Lithium Batteries. Chem. Lett. 2010, 39, 950–952. [Google Scholar] [CrossRef]
- Yao, M.; Kuratani, K.; Kojima, T.; Takeichi, N.; Senoh, H.; Kiyobayashi, T. Indigo Carmine: An Organic Crystal as a Positive-Electrode Material for Rechargeable Sodium Batteries. Sci. Rep. 2015, 4. [Google Scholar] [CrossRef]
- Deunf, E.; Poizot, P.; Lestriez, B. Aqueous Processing and Formulation of Indigo Carmine Positive Electrode for Lithium Organic Battery. J. Electrochem. Soc. 2019, 166, A747–A753. [Google Scholar] [CrossRef]
- Kato, M.; Sano, H.; Kiyobayashi, T.; Takeichi, N.; Yao, M. Improvement of the Battery Performance of Indigo, an Organic Electrode Material, Using PEDOT/PSS with d-Sorbitol. ACS Omega 2020, 5, 18565–18572. [Google Scholar] [CrossRef]
- Poizot, P.; Laruelle, S.; Grugeon, S.; Dupont, L.; Tarascon, J.-M. From the Vanadates to 3d-Metal Oxides Negative Electrodes. Ionics 2000, 6, 321–330. [Google Scholar] [CrossRef]
- Delacourt, C.; Poizot, P.; Morcrette, M.; Tarascon, J.-M.; Masquelier, C. One-Step Low-Temperature Route for the Preparation of Electrochemically Active LiMnPO4 Powders. Chem. Mater. 2004, 16, 93–99. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lécuyer, M.; Deschamps, M.; Guyomard, D.; Gaubicher, J.; Poizot, P. Electrochemical Assessment of Indigo Carmine Dye in Lithium Metal Polymer Technology. Molecules 2021, 26, 3079. https://doi.org/10.3390/molecules26113079
Lécuyer M, Deschamps M, Guyomard D, Gaubicher J, Poizot P. Electrochemical Assessment of Indigo Carmine Dye in Lithium Metal Polymer Technology. Molecules. 2021; 26(11):3079. https://doi.org/10.3390/molecules26113079
Chicago/Turabian StyleLécuyer, Margaud, Marc Deschamps, Dominique Guyomard, Joël Gaubicher, and Philippe Poizot. 2021. "Electrochemical Assessment of Indigo Carmine Dye in Lithium Metal Polymer Technology" Molecules 26, no. 11: 3079. https://doi.org/10.3390/molecules26113079
APA StyleLécuyer, M., Deschamps, M., Guyomard, D., Gaubicher, J., & Poizot, P. (2021). Electrochemical Assessment of Indigo Carmine Dye in Lithium Metal Polymer Technology. Molecules, 26(11), 3079. https://doi.org/10.3390/molecules26113079





