Insights into Layered Oxide Cathodes for Rechargeable Batteries
Abstract
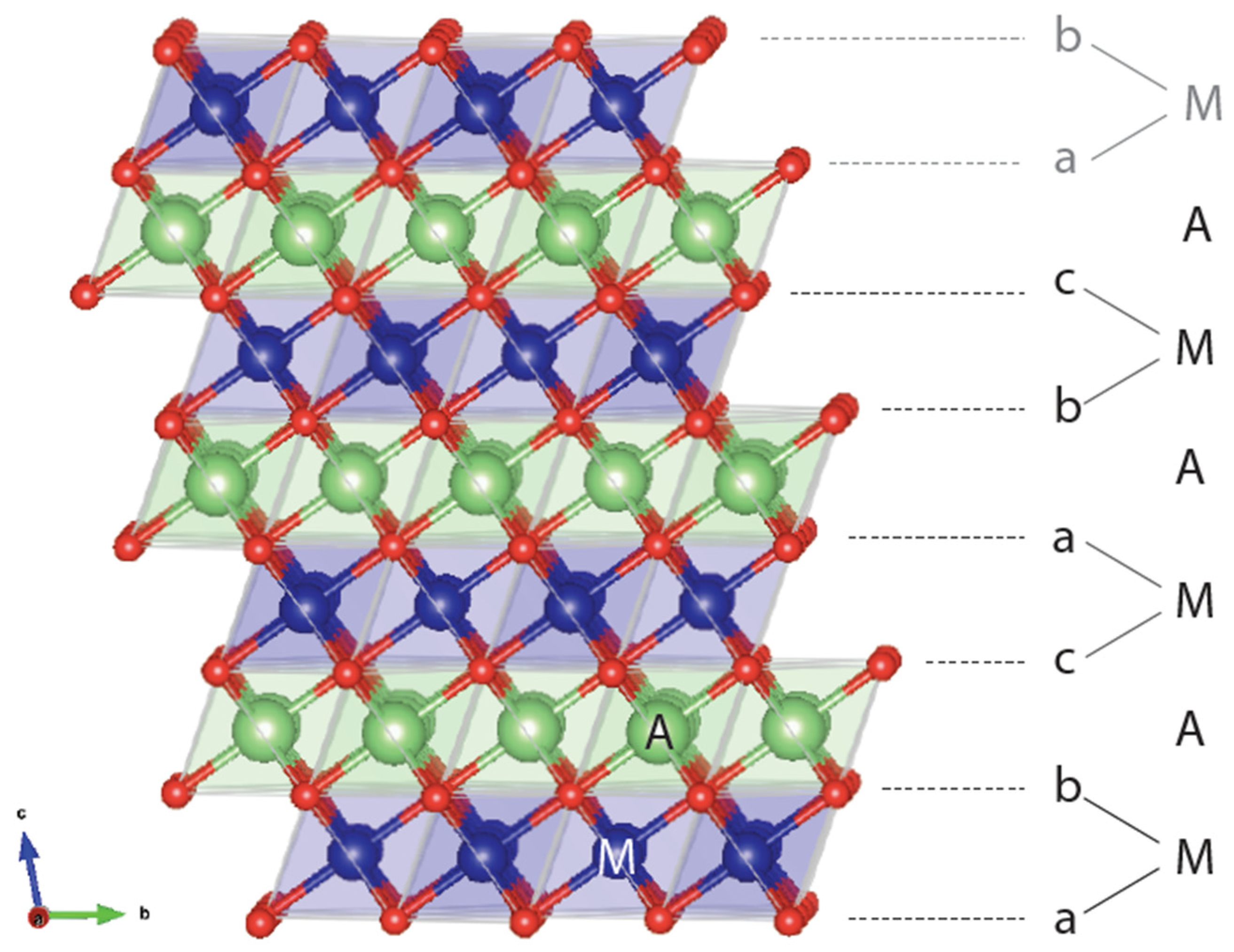
1. Introduction to the O3 Structure
2. Evolution from LiCoO2 to NMC
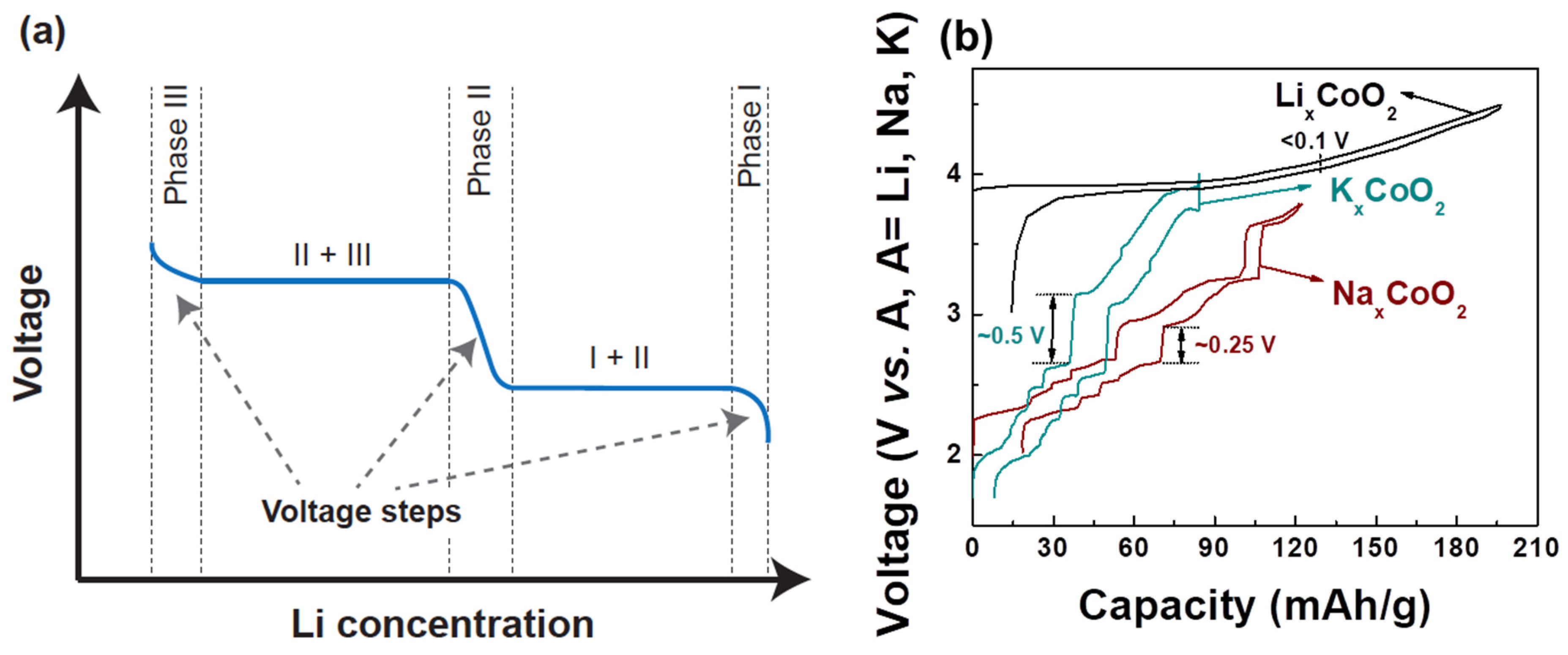
3. Alkali–Alkali Interactions, Alkali/Vacancy Ordering, and Voltage Slope
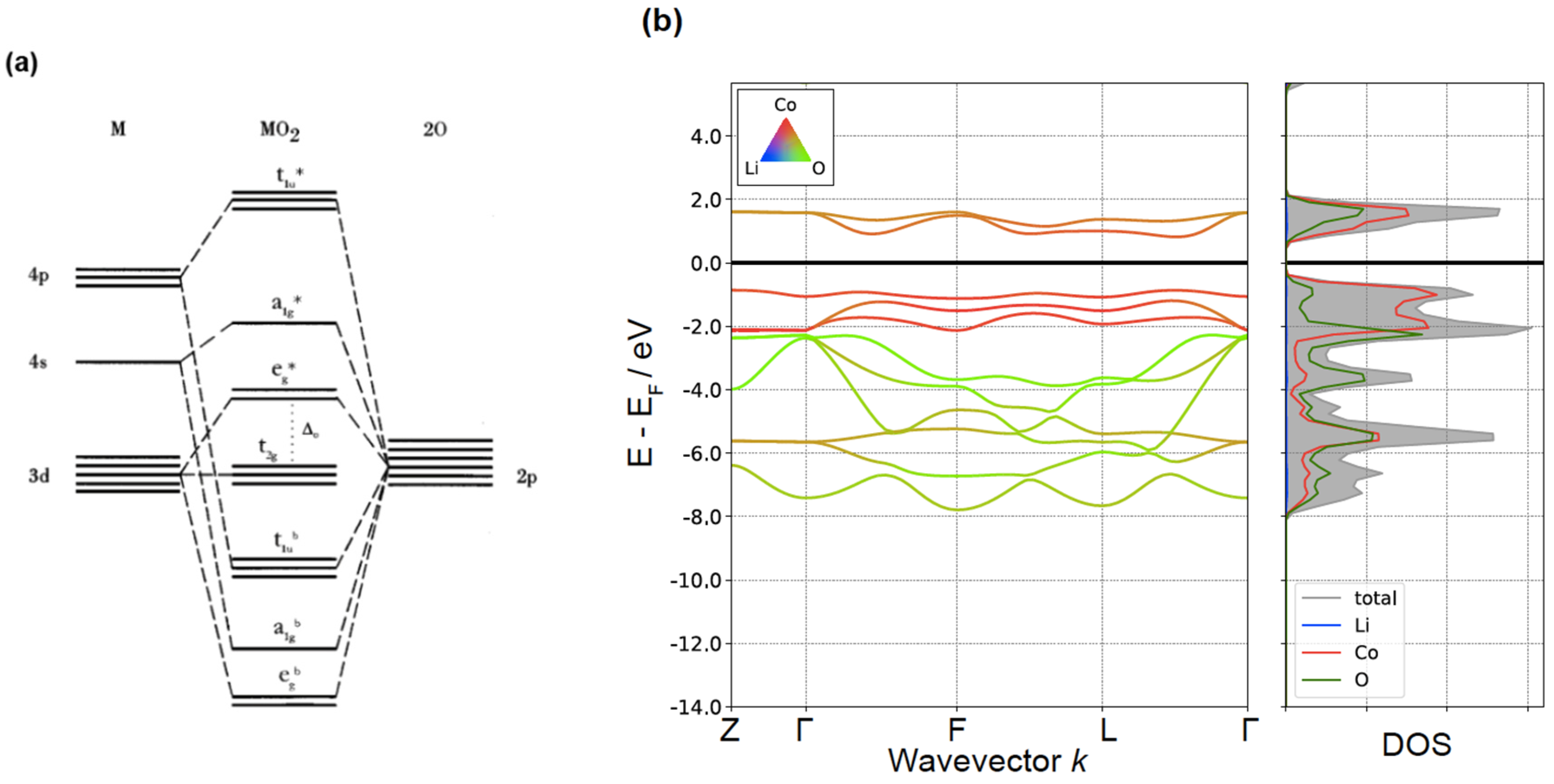
4. Electronic Structure of LiCoO2
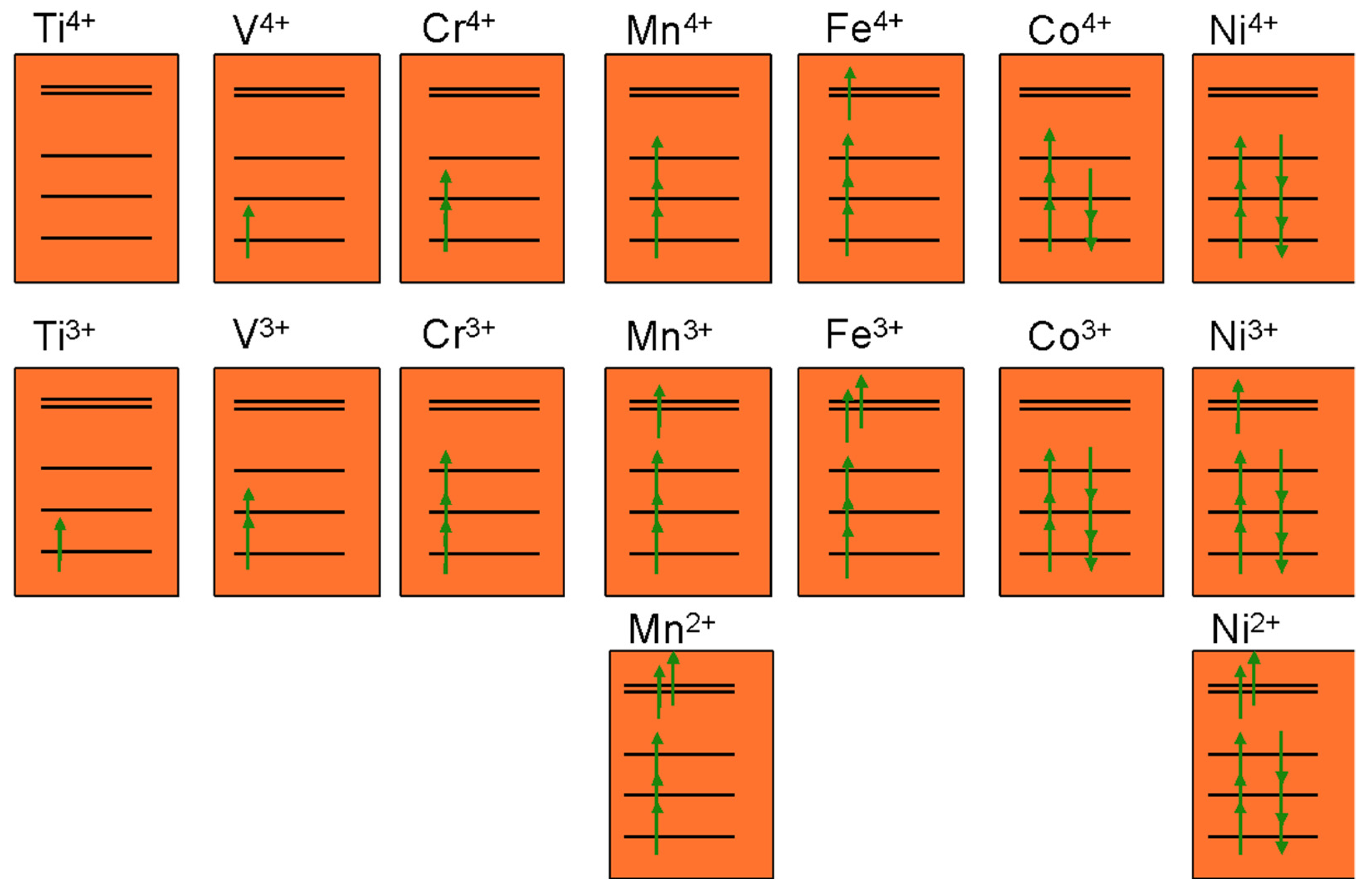
5. Electronic Structure Trends in Layered LixMO2 Oxides
6. Implications of Electronic Structure on Layered Stability
7. Diffusion Mechanism
8. Beyond Layered Materials: DRX
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gamble, F.R.; Osiecki, J.H.; Cais, M.; Pisharody, R.; Disalvo, F.J.; Geballe, T.H. Intercalation complexes of lewis bases and layered sulfides: A large class of new superconductors. Science 1971, 174, 493–497. [Google Scholar] [CrossRef]
- Whittingham, M.S. Electrical Energy Storage and Intercalation Chemistry. Science 1976, 192, 1126–1127. [Google Scholar] [CrossRef]
- Mizushima, K.; Jones, P.C.; Wiseman, P.J.; Goodenough, J.B. LixCoO2 (0 < x < −1): A new cathode material for batteries of high energy density. Mater. Res. Bull. 1980, 15, 783–789. [Google Scholar] [CrossRef]
- Delmas, C.; Fouassier, C.; Hagenmuller, P. Structural classification and properties of the layered oxides. Physica B+C 1980, 99, 81–85. [Google Scholar] [CrossRef]
- Ceder, G.; Van der Ven, A.; Marianetti, C.; Morgan, D. First-principles alloy theory in oxides. Model. Simul. Mater. Sci. Eng. 2000, 8, 311–321. [Google Scholar] [CrossRef]
- Wu, E.J.; Tepesch, P.D.; Ceder, G. Size and charge effects on the structural stability of LiMO2 (M = transition metal) compounds. Philos. Mag. B 1998, 77, 1039–1047. [Google Scholar] [CrossRef]
- Hewston, T.A.; Chamberland, B.L. A Survey of first-row ternary oxides LiMO2 (M = Sc-Cu). J. Phys. Chem. Solids 1987, 48, 97–108. [Google Scholar] [CrossRef]
- Kim, S.; Ma, X.; Ong, S.P.; Ceder, G. A comparison of destabilization mechanisms of the layered NaxMO2 and LixMO2 compounds upon alkali de-intercalation. Phys. Chem. Chem. Phys. 2012, 14, 15571–15578. [Google Scholar] [CrossRef] [PubMed]
- Orman, H.J.; Wiseman, P.J. Cobalt(III) lithium oxide, CoLiO2: Structure refinement by powder neutron diffraction. Acta Crystallogr. Sect. C 1984, 40, 12–14. [Google Scholar] [CrossRef]
- Rüdorff, W.; Becker, H. Notizen: Die Strukturen von LiVO2, NaVO2, LiCrO2 und NaCrO2. Z. Nat. B 1954, 9, 614–615. [Google Scholar] [CrossRef]
- Dyer, L.D.; Borie, B.S.; Smith, G.P. Alkali Metal-Nickel Oxides of the Type MNiO2. J. Am. Chem. Soc. 1954, 76, 1499–1503. [Google Scholar] [CrossRef]
- Manthiram, A.; Goodenough, J.B. Layered lithium cobalt oxide cathodes. Nat. Energy 2021, 6, 323. [Google Scholar] [CrossRef]
- Fu, X.; Beatty, D.N.; Gaustad, G.G.; Ceder, G.; Roth, R.; Kirchain, R.E.; Olivetti, E.A. Perspectives on Cobalt Supply through 2030 in the Face of Changing Demand. Environ. Sci. Technol. 2020, 54, 2985–2993. [Google Scholar] [CrossRef] [PubMed]
- Delmas, C.; Saadoune, I. Electrochemical and physical properties of the LixNi1−yCoyO2 phases. Solid State Ion. 1992, 53–56, 370–375. [Google Scholar] [CrossRef]
- Ceder, G. The Stability of Orthorhombic and Monoclinic-Layered LiMnO2. Electrochem. Solid-State Lett. 1999, 2, 550. [Google Scholar] [CrossRef]
- Capitaine, F.; Gravereau, P.; Delmas, C. A new variety of LiMnO2 with a layered structure. Solid State Ion. 1996, 89, 197–202. [Google Scholar] [CrossRef]
- Armstrong, A.R.; Bruce, P.G. Synthesis of layered LiMnO2 as an electrode for rechargeable lithium batteries. Nature 1996, 381, 499–500. [Google Scholar] [CrossRef]
- Reed, J.; Ceder, G.; Van Der Ven, A. Layered-to-Spinel Phase Transition in LixMnO2. Electrochem. Solid-State Lett. 2001, 4, A78. [Google Scholar] [CrossRef]
- Shao-Horn, Y.; Hackney, S.A.; Armstrong, A.R.; Bruce, P.G.; Gitzendanner, R.; Johnson, C.S.; Thackeray, M.M. Structural Characterization of Layered LiMnO2 Electrodes by Electron Diffraction and Lattice Imaging. J. Electrochem. Soc. 1999, 146, 2404–2412. [Google Scholar] [CrossRef]
- Ceder, G.; Van der Ven, A. Phase diagrams of lithium transition metal oxides: Investigations from first principles. Electrochim. Acta 1999, 45, 131–150. [Google Scholar] [CrossRef]
- Jang, Y.; Huang, B.; Wang, H.; Sadoway, D.R.; Ceder, G.; Chiang, Y.; Liu, H.; Tamura, H. LiAlyCo1 − yO2 (R-3m) Intercalation Cathode for Rechargeable Lithium Batteries. J. Electrochem. Soc. 1999, 146, 862–868. [Google Scholar] [CrossRef]
- Dahn, J.R.; Zheng, T.; Thomas, C.L. Structure and Electrochemistry of Li2CrxMn2−xO4 for 1.0 ≤ x ≤ 1.5. J. Electrochem. Soc. 1998, 145, 851–859. [Google Scholar] [CrossRef]
- Davidson, I.J.; McMillan, R.S.; Murray, J.J.; Greedan, J.E. Lithium-ion cell based on orthorhombic LiMnO2. J. Power Sources 1995, 54, 232–235. [Google Scholar] [CrossRef]
- Reed, J.S. Ab-Initio Study of Cathode Materials for Lithium Batteries. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2003. [Google Scholar]
- Ohzuku, T.; Makimura, Y. Layered Lithium Insertion Material of LiCo1/3Ni1/3Mn1/3O2 for Lithium-Ion Batteries. Chem. Lett. 2001, 30, 642–643. [Google Scholar] [CrossRef]
- Ohzuku, T.; Makimura, Y. Layered Lithium Insertion Material of LiNi1/2Mn1/2O2: A Possible Alternative to LiCoO2 for Advanced Lithium-Ion Batteries. Chem. Lett. 2001, 30, 744–745. [Google Scholar] [CrossRef]
- Lu, Z.; MacNeil, D.D.; Dahn, J.R. Layered Li[NixCo1-2xMnx]O2 Cathode Materials for Lithium-Ion Batteries. Electrochem. Solid-State Lett. 2001, 4, A200. [Google Scholar] [CrossRef]
- Lu, Z.; MacNeil, D.D.; Dahn, J.R. Layered Cathode Materials Li[NixLi1/3−2x/3Mn2/3−x/3]O2 for Lithium-Ion Batteries. Electrochem. Solid-State Lett. 2001, 4, A191. [Google Scholar] [CrossRef]
- Reed, J.; Ceder, G. Charge, Potential, and Phase Stability of Layered Li(Ni0.5Mn0.5)O2. Electrochem. Solid-State Lett. 2002, 5, A145. [Google Scholar] [CrossRef]
- Manthiram, A.; Song, B.; Li, W. A perspective on nickel-rich layered oxide cathodes for lithium-ion batteries. Energy Storage Mater. 2017, 6, 125–139. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.; Cha, H.; Yoon, M.; Park, M.; Cho, J. Prospect and Reality of Ni-Rich Cathode for Commercialization. Adv. Energy Mater. 2018, 8, 1702028. [Google Scholar] [CrossRef]
- Zhu, L.; Bao, C.; Xie, L.; Yang, X.; Cao, X. Review of synthesis and structural optimization of LiNi1/3Co1/3Mn1/3O2 cathode materials for lithium-ion batteries applications. J. Alloys Compd. 2020, 831, 154864. [Google Scholar] [CrossRef]
- Aydinol, M.K.; Ceder, G. First-Principles Prediction of Insertion Potentials in Li-Mn Oxides for Secondary Li Batteries. J. Electrochem. Soc. 1997, 144, 3832–3835. [Google Scholar] [CrossRef]
- Li, W.; Reimers, J.N.; Dahn, J.R. Lattice-gas-model approach to understanding the structures of lithium transition-metal oxides LiMO2. Phys. Rev. B 1994, 49, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Van der Ven, A.; Aydinol, M.K.; Ceder, G.; Kresse, G.; Hafner, J. First-principles investigation of phase stability in LixCoO2. Phys. Rev. B 1998, 58, 2975–2987. [Google Scholar] [CrossRef]
- Reimers, J.N.; Dahn, J.R. Electrochemical and In Situ X-ray Diffraction Studies of Lithium Intercalation in LixCoO2. J. Electrochem. Soc. 1992, 139, 2091–2097. [Google Scholar] [CrossRef]
- Shao-Horn, Y.; Levasseur, S.; Weill, F.; Delmas, C. Probing Lithium and Vacancy Ordering in O3 Layered LixCoO2 (x ≈ 0.5). J. Electrochem. Soc. 2003, 150, A366. [Google Scholar] [CrossRef]
- Li, W.; Reimers, J.N.; Dahn, J.R. In situ x-ray diffraction and electrochemical studies of Li1−xNiO2. Solid State Ion. 1993, 67, 123–130. [Google Scholar] [CrossRef]
- Peres, J.P.; Weill, F.; Delmas, C. Lithium/vacancy ordering in the monoclinic LixNiO2 (0.50 ≤ x ≤ 0.75) solid solution. Solid State Ion. 1999, 116, 19–27. [Google Scholar] [CrossRef]
- Arai, H.; Okada, S.; Ohtsuka, H.; Ichimura, M.; Yamaki, J. Characterization and cathode performance of Li1−xNi1+xO2 prepared with the excess lithium method. Solid State Ion. 1995, 80, 261–269. [Google Scholar] [CrossRef]
- Kim, H.; Ji, H.; Wang, J.; Ceder, G. Next-Generation Cathode Materials for Non-aqueous Potassium-Ion Batteries. Trends Chem. 2019, 1, 682–692. [Google Scholar] [CrossRef]
- Lee, W.; Kim, J.; Yun, S.; Choi, W.; Kim, H.; Yoon, W.-S. Multiscale factors in designing alkali-ion (Li, Na, and K) transition metal inorganic compounds for next-generation rechargeable batteries. Energy Environ. Sci. 2020, 13, 4406–4449. [Google Scholar] [CrossRef]
- Tian, Y.; Zeng, G.; Rutt, A.; Shi, T.; Kim, H.; Wang, J.; Koettgen, J.; Sun, Y.; Ouyang, B.; Chen, T.; et al. Promises and Challenges of Next-Generation “Beyond Li-ion” Batteries for Electric Vehicles and Grid Decarbonization. Chem. Rev. 2021, 121, 1623–1669. [Google Scholar] [CrossRef] [PubMed]
- Berthelot, R.; Carlier, D.; Delmas, C. Electrochemical investigation of the P2–NaxCoO2 phase diagram. Nat. Mater. 2011, 10, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lee, D.A.E.H.O.E.; Meng, Y.S. Recent advances in sodium intercalation positive electrode materials for sodium ion batteries. Funct. Mater. Lett. 2013, 6, 1330001. [Google Scholar] [CrossRef]
- Hironaka, Y.; Kubota, K.; Komaba, S. P2- and P3-KxCoO2 as an electrochemical potassium intercalation host. Chem. Commun. 2017, 53, 3693–3696. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, J.C.; Bo, S.-H.; Shi, T.; Kwon, D.-H.; Ceder, G. K-Ion Batteries Based on a P2-Type K0.6CoO2 Cathode. Adv. Energy Mater. 2017, 7, 1700098. [Google Scholar] [CrossRef]
- Sun, Y.-K.; Han, J.-M.; Myung, S.-T.; Lee, S.-W.; Amine, K. Significant improvement of high voltage cycling behavior AlF3-coated LiCoO2 cathode. Electrochem. Commun. 2006, 8, 821–826. [Google Scholar] [CrossRef]
- Radin, M.D.; Van der Ven, A. Stability of Prismatic and Octahedral Coordination in Layered Oxides and Sulfides Intercalated with Alkali and Alkaline-Earth Metals. Chem. Mater. 2016, 28, 7898–7904. [Google Scholar] [CrossRef]
- DeKock, R.L.; Gray, H.B. Chemical Structure and Bonding; The Benjamin/Cummings Publishing Company: Menlo Park, CA, USA, 1980. [Google Scholar]
- Sun, J.; Ruzsinszky, A.; Perdew, J.P. Strongly Constrained and Appropriately Normed Semilocal Density Functional. Phys. Rev. Lett. 2015, 115, 36402. [Google Scholar] [CrossRef]
- Aydinol, M.K.; Kohan, A.F.; Ceder, G.; Cho, K.; Joannopoulos, J. Ab initio study of lithium intercalation in metal oxides and metal dichalcogenides. Phys. Rev. B 1997, 56, 1354–1365. [Google Scholar] [CrossRef]
- Marianetti, C.A.; Morgan, D.; Ceder, G. First-principles investigation of the cooperative Jahn-Teller effect for octahedrally coordinated transition-metal ions. Phys. Rev. B 2001, 63, 224304. [Google Scholar] [CrossRef]
- Akimoto, J.; Gotoh, Y.; Oosawa, Y. Synthesis and Structure Refinement of LiCoO2 Single Crystals. J. Solid State Chem. 1998, 141, 298–302. [Google Scholar] [CrossRef]
- Wolverton, C.; Zunger, A. First-Principles Prediction of Vacancy Order-Disorder and Intercalation Battery Voltages in LiCoO2. Phys. Rev. Lett. 1998, 81, 606–609. [Google Scholar] [CrossRef]
- Yin, S.-C.; Rho, Y.-H.; Swainson, I.; Nazar, L.F. X-ray/Neutron Diffraction and Electrochemical Studies of Lithium De/Re-Intercalation in Li1-xCo1/3Ni1/3Mn1/3O2 (x = 0 → 1). Chem. Mater. 2006, 18, 1901–1910. [Google Scholar] [CrossRef]
- Reed, J.; Ceder, G. Role of Electronic Structure in the Susceptibility of Metastable Transition-Metal Oxide Structures to Transformation. Chem. Rev. 2004, 104, 4513–4534. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-S.; Hwang, S.-J.; Choy, J.-H. Relationship between Chemical Bonding Character and Electrochemical Performance in Nickel-Substituted Lithium Manganese Oxides. J. Phys. Chem. B 2001, 105, 4860–4866. [Google Scholar] [CrossRef]
- Chernova, N.A.; Roppolo, M.; Dillon, A.C.; Whittingham, M.S. Layered vanadium and molybdenum oxides: Batteries and electrochromics. J. Mater. Chem. 2009, 19, 2526–2552. [Google Scholar] [CrossRef]
- Radin, M.D.; Vinckeviciute, J.; Seshadri, R.; Van der Ven, A. Manganese oxidation as the origin of the anomalous capacity of Mn-containing Li-excess cathode materials. Nat. Energy 2019, 4, 639–646. [Google Scholar] [CrossRef]
- Vinckeviciute, J.; Kitchaev, D.A.; Van der Ven, A. A Two-Step Oxidation Mechanism Controlled by Mn Migration Explains the First-Cycle Activation Behavior of Li2MnO3-Based Li-Excess Materials. Chem. Mater. 2021, 33, 1625–1636. [Google Scholar] [CrossRef]
- Olivetti, E.A.; Ceder, G.; Gaustad, G.G.; Fu, X. Lithium-Ion Battery Supply Chain Considerations: Analysis of Potential Bottlenecks in Critical Metals. Joule 2017, 1, 229–243. [Google Scholar] [CrossRef]
- Van der Ven, A. Lithium Diffusion in Layered LixCoO2. Electrochem. Solid-State Lett. 1999, 3, 301. [Google Scholar] [CrossRef]
- Xia, H.; Lu, L.; Ceder, G. Substrate effect on the microstructure and electrochemical properties of LiCoO2 thin films grown by PLD. J. Alloys Compd. 2006, 417, 304–310. [Google Scholar] [CrossRef]
- Jang, Y.-I.; Neudecker, B.J.; Dudney, N. Lithium diffusion in LixCoO2 (0.45 < x < 0.7) Intercalation cathodes. Electrochem. Solid-State Lett. 2001, 4, A74. [Google Scholar] [CrossRef]
- Ohzuku, T.; Ueda, A. Solid-State Redox Reactions of LiCoO2 (R3m) for 4 Volt Secondary Lithium Cells. J. Electrochem. Soc. 1994, 141, 2972–2977. [Google Scholar] [CrossRef]
- Amatucci, G.G.; Tarascon, J.M.; Klein, L.C. CoO2, the End Member of the LixCoO2 Solid Solution. J. Electrochem. Soc. 1996, 143, 1114–1123. [Google Scholar] [CrossRef]
- Van der Ven, A.; Aydinol, M.K.; Ceder, G. First-Principles Evidence for Stage Ordering in LixCoO2. J. Electrochem. Soc. 1998, 145, 2149–2155. [Google Scholar] [CrossRef]
- Van der Ven, A.; Ceder, G. Lithium diffusion mechanisms in layered intercalation compounds. J. Power Sources 2001, 97–98, 529–531. [Google Scholar] [CrossRef]
- Urban, A.; Lee, J.; Ceder, G. The Configurational Space of Rocksalt-Type Oxides for High-Capacity Lithium Battery Electrodes. Adv. Energy Mater. 2014, 4, 1400478. [Google Scholar] [CrossRef]
- Van der Ven, A.; Bhattacharya, J.; Belak, A.A. Understanding Li Diffusion in Li-Intercalation Compounds. Acc. Chem. Res. 2013, 46, 1216–1225. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Ceder, G. Factors that affect Li mobility in layered lithium transition metal oxides. Phys. Rev. B 2006, 74, 94105. [Google Scholar] [CrossRef]
- Kang, K.; Meng, Y.S.; Bréger, J.; Grey, C.P.; Ceder, G. Electrodes with High Power and High Capacity for Rechargeable Lithium Batteries. Science 2006, 311, 977–980. [Google Scholar] [CrossRef]
- Lee, J.; Urban, A.; Li, X.; Su, D.; Hautier, G.; Ceder, G. Unlocking the Potential of Cation-Disordered Oxides for Rechargeable Lithium Batteries. Science 2014, 343, 519–522. [Google Scholar] [CrossRef]
- Ji, H.; Urban, A.; Kitchaev, D.A.; Kwon, D.-H.; Artrith, N.; Ophus, C.; Huang, W.; Cai, Z.; Shi, T.; Kim, J.C.; et al. Hidden structural and chemical order controls lithium transport in cation-disordered oxides for rechargeable batteries. Nat. Commun. 2019, 10, 592. [Google Scholar] [CrossRef]
- Lun, Z.; Ouyang, B.; Kwon, D.-H.; Ha, Y.; Foley, E.E.; Huang, T.-Y.; Cai, Z.; Kim, H.; Balasubramanian, M.; Sun, Y.; et al. Cation-disordered rocksalt-type high-entropy cathodes for Li-ion batteries. Nat. Mater. 2021, 20, 214–221. [Google Scholar] [CrossRef]
- Clément, R.J.; Lun, Z.; Ceder, G. Cation-disordered rocksalt transition metal oxides and oxyfluorides for high energy lithium-ion cathodes. Energy Environ. Sci. 2020, 13, 345–373. [Google Scholar] [CrossRef]
- Baur, C.; Källquist, I.; Chable, J.; Chang, J.H.; Johnsen, R.E.; Ruiz-Zepeda, F.; Mba, J.M.A.; Naylor, A.J.; Garcia-Lastra, J.M.; Vegge, T.; et al. Improved cycling stability in high-capacity Li-rich vanadium containing disordered rock salt oxyfluoride cathodes. J. Mater. Chem. A 2019, 7, 21244–21253. [Google Scholar] [CrossRef]
- Chen, R.; Ren, S.; Yavuz, M.; Guda, A.A.; Shapovalov, V.; Witter, R.; Fichtner, M.; Hahn, H. Li+ intercalation in isostructural Li2VO3 and Li2VO2F with O2− and mixed O2−/F− anions. Phys. Chem. Chem. Phys. 2015, 17, 17288–17295. [Google Scholar] [CrossRef]
- Lee, J.; Kitchaev, D.A.; Kwon, D.-H.; Lee, C.-W.; Papp, J.K.; Liu, Y.-S.; Lun, Z.; Clement, R.J.; Shi, T.; McCloskey, B.D.; et al. Reversible Mn2+/Mn4+ double redox in lithium-excess cathode materials. Nature 2018, 556, 185–190. [Google Scholar] [CrossRef]
- Takeda, N.; Hoshino, S.; Xie, L.; Chen, S.; Ikeuchi, I.; Natsui, R.; Nakura, K.; Yabuuchi, N. Reversible Li storage for nanosize cation/anion-disordered rocksalt-type oxyfluorides: LiMoO2–x LiF (0 ≤ x ≤ 2) binary system. J. Power Sources 2017, 367, 122–129. [Google Scholar] [CrossRef]
- Glazier, S.L.; Li, J.; Zhou, J.; Bond, T.; Dahn, J.R. Characterization of Disordered Li(1+x)Ti2xFe(1–3x)O2 as Positive Electrode Materials in Li-Ion Batteries Using Percolation Theory. Chem. Mater. 2015, 27, 7751–7756. [Google Scholar] [CrossRef]
- Cambaz, M.A.; Vinayan, B.P.; Euchner, H.; Pervez, S.A.; Geßwein, H.; Braun, T.; Gross, A.; Fichtner, M. Design and Tuning of the Electrochemical Properties of Vanadium-Based Cation-Disordered Rock-Salt Oxide Positive Electrode Material for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 39848–39858. [Google Scholar] [CrossRef] [PubMed]
- Urban, A.; Abdellahi, A.; Dacek, S.; Artrith, N.; Ceder, G. Electronic-Structure Origin of Cation Disorder in Transition-Metal Oxides. Phys. Rev. Lett. 2017, 119, 176402. [Google Scholar] [CrossRef] [PubMed]
- Lun, Z.; Ouyang, B.; Kitchaev, D.A.; Clément, R.J.; Papp, J.K.; Balasubramanian, M.; Ceder, G. Improved Cycling Performance of Li-Excess Cation-Disordered Cathode Materials upon Fluorine Substitution. Adv. Energy Mater. 2019, 9, 1802959. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.H.; Kim, H.; Ceder, G. Insights into Layered Oxide Cathodes for Rechargeable Batteries. Molecules 2021, 26, 3173. https://doi.org/10.3390/molecules26113173
Yang JH, Kim H, Ceder G. Insights into Layered Oxide Cathodes for Rechargeable Batteries. Molecules. 2021; 26(11):3173. https://doi.org/10.3390/molecules26113173
Chicago/Turabian StyleYang, Julia H., Haegyeom Kim, and Gerbrand Ceder. 2021. "Insights into Layered Oxide Cathodes for Rechargeable Batteries" Molecules 26, no. 11: 3173. https://doi.org/10.3390/molecules26113173
APA StyleYang, J. H., Kim, H., & Ceder, G. (2021). Insights into Layered Oxide Cathodes for Rechargeable Batteries. Molecules, 26(11), 3173. https://doi.org/10.3390/molecules26113173






