Fast Li-Ion Conduction in Spinel-Structured Solids
Abstract
1. Introduction
2. Results
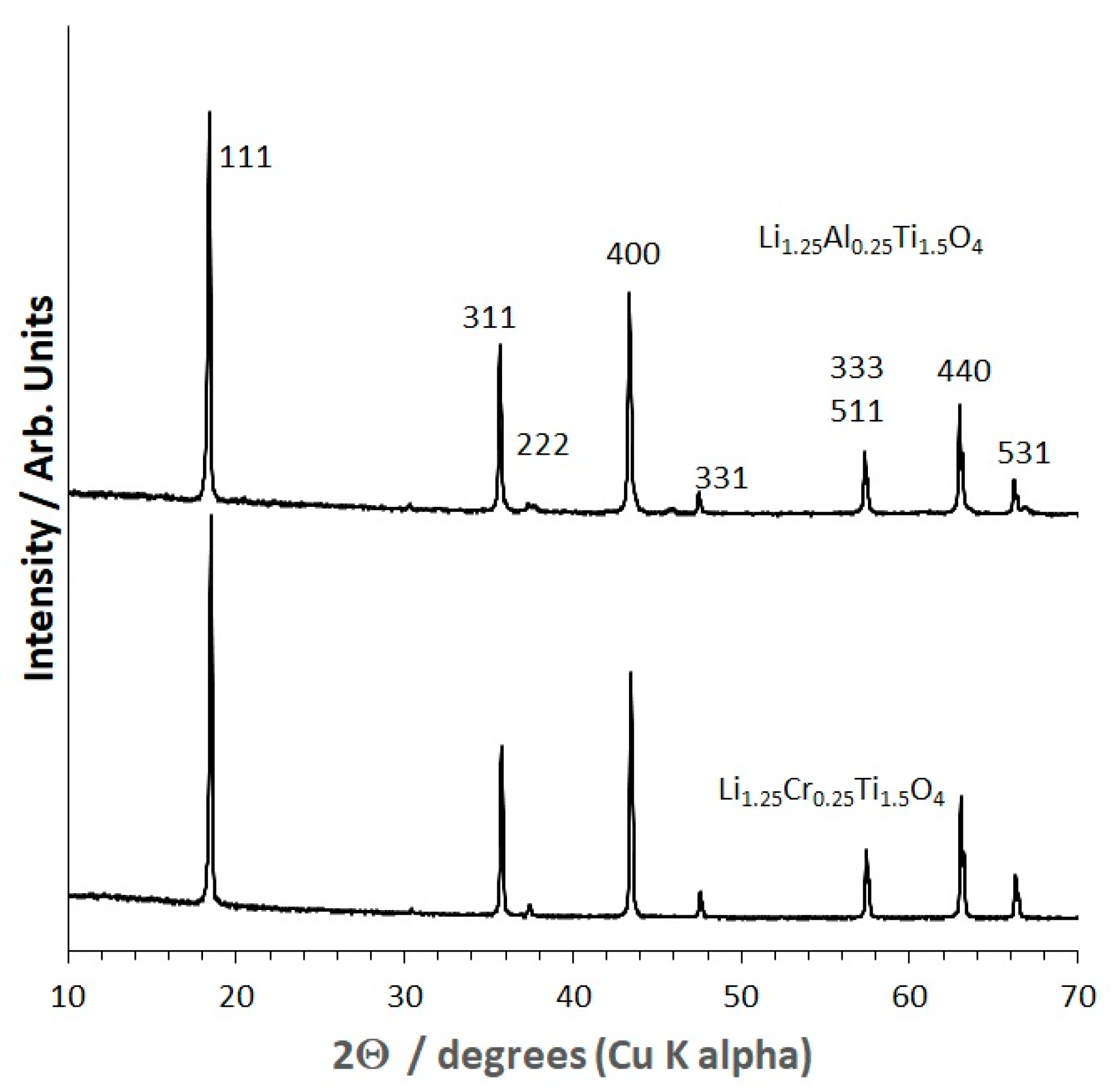
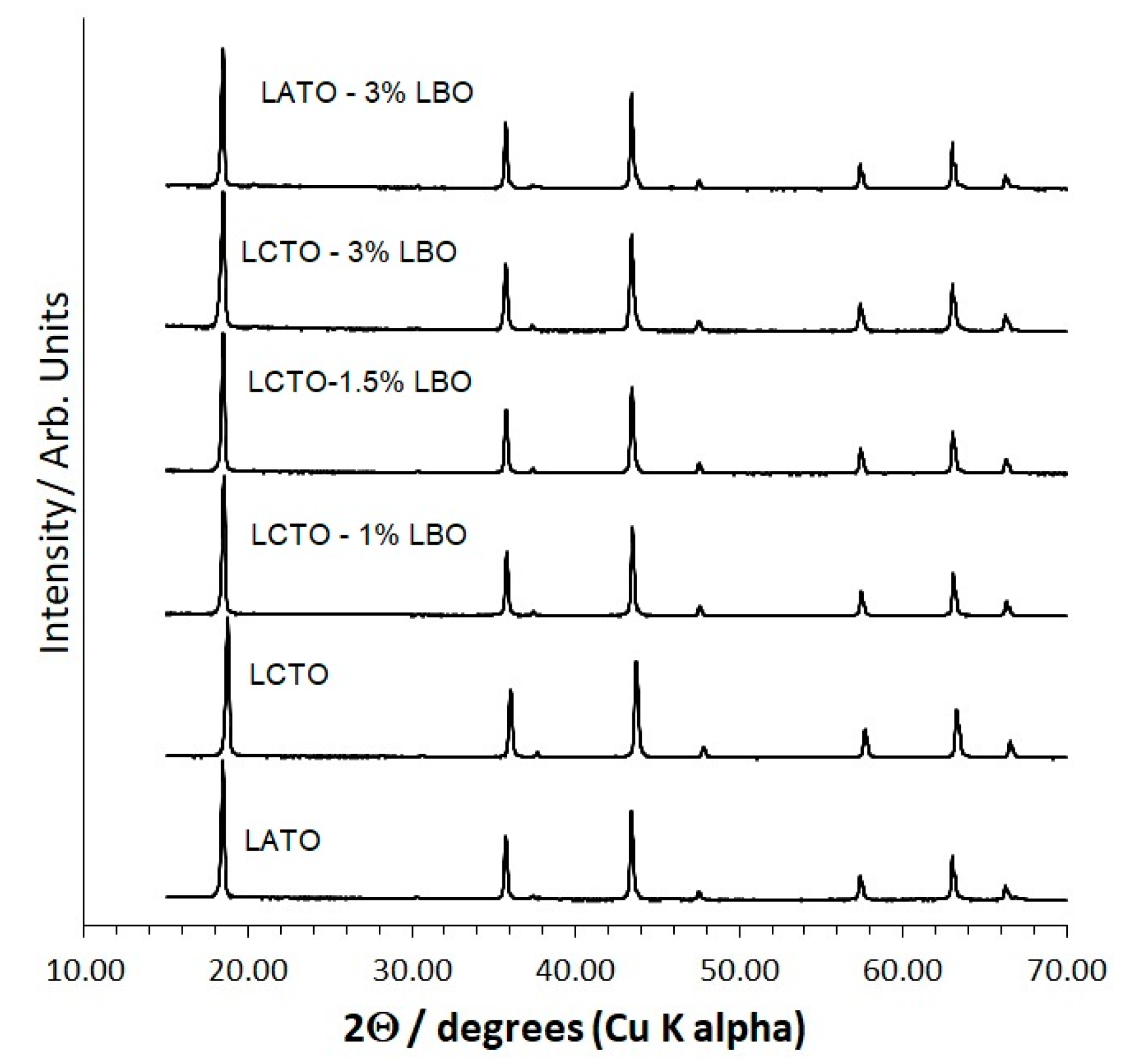
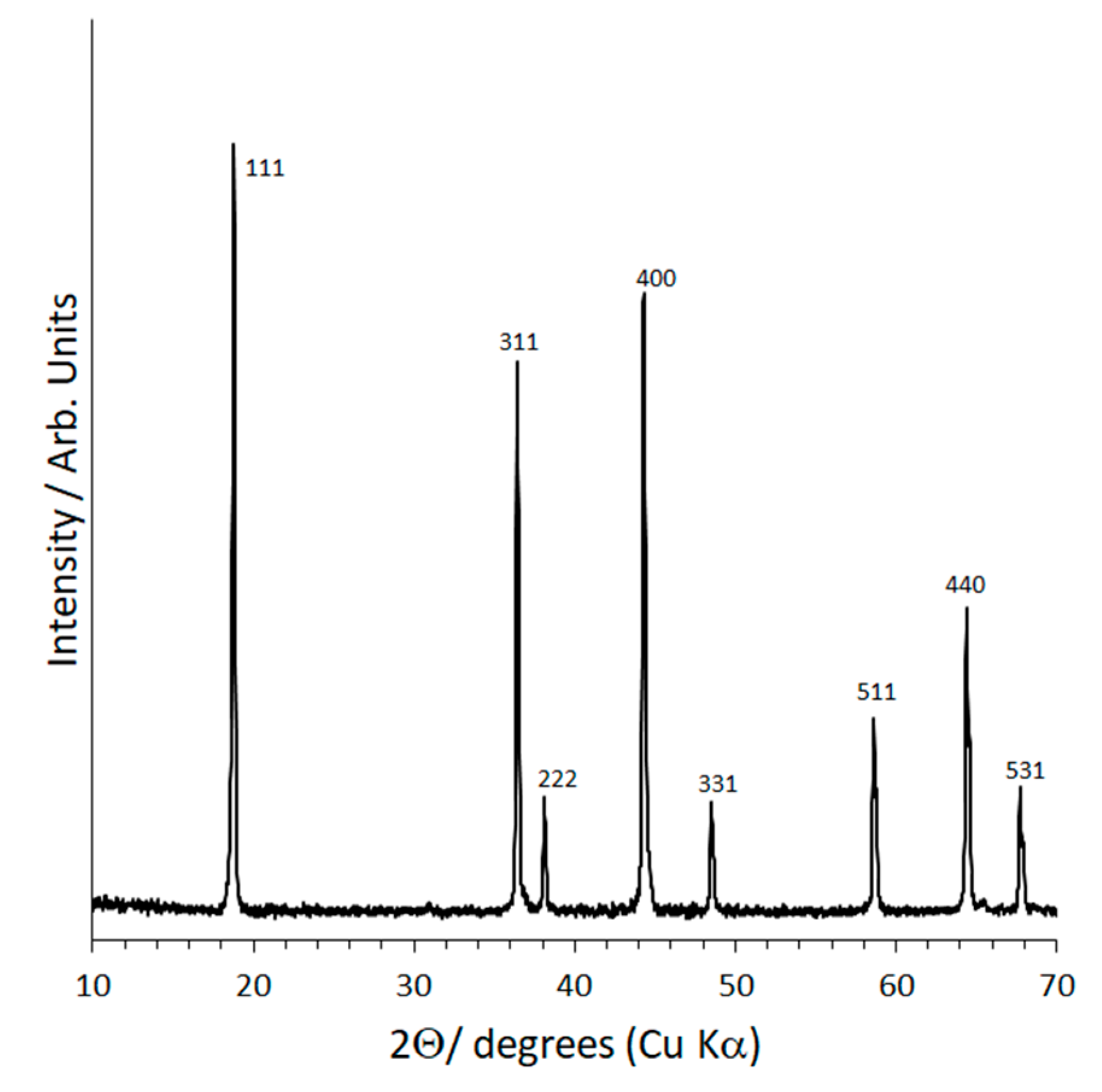
2.1. X-ray Diffraction (XRD) and Structural Refinement
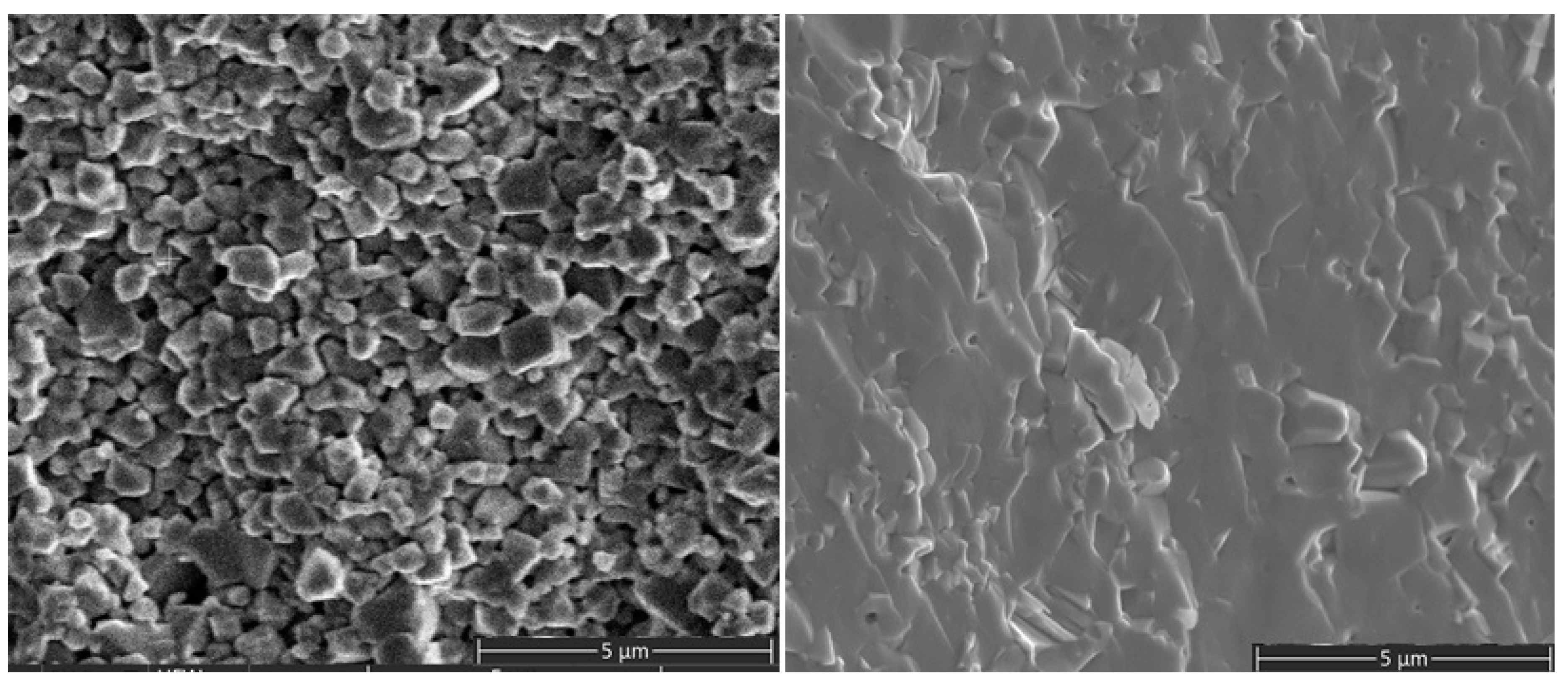
2.2. Microstructure
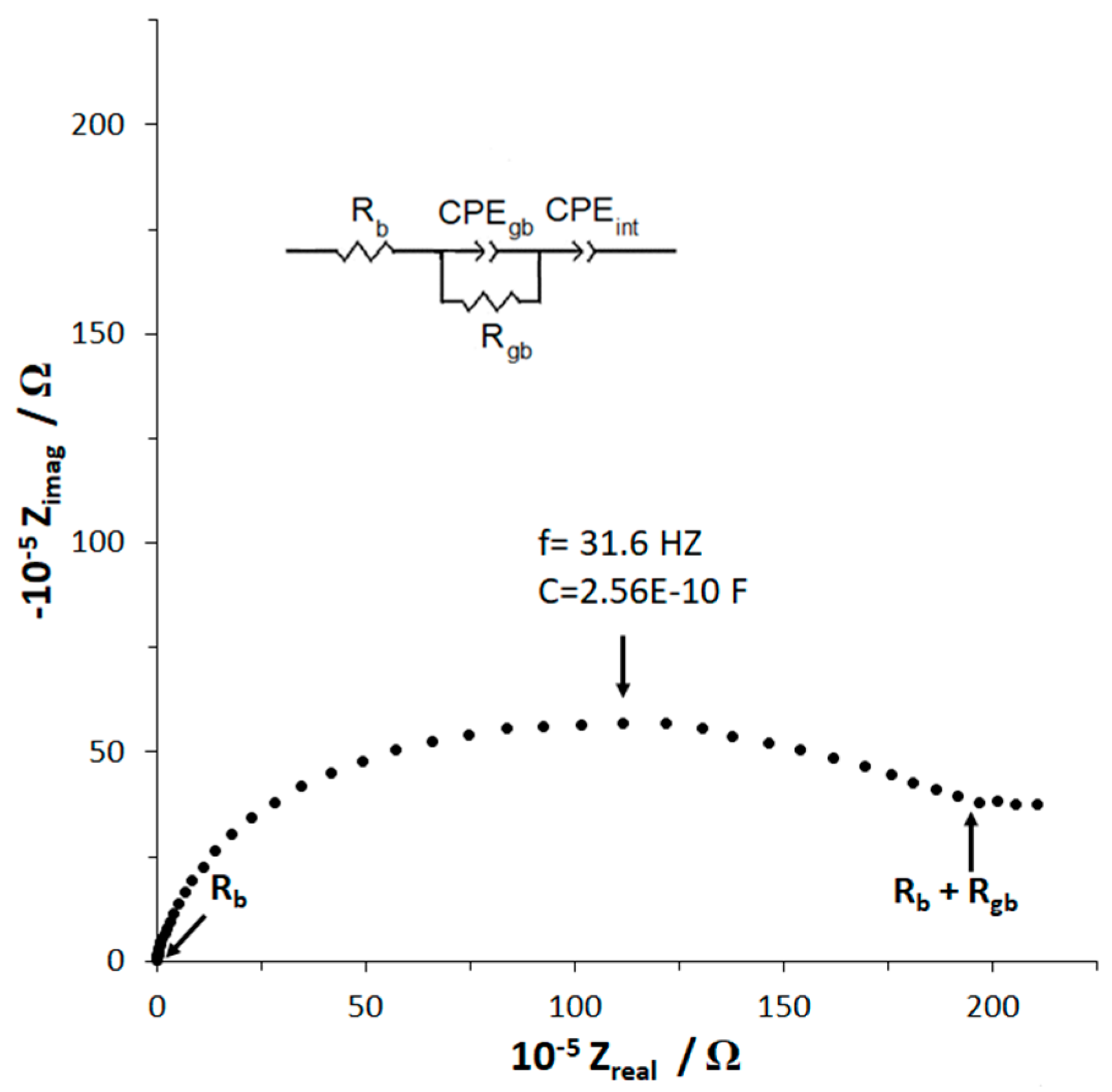
2.3. Conductivity
2.4. Electrochemical Properties of Solid Solutions of LiNi0.5Mn1.5O4 and “Li1.25Cr0.25Mn1.5O4”
2.5. Composition
3. Discussion
4. Materials and Methods
4.1. Powder Preparation
4.2. Consolidation of Samples for Conductivity Measurements
4.3. X-ray Diffraction
4.4. Elemental Analysis
4.5. Microstructure
4.6. Conductivity
4.7. Electrochemical Measurements
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- Murugan, R.; Thangadurai, V.; Weppner, W. Fast Lithium Ion Conduction in Garnet-type Li7La3Zr2O12. Angew. Chem. Int. Ed. 2007, 46, 7778–7781. [Google Scholar] [CrossRef] [PubMed]
- Thackeray, M.M.; David, W.I.F.; Bruce, P.G.; Goodenough, J.B. Lithium Insertion into Manganese Spinels. Mater. Res. Bull. 1983, 11, 461–472. [Google Scholar] [CrossRef]
- Ota, T.; Yamai, I. Thermal Expansion Behavior of NaZr2(PO4)3Type Compounds. J. Am. Ceram. Soc. 1986, 69, 1–6. [Google Scholar] [CrossRef]
- Yamai, I.; Ota, T. Grain Size-Microcracking Relation for NaZr2(PO4)3 Family Ceramics. J. Am. Ceram. Soc. 1993, 76, 487–491. [Google Scholar] [CrossRef]
- Smith, S.; Thompson, T.; Sakamoto, J.; Allen, J.L.; Baker, D.R.; Wolfenstine, J. Electrical, mechanical and chemical behavior of Li1.2Zr1.9Sr0.1(PO4)3. Solid-State Ion. 2017, 300, 38–45. [Google Scholar] [CrossRef]
- Case, E.D. The effect of microcracking upon the Poisson’s ratio for brittle materials. J. Mater. Sci. Lett. 1984, 19, 3702–3712. [Google Scholar]
- Kim, Y.; Case, E.D.; Gaynor, S. The effect of surface-limited microcracks on the effective Young’s modulus of ceramics. J. Mater. Sci. 1993, 28, 1910–1918. [Google Scholar] [CrossRef]
- Jackman, S.D.; Cutler, R.A. The effect of microcracking on ionic conductivity in LATP. J. Power Sources 2012, 218, 65–72. [Google Scholar] [CrossRef]
- Hupfer, T.; Bucharsky, E.C.; Schell, K.G.; Senyshyn, A.; Monchak, M.; Hoffmann, M.J.; Ehrenberg, H. Evolution of microstructure and its relation to ionic conductivity in Li1+xAlxTi2-x(PO4)3. Solid-State Ion. 2016, 288, 235–239. [Google Scholar] [CrossRef]
- Kawai, H.; Tabuchi, M.; Nagata, M.; Tukamoto, H.; West, A.R. Crystal chemistry and physical properties of complex lithium spinels Li2MM’3O8 (M = Mg, Co, Ni, Zn; M’ = Ti, Ge). J. Mater. Chem. 1998, 8, 1273–1280. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Goodenough, J.B. Solid State Cell Wherein an Anode, Solid Electrolyte and Cathode Each Comprise a Cu-Bic-Close-Packed Framework Structure. U.S. Patent No. 4,507,371, 26 March 1985. [Google Scholar]
- Rosciano, F.; Pescarmona, P.P.; Houthoofd, K.; Persoons, A.; Bottke, P.; Wilkening, M. Towards a Lattice-Matching Solid-State Battery: Synthesis of a New Class of Lithium-Ion Conductors with the Spinel Structure. Phys. Chem. Chem. Phys. 2013, 15, 6107–6112. [Google Scholar] [CrossRef] [PubMed]
- Djenadic, R.; Botros, M.; Hahn, H. Is Li-Doped MgAl2O4 a Potential Solid Electrolyte for an All-Spinel Li-Ion Battery? Solid State Ion. 2016, 287, 71–76. [Google Scholar] [CrossRef]
- O’Callaghan, M.P.; Lynham, D.R.; Cussen, E.J.; Chen, G.Z. Structure and Ionic-Transport Properties of Lithium-Containing Garnets Li3Ln3Te2O12(Ln = Y, Pr, Nd, Sm−Lu). Chem. Mater. 2006, 18, 4681–4689. [Google Scholar] [CrossRef]
- Thangadurai, V.; Weppner, W. Li6ALa2Ta2O12 (A = Sr, Ba): Novel Garnet-like Oxides for Fast Lithium Ion Conduction. Adv. Funct. Mater. 2005, 15, 107–112. [Google Scholar] [CrossRef]
- Goodenough, J.B. Fast Ionic Conduction in Solids. Proc. R. Soc. A 1984, 393, 215–234. [Google Scholar] [CrossRef]
- Xie, H.; Li, Y.; Goodenough, J.B. NASICON-Type Li1+2xZr2−xCax(PO4)3 with High Ionic Conductivity at Room Temperature. RSC Adv. 2011, 1, 1728–1731. [Google Scholar] [CrossRef]
- Kerman, K.; Luntz, A.; Viswanathan, V.; Chiang, Y.-M.; Chen, Z. Review—Practical Challenges Hindering the Development of Solid-state Li Ion Batteries. J. Electrochem. Soc. 2017, 161, A1731–A1744. [Google Scholar] [CrossRef]
- Bielefeld, A.; Weber, D.A.; Janek, J. Microstructural Modeling of Composite Cathodes for All-Solid-State Batteries. J. Phys. Chem. C Nanomater. Interfaces 2019, 121, 1626–1634. [Google Scholar] [CrossRef]
- Kotobuki, M.; Munakata, H.; Kanamura, K.; Sato, Y.; Yoshida, T. Compatibility of Li7La3Zr2O12 Solid Electrolyte to All-Solid-State Battery Using Li Metal Anode. J. Electrochem. Soc. 2010, 151, A1076–A1079. [Google Scholar] [CrossRef]
- Shannon, R.D.; Prewitt, C.T. Effective Ionic Radii in Oxides and Fluorides. Acta Crystallogr. B 1969, 21, 925–946. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. A 1976, 31, 751–767. [Google Scholar] [CrossRef]
- Burdett, J.K.; Price, G.D.; Price, S.L. Role of the Crystal-Field Theory in Determining the Structures of Spinels. J. Am. Chem. Soc. 1982, 101, 92–95. [Google Scholar] [CrossRef]
- Le, M.-L.-P.; Strobel, P.; Colin, C.V.; Pagnier, T.; Alloin, F. Spinel-Type Solid Solutions Involving Mn4+ and Ti4+: Crystal Chemistry, Magnetic and Electrochemical Properties. J. Phys. Chem. Solids 2011, 71, 124–135. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent Advances in Magnetic Structure Determination by Neutron Powder Diffraction. Phys. B Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Roisnel, T.; Rodríquez-Carvajal, J. WinPLOTR: A Windows Tool for Powder Diffraction Pattern Analysis. Available online: https://www.scientific.net/MSF.378-381.118 (accessed on 28 April 2021).
- Hill, R.J.; Craig, J.R.; Gibbs, G.V. Systematics of the Spinel Structure Type. Phys. Chem. Miner. 1979, 1, 317–339. [Google Scholar]
- Huggins, R.A. Simple Method to Determine Electronic and Ionic Components of the Conductivity in Mixed Conductors a Review. Ionics (Kiel) 2002, 8, 300–313. [Google Scholar] [CrossRef]
- Thangadurai, V.; Huggins, R.A.; Weppner, W. Use of Simple Ac Technique to Determine the Ionic and Electronic Conductivities in Pure and Fe-Substituted SrSnO3 Perovskites. J. Power Sources 2002, 108, 64–69. [Google Scholar] [CrossRef]
- Bauerle, J.E. Study of Solid Electrolyte Polarization by a Complex Admittance Method. J. Phys. Chem. Solids 1969, 31, 2657–2670. [Google Scholar] [CrossRef]
- Irvine, J.T.S.; Sinclair, D.C.; West, A.R. Electroceramics: Characterization by Impedance Spectroscopy. Adv. Mater. 1990, 1, 132–138. [Google Scholar] [CrossRef]
- Kennedy, J.H.; Kimura, N.; Stuber, S.M. Measurement of electronic conductivity in Fe-doped β-alumina. J. Electrochem. Soc. 1982, 129, 1968–1973. [Google Scholar] [CrossRef]
- Hema, M.; Selvasekerapandian, S.; Hirankumar, G.; Sakunthala, A.; Arunkumar, D.; Nithya, H. Structural and Thermal Studies of PVA:NH4I. J. Phys. Chem. Solids 2009, 71, 1098–1103. [Google Scholar] [CrossRef]
- Wu, Z.; Xie, Z.; Yoshida, A.; Wang, Z.; Hao, X.; Abudula, A.; Guan, G. Utmost Limits of Various Solid Electrolytes in All-Solid-State Lithium Batteries: A Critical Review. Renew. Sustain. Energy Rev. 2019, 109, 367–385. [Google Scholar] [CrossRef]
- Bayard, M.A. Complex Impedance Analysis of the Ionic Conductivity of Na1+xZr2SixP3−xO12 Ceramics. J. Electroanal. Chem. 1978, 91, 201–209. [Google Scholar] [CrossRef]
- Allen, J.L.; Wolfenstine, J.; Rangasamy, E.; Sakamoto, J. Effect of Substitution (Ta, Al, Ga) on the Conductivity of Li7La3Zr2O12. J. Power Sources 2012, 206, 315–319. [Google Scholar] [CrossRef]
- Knauth, P. Inorganic Solid Li Ion Conductors: An Overview. Solid-State Ion. 2009, 18, 911–916. [Google Scholar] [CrossRef]
- Harada, Y.; Ishigaki, T.; Kawai, H.; Kuwano, J. Lithium ion conductivity of polycrystalline perovskite La0.67−xLi3xTiO3 with ordered and disordered arrangements of the A-site ions. Solid-State Ion. 1998, 108, 407–413. [Google Scholar] [CrossRef]
- Adachi, G.-Y.; Imanaka, N.; Aono, H. Fast Li⊕ Conducting Ceramic Electrolytes. Adv. Mater. 1996, 1, 127–135. [Google Scholar] [CrossRef]
- Wagner, C. The theory of the warm-up process. Z. Phys. Chem. 1933, 21, 25–41. [Google Scholar]
- Weppner, W.; Huggins, R.A. Determination of the Kinetic Parameters of Mixed-conducting Electrodes and Application to the System Li3Sb. J. Electrochem. Soc. 1977, 121, 1569–1578. [Google Scholar] [CrossRef]
- West, A.R. Solid-State Chemistry and Its Applications, 2nd ed.; John Wiley & Sons: West Sussex, UK, 2014; p. 421. [Google Scholar]
- Alcántara, R.; Jaraba, M.; Lavela, P.; Tirado, J.L.; Biensan, P.; de Guibert, A.; Jordy, C.; Peres, J.P. Structural and Electrochemical Study of New LiNi0.5TixMn1.5-XO4 Spinel Oxides for 5-V Cathode Materials. Chem. Mater. 2003, 11, 2376–2382. [Google Scholar] [CrossRef]
- Liu, D.; Lu, Y.; Goodenough, J.B. Rate Properties and Elevated-Temperature Performances of LiNi0.5−xCr2xMn1.5−xO4 (0≤2x≤0.8) as 5 V Cathode Materials for Lithium-Ion Batteries. J. Electrochem. Soc. 2010, 151, A1269. [Google Scholar] [CrossRef]
- Thompson, T.; Sharafi, A.; Johannes, M.D.; Huq, A.; Allen, J.L.; Wolfenstine, J.; Sakamoto, J. A Tale of Two Sites: On Defining the Carrier Concentration in Garnet-Based Ionic Conductors for Advanced Li Batteries. Adv. Energy Mater. 2015, 1, 1500096. [Google Scholar] [CrossRef]
- Aono, H.; Sugimoto, E.; Sadaoka, Y.; Imanaka, N.; Adachi, G. Ionic Conductivity of Solid Electrolytes Based on Lithium Titanium Phosphate. J. Electrochem. Soc. 1990, 131, 1023–1027. [Google Scholar] [CrossRef]
- Monchak, M.; Hupfer, T.; Senyshyn, A.; Boysen, H.; Chernyshov, D.; Hansen, T.; Schell, K.G.; Bucharsky, E.C.; Hoffmann, M.J.; Ehrenberg, H. Lithium Diffusion Pathway in Li1.3Al0.3Ti1.7(PO4)3 (LATP) Superionic Conductor. Inorg. Chem. 2016, 51, 2941–2945. [Google Scholar] [CrossRef] [PubMed]
- Wilkening, M.; Amade, R.; Iwaniak, W.; Heitjans, P. Ultraslow Li Diffusion in Spinel-Type Structured Li4Ti5O12—a Comparison of Results from Solid-state NMR and Impedance Spectroscopy. Phys. Chem. Chem. Phys. 2007, 1, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Wagemaker, M.; van Eck, E.R.H.; Kentgens, A.P.M.; Mulder, F.M. Li-Ion Diffusion in the Equilibrium Nanomorphology of Spinel Li4+xTi5O12. J. Phys. Chem. B 2009, 111, 224–230. [Google Scholar] [CrossRef]
- Ganapathy, S.; Vasileiadis, A.; Heringa, J.R.; Wagemaker, M. The Fine Line between a Two-Phase and Solid-Solution Phase Transformation and Highly Mobile Phase Interfaces in Spinel Li4+xTi5O12. Adv. Energy Mater. 2017, 1, 1601781. [Google Scholar] [CrossRef]
- Wang, G.X.; Bradhurst, D.H.; Dou, S.X.; Liu, H.K. Spinel Li[Li1/3Ti5/3]O4 as an Anode Material for Lithium Ion Batteries. J. Power Sources 1999, 83, 156–161. [Google Scholar] [CrossRef]
- Panero, S.; Reale, P.; Ronci, F.; Albertini, V.R.; Scrosati, B. Structural and Electrochemical Study on Li(Li1/3Ti5/3)O4 Anode Material for Lithium Ion Batteries. Ionics (Kiel) 2000, 6, 461–465. [Google Scholar] [CrossRef]
- Sugiyama, J.; Nozaki, H.; Umegaki, I.; Mukai, K.; Miwa, K.; Shiraki, S.; Hitosugi, T.; Suter, A.; Prokscha, T.; Salman, Z.; et al. Li-Ion Diffusion In Li4Ti5O12 and LiTi2O4 battery Materials Detected by Muon Spin Spectroscopy. Phys. Rev. B Condens. Matter Mater. Phys. 2015, 91. [Google Scholar] [CrossRef]
- Miara, L.J.; Richards, W.D.; Wang, Y.E.; Ceder, G. First-Principles Studies on Cation Dopants and Electrolyte|cathode Interphases for Lithium Garnets. Chem. Mater. 2015, 21, 4040–4047. [Google Scholar] [CrossRef]
- Zhu, Y.; He, X.; Mo, Y. First Principles Study on Electrochemical and Chemical Stability of Solid Electrolyte–Electrode Interfaces in All-Solid-State Li-Ion Batteries. J. Mater. Chem. A Mater. Energy Sustain. 2016, 4, 3253–3266. [Google Scholar] [CrossRef]
- Hänsel, C.; Afyon, S.; Rupp, J.L.M. Investigating the All-Solid-State Batteries Based on Lithium Garnets and a High Potential Cathode—LiMn1.5Ni0.5O4. Nanoscale 2016, 1, 18412–18420. [Google Scholar] [CrossRef]
- Ohta, N.; Takada, K.; Sakaguchi, I.; Zhang, L.; Ma, R.; Fukuda, K.; Osada, M.; Sasaki, T. LiNbO3-Coated LiCoO2 as Cathode Material for All Solid-State Lithium Secondary Batteries. Electrochem. Commun. 2007, 9, 1486–1490. [Google Scholar] [CrossRef]
- Xia, H.; Meng, Y.S.; Lu, L.; Ceder, G. Electrochemical Properties of Nonstoichiometric LiNi0.5Mn1.5O4−δ Thin-Film Electrodes Prepared by Pulsed Laser Deposition. J. Electrochem. Soc. 2007, 151, A737. [Google Scholar] [CrossRef]
- Gover, R.K.B.; Irvine, J.T.S.; Finch, A.A. Transformation of LiTi2O4 from Spinel to Ramsdellite on Heating. J. Solid-State Chem. 1997, 131, 382–388. [Google Scholar] [CrossRef]
- Rietveld, H.M. A Profile Refinement Method for Nuclear and Magnetic Structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Allen, J.L.; Allen, J.L.; Thompson, T.; Delp, S.A.; Wolfenstine, J.; Jow, T.R. Cr and Si Substituted-LiCo0.9Fe0.1PO4: Structure, Full and Half Li-Ion Cell Performance. J. Power Sources 2016, 327, 229–234. [Google Scholar] [CrossRef]

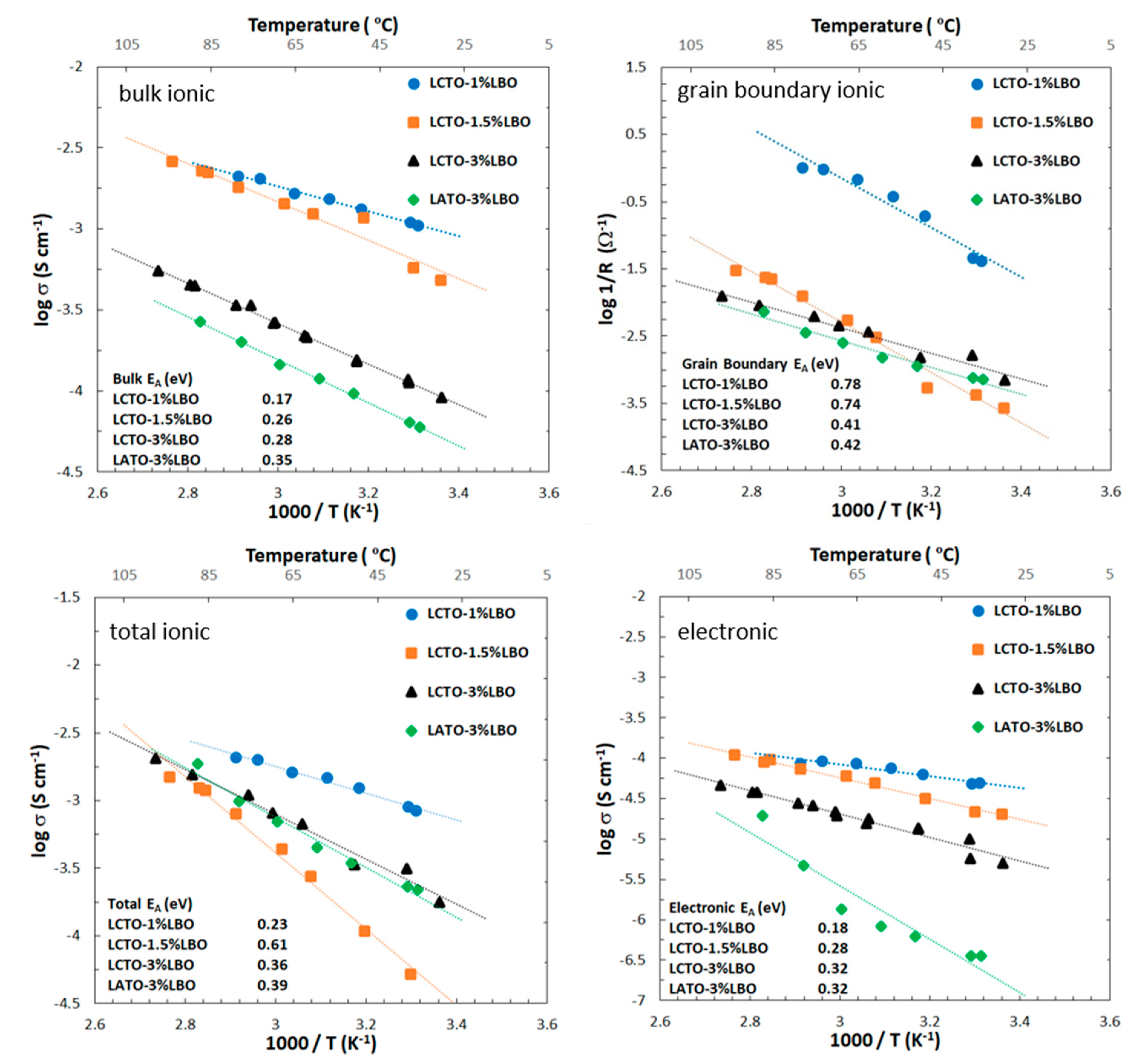

| Sample | σbulk (S cm−1) | Zgb/Ztot (%) | σion (S cm−1) | σelec (S cm−1) | D (%) |
|---|---|---|---|---|---|
| LCTO | 1.63 × 10−4 | 99.9 | 1.19 × 10−7 | 1.84 × 10−8 | 94 |
| LATO | 5.11 × 10−5 | 99.2 | 4.08 × 10−7 | 9.79 × 10−8 | 97 |
| LCTO/1% LBO | 6.77 × 10−4 | 19.3 | 4.17 × 10−4 | 3.76 × 10−4 | 98 |
| LCTO/1.5% LBO | 4.01 × 10−4 | 94.0 | 1.96 × 10−4 | 1.78 × 10−4 | 99 |
| LCTO/3% LBO | 8.75 × 10−5 | 51.9 | 5.32 × 10−5 | 4.06 × 10−5 | 97 |
| LATO/3% LBO | 5.00 × 10−5 | 27.4 | 1.78 × 10−5 | 1.20 × 10−7 | 99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allen, J.L.; Crear, B.A.; Choudhury, R.; Wang, M.J.; Tran, D.T.; Ma, L.; Piccoli, P.M.; Sakamoto, J.; Wolfenstine, J. Fast Li-Ion Conduction in Spinel-Structured Solids. Molecules 2021, 26, 2625. https://doi.org/10.3390/molecules26092625
Allen JL, Crear BA, Choudhury R, Wang MJ, Tran DT, Ma L, Piccoli PM, Sakamoto J, Wolfenstine J. Fast Li-Ion Conduction in Spinel-Structured Solids. Molecules. 2021; 26(9):2625. https://doi.org/10.3390/molecules26092625
Chicago/Turabian StyleAllen, Jan L., Bria A. Crear, Rishav Choudhury, Michael J. Wang, Dat T. Tran, Lin Ma, Philip M. Piccoli, Jeff Sakamoto, and Jeff Wolfenstine. 2021. "Fast Li-Ion Conduction in Spinel-Structured Solids" Molecules 26, no. 9: 2625. https://doi.org/10.3390/molecules26092625
APA StyleAllen, J. L., Crear, B. A., Choudhury, R., Wang, M. J., Tran, D. T., Ma, L., Piccoli, P. M., Sakamoto, J., & Wolfenstine, J. (2021). Fast Li-Ion Conduction in Spinel-Structured Solids. Molecules, 26(9), 2625. https://doi.org/10.3390/molecules26092625








