Triple-Knockout, Synuclein-Free Mice Display Compromised Lipid Pattern
Abstract
1. Introduction
2. Results
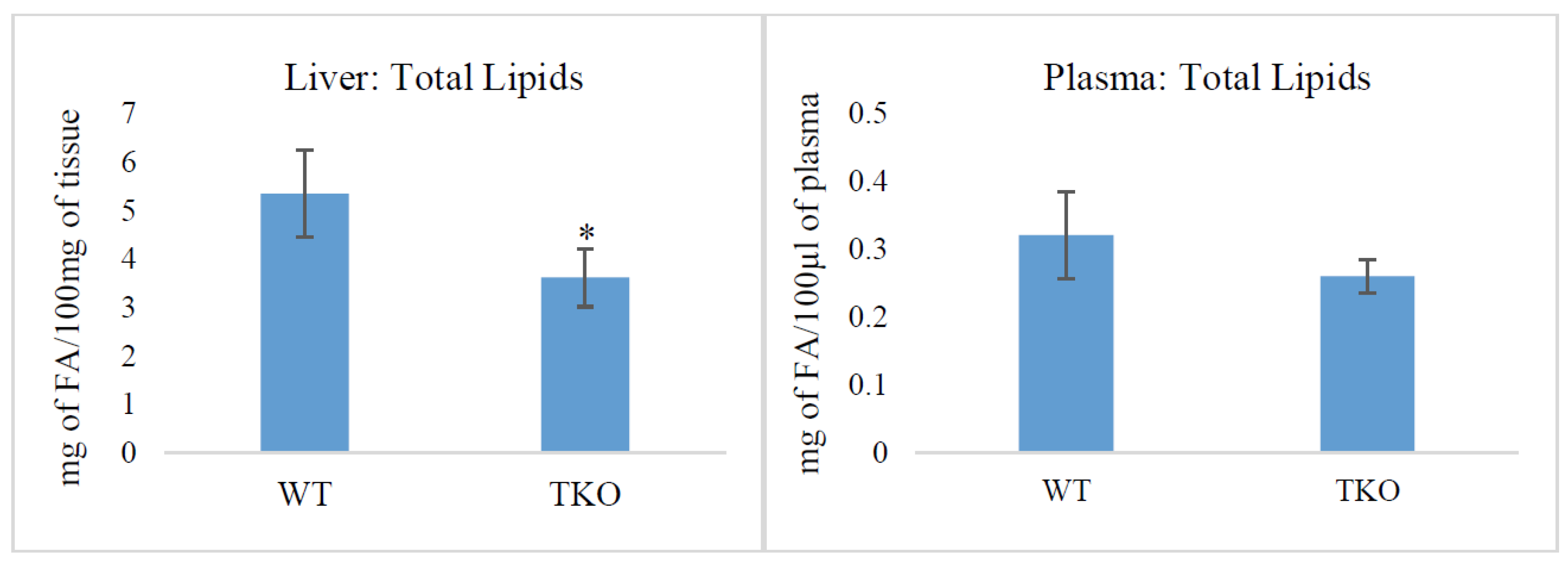
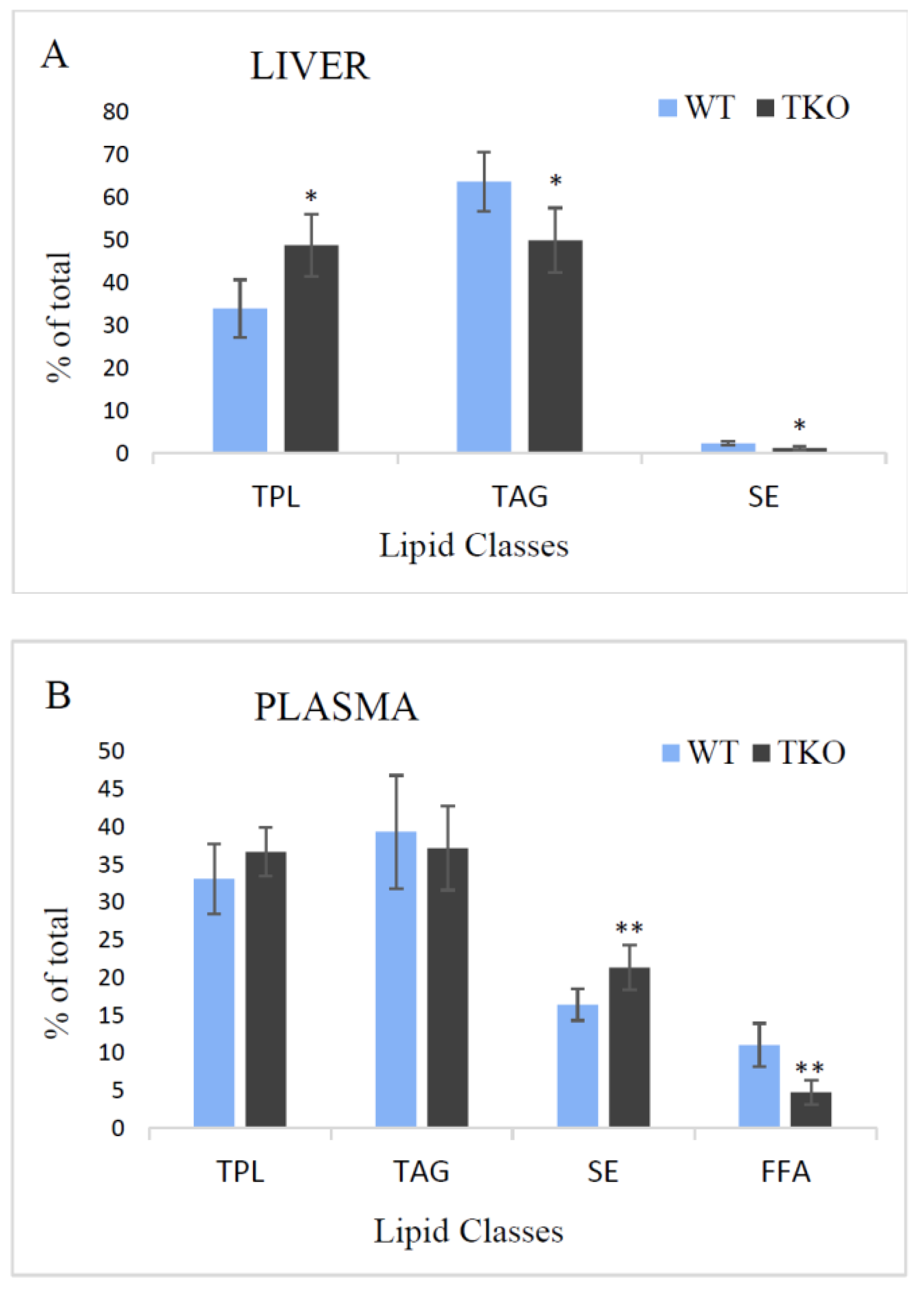
2.1. Total Lipids and Lipid Classes in Liver and Plasma from WT and TKO Mice
2.2. Fatty Acid Composition of Total Lipids in Liver and Plasma from WT and TKO Mice
2.3. Fatty Acid Composition of Lipid Classes in Liver and Plasma from WT and TKO Mice
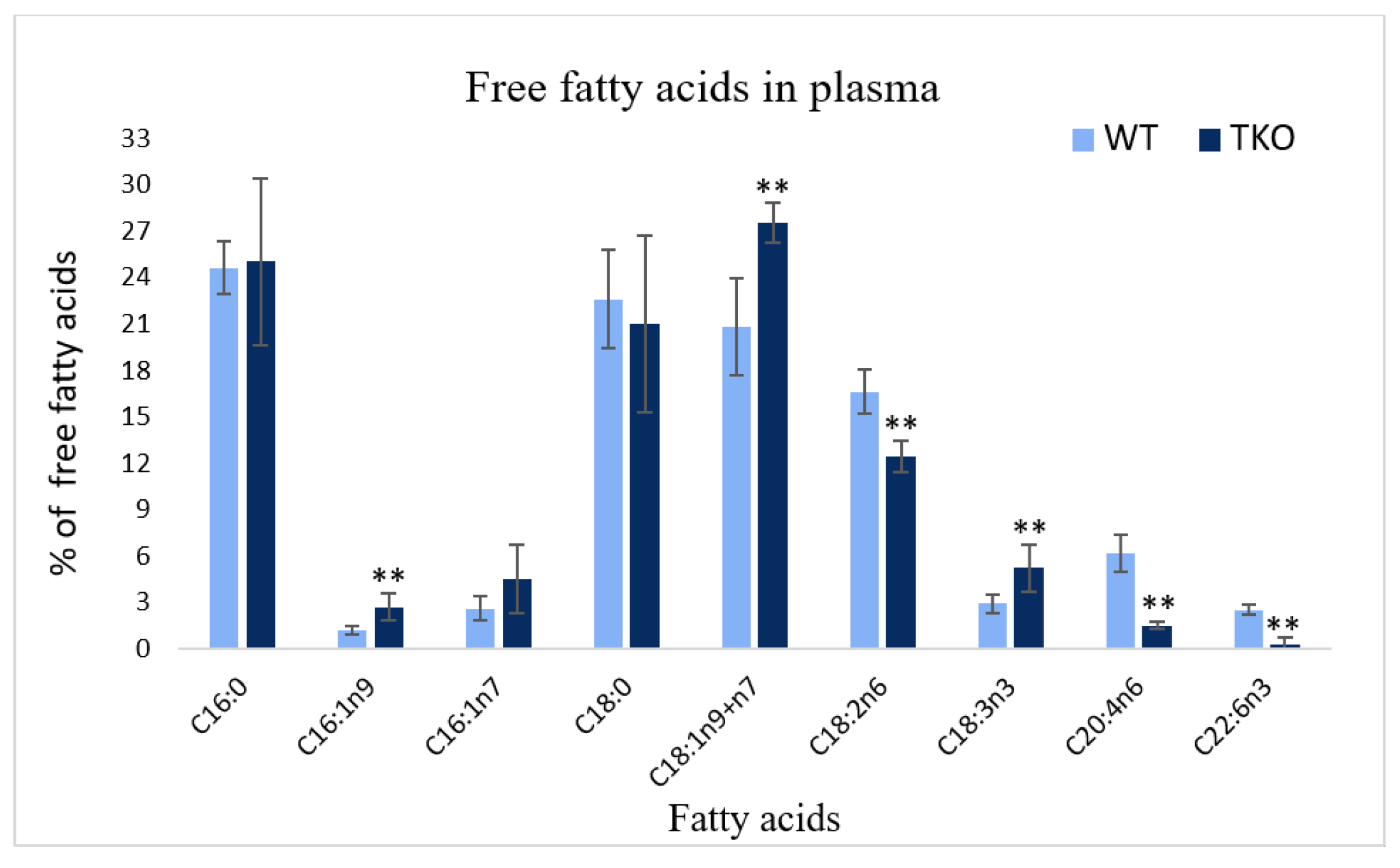
2.4. Composition of Free Fatty Acids in Plasma
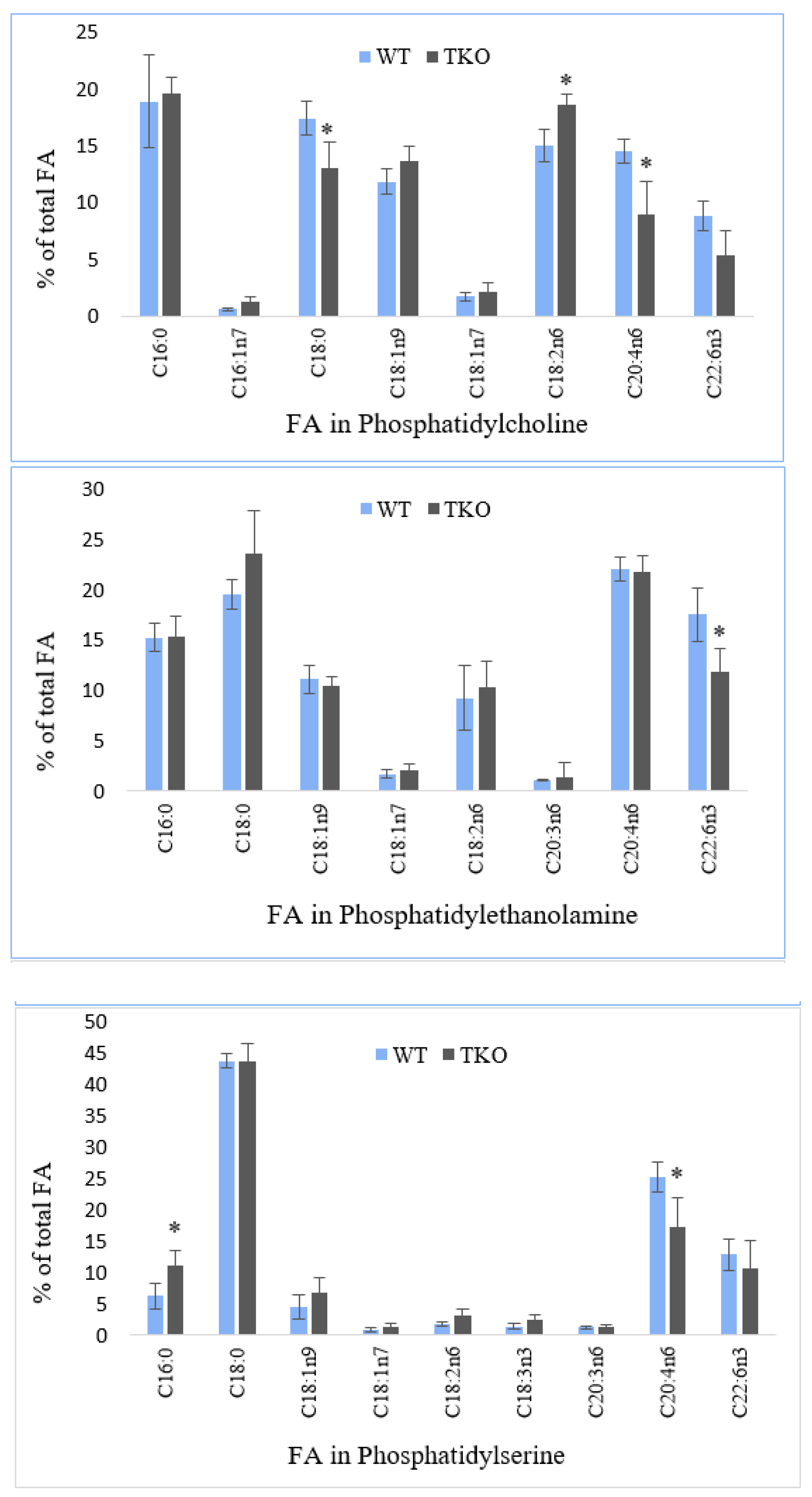
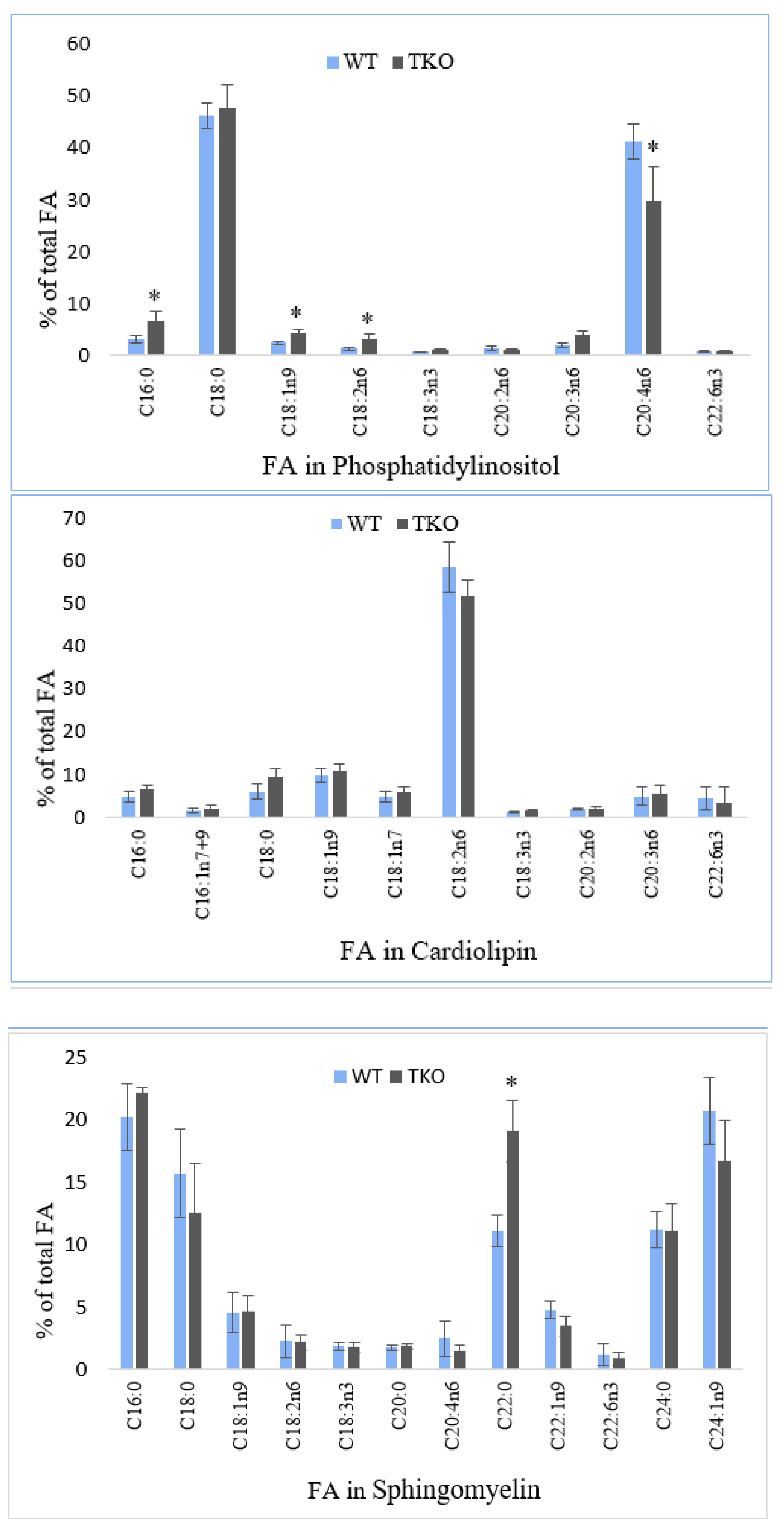
2.5. Fatty Acid Composition of Individual Phospholipids in Liver from WT and TKO Mice
2.6. Fatty Acid Composition of Total Lipids in Midbrain and Cortex from WT and TKO Mice
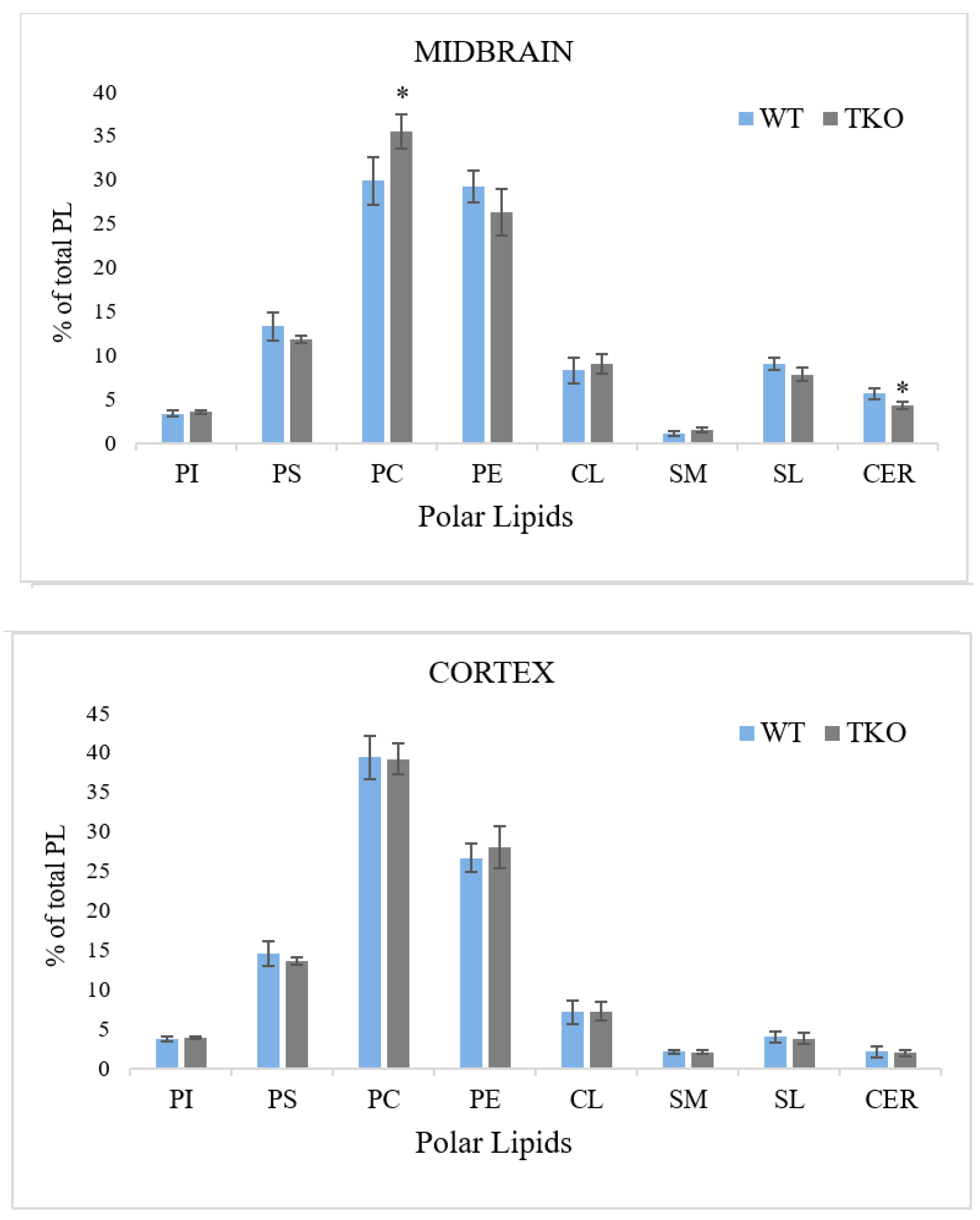
2.7. Composition of Individual Phospholipids in Midbrain and Cortex from WT and TKO Mice
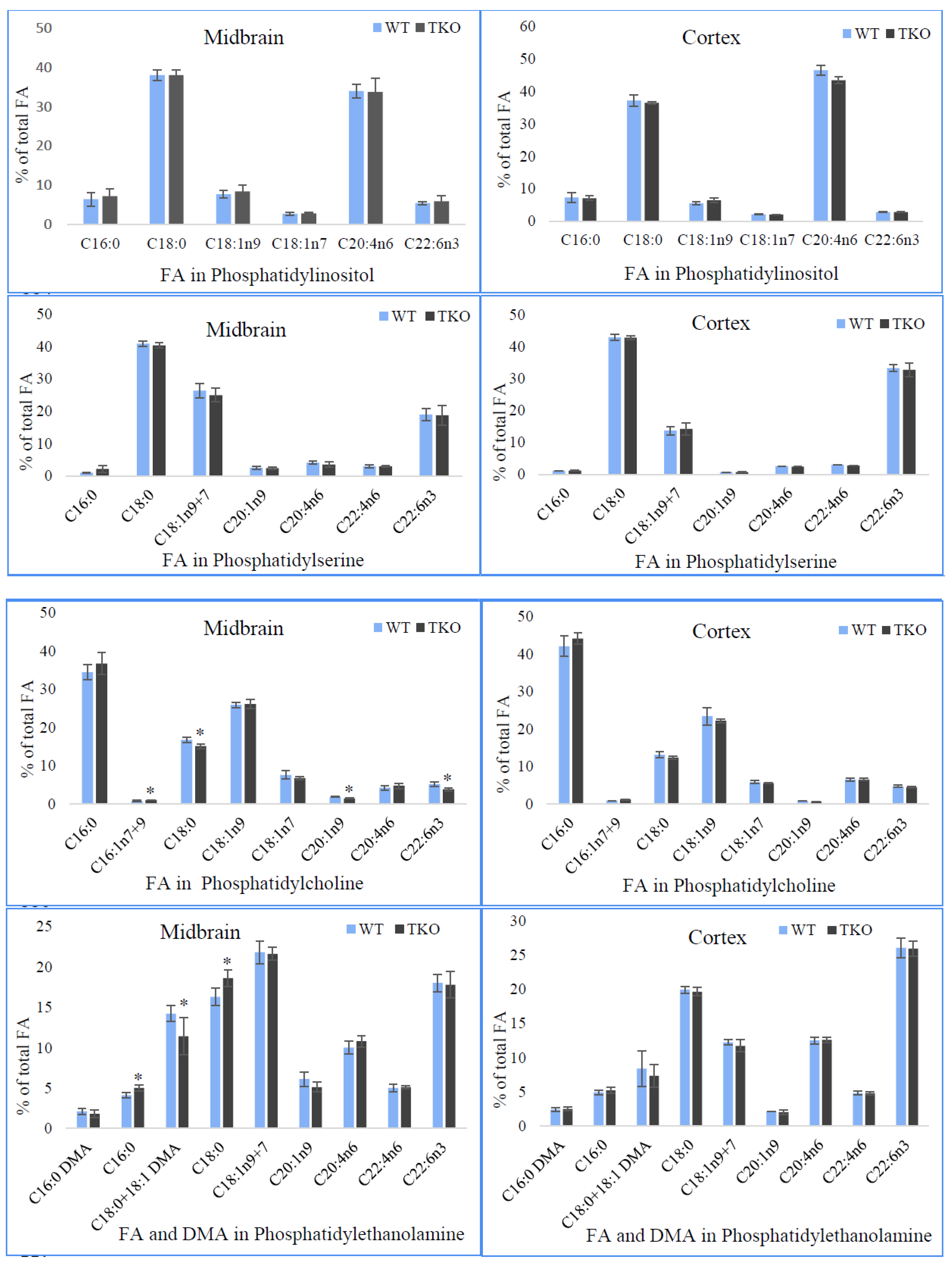
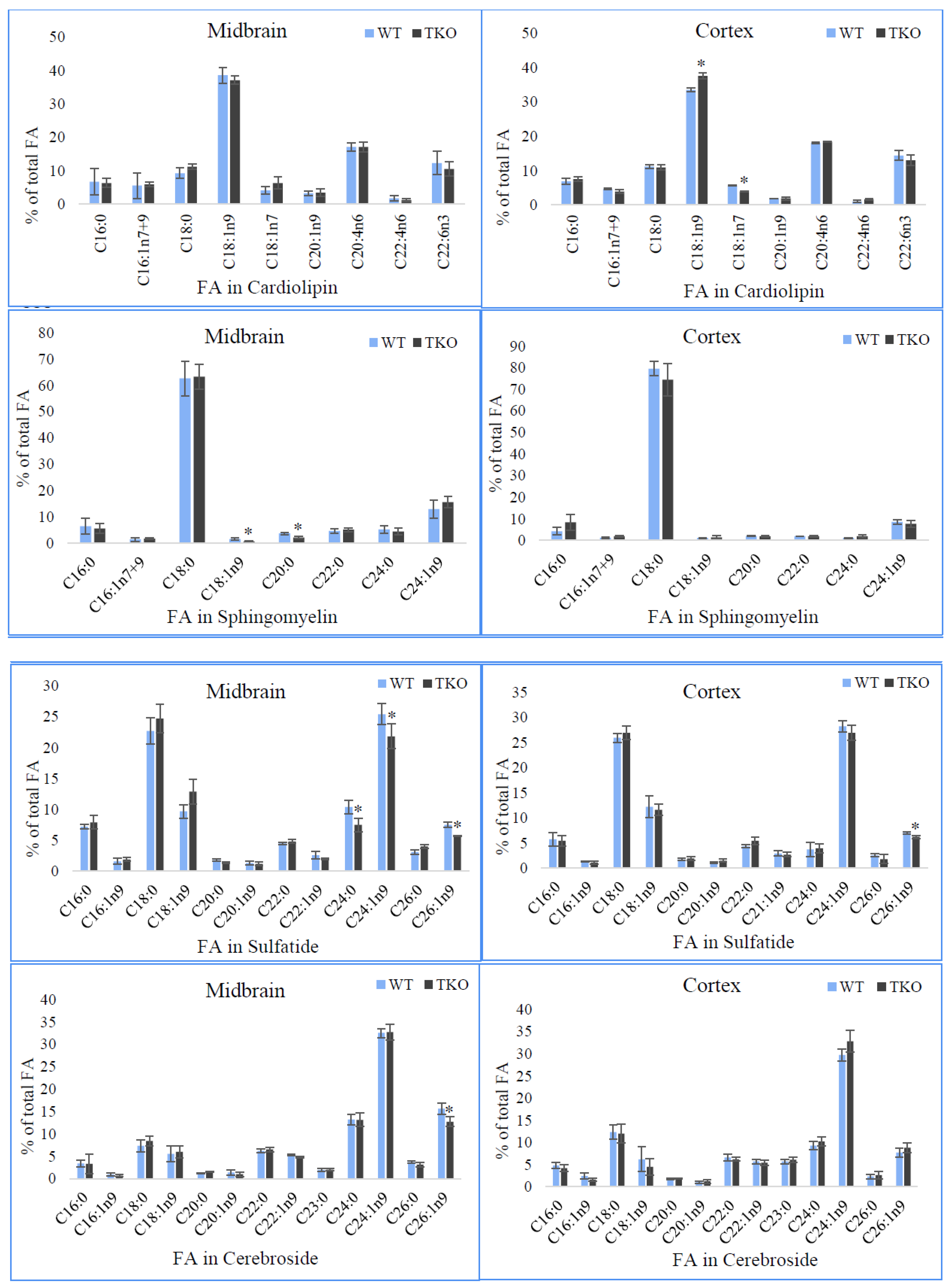
2.8. Fatty Acid Composition of Individual Phospholipids in Midbrain and Cortex from WT and TKO Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Lipid Analysis
4.3. Fatty Acid Analysis
4.4. Gas Chromatography
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACSL | long chain-acyl-CoA synthetases |
| ALA | α-linolenic acid |
| ARA | arachidonic acid |
| ER | cerebrosides |
| ChREBP | carbohydrate-responsive element-binding protein |
| CL | cardiolipin |
| DGLA | dihomo-γ-linolenic acid |
| DHA | docosahexaenoic acid |
| DMA | dimethyl acetals |
| EPA | eicosapentaenoic acid |
| FA | fatty acids |
| FABS | fatty acid binding protein |
| FAME | fatty acid methyl esters |
| GC | gas chromatography |
| HPLC-MS-MS | high-performance liquid chromatography-mass spectrometry |
| LA | linoleic acid |
| LCPUFA | long-chain polyunsaturated fatty acids |
| LP | lipoproteins |
| MLX | max-like protein X |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| NAC | non-Aβ component |
| PC | phosphatidylcholine |
| PD | Parkinson’s disease |
| PE | phosphatidylethanolamine |
| PI | phosphatidylinositol |
| PPAR-α | peroxisome proliferator-activated receptor alpha |
| PS | phosphatidylserine |
| SE | steryl esters |
| SL | sulfatide |
| SM | sphingomyelin |
| SNAP23 | synaptosomal-associated protein 23 |
| SNARE | N- ethylmaleimide-sensitive factor attachment protein receptors |
| SREBP-1 | sterol regulatory element-binding protein 1 |
| SV | synaptic vesicle |
| TAG | triacylglycerol |
| TKO | triple synuclein-knockout |
| TPL | total polar lipids |
| VLDL | very low-density lipoproteins |
| WT+13:1311:15:39 | wild type |
References
- George, J.M. The synucleins. Gen. Biol. 2001, 3, 3002.1–3002.6. [Google Scholar] [CrossRef]
- Bendor, J.; Logan, T.; Edwards, R.H. The functions of α-synuclein. Neuron 2013, 79, 1044–1066. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Zhao, Y. Evolutionary aspects of the synuclein super-family and sub-families based on large-scale phylogenetic and group-discrimination analysis. Biochem. Biophys. Res. Commun. 2013, 441, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Connor-Robson, N.; Peters, O.; Millership, S.; Ninkina, N.; Buchman, V.L. Combinational losses of synucleins reveal their differential requirements for compensating age-dependent alterations in motor behaviour and dopamine metabolism. Neurobiol. Aging 2016, 46, 107–112. [Google Scholar] [CrossRef]
- Longhena, F.; Faustini, G.; Spillantini, M.G.; Bellucci, A. Living in promiscuity: The multiple partners of alpha-synuclein at the synapse in physiology and pathology. Int. J. Mol. Sci. 2019, 20, 141. [Google Scholar] [CrossRef]
- Maroteaux, L.; Campanelli, J.T.; Scheller, R.H. Synuclein: A neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J. Neurosci. 1988, 8, 2804–2815. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.W.P.; Buell, A.K.; Michaels, T.C.T.; Meisl, G.; Carozza, J.; Flagmeier, P.; Vendruscolo, M.; Knowles, T.P.J.; Dobson, C.M.; Galvagnion, C. β-Synuclein suppresses both the initiation and amplification steps of α-synuclein aggregation via competitive binding to surfaces. Sci. Rep. 2016, 6, 36010. [Google Scholar] [CrossRef] [PubMed]
- Fusco, G.; Sanz-Hernandes, M.; De Simone, A. Order and disorder in the physiological membrane binding of α-synuclein. Curr. Opin. Struct. Biol. 2018, 48, 49–57. [Google Scholar] [CrossRef]
- Ohtake, H.; Limprasert, P.; Fan, Y.; Onodera, O.; Kakita, A.; Takahashi, H.; Bonner, L.T.; Tsuang, D.W.; Murray, I.V.; Lee, V.M.; et al. Beta-synuclein gene alterations in dementia with Lewy bodies. Neurology 2004, 63, 805–811. [Google Scholar] [CrossRef]
- Ducas, V.C.; Rhoades, E. Quantifying interactions of β-synuclein and γ-synuclein with model membranes. J. Mol. Biol. 2012, 423, 528–539. [Google Scholar] [CrossRef]
- Raina, A.; Leite, K.; Chakrabarti, K.S.; Guerin, S.; Mahajani, S.U.; Voll, D.; Becker, S.; Griesinger, C.; Bähr, M.; Kügler, S. Dopamine promotes the neurodegenerative potential of β-synuclein. J. Neurochem. 2021, 156, 674–691. [Google Scholar] [CrossRef]
- Biere, A.L.; Wood, S.J.; Wypych, J.; Steavenson, S.; Jiang, Y.; Anafi, D.; Jacobsen, F.W.; Jarosinski, M.A.; Wu, G.M.; Louis, J.C.; et al. Parkinson’s disease-associated alpha-synuclein is more fibrillogenic than beta- and gamma-synuclein and cannot cross-seed its homologs. J. Biol. Chem. 2000, 275, 34574–34579. [Google Scholar] [CrossRef]
- Sung, Y.-H.; Eliezer, D. Secondary structure and dynamics of micelle bound β- and γ-synuclein. Protein Sci. 2006, 15, 1162–1174. [Google Scholar] [CrossRef]
- Mochizuki, H.; Choong, C.-J.; Masliah, E. A refined concept: α-synuclein dysregulation disease. Neurochem. Int. 2018, 119, 84–96. [Google Scholar] [CrossRef]
- Roberts, H.L.; Brown, D.R. Seeking a mechanism for the toxicity and oligomeric α-synuclein. Biomolecules 2015, 5, 282–305. [Google Scholar] [CrossRef]
- Burré, J.; Sharma, M.; Südhof, T.C. Systematic mutagenesis of α-synuclein reveals distinct sequence requirements for physiological and pathological activities. J. Neurosci. 2012, 32, 15227–15242. [Google Scholar] [CrossRef]
- Burré, J.; Sharma, M.; Tsetsenis, T.; Buchman, V.; Etherton, M.R.; Südhof, T.C. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science 2010, 329, 1663–1667. [Google Scholar] [CrossRef]
- Stefanis, L. a-Synuclein in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009399. [Google Scholar] [CrossRef]
- Iyer, A.; Glaessens, M.A.E. Disruptive membrane interactions of alpha-synuclein aggregates. Biochem. Biophys. Acta Proteins Proteom. 2018, 1867, 468–482. [Google Scholar] [CrossRef]
- Ho, G.H.H.; Ramalingam, N.; Imberdis, T.; Wilkie, E.C.; Dettmer, U.; Selkoe, D.J. Upregulation of cellular palmitoylation mitigates α-synuclein accumulation and neurotoxicity. Mov. Disord. 2021, 36, 348–359. [Google Scholar] [CrossRef]
- Kaur, U.; Lee, J.C. Unroofing site-specific α-synuclein-lipid interactions at the plasma membrane. Proc. Natl. Acad. Sci. USA 2020, 117, 18977–18983. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.R.; Rhoades, E. Effects of curvature and composition of α-synuclein binding to lipid vesicles. Biophys. J. 2010, 99, 2279–2288. [Google Scholar] [CrossRef]
- Lee, H.J.; Cho, E.D.; Lee, K.W.; Kim, J.H.; Cho, S.G.; Lee, S.J. Autophagic failure promotes the exocytosis and intercellular transfer of α-synuclein. Exp. Mol. Med. 2013, 45, e22. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, E.I.; Jiang, Z.; Strub, M.P.; Lee, J.C. Effects of phosphatidylcholine membrane fluidity on the conformation and aggregation of N-terminally acetylated α-synuclein. J. Biol. Chem. 2018, 293, 11195–11205. [Google Scholar] [CrossRef] [PubMed]
- Fusco, G.; De Simone, A.; Tata, G.; Vostrikov, V.; Vendruscolo, M.; Dobson, C.M.; Veglia, G. Direct observation of the three regions in α-synuclein that determine its membrane-bound behaviour. Nat. Commun. 2014, 5, 3827. [Google Scholar] [CrossRef]
- Jao, C.C.; Der-Sarkissian, A.; Chen, J.; Langen, R. Structure of membrane-bound alpha-synuclein studied by site-directed spin labeling. Proc. Natl. Acad. Sci. USA 2004, 3, 8331–8336. [Google Scholar] [CrossRef] [PubMed]
- Jao, C.C.; Hegde, B.G.; Chen, J.; Haworth, I.S.; Langen, R. Structure of membrane-bound α-synuclein from site-directed spin labelling and computational refinement. Proc. Natl. Acad. Sci. USA 2008, 105, 19666–19671. [Google Scholar] [CrossRef]
- Wietek, J.; Haralampiev, I.; Amoussouvi, A.; Herrmann, A.; Stöckl, M. Membrane bound α-synuclein in fully embedded in the lipid bilayer while segments with higher flexibility remain. FEBS Lett. 2013, 587, 2572–2577. [Google Scholar] [CrossRef] [PubMed]
- Westphal, C.H.; Chandra, S.S. Monomeric synucleins generate membrane curvature. J. Biol. Chem. 2013, 288, 1829–1840. [Google Scholar] [CrossRef] [PubMed]
- Varkey, J.; Isas, J.M.; Mizuno, N.; Jensen, M.B.; Bhatia, V.K.; Jao, C.C.; Petrlova, J.; Voss, J.C.; Stamou, D.G.; Steven, A.C.; et al. Membrane curvature induction and tabulation are common features of synucleins and apolipoproteins. J. Biol. Chem. 2010, 285, 32486–32493. [Google Scholar] [CrossRef]
- Ouberai, M.M.; Wang, J.; Swann, M.J.; Galvagnion, C.; Guilliam, T.; Dobson, C.M.; Welland, M.E. α-Synuclein senses lipid packing defects and induces lateral expansion of lipids leading to membrane remodelling. J. Biol. Chem. 2013, 288, 20883–20895. [Google Scholar] [CrossRef]
- Braun, A.R.; Lacy, M.M.; Ducas, V.C.; Rhoades, E.; Sachs, J.N. α-Synuclein’s uniquely long amphipathic helix enhances its membrane binding and remodeling capacity. J. Membr. Biol. 2017, 250, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Burré, J.; Sharma, M.; Südhof, T.C. Alpha-synuclein assembles into higher-order multimers upon membrane binding to promote SNARE complex formation. Proc. Natl. Acad. Sci. USA 2014, 111, E4274–E4283. [Google Scholar] [CrossRef] [PubMed]
- Hannestag, J.K.; Rocha, S.; Agnarsson, B.; Zhdanov, V.P.; Wittung-Strafshede, P.; Höök, F. Single-vesicle imaging reveals lipid-selective and stepwise membrane disruption by monomeric α-synuclein. Proc. Natl. Acad. Sci. USA 2020, 117, 14178–14186. [Google Scholar]
- Fusco, G.; Chen, S.W.; Williamson, P.T.F.; Cascella, R.; Perni, M.; Jarvis, J.A.; Cecchi, C.; Vendruscolo, M.; Chiti, F.; Cremades, N.; et al. Structure basis of membrane disruption and cellular toxicity by α-synuclein oligomers. Science 2017, 358, 1440–1443. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, M.; Gue, K.; Emond, V.; Julien, P.; Kang, J.X.; Cicchetti, F.; Calon, F. Transgenic conversion of omega-6 into omega-3 fatty acids in a mouse model of Parkinson’s disease. J. Lipid Res. 2011, 52, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Galvagnion, C. The role of lipids interacting with α-synuclein in the pathogenesis of Parkinson’s disease. J. Parkinsons Dis. 2017, 7, 433–450. [Google Scholar] [CrossRef]
- De Franceschi, G.; Fecchio, C.; Sharon, R.; Schapira, A.H.V.; Proukakis, C.; Bellotti, V.; De Laureto, P.P. α-Synuclein structural features inhibit harmful polyunsaturated fatty acid oxidation, suggesting roles in neuroprotection. J. Biol. Chem. 2017, 292, 6927–6937. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Sango, K.; Wada, K.; Nagai, Y. Pathological role of lipid interaction with α-synuclein in Parkinson’s disease. Neurochem. Int. 2018, 119, 97–106. [Google Scholar] [CrossRef]
- Alza, N.P.; Iglesias, P.A.; Conde, M.A.; Uranga, R.M.; Salvador, G.A. Lipids at the crossroad of α-synuclein function and dysfunction; biological and pathological implications. Front. Cell Neurosci. 2019, 13, 175. [Google Scholar] [CrossRef]
- Kiechle, V.; Grozdanov, V.; Danzer, K.M. The role of lipids in the initiation of α-synuclein misfolding. Front. Cell Dev. Biol. 2020, 8, 562241. [Google Scholar] [CrossRef]
- Fanning, S.; Selkoe, D.; Dettmer, U. Vesicle trafficking and lipid metabolism in synucleonopathy. Acta Neuropath. 2021, 141, 491–510. [Google Scholar] [CrossRef]
- Mori, A.; Hattori, N. Lipids: Key players that modulate α-synuclein toxicity and neurodegeneration in Parkinson’s disease. Int. J. Mol. Sci. 2020, 21, 3301. [Google Scholar] [CrossRef]
- Ugalde, C.L.; Lawson, V.A.; Finkelstein, D.I.; Hill, A.F. The role of lipids in α-synuclein misfolding and neurotoxicity. J. Biol. Chem. 2019, 294, 9016–9028. [Google Scholar] [CrossRef]
- Sánchez Campos, S.; Alza, N.P.; Salvador, G.A. Lipid metabolism alterations in the neuronal response to A53T α-synuclein and Fe-induced injury. Arch. Biochem. Biophys. 2018, 655, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Golovko, M.Y.; Faergeman, N.J.; Cole, N.B.; Castagnet, P.I.; Nussbaum, R.L.; Murphy, E.R. Alpha-synuclein gene deletion decreases brain palmitate uptake and alters the palmitate metabolism in the absence of alpha-synuclein palmitate binding. Biochemistry 2005, 44, 8251–8259. [Google Scholar] [CrossRef]
- Golovko, M.Y.; Rosenberger, T.A.; Feddersen, S.; Faergeman, N.J.; Murphy, E.J. Alpha-synuclein gene ablation increases docosahexaenoic acid incorporation and turnover in brain phospholipids. J. Neurochem. 2007, 101, 201–211. [Google Scholar] [CrossRef]
- Guschina, I.; Millership, S.; O’Donnell, V.; Ninkina, N.; Harwood, J.; Buchman, V.L. Lipid classes and fatty acid patterns are altered in the brain of γ-synuclein null mutant mice. Lipids 2011, 46, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Millership, S.J.; Ninkina, N.; Guschina, I.; Norton, J.; Brambilla, R.; Oort, P.; Adams, S.; Dennis, R.J.; Voshol, P.; Rochford, J.; et al. Increased lipolysis and altered lipid homeostasis protect γ-synuclein null mutant mice from diet-induced obesity. Proc. Natl. Acad. Sci. USA 2012, 109, 20943–20948. [Google Scholar] [CrossRef]
- Millership, S.; Ninkina, N.; Rochford, J.J.; Buchman, V.L. Gamma-synuclein is a novel player in the control of body lipid metabolism. Adipocyte 2013, 2, 276–280. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Greten-Harrison, B.; Polydoro, M.; Morimoto-Tomita, M.; Diao, L.; Williams, A.M.; Nie, E.H.; Makani, S.; Tian, N.; Castillo, P.E.; Buchman, V.L.; et al. αβɣ-Synuclein triple knockout mice reveal age-dependent neuronal dysfunction. Proc. Natl. Acad. Sci. USA 2010, 107, 19573–19578. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Peters, O.; Millership, S.; Ninkina, N.; Doig, N.; Connor-Robson, N.; Threlfell, S.; Kooner, G.; Deacon, R.M.; Bannerman, D.M.; et al. Functional alterations to the nigrostriatal system in mice lacking all three members of the synuclein family. J. Neurosci. 2011, 31, 7264–7274. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Brietzke, E. Recent advances in lipidomics: Analytical and clinical perspectives. Prostag. Oth. Lipid Med. 2017, 128–129, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Gross, R.W. The evolution of lipidomics through space and time. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 8, 731–739. [Google Scholar] [CrossRef]
- Aureli, M.; Grassi, S.; Prioni, S.; Sonnino, S.; Prinetti, A. Lipid membrane domain in the brain. Biochim. Biophys. Acta 2015, 1851, 1006–1016. [Google Scholar] [CrossRef]
- Selkoe, D.; Dettmer, U.; Luth, E.; Kim, N.; Newman, A. Defining the native state of molecular aspects of medicine. Neurodegener. Dis. 2014, 13, 114–117. [Google Scholar]
- Alecu, I.; Bennett, S.A.L. Dysregulated lipid metabolism and its role in α-synucleinopathy in Parkinson’s disease. Front. Neurosci. 2019, 13, 328. [Google Scholar] [CrossRef]
- Sundaram, M.; Yao, Z. Recent progress in understanding protein and lipid factors affecting hepatic VLDL assembly and secretion. Nutr. Metab. 2010, 27, 7–35. [Google Scholar] [CrossRef]
- Mason, T.M. The role of factors that regulate the synthesis and secretion of very-low-density lipoprotein by hepatocytes. Crit. Rev. Clin. Lab. Sci. 1998, 35, 461–487. [Google Scholar] [CrossRef]
- Golovko, M.Y.; Barceló-Coblijn, G.; Castagnet, P.I.; Austin, S.; Combs, C.K.; Murphy, E.J. The role of α-synuclein in brain lipid metabolism: A downstream impact on brain inflammatory response. Mol. Cell. Biol. 2009, 326, 55–66. [Google Scholar] [CrossRef]
- Sharon, R.; Bar-Joseph, I.; Mirick, G.E.; Serhan, C.N.; Selkoe, D.J. Altered fatty acid composition of dopaminergic neurons expressing alpha-synuclein and human brains with alpha-synucleinopathies. J. Biol. Chem. 2003, 278, 49874–49881. [Google Scholar] [CrossRef] [PubMed]
- Sepe, F.N.; Chiasserini, D.; Parnetti, L. Role of FABP3 as biomarker in Alzheimer’s disease and synucleinopathies. Future Neurol. 2018, 13, 199–207. [Google Scholar] [CrossRef]
- Grevengoed, T.J.; Klett, E.L.; Coleman, R.A. Acyl-CoA metabolism and partitioning. Annu. Rev. Nutr. 2014, 34, 1–30. [Google Scholar] [CrossRef]
- Young, P.A.; Senkal, C.E.; Suchanek, A.L.; Grevengoed, T.J.; Lin, D.D.; Zhao, L.; Crunk, A.E.; Klett, E.L.; Füllekrug, J.; Obeid, L.M.; et al. Long-chain acyl-CoA synthetase 1 interacts with key proteins that activate and direct fatty acids into niche hepatic pathways. J. Biol. Chem. 2018, 26, 6724–16740. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Arasaki, K.; Ohsaki, Y.; Fujimoto, T.; Ohtomo, T.; Yamada, J.M.; Tagaya, M. Syntaxin 17 promotes lipid droplet formation by regulating the distribution of acyl-CoA synthetase 3. J. Lipid Res. 2018, 59, 805–819. [Google Scholar] [CrossRef]
- Ramasamy, I. Recent advances in physiological lipoprotein metabolism. Clin. Chem. Lab. Med. 2014, 52, 1695–1727. [Google Scholar] [CrossRef]
- Emamzadeh, F.N.; Allsop, D. α-Synuclein interactions with lipoproteins in plasma. J. Mol. Neurosci. 2017, 63, 165–172. [Google Scholar] [CrossRef]
- Surguchov, A. Commentary: α-synuclein interactions with lipoproteins in plasma. Front. Mol. Neurosci. 2017, 10, 362. [Google Scholar] [CrossRef]
- Kim, H.Y.; Spector, A.A. N-Docosahexaenoylethanolamine: A neurotrophic and neuroprotective metabolite of docosahexaenoic acid. Mol. Asp. Med. 2018, 64, 34–44. [Google Scholar] [CrossRef]
- Lacombe, R.J.S.; Chouinard-Watkins, R.; Bazinet, R.P. Brain docosahexaenoic acid uptake and metabolism. Mol. Asp. Med. 2018, 64, 109–134. [Google Scholar] [CrossRef]
- Moore, S.A.; Yoder, E.; Murphy, S.; Dutton, G.R.; Spector, A.A. Astrocytes, not neurons, produce docosahexaenoic acid (22:6 omega-3) and arachidonic acid (20:4 omega-6). J. Neurochem. 1991, 56, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, K.; Rodriguez-Rodriguez, R.; Gaebler, A.; Casals, N.; Scheller, A.; Kuerschner, L. Astrocytes and oligodendrocytes in grey and white matter regions of the brain metabolize fatty acids. Sci. Rep. 2017, 7, 10779. [Google Scholar] [CrossRef]
- Liu, J.J.; Green, P.; Mann, J.J.; Rapoport, S.I.; Sublette, M.E. Pathways of polyunsaturated fatty acid utilization: Implications for brain function in neuropsychiatric health and disease. Brain Res. 2015, 1597, 220–246. [Google Scholar] [CrossRef]
- Jump, D.B. N-3 polyunsaturated fatty acid regulation of hepatic gene transcription. Curr. Opin. Lipidol. 2008, 19, 242–247. [Google Scholar] [CrossRef]
- Celver, J.; Scharma, M.; Kovoor, A. D (2)-Dopamine receptors target regulator of G protein signaling 9–2 to detergent-resistant membrane fractions. J. Neurochem. 2012, 120, 56–69. [Google Scholar] [CrossRef]
- Ferreira, N.S.; Engelsby, H.; Neess, D.; Kelly, S.L.; Volpert, G.; Merrill, A.H.; Futerman, A.H.; Færgeman, N.J. Regulation of very-long acyl chain ceramide synthesis by acyl-CoA-binding protein. J. Biol. Chem. 2017, 292, 7588–7597. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.; Kim, J.; Hawk, B.J.; Shin, Y.K. α-Synuclein may cross-bridge v-SNARE and acidic phospholipids to facilitate SNARE-dependent vesicle docking. Biochem. J. 2017, 474, 2039–2049. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, D.; Xu, J.; Yanagita, T.; Xue, C.; Zhang, T. DHA enriched phospholipids with different polar groups (PC and PS) had different improvements on MPTP-induced mice with Parkinson’s disease. J. Funct. Foods 2018, 45, 417–426. [Google Scholar] [CrossRef]
- Scherma, M.; Masia, P.; Satta, V.; Fratta, W.; Fadda, P.; Tanda, G. Brain activity of anandamide: A rewarding bliss? Acta Pharmacol. Sin. 2019, 40, 309–323. [Google Scholar] [PubMed]
- Darios, F.; Ruipérez, V.; López, I.; Villanueva, J.; Gutierrez, L.M.; Davletov, B. Alpha-synuclein sequesters arachidonic acid to modulate SNARE-mediated exocytosis. EMBO Rep. 2010, 11, 528–533. [Google Scholar] [CrossRef]
- Dorninger, F.; Forss-Petter, S.; Berger, J. From peroxisomal disorders to common neurodegenerative diseases–the role of ether lipids in the nervous system. FEBS Lett 2017, 591, 2761–2788. [Google Scholar] [CrossRef] [PubMed]
- Dean, J.M.; Lodhi, I.J. Structural and functional roles of ether lipids. Protein Cell 2018, 9, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Fabelo, N.; Martín, V.; Santpere, G.; Marín, R.; Torrent, L.; Ferrer, I.; Díaz, M. Severe alterations in lipid composition of frontal cortex lipid rafts from Parkinson’s disease and incidental Parkinson’s disease. Mol. Med. 2011, 17, 1107–1118. [Google Scholar] [CrossRef] [PubMed]
- Honsho, M.; Fujiki, Y. Plasmalogen homeostasis - regulation of plasmalogen biosynthesis and its physiological consequence in mammals. FEBS Lett. 2017, 591, 2720–2729. [Google Scholar] [CrossRef] [PubMed]
- Miville-Godbout, E.; Bourque, M.; Morissette, M.; Al-Sweidi, S.; Smith, T.; Mochizuki, A.; Senanayake, V.; Jayasinghe, D.; Wang, L.; Goodenowe, D.; et al. Plasmalogen augmentation reserves striatal dopamine loss in MPTP mice. PLoS ONE 2016, 11, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Levental, I.; Levental, K.; Heberle, F. Lipid rafts: Controversies resolved, mysteries remain. Trends Cell Biol. 2020, 30, 341–353. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Kates, M. Techniques of Lipidology: Isolation, Analysis and Identification of Lipids; Elsevier Science Publishing Company: New York, NY, USA, 1986. [Google Scholar]
| Fatty Acid | PLASMA | LIVER | ||
|---|---|---|---|---|
| WT | TKO | WT | TKO | |
| C16:0 | 23.6 ± 1.2 | 44.2 ± 5.5 * | 21.6 ± 1.5 | 27.7 ± 3.4 * |
| C16:1n7 | 0.7 ± 0.4 | 1.8 ± 0.6 | 0.8 ± 0.4 | 1.2 ± 0.8 |
| C18:0 | 24.1 ± 1.0 | 28.4 ± 4.7 | 22.4 ± 1.9 | 21.2 ± 2.8 |
| C18:1n9 | 12.7 ± 0.9 | 18.1 ± 1.0 * | 13.0 ± 1.0 | 15.2 ± 0.8 * |
| C18:2n6 | 19.2 ± 1.4 | 2.7 ± 0.8 * | 13.5 ± 1.1 | 14.4 ± 0.7 |
| C18:3n3 | 0.9 ± 0.2 | 0.8 ± 0.1 | tr. | tr. |
| C20:3n6 | 1.6 ± 0.2 | tr. | 1.7 ± 0.3 | 1.8 ± 0.3 |
| C20:4n6 | 10.0 ± 1.3 | tr. | 15.7 ± 1.7 | 10.4 ± 2.7 * |
| C22:6n3 | 4.0 ± 0.6 | tr. | 7.6 ± 1.4 | 4.2 ± 2.2 |
| C24:1n9 | 1.4 ± 0.2 | 1.3 ± 0.4 | 0.9 ± 0.1 | 0.7 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guschina, I.A.; Ninkina, N.; Roman, A.; Pokrovskiy, M.V.; Buchman, V.L. Triple-Knockout, Synuclein-Free Mice Display Compromised Lipid Pattern. Molecules 2021, 26, 3078. https://doi.org/10.3390/molecules26113078
Guschina IA, Ninkina N, Roman A, Pokrovskiy MV, Buchman VL. Triple-Knockout, Synuclein-Free Mice Display Compromised Lipid Pattern. Molecules. 2021; 26(11):3078. https://doi.org/10.3390/molecules26113078
Chicago/Turabian StyleGuschina, Irina A., Natalia Ninkina, Andrei Roman, Mikhail V. Pokrovskiy, and Vladimir L. Buchman. 2021. "Triple-Knockout, Synuclein-Free Mice Display Compromised Lipid Pattern" Molecules 26, no. 11: 3078. https://doi.org/10.3390/molecules26113078
APA StyleGuschina, I. A., Ninkina, N., Roman, A., Pokrovskiy, M. V., & Buchman, V. L. (2021). Triple-Knockout, Synuclein-Free Mice Display Compromised Lipid Pattern. Molecules, 26(11), 3078. https://doi.org/10.3390/molecules26113078






