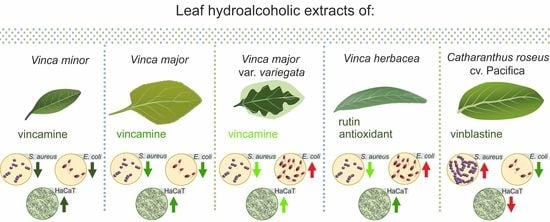
The Phytochemical Analysis of Vinca L. Species Leaf Extracts Is Correlated with the Antioxidant, Antibacterial, and Antitumor Effects
Abstract
:1. Introduction
2. Results
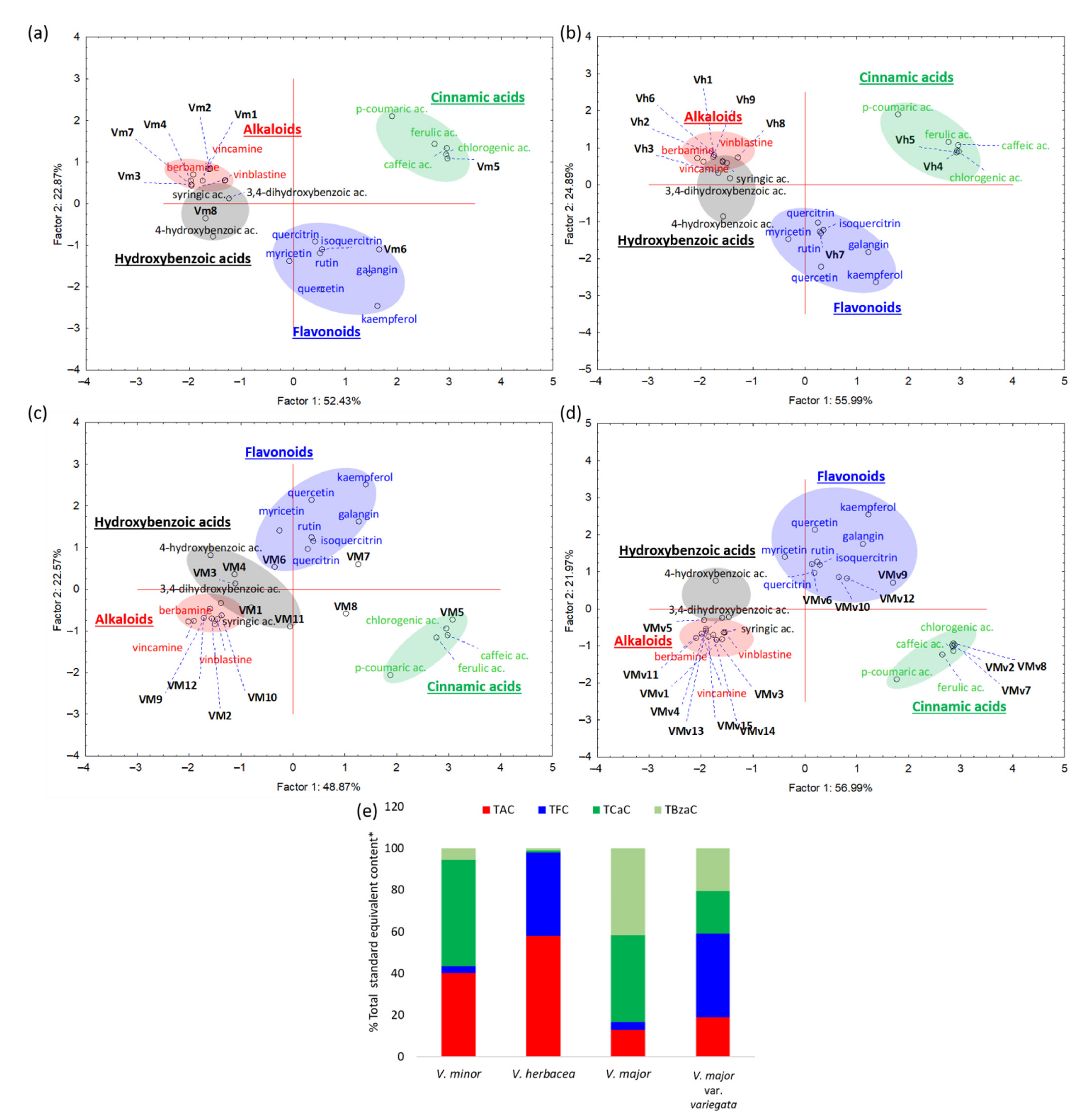
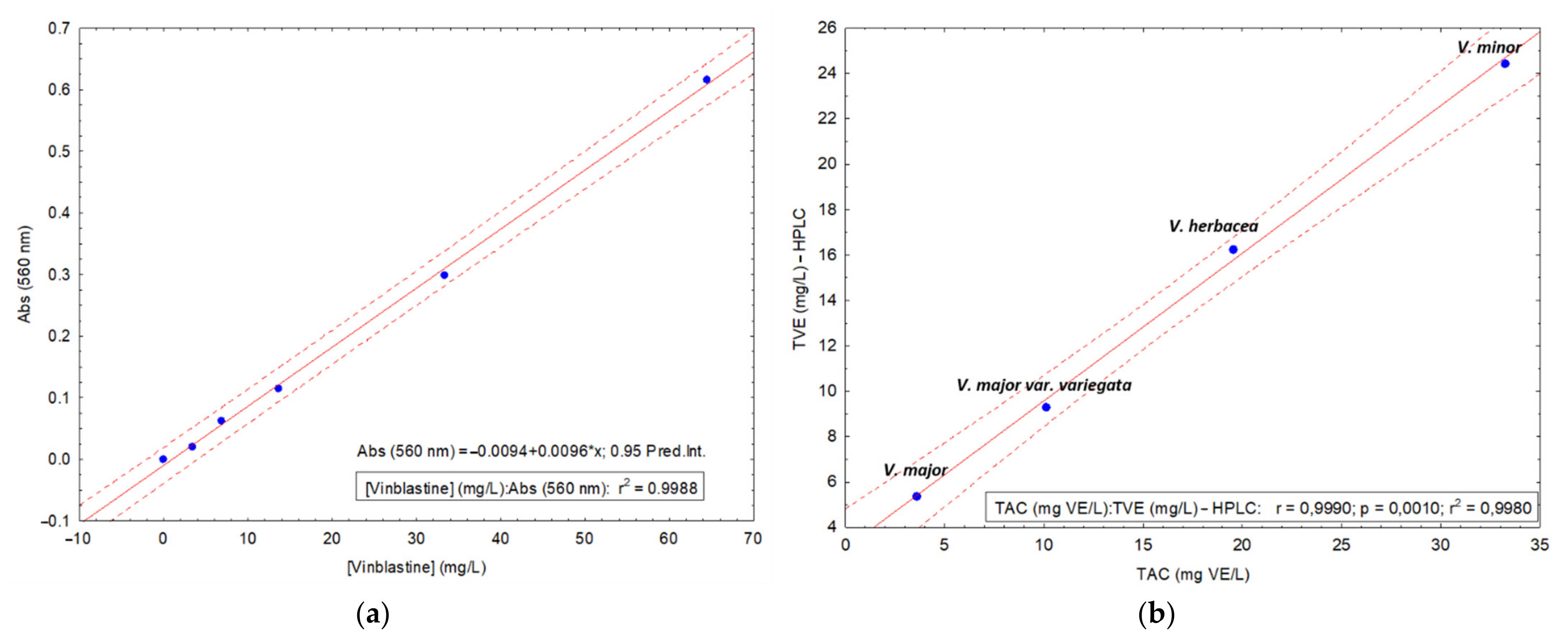
2.1. Phytochemical Analyses
HPLC-DAD Method
2.2. Colorimetric Determination of Total Phenolic and Flavonoid Content
2.3. Determination of Total Alkaloid Content
2.4. Antioxidant Activity
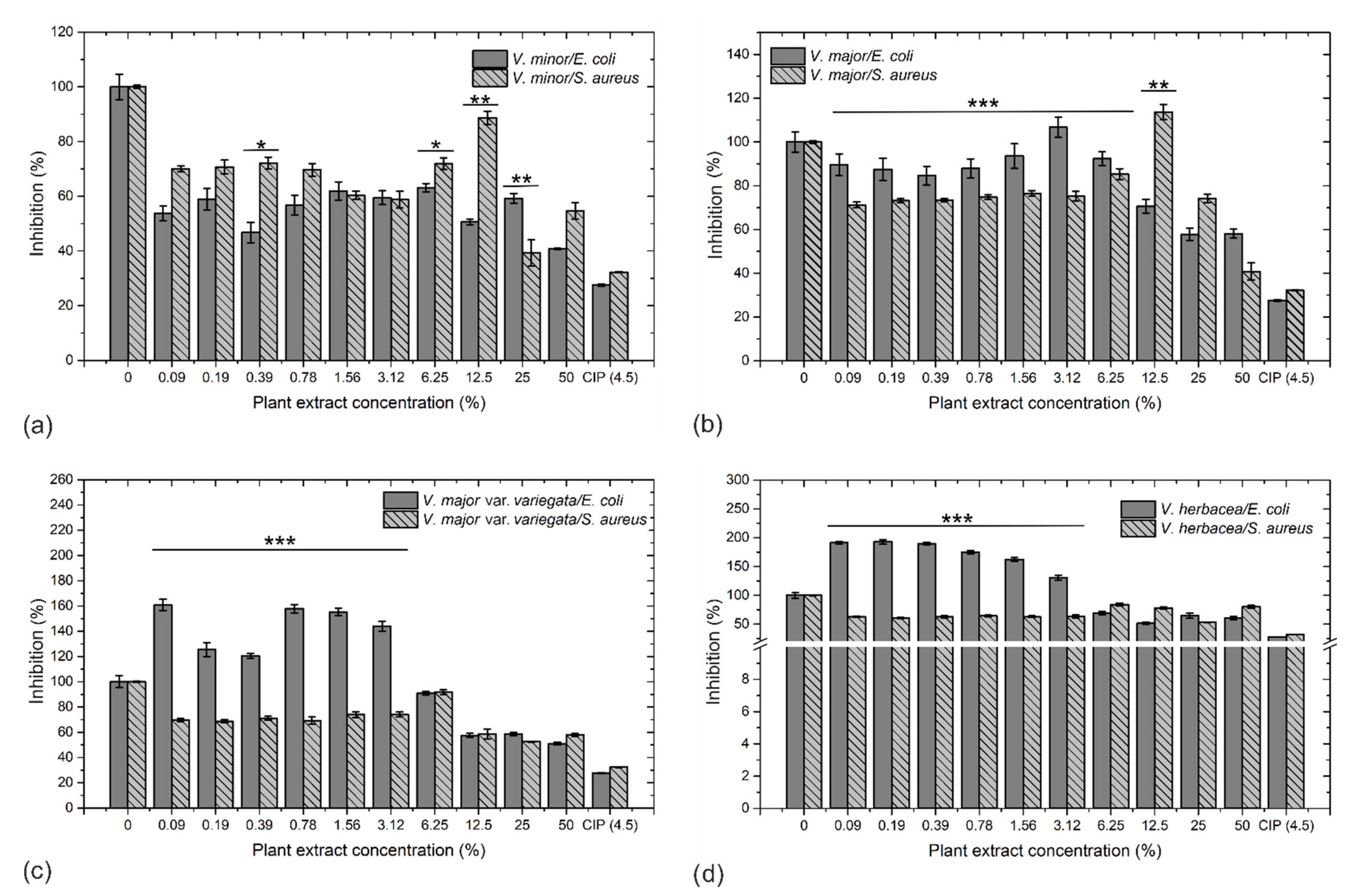
2.5. Antibacterial Activity and Morphological Examination
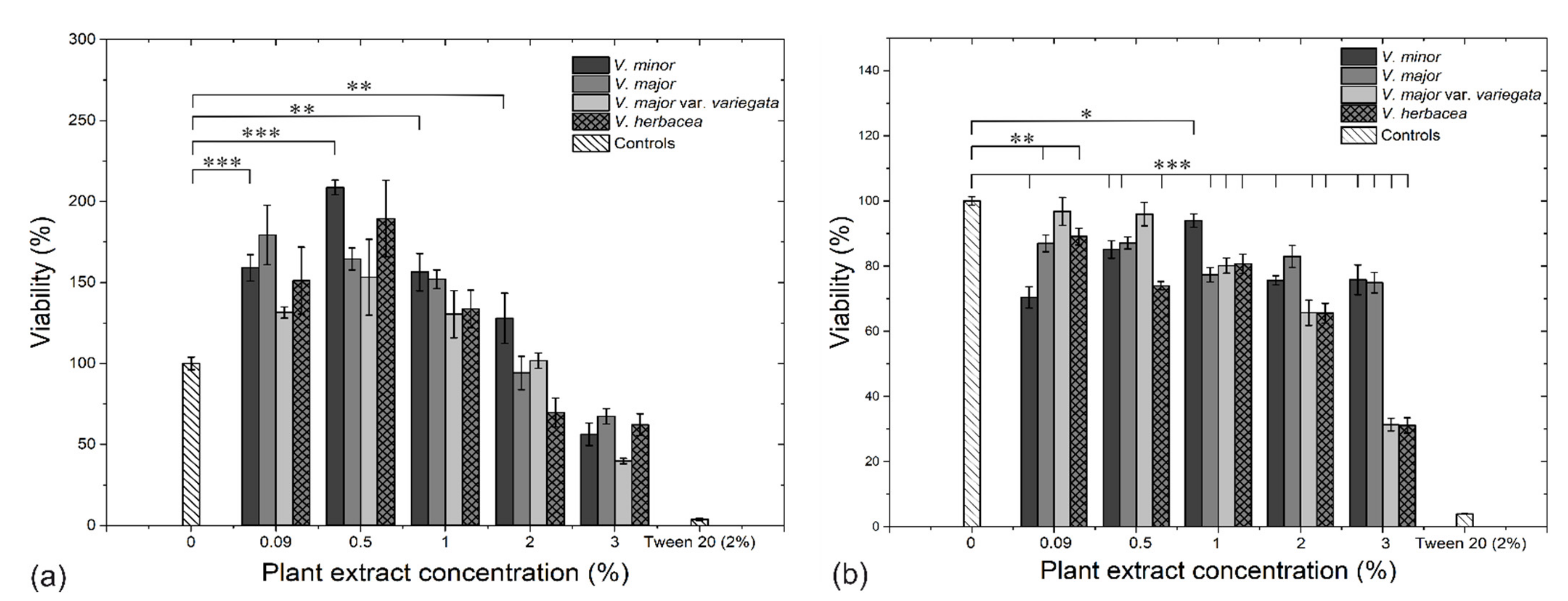
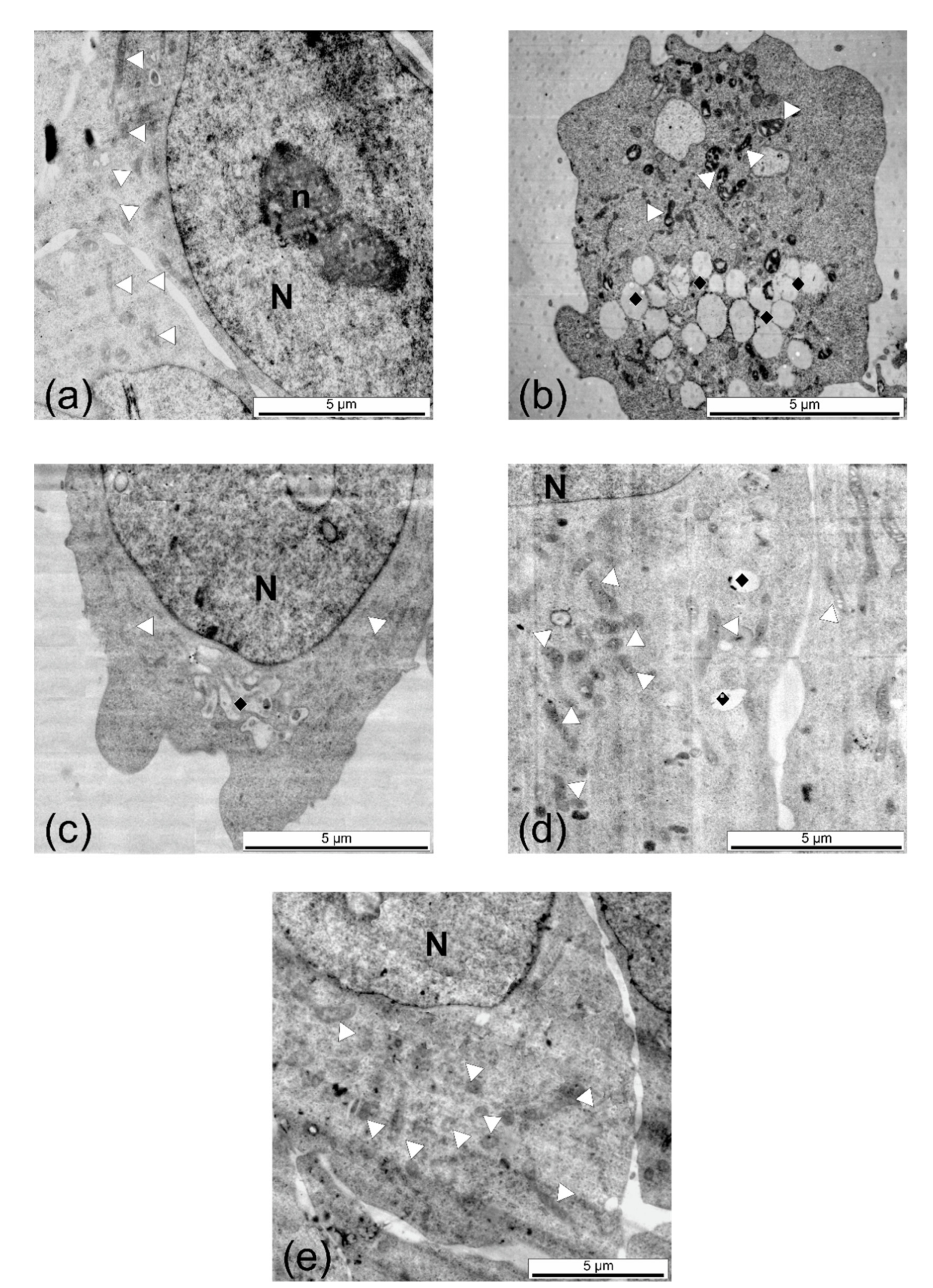
2.6. Cytotoxicity Assays and Ultrastructural Examination
3. Discussion
4. Materials and Methods
4.1. Plant Material and Extract Preparation
4.2. Phytochemical Analyses of the Vinca Leaf Extracts
4.2.1. HPLC-DAD Method
4.2.2. Alkaloid Content
4.2.3. Colorimetric Determination of Phytoconstituents in the Vinca Leaf Extracts
4.3. In Vitro Antioxidant Activity
4.4. Antibacterial Activities
4.4.1. Agar-Well Diffusion Method
4.4.2. Microdilution Method
4.4.3. Morphological Examination through Scanning Electron Microscopy
4.5. In Vitro Cytotoxicity Assays
4.5.1. Biochemical Assays
4.5.2. Ultrastructural Investigation through Transmission Electron Microscopy
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jhanji, R.; Bhati, V.; Singh, A.; Kumar, A. Phytomolecules against bacterial biofilm and efflux pump: An in silico and in vitro study. J. Biomol. Struct. Dyn. 2019. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Costa, V.M.; King, C.E.; Kalan, L.; Morar, M.; Sung, W.W.L.; Schwarz, C.; Froese, D.; Zazula, G.; Calmels, F.; Debruyne, R.; et al. Antibiotic resistance is ancient. Nature 2011, 477, 457–461. [Google Scholar] [CrossRef] [PubMed]
- AlSalhi, M.S.; Elangovan, K.; Ranjitsingh, A.J.A.; Murali, P.; Devanesan, S. Synthesis of silver nanoparticles using plant derived 4-N-methyl benzoic acid and evaluation of antimicrobial, antioxidant and antitumor activity. Saudi J. Biol. Sci. 2019, 26, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Deepika, M.S.; Thangam, R.; Vijayakumar, T.S.; Sasirekha, R.; Vimala, R.T.V.; Sivasubramanian, S.; Arun, S.; Babu, M.D.; Thirumurugan, R. Antibacterial synergy between rutin and florfenicol enhances therapeutic spectrum against drug resistant Aeromonas hydrophila. Microb. Pathogen. 2019, 135. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Mehta, A. Anticancer potential of medicinal plants and their phytochemicals: A review. Braz. J. Bot. 2015, 38, 199–210. [Google Scholar] [CrossRef]
- Chagas, C.M.; Alisaraie, L. Metabolites of Vinca alkaloid vinblastine: Tubulin binding and activation of nausea-associated receptors. ACS Omega 2019, 4, 9784–9799. [Google Scholar] [CrossRef] [PubMed]
- Ganeshpurkar, A.; Saluja, A.K. The pharmacological potential of rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef] [Green Version]
- Pârvu, M.; Vlase, L.; Fodorpataki, L.; Pârvu, O.; Bartha, C.; Roșca-Casian, O.; Barbu-Tudoran, L.; Pârvu, A.E. Chemical composition of celandine (Chelidonium majus L.) extract and its effects on Botrytis tulipae (Lib.) lind fungus and the tulip. Not. Bot. Hort. Agrobot. 2013, 41, 414–426. [Google Scholar] [CrossRef] [Green Version]
- Meira, C.S.; Guimarães, E.T.; dos Santos, J.A.F.; Moreira, D.R.M.; Nogueira, R.C.; Tomassini, T.C.B.; Ribeiro, I.M.; de Souza, C.V.C.; dos Santos, R.R.; Soares, M.B.P. In vitro and in vivo antiparasitic activity of Physalis angulata L. concentrated ethanolic extract against Trypanosoma cruzi. Phytomedicine 2015, 22, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, H.; Naseri, G.; Rezaee, R.; Mohammadi, M.; Banikazemi, Z.; Mirzaei, H.R.; Salehi, H.; Peyvandi, M.; Pawelek, J.M.; Sahebkar, A. Curcumin: A new candidate for melanoma therapy? Int. J. Can. 2016, 139, 1683–1695. [Google Scholar] [CrossRef] [PubMed]
- PLANTS. United States Department of Agriculture. PLANTS Database. Available online: https://plants.sc.egov.usda.gov (accessed on 14 May 2021).
- EEA. European Environment Agency. Available online: https://eunis.eea.europa.eu (accessed on 14 May 2021).
- Koyuncu, M. A new species of Vinca (Apocynaceae) from eastern Anatolia, Turkey. Turk. J. Bot. 2012, 36, 247–251. [Google Scholar] [CrossRef]
- POW. Plants of the World Online. Available online: http://powo.science.kew.org/ (accessed on 14 May 2021).
- Cheng, G.-G.; Zhao, H.-Y.; Liu, L.; Zhao, Y.-L.; Song, C.-W.; Gu, J.; Sun, W.-B.; Liu, Y.-P.; Luo, X.-D. Non-alkaloid constituents of Vinca major. Chin. J. Nat. Med. 2016, 14, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Sukhdev, S.; Shamsher, K.S.; Indu, K. Antilipase activity guided fractionation of Vinca major. J. King Saud Univ. Sci. 2017. [Google Scholar] [CrossRef]
- Abouzeid, S.; Hijazin, T.; Lewerenz, L.; Hansch, R.; Selmar, D. The genuine localization of indole alkaloids in Vinca minor and Catharanthus roseus. Phytochemistry 2019, 168, 112110. [Google Scholar] [CrossRef] [PubMed]
- Boga, M.; Kolak, U.; Topcu, G.; Bahadori, F.; Kartal, M.; Farnsworth, N.R. Two new indole alkaloids from Vinca herbacea L. Phytochem. Lett. 2011, 4, 399–403. [Google Scholar] [CrossRef]
- Boyadzhiev, L.; Yordanov, B. Pertraction of indole alkaloids from Vinca minor L. Sep. Sci. Technol. 2004, 39, 1321–1329. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Pan, Y.-j.; Zu, Y.-G.; Tang, Z.-H. Determination of alkaloids in Catharanthus roseus and Vinca minor by high-performance liquid chromatography–tandem mass spectrometry. Anal. Lett. 2015. [Google Scholar] [CrossRef]
- Ciorîță, A.; Tripon, S.C.; Mircea, I.G.; Podar, D.; Barbu-Tudoran, L.; Mircea, C.; Pârvu, M. The Morphological and Anatomical Traits of the Leaf in Representative Vinca Species Observed on Indoor- and Outdoor-Grown Plants. Plants 2021, 10, 622. [Google Scholar] [CrossRef] [PubMed]
- Almagro, L.; Fernández-Pérez, F.; Pedreño, M.A. Indole alkaloids from Catharanthus roseus: Bioproduction and their effect on human health. Molecules 2015, 20, 2973–3000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, P.; Sharma, A.; Khan, S.A.; Shanker, K.; Mathur, A.K. Over-expression of Catharanthus roseus tryptophan decarboxylase and strictosidine synthase in rol gene integrated transgenic cell suspensions of Vinca minor. Protoplasma 2015, 252, 373–381. [Google Scholar] [CrossRef]
- Moudi, M.; Go, R.; Seok Yien, C.Y.; Nazre, M. Vinca alkaloids. Int. J. Prevent. Med. 2013, 41, 1231–1235. [Google Scholar]
- Lucas, H. Ueber den Bitterstoff der Blätter von Vinca minor L. Phys. Chem. Prakt. Pharm. 1859, 147, 147–150. [Google Scholar] [CrossRef] [Green Version]
- Scheindlin, S.; Rubin, N. Isolation of an alkaloid from Vinca minor. J. Am. Pharm. Assoc. 1955, 44, 330–332. [Google Scholar] [CrossRef]
- Mokry, J.; Kompis, I.; Dubravkova, L.; Sefcovic, P. Alkaloids of Vinca minor L. The structure of vincadine and vincaminoreine. Tetrahedron Lett. 1962, 25, 1185–1188. [Google Scholar] [CrossRef]
- Pyuskyulev, B.; Ognyanov, I.; Panov, P. Alkaloide aus Vinca herbacea W. K. II Norfluorocurarin. Tetrahedron Lett. 1967, 46, 4559–4562. [Google Scholar] [CrossRef]
- Cheng, G.-G.; Zhao, Y.-L.; Zhang, Y.; Lunga, P.-K.; Hu, D.-B.; Li, Y.; Gu, J.; Song, C.-W.; Sun, W.-B.; Liu, Y.-P.; et al. Indole alkaloids from cultivated Vinca major. Tetrahedron 2014, 70, 8723–8729. [Google Scholar] [CrossRef]
- Tulyaganov, T.S.N. A M Alkaloids of Vinca minor. Chem. Nat. Comp. 2000, 36, 540. [Google Scholar] [CrossRef]
- Verma, P.; Khan, S.A.; Masood, N.; Manika, N.; Sharma, A.; Verma, N.; Luqman, S.; Mathur, A.K. Differential rubisco content and photosynthetic efficiency of rol gene integrated Vinca minor transgenic plant: Correlating factors associated with morpho-anatomical changes, gene expression and alkaloid productivity. J. Plant Physiol. 2017, 219, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Farahanikia, B.; Akbarzadeh, T.; Jahangirzadeh, A.; Yassa, N.; Shams Ardekani, M.R.; Mirnezami, T.; Hadjiakhoondi, A.; Khanavi, M. Phytochemical investigation of Vinca minor cultivated in Iran. Iran. J. Pharm. Res. 2011, 10, 777–785. [Google Scholar]
- Demessie, Z.; Woolfson, K.N.; Yu, F.; Qu, Y.; De Luca, V. The ATP binding cassette transporter, VmTPT2/VmABCG1, is involved in export of the monoterpenoid indole alkaloid, vincamine in Vinca minor leaves. Phytochemistry 2017, 140, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Koel, M.; Kuhtinskaja, M.; Vaher, M. Extraction of bioactive compounds from Catharanthus roseus and Vinca minor. Sep. Purif. Technol. 2020, 252. [Google Scholar] [CrossRef]
- Boyadzhiev, L.; Mecheva, D.; Yordanov, B. Extraction of vincamine from Periwinkle (Vinca minor L.): Obtaining of total extract. C. R. Acad. Bulg. Sci. 2002, 55, 49–52. [Google Scholar]
- El-Dessouki, A.M.; El Fattah, M.A.; Awad, A.S.; Zak, H.F. Zafirlukast and vincamine ameliorate tamoxifen-induced oxidative stress and inflammation: Role of the JNK/ERK pathway. Life Sci. 2018, 202, 78–88. [Google Scholar] [CrossRef]
- Hasa, D.; Perissutti, B.; Dall’Acqua, S.; Chierotti, M.R.; Gobetto, R.; Grabnar, I.; Cepek, C.; Voinovich, D. Rationale of using Vinca minor Linne dry extract phytocomplex as a vincamine’s oral bioavailability enhancer. Eur. J. Pharm. Biopharm. 2013. [Google Scholar] [CrossRef]
- Wang, J.; Lv, X.; Xu, J.; Liu, X.; Du, T.; Sun, G.; Chen, J.; Shen, X.; Wang, J.; Hu, L. Design, synthesis and biological evaluation of vincamine derivatives as potential pancreatic B-cells protective agents for the treatment of type 2 diabetes mellitus. Eur. J. Med. Chem. 2020, 188. [Google Scholar] [CrossRef] [PubMed]
- Söhretoglu, D.; Masullo, M.; Piacente, S.; Kirmizibekmez, H. Iridoids, monoterpenoid glucoindole alkaloids and flavonoids from Vinca major. Biochem. Syst. Ecol. 2013, 49, 69–72. [Google Scholar] [CrossRef]
- Roepke, J.; Salim, V.; Wu, M.; Thamm, A.M.; Murata, J.; Ploss, K.; Boland, W.; De Luca, V. Vinca drug components accumulate exclusively in leaf exudates of Madagascar periwinkle. Proc. Natl. Acad. Sci. USA 2010, 107, 15287–15292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foddai, M.; Maldini, M.; Addis, R.; Petretto, G.L.; Chessa, M.; Pintore, G. Profiling of the bioactive compounds in flowers, leaves and roots of Vinca sardoa. Nat. Prod. Commun. 2017, 12, 933–936. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Lu, X.; Guo, X.; Guo, Q.; Li, D. Metabolomics Characterization of Two Apocynaceae Plants, Catharanthus roseus and Vinca minor, Using GC-MS and LC-MS Methods in Combination. Molecules 2017, 22, 997. [Google Scholar] [CrossRef] [Green Version]
- Macavei, S.G.; Suciu, M.; Craciunescu, I.; Barbu-Tudoran, L.; Tripon, S.C.; Balan, R. Hyperthermia effects on normal and tumor skin cells. Ann. RSCB 2016, 21, 11–21. [Google Scholar]
- Bryan, N.S.; Grisham, M.B. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic. Biol. Med. 2007, 43, 645–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, B.; Lustberg, M.; Staff, N.P.; Kolb, N.; Alberti, P.; Argyriou, A.A. Vinca alkaloids, thalidomide and eribulin-induced peripheral neurotoxicity: From pathogenesis to treatment. J. Peripher. Nerv. Soc. 2019, 24 (Suppl. 2), S63–S73. [Google Scholar] [CrossRef]
- Liao, F.-Y.; Xie, Y.; Jiang, H. The effect of water stress on the physiology of Vinca major ‘variegata’. Appl. Mech. Mater. 2013, 409–410, 782–787. [Google Scholar] [CrossRef]
- Jaleel, A.C.; Gopi, R.; Manivannan, P.; Gomathinayagam, M.; Sridharan, R.; Panneerselvam, R. Antioxidant potential and indole alkaloid profile variations with water deficits along different parts of two varieties of Catharanthus roseus. Colloid Surf. B 2008, 62, 312–318. [Google Scholar] [CrossRef]
- Bahadori, F.; Topçu, G.; Boğa, M.; Türkekul, A.; Kolak, U.; Kartal, M. Indole alkaloids from Vinca major and V. minor growing in Turkey. Nat. Prod. Commun. 2012, 7, 731–734. [Google Scholar] [CrossRef] [Green Version]
- Barrales-Cureño, H.J.; Reyes, C.R.; García, I.V.; Valdez, L.G.L.; De Jesús, A.G.; Cortés Ruíz, J.A.; Sánchez Herrera, L.M.; Calderón Caballero, M.C.; Magallón, J.A.S.; Espinoza Perez, J.; et al. Alkaloids of pharmacological importance in Catharanthus roseus. In Alkaloids—Their Importance in Nature and Human Life; Kurek, J., Ed.; Intech Open Ltd.: London, UK, 2019; Volume 1, p. 18. [Google Scholar]
- Verma, P.; Mathur, A.K.; Shanker, K. Enhanced vincamine production in selected tryptophan-overproducing shoots of Vinca minor. Plant Cell Tiss. Org. Cult. 2012, 111, 239–245. [Google Scholar] [CrossRef]
- O’Connor, S.E. Alkaloid biosynthesis. Wiley Encycl. Chem. Biol. 2008, 1, 17–33. [Google Scholar] [CrossRef]
- Yin, H.; Sun, Y.-H. Vincamine-producing endophytic fungus isolated from Vinca minor. Phytomedicine 2011, 18, 802–805. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, H.-L.; Li, Y.; Gu, H.-W.; Yin, X.-L.; Xie, L.-X.; Yu, R.-Q. Rapid and simultaneous determination of five Vinca alkaloids in Catharanthus roseus and human serum using trilinear component modeling of liquid chromatography–diode array detection data. J. Chromat. B 2016, 1026, 114–123. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Tapias, E.C.; Kim, H.K.; Lefeber, A.W.M.; Erkelens, C.; Verhoeven, J.T.J.; Brzin, J.; Zel, J.; Verpoorte, R. Metabolic discrimination of Catharanthus roseus leaves infected by Phytoplasma using 1H-NMR spectroscopy and multivariate data analysis. Plant Physiol. 2004, 135, 2398–2410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Vrieling, K.; Klinkhamer, P.G.L. Interactions between plant metabolites affect herbivores: A study with pyrrolizidine alkaloids and chlorogenic acid. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Ye, M.; Zhang, J. Vincamine prevents lipopolysaccharide induced inflammation and oxidative stress via thioredoxin reductase activation in human corneal epithelial cells. Am. J. Transl. Res. 2018, 10, 2195–2204. [Google Scholar] [PubMed]
- Stagos, D. Antioxidant activity of polyphenolic plant extracts. Antioxidants 2020, 9, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grujić, S.M.; Radojević, I.D.; Vasić, S.M.; Čomić, L.R.; Topuzović, M.D. Antimicrobial activity and some phytochemical analysis of two extracts Vinca minor L. Krag. J. Sci. 2014, 3, 145–154. [Google Scholar] [CrossRef] [Green Version]
- Șimșek Sezer, E.N.; Uysal, T. Volatile and phenolic compositions of the leaves of two Vinca L. species from Turkey. Curr. Perspect. Med. Arom. Plants 2018, 1, 103–110. [Google Scholar]
- Özçelik, B.; Kartal, M.; Orhan, I. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm. Biol. 2011, 49, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial Activity of Some Flavonoids and Organic Acids Widely Distributed in Plants. J. Clin. Med. 2020, 9, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubey, S.; Ganeshpurkar, A.; Bansal, D.; Dubey, N. Experimental studies on bioactive potential of rutin. Chron. Young Sci. 2013, 4, 153–157. [Google Scholar] [CrossRef]
- Suwalak, S.; Voravuthikunchai, S.P. Morphological and ultrastructural changes in the cell structure of enterohaemorrhagic Escherichia coli O157:H7 following treatment with Quercus infectoria nut galls. J. Electron Microsc. 2009, 58, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Aruldass, C.A.; Marimuthu, M.M.; Ramanathan, S.; Mansor, S.M.; Murugaiyah, V. Effects of Mesua ferrea Leaf and Fruit Extracts on Growth and Morphology of Staphylococcus aureus. Microsc. Microanal. 2013, 19, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Mahajan, S.; Sharma, R. Evaluation of antimicrobial activity of Curcuma longa rhizome extract against Staphylococcus aureus. Biotechnol. Rep. 2015, 6, 51–55. [Google Scholar] [CrossRef] [Green Version]
- Khanavi, M.; Pourmoslemi, S.; Farahanikia, B.; Hadjiakhoondi, A.; Ostad, S.N. Cytotoxicity of Vinca minor. Pharm. Biol. 2010, 48, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Amos, L.A. What tubulin drugs tell us about microtubule structure and dynamics. Semin. Cell Dev. Biol. 2011, 22, 916–926. [Google Scholar] [CrossRef]
- Khattak, S.; Khan, H. Anti-cancer potential of phyto-alkaloids: A prospective review. Curr. Cancer Ther. Rev. 2016, 12, 66–75. [Google Scholar] [CrossRef]
- Zhu, W.; Gao, J. The Use of Botanical Extracts as Topical Skin-Lightening Agents for the Improvement of Skin Pigmentation Disorders. J. Invest. Dermat. Symp. Proceed. 2008, 13, 20–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arunkumar, R.; Abraham, A.N.; Shukla, R.; Drummond, C.J.; Greaves, T.L. Cytotoxicity of protic ionic liquids towards the HaCat cell line derived from human skin. J. Mol. Liq. 2020, 314, 113602. [Google Scholar] [CrossRef]
- Danciu, C.; Zupko, I.; Bor, A.; Schwiebs, A.; Radeke, H.; Hancianu, M.; Cioanca, O.; Alexa, E.; Oprean, C.; Bojin, F.; et al. Botanical Therapeutics: Phytochemical Screening and Biological Assessment of Chamomile, Parsley and Celery Extracts against A375 Human Melanoma and Dendritic Cells. Int. J. Mol. Sci. 2018, 19, 3624. [Google Scholar] [CrossRef] [Green Version]
- Andreicut, A.-D.; Pârvu, A.E.; Mot, A.C.; Pârvu, M.; Fischer Fodor, E.; Cătoi, A.F.; Feldrihan, V.; Cecan, M.; Irimie, A. Phytochemical analysis of anti-inflammatory and antioxidant effects of Mahonia aquifolium flower and fruit extracts. Oxid. Med. Cell Longev. 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- Farcaș, A.D.; Moț, A.C.; Zăgrean-Tuza, C.; Toma, V.; Cimpoiu, C.; Hosu, A.; Pârvu, M.; Roman, I.; Silaghi-Dumitrescu, R. Chemo-mapping and biochemicalmodulatory and antioxidant/prooxidant effect of Galium verum extract during acute restraint and dark stress in female rats. PLoS ONE 2018, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sreevidya, N.; Mehrotra, S. Spectrophotometric method for estimation of alkaloids precipitable with Dragendorff’s reagent in plant materials. J. AOAC Int. 2003, 86, 1124–1127. [Google Scholar] [CrossRef] [Green Version]
- Blainski, A.; Lopes, G.C.; de Mello, J.C.P. Application and analysis of the Folin Ciocâlteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pękal, A.; Pyrzynska, K. Evaluation of aluminium complexation reaction for flavonoid content assay. Food Anal. Met. 2014, 7, 1776–1782. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moț, A.C.; Bischin, C.; Mureșan, B.; Pârvu, M.; Damian, G.; Vlase, L.; Silaghi-Dumitrescu, R. Antioxidant activity evaluation by physiologically relevant assays based on haemoglobin peroxidase activity and cytochrome c-induced oxidation of liposomes. Nat. Prod. Res. 2016, 30, 1315–1319. [Google Scholar] [CrossRef]
- Carpa, R.; Dragan-Bularda, M.; Muntean, V. General Microbiology. Practical Guide; Presa Universitara Clujeana: Cluj-Napoca, Romania, 2014; pp. 58–61. [Google Scholar]
- Leclercq, R.; Canton, R.; Brown, D.F.J.; Giske, C.G.; Heisig, P.; MacGowan, A.P.; Mouton, J.W.; Nordmann, P.; Rodloff, A.C.; Rossolini, G.M.; et al. EUCAST expert rules in antimicrobial susceptibility testing. Clin. Microbiol. Infect. 2013, 19, 141–160. [Google Scholar] [CrossRef] [Green Version]
- Erlandsen, S.L.; Kristich, C.J.; Dunny, G.M.; Wells, C.L. High-resolution visualization of the microbial glycocalyx with low-voltage scanning electron microscopy: Dependence on cationic dyes. J. Histochem. Cytochem. 2004, 52, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Macavei, S.G.; Suciu, M.; Craciunescu, I.; Barbu-Tudoran, L.; Tripon, S.C.; Leostean, C.; Balan, R. SPION size dependent effects on normal and cancer cells. Stud. Univ. Babes Bolyai Biol. 2017, LXII, 29–42. [Google Scholar] [CrossRef]
- Hayat, M.A. Principles and Techniques of Electron Microscopy. Biological Applications, 4th ed.; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
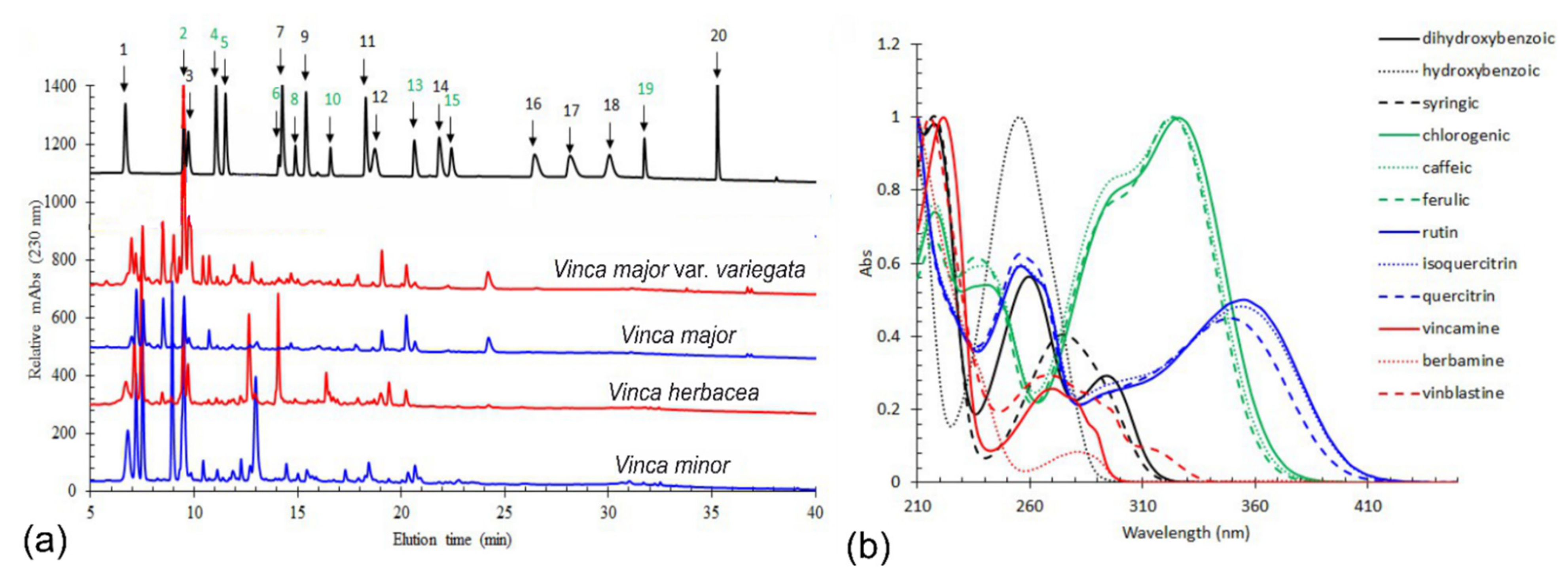


| Compound | Compound Concentration Expresses in μg/g | |||
|---|---|---|---|---|
| V. minor | V. major | V. major var. variegata | V. herbacea | |
| Chlorogenic acid | 4112 ± 13 ** | 675 ± 160 | 932 ± 260 | 1538 ± 200 * |
| Caffeic acid | 229 ± 2 *** | 13 ± 2 | 182 ± 23 ** | 13 ± 1 |
| Rutin | 73 ± 1 ** | 11 ± 1 | 94 ± 10 ** | 2528 ± 160 *** |
| Isoquercitrin | 12 ± 1 | 12 ± 1 | 38 ± 4 * | 87 ± 4 *** |
| Quercitrin | 52 ± 4 * | 11 ± 1 | 45 ± 8 * | 109 ± 10 *** |
| Vincamine | 65 ± 1 *** | 42 ± 3 | 31 ± 2 | n.d. |
| Quercetin | 21 ± 2 | 14 ± 2 | 28 ± 2 | 20 ± 3 |
| Extract | REAC | CUPRAC | LipPx | TPC | TFC | TAC |
|---|---|---|---|---|---|---|
| mg rutin/g | mg rutin/g | mg rutin/g | mg gallic/g | mg rutin/g | mg vinblastine/g | |
| V. minor | 106 ± 53 * | 123 ± 18 * | 43 ± 9 | 51 ± 0 * | 29 ± 1 | 332 ± 33 *** |
| V. major | 35 ± 13 | 50 ± 2 | 153 ± 12 ** | 21 ± 2 | 11 ± 3 | 35 ± 11 |
| V. major var. variegata | 335 ± 10 ** | 463 ± 37 ** | 229 ± 20 ** | 93 ± 7 ** | 179 ± 2 *** | 101 ± 6 * |
| V. herbacea | 405 ± 29 ** | 583 ± 48 ** | 267 ± 23 ** | 100 ± 6 ** | 151 ± 1 *** | 195 ± 9 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciorîță, A.; Zăgrean-Tuza, C.; Moț, A.C.; Carpa, R.; Pârvu, M. The Phytochemical Analysis of Vinca L. Species Leaf Extracts Is Correlated with the Antioxidant, Antibacterial, and Antitumor Effects. Molecules 2021, 26, 3040. https://doi.org/10.3390/molecules26103040
Ciorîță A, Zăgrean-Tuza C, Moț AC, Carpa R, Pârvu M. The Phytochemical Analysis of Vinca L. Species Leaf Extracts Is Correlated with the Antioxidant, Antibacterial, and Antitumor Effects. Molecules. 2021; 26(10):3040. https://doi.org/10.3390/molecules26103040
Chicago/Turabian StyleCiorîță, Alexandra, Cezara Zăgrean-Tuza, Augustin C. Moț, Rahela Carpa, and Marcel Pârvu. 2021. "The Phytochemical Analysis of Vinca L. Species Leaf Extracts Is Correlated with the Antioxidant, Antibacterial, and Antitumor Effects" Molecules 26, no. 10: 3040. https://doi.org/10.3390/molecules26103040
APA StyleCiorîță, A., Zăgrean-Tuza, C., Moț, A. C., Carpa, R., & Pârvu, M. (2021). The Phytochemical Analysis of Vinca L. Species Leaf Extracts Is Correlated with the Antioxidant, Antibacterial, and Antitumor Effects. Molecules, 26(10), 3040. https://doi.org/10.3390/molecules26103040