The Analysis of Phenolic Compounds in Walnut Husk and Pellicle by UPLC-Q-Orbitrap HRMS and HPLC
Abstract
1. Introduction
2. Results and Discussion
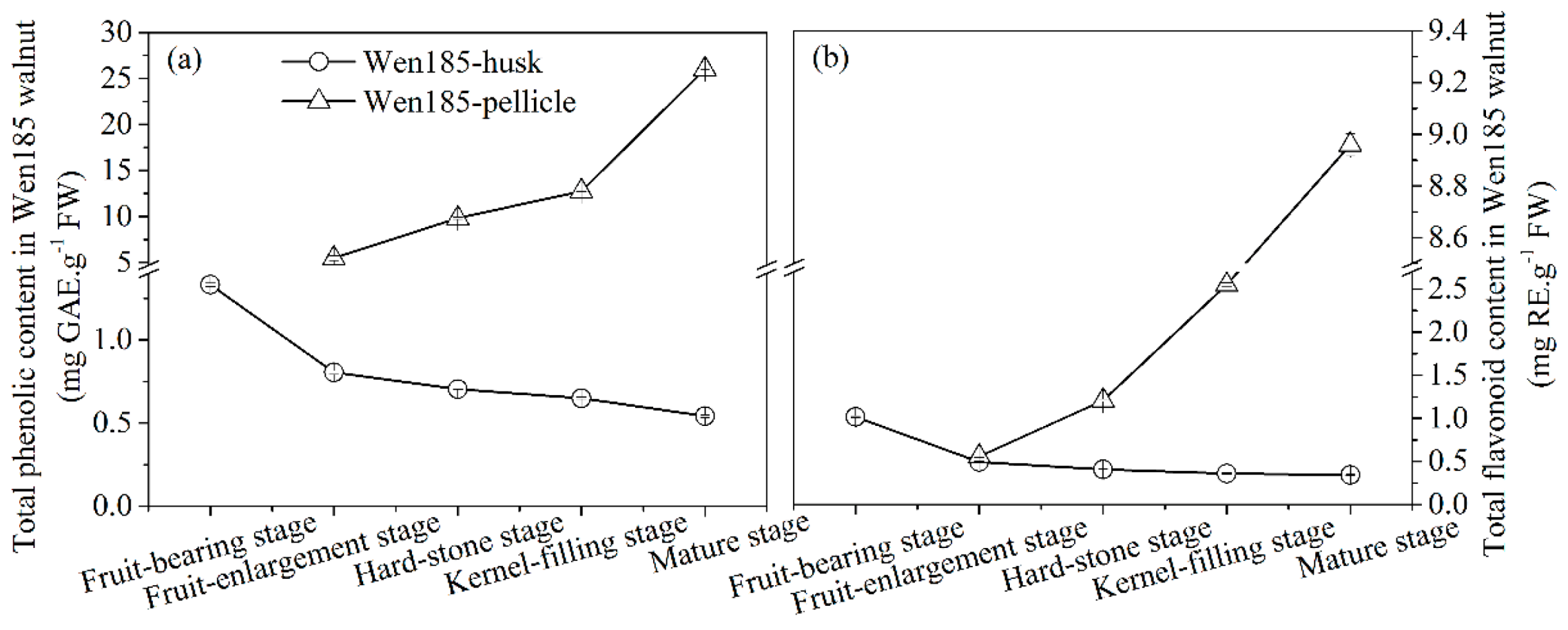
2.1. Changes of Phenols and Flavonoid Content of Walnut Husk and Pellicle
2.2. Characterization of Phenolic Components in Walnut Husk and Pellicle
2.2.1. Hydrolysable Tannins and Related Compounds
2.2.2. Flavonoids
2.2.3. Phenolic Acid and Derivatives
2.2.4. Identification of Quinones
2.3. Comparative Analysis of Components in Walnut Husk and Pellicle
2.4. Content Variation of Main Phenolic Compounds during Walnut Ripening
3. Materials and Methods
3.1. Materials
3.2. Standards and Reagents
3.3. Polyphenols Extraction
3.4. Preparation Standard Solution
3.5. Qualitative and Quantitative Components in Walnut Husk and Pellicle
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Jahanban-Esfahlan, A.; Ostadrahimi, A.; Tabibiazar, M.; Amarowicz, R. A Comprehensive review on the chemical constituents and functional uses of walnut (Juglans spp.) Husk. Int. J. Mol. Sci. 2019, 20, 3920. [Google Scholar] [CrossRef]
- Grace, M.H.; Warlick, C.W.; Neff, S.A.; Lila, M.A. Efficient preparative isolation and identification of walnut bioactive components using high-speed counter-current chromatography and LC-ESI-IT-TOF-MS. Food Chem. 2014, 158, 229–238. [Google Scholar] [CrossRef]
- Hardman, W.E. Walnuts have potential for cancer prevention and treatment in mice. J. Nutr. 2014, 144, 555S–560S. [Google Scholar] [CrossRef]
- Puupponen-Pimiä, R.; Nohynek, L.; Meier, C.; Kähkönen, M.; Heinonen, M.; Hopia, A.; Oksman-Caldentey, K.M. Antimicrobial properties of phenolic compounds from berries. J. Appl. Microbiol. 2001, 90, 494–507. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Trandafir, I.; Cosmulescu, S.; Nour, V. Phenolic profile and antioxidant capacity of walnut extract as influenced by the extraction method and solvent. Int. J. Food Eng. 2017, 13, 1–8. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Amarowicz, R. Walnut (Juglans regia L.) shell pyroligneous acid: Chemical constituents and functional applications. RSC Adv. 2018, 8, 22376–22391. [Google Scholar] [CrossRef]
- Martínez, M.L.; Labuckas, D.O.; Lamarque, A.L.; Maestri, D.M. Walnut (Juglans regia L.): Genetic resources, chemistry, by-products. J. Sci. Food Agric. 2010, 90, 1959–1967. [Google Scholar] [CrossRef]
- Persic, M.; Mikulic-Petkovsek, M.; Slatnar, A.; Solar, A.; Veberic, R. Changes in phenolic profiles of red-colored pellicle walnut and hazelnut kernel during ripening. Food Chem. 2018, 252, 349–355. [Google Scholar] [CrossRef]
- Colaric, M.; Veberic, R.; Solar, A.; Hudina, M.; Stampar, F. Phenolic acids, syringaldehyde, and juglone in fruits of different cultivars of Juglans regia L. J. Agric. Food Chem. 2005, 53, 6390–6396. [Google Scholar] [CrossRef]
- Liu, P.Z.; Li, L.L.; Song, L.J.; Sun, X.T.; Yan, S.J.; Huang, W.J. Characterisation of phenolics in fruit septum of Juglans regia Linn. by ultra performance liquid chromatography coupled with Orbitrap mass spectrometer. Food Chem. 2019, 286, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Koleckar, V.; Kubikova, K.; Rehakova, Z.; Kuca, K.; Jun, D.; Jahodar, L.; Opletal, L. Condensed and hydrolysable tannins as antioxidants influencing the health. Mini-Rev. Med. Chem. 2008, 8, 436–447. [Google Scholar] [CrossRef]
- Blum, F. High performance liquid chromatography. Br. J. Hosp. Med. 2014, 75, C18–C21. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.B.; Zhang, W.E.; Li, X.; Pan, X.J. Seasonal variations of phenolic profiles and antioxidant activity of walnut (Juglans sigillata Dode) green husks. Int. J. Food Prop. 2017, 20, S2635–S2646. [Google Scholar] [CrossRef]
- Cosmulescu, S.; Trandafir, I. Seasonal variation of total phenols in leaves of walnut (Juglans regia L.). J. Med. Plants Res. 2011, 5, 4938–4942. [Google Scholar]
- Yu, W.J.; Wang, M.M.; Han, F.; Li, M.C. Comparison of polyphenols content in different parts of different walnut of Xinjiang. Fram Prod. Process. 2018, 11, 56–58. [Google Scholar]
- Vu, D.C.; Vo, P.H.; Coggeshall, M.V.; Lin, C.H. Identification and characterization of phenolic compounds in black walnut kernels. J. Agric. Food Chem. 2018, 66, 4503–4511. [Google Scholar] [CrossRef]
- Liu, R.X.; Zhao, Z.Y.; Dai, S.J.; Che, X.; Liu, W.H. Identification and quantification of bioactive compounds in Diaphragma juglandis fructus by UHPLC-Q-Orbitrap HRMS and UHPLC-MS/MS. J. Agric. Food Chem. 2019, 67, 3811–3825. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.D.; Luo, H.T.; Xu, M.Y.; Zhai, M.; Guo, Z.R.; Qiao, Y.S.; Wang, L.J. Dynamic changes in phenolics and antioxidant capacity during pecan (Carya illinoinensis) kernel ripening and its phenolics profiles. Molecules 2018, 23, 435. [Google Scholar] [CrossRef]
- Escobar-Avello, D.; Lozano-Castellón, J.; Mardones, C.; Pérez, A.J.; Saéz, V.; Riquelme, S.; Baer, D.V.; Vallverdú-Queralt, A. Phenolic profile of grape canes: Novel compounds identified by LC-ESI-LTQ-Orbitrap-MS. Molecules 2019, 24, 3763. [Google Scholar] [CrossRef]
- Vasić, V.; Gašić, U.; Stanković, D.; Lušić, D.; Vukić-Lušić, D.; Milojković-Opsenica, D.; Tešić, Ž.; Trifković, J. Towards better quality criteria of European honeydew honey: Phenolic profile and antioxidant capacity. Food Chem. 2019, 274, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.D.; Huo, J.H.; Wang, G.L.; Wang, W.M. Identification and characterization of chemical constituents in Cortex Juglandis Mandshuricae based on UPLC-Q-TOF/MS. Chin. Tradit. Herb. Drugs 2017, 48, 657–667. [Google Scholar]
- Regueiro, J.; Sánchez-González, C.; Vallverdú-Queralt, A.; Simal-Gándara, J.; Lamuela-Raventós, R.; Izquierdo-Pulido, M. Comprehensive identification of walnut polyphenols by liquid chromatography coupled to linear ion trap-Orbitrap mass spectrometry. Food Chem. 2014, 152, 340–348. [Google Scholar] [CrossRef]
- Hilary, S.; Tomás-Barberán, F.A.; Martinez-Blazquez, J.A.; Kizhakkayi, J.; Souka, U.; Al-Hammadi, S.; Habib, H.; Ibrahim, W.; Platat, C. Polyphenol characterisation of Phoenix dactylifera L. (date) seeds using HPLC-mass spectrometry and its bioaccessibility using simulated in-vitro digestion/Caco-2 culture model. Food Chem. 2020, 311, 125969.1–125969.9. [Google Scholar] [CrossRef]
- Yamada, H.; Wakamori, S.; Hirokane, T.; Ikeuchi, K.; Matsumoto, S. Structural revisions in natural ellagitannins. Molecules 2018, 23, 1901. [Google Scholar] [CrossRef]
- Gómez-Caravaca, A.M.; Verardo, V.; Segura-Carretero, A.; Caboni, M.F.; Fernández-Gutiérrez, A. Development of a rapid method to determine phenolic and other polar compounds in walnut by capillary electrophoresis-electrospray ionization time-of-flight mass spectrometry. J. Chromatogr. A 2008, 1209, 238–245. [Google Scholar] [CrossRef]
- Terra, X.; Pallarés, V.; Ardèvol, A.; Bladé, C.; Fernández-Larrea, J.; Pujadas, G.; Salvadó, J.; Arola, L.; Blay, M. Modulatory effect of grape-seed procyanidins on local and systemic inflammation in diet-induced obesity rats. J. Nutr. Biochem. 2011, 22, 380–387. [Google Scholar] [CrossRef]
- Leonetti, D.; Soleti, R.; Clere, N.; Vergori, L.; Jacques, C.; Duluc, L.; Dourguia, C.; Maitinez, M.C.; Andriantsitohaina, R. Extract enriched in flavan-3-ols and mainly procyanidin dimers improves metabolic alterations in a mouse model of obesity-related disorders partially via estrogen receptor alpha. Front. Pharmacol. 2018, 9, 406–419. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Boix, N.; Piqué, E.; Gómez-Catalan, J.; Medina-Remon, A.; Sasot, G.; Mercader-Martí, M.; Llobet, J.M.; Lamuela-Raventos, R.M. Identification of phenolic compounds in red wine extract samples and zebrafish embryos by HPLC-ESI-LTQ-Orbitrap-MS. Food Chem. 2015, 181, 146–151. [Google Scholar] [CrossRef]
- Cao, L.J.; Zhang, X.; Chen, C.Y.; Li, J.; Zhao, S.L. Research on the chemical components and pharmaceutical actions of walnut green husk. Hubei Agric. Sci. 2016, 55, 4630–4633. [Google Scholar]
- Shimoda, H.; Tanaka, J.; Kikuchi, M.; Fukuda, T.; Ito, H.; Hatano, T.; Yoshida, T. Effect of polyphenol-rich extract from walnut on diet-induced hypertriglyceridemia in mice via enhancement of fatty acid oxidation in the liver. J. Agric. Food Chem. 2009, 57, 1786–1792. [Google Scholar] [CrossRef] [PubMed]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar]
- Jia, Z.S.; Tang, M.C.; Wu, J.M. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar]
| No. | RT (min) | Formula | Ion Mode | Measured Mass (m/z) | MS/MS Fragments (m/z) | Error (ppm) | Compound Identification | Reference | Classification | Walnut | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Husk | Pellicle | ||||||||||
| 1 | 0.744 | C20H18O14 | M−H | 481.06317 | 301.000, 275.020, 229.014, 257.010 | 1.666 | HHDP-glucose isomer | [11] | Hydrolysable Tannins | − | + |
| 2 | 0.895 | C7H12O6 | M−H | 191.05539 | 85.028, 93.033, 87.008, 59.013, 173.045 | −3.772 | quinic acid | [17] | Hydrolysable Tannins | + | + |
| 3 | 1.162 | C13H16O10 | M−H | 331.06760 | 169.014, 271.046, 211.025, 125.023 | 1.620 | monogalloyl-glucose isomer | [11] | Hydrolysable Tannins | + | + |
| 4 | 1.307 | C7H6O5 | M−H | 169.01352 | 125.023, 107.013 | −4.264 | gallic acid | [11] | Phenolic Acid | + | + |
| 5 | 1.361 | C8H8O4 | M−H | 167.03427 | 123.044, 139.003 | −4.243 | hydroxymandelic acid | [18] | Phenolic Acid | + | + |
| 6 | 2.073 | C13H16O10 | M−H | 331.06760 | 169.014, 125.023, 271.046, 211.025 | 1.620 | gallic acid hexoside | [19] | Phenolic Acid | − | + |
| 7 | 2.699 | C13H16O9 | M−H | 315.07281 | 108.021, 152.011 | 2.110 | protocatechuic acid-O-hexoside | [20] | Phenolic Acid | + | + |
| 8 | 3.749 | C8H8O4 | M+H | 169.04951 | 111.044, 65.039, 125.060, 169.050, 151.039, 93.034 | −3.284 | isovanillic acid | Phenolic Acid | + | + | |
| 9 | 3.786 | C7H6O3 | M−H | 137.02347 | 108.021, 93.033 | −2.861 | protocatechualdehyde | [18] | Phenolic Acid | + | − |
| 10 | 3.971 | C16H18O9 | M−H | 353.08832 | 191.056, 135.044, 179.034 | 1.467 | neochlorogenic acid | [11] | Phenolic Acid | + | + |
| 11 | 4.090 | C9H6O3 | M+H | 163.03888 | 163.039, 107.049, 95.050 | −3.377 | 7-hydroxycoumarine | Other | + | + | |
| 12 | 4.102 | C14H18O10 | M−H | 345.08307 | 168.006, 124.016 | 1.040 | methyl galloyl hexoside | [18] | Hydrolysable Tannins | + | + |
| 13 | 4.171 | C7H6O4 | M−H | 153.01846 | 109.028, 81.033 | −5.665 | protocatehuic acid | [18] | Phenolic Acid | + | + |
| 14 | 4.270 | C15H20O10 | M−H | 359.09897 | 197.045, 153.055 | 1.667 | dimethyl galloyl hexoside | [18] | Hydrolysable Tannins | + | + |
| 15 | 4.447 | C15H18O9 | M−H | 341.08826 | 177.055, 135.044 | 1.662 | caffeic acid hexoside II | [21] | Phenolic Acid | + | + |
| 16 | 4.448 | C8H8O5 | M−H | 183.02930 | 168.006, 124.016 | −3.240 | methyl gallate | [19] | Other | + | + |
| 17 | 4.470 | C13H15O8 | M−H | 299.07767 | 137.024 | 1.872 | hydroxybenzoyl hexoside | [20] | Phenolic Acid | + | + |
| 18 | 4.485 | C30H26O12 | M−H | 577.13678 | 125.024, 289.072, 407.078, 425.089, 109.029, 137.024 | 2.841 | B-type procyanidin dimer isomer | [11] | Flavonoid | − | + |
| 19 | 4.814 | C21H22O12 | M−H | 465.10535 | 303.051, 285.039, 201.113 | 3.239 | epicatechin-3-O-glucoside isomer | [18] | Flavonoid | + | + |
| 20 | 4.911 | C7H6O3 | M−H | 137.02348 | 93.033, 108.021, 137.024 | −6.763 | p-hydroxybenzoic acid | [19] | Phenolic Acid | + | + |
| 21 | 4.912 | C15H14O6 | M−H | 289.07224 | 109.029, 245.082, 123.044, 125.024, 203.071, 137.024, 151.039, 205.050, 97.028 | 1.644 | (+)-catechin | [11] | Flavonoid | + | + |
| 22 | 4.930 | C14H10O9 | M−H | 321.02539 | 169.014, 125.024 | 0.594 | m-digallate | [18] | Phenolic Acid | + | + |
| 23 | 4.930 | C10H6O3 | M−H | 173.02376 | 173.024, 145.029, 117.034 | 3.225 | 2-hydroxy-1, 4-naphthoquinone | Quinones | + | + | |
| 24 | 5.099 | C20H20O14 | M−H | 483.07886 | 169.014, 125.024, 271.047, 211.025, 313.057, 331.068 | 1.733 | digalloyl-glucose isomer | [11] | Hydrolysable Tannins | − | + |
| 25 | 5.102 | C8H8O4 | M−H | 167.03427 | 152.011, 108.021, 123.044 | −4.243 | vanillic acid | [17] | Phenolic Acid | + | + |
| 26 | 5.175 | C16H18O8 | M−H | 337.09344 | 163.039, 119.049, 173.045, 93.033 | 1.646 | 3-p-coumaroylquinic acid | [11] | Phenolic Acid | + | + |
| 27 | 5.199 | C45H38O18 | M−H | 865.19952 | 125.024, 289.072, 407.078, 151.039 | 1.149 | B-type procyanidin trimer isomer | [11] | Flavonoid | − | + |
| 28 | 5.266 | C10H10O3 | M+H | 179.07021 | 133.065, 147.044 | −0.415 | juglanoside isomer | [18] | Quinones | + | + |
| 29 | 5.352 | C60H50O24 | M−2H | 576.12830 | 289.072, 287.057, 407.079 | 2.171 | B-type procyanidin tetramer isomer | [11] | Flavonoid | + | + |
| 30 | 5.452 | C9H8O4 | M−H | 179.03435 | 135.044, 107.049 | −3.533 | caffeic acid | [17] | Phenolic Acid | + | + |
| 31 | 5.473 | C27H22O18 | M−H | 633.07495 | 301.000, 275.020, 125.023, 229.014, 257.010 | 2.561 | galloyl-HHDP-glucose | [11] | Hydrolysable Tannins | − | + |
| 32 | 5.701 | C9H10O5 | M−H | 197.04512 | 182.022, 123.008, 166.998, 153.055, 138.031 | −2.130 | syringic acid | [17] | Phenolic Acid | + | + |
| 33 | 5.777 | C41H28O26 | M−H | 935.08185 | 275.020, 301.000, 229.014, 257.010, 299.020 | 2.500 | galloyl-bis-HHDP-glucose | [18] | Hydrolysable Tannins | + | − |
| 34 | 5.887 | C15H22O5 | M−H | 281.13980 | 237.150, 171.118, 123.080, 189.129 | 1.284 | dihydrophaseic acid | [18] | Other | + | + |
| 35 | 5.927 | C10H10O3 | M−H | 177.05504 | 177.055, 159.044, 149.060, 131.049 | −3.793 | isosclerone isomer | [18] | Other | + | + |
| 36 | 5.951 | C10H8O3 | M+H | 177.05447 | 177.055, 149.060, 131.049, 121.065, 159.044 | −3.977 | 5-hydroxy-1, 4-naphthaoquinon | [22] | Quinones | + | + |
| 37 | 5.961 | C16H18O9 | M−H | 353.08832 | 135.044, 179.034, 191.056 | 1.467 | chlorogenic acid | [18] | Phenolic Acid | + | + |
| 38 | 6.177 | C15H18O8 | M−H | 325.09341 | 265.072, 119.049, 163.039, 205.050, 235.061, 145.029 | 1.613 | coumaric acid hexoside isomer | [11] | Phenolic Acid | + | + |
| 39 | 6.185 | C10H10O2 | M+H | 163.07530 | 131.049, 103.055, 163.075 | −3.421 | methyl cinnamate | Phenolic Acid | + | + | |
| 40 | 6.205 | C11H12O5 | M+H | 225.07591 | 175.039, 91.055, 119.049, 147.044, 207.101 | −2.477 | sinapinic acid | Phenolic Acid | + | + | |
| 41 | 6.342 | C15H14O6 | M−H | 289.07224 | 109.029, 245.082, 123.044, 125.024, 203.071, 137.024, 151.039 | 1.644 | (−)-epicatechin | [18] | Flavonoid | + | + |
| 42 | 6.344 | C8H8O3 | M−H | 151.03922 | 136.016, 108.021, 123.045 | −5.594 | vanillin | [18] | Other | + | + |
| 43 | 6.387 | C21H22O13 | M−H | 481.09924 | 169.014, 125.024, 300.999 | 1.004 | galloyl methylgalloyl dexoyhexoside isomer | [18] | Hydrolysable Tannins | − | + |
| 44 | 6.400 | C16H18O8 | M+H | 339.10748 | 177.055, 145.028, 321.097, 117.034 | 0.097 | trihydroxynaphthaline glucoside | [18] | Quinones | + | + |
| 45 | 6.510 | C40H28O25 | M−H | 907.08575 | 301.000, 275.020, 783.070 | 1.769 | heterophylliin E isomer | [23] | Hydrolysable Tannins | − | + |
| 46 | 6.546 | C10H6O3 | M+H | 175.07520 | 119.086, 147.080, 129.070 | −2.280 | juglone | [22] | Quinones | + | + |
| 47 | 6.571 | C14H18O9 | M−H | 329.08835 | 167.034, 123.044 | 3.307 | vanillic acid hexoside | Phenolic Acid | + | + | |
| 48 | 6.743 | C21H10O13 | M−H | 469.00540 | 425.016, 301.000 | 1.155 | valoneic acid dilactone | [23] | Other | − | + |
| 49 | 6.854 | C10H8O3 | M−H | 175.03929 | 147.044, 131.049 | −4.391 | 7-hydroxy-methylcoumarin | [18] | Phenolic Acid | + | − |
| 50 | 6.920 | C21H22O12 | M−H | 465.10403 | 285.041 | 0.418 | quercetin-3-O-galactopyranoside isomer | [18] | Flavonoid | + | + |
| 51 | 6.953 | C20H16O13 | M−H | 463.05298 | 300.999, 301.072 | 3.729 | ellagic acid hexoside isomer | [11] | Phenolic Acid | − | + |
| 52 | 7.085 | C9H10O4 | M−H | 181.05002 | 166.027, 151.003, 123.008 | −3.379 | syringaldehyde | [18] | Phenolic Acid | + | + |
| 53 | 7.103 | C41H28O27 | M−H | 951.07581 | 301.000, 275.020, 783.071, 299.992, 229.014, 257.010, 907.087 | 1.362 | trigalloyl-HHDP-glucose | [11] | Hydrolysable Tannins | − | + |
| 54 | 7.135 | C9H8O3 | M−H | 163.03932 | 119.049, 93.033 | −4.527 | o-coumaric acid | [18] | Phenolic Acid | + | + |
| 55 | 7.299 | C27H22O19 | M−H | 649.06903 | 300.999 | 1.203 | valoneoyl-glucose | [19] | Hydrolysable Tannins | + | + |
| 56 | 7.384 | C22H18O10 | M−H | 441.08365 | 289.072, 109.029, 245.082, 125.024, 203.071, 151.039, 205.050 | 2.128 | (−)-epicatechin 3-O-gallate | [11] | Flavonoid | − | + |
| 57 | 7.460 | C27H24O18 | M−H | 635.09070 | 169.014, 125.024, 483.079, 313.057, 465.068, 211.025 | 2.705 | trigalloyl-glucose isomer | [11] | Hydrolysable Tannins | − | + |
| 58 | 7.527 | C19H14O12 | M−H | 433.04199 | 299.992, 301.000, 229.014 | 1.737 | ellagic acid pentoside isomer | [11] | Phenolic Acid | − | + |
| 59 | 7.547 | C34H26O22 | M−H | 785.08685 | 301.000, 275.020, 229.014, 125.023, 257.010, 169.014 | 3.265 | digalloyl-HHDP-glucose | [11] | Hydrolysable Tannins | − | + |
| 60 | 7.696 | C21H20O13 | M−H | 479.08334 | 316.023, 317.031 | 0.487 | myricetin-O-hexoside | [20] | Flavonoid | + | + |
| 61 | 7.802 | C21H20O13 | M+H | 481.09705 | 319.045, 153.018, 85.029, 91.039 | −1.152 | myricetin-3-O-beta-D-galactopyranoside | [22] | Flavonoid | + | + |
| 62 | 7.835 | C20H20O11 | M−H | 435.09390 | 151.003, 285.041, 107.013 | 1.431 | taxifolin-3-O-arabinofuranoside isomer | [18] | Flavonoid | + | − |
| 63 | 7.874 | C9H10O3 | M+H | 167.07022 | 167.070, 123.044, 149.096, 121.029 | −3.286 | ethyl paraben | Phenolic Acid | + | + | |
| 64 | 7.878 | C9H10O3 | M−H | 165.05498 | 121.029, 108.021 | −4.438 | hydroxyphenyl-propionic acid | [18] | Phenolic Acid | + | + |
| 65 | 8.121 | C28H24O16 | M−H | 615.10004 | 300.028, 301.036, 271.025, 169.014, 125.024 | 1.441 | quercetin galloyl hexoside isomer | [11] | Flavonoid | + | + |
| 66 | 8.140 | C34H24O22 | M−H | 783.07117 | 301.000, 275.020, 229.014, 257.009 | 3.227 | pedunculagin/casuariin isomer (bis-HHDP-glucose) | [11] | Hydrolysable Tannins | − | + |
| 67 | 8.298 | C14H6O8 | M−H | 300.99945 | 229.015, 283.997, 257.010, 185.024 | 1.531 | ellagic acid | [11] | Phenolic Acid | + | + |
| 68 | 8.320 | C15H12O7 | M−H | 303.05136 | 125.024, 285.041, 177.019 | 1.980 | taxifolin isomer | [18] | Flavonoid | + | − |
| 69 | 8.356 | C10H10O4 | M−H | 193.05016 | 175.039, 147.044, 178.027, 131.049 | −2.457 | ferulic acid | [18] | Phenolic Acid | + | + |
| 70 | 8.429 | C9H8O3 | M−H | 163.03925 | 119.049, 93.033 | −4.995 | p-coumaric acid | [17] | Phenolic Acid | + | + |
| 71 | 8.502 | C21H18O13 | M−H | 477.06006 | 299.992, 315.016 | 1.150 | methyl ellagic acid hexoside | [19] | Phenolic Acid | + | + |
| 72 | 8.704 | C10H8O2 | M+H | 161.05965 | 133.065, 105.070, 115.054, 91.055 | −0.394 | naphthalened iol isomer | [18] | Quinones | + | + |
| 73 | 8.718 | C34H28O22 | M−H | 787.10297 | 169.014, 125.024, 617.080, 465.069, 313.057 | 3.845 | tetragalloyl-glucose isomer | [11] | Hydrolysable Tannins | − | + |
| 74 | 8.779 | C21H20O12 | M−H | 463.08939 | 271.025, 151.003, 178.998 | 3.764 | myricitrin | [18] | Flavonoid | + | + |
| 75 | 9.040 | C21H22O11 | M−H | 449.10910 | 151.003, 285.041, 125.023, 107.013, 303.051, 178.998 | 0.379 | astilbin isomer | [18] | Flavonoid | + | + |
| 76 | 9.116 | C15H10O6 | M+H | 287.05499 | 287.055, 213.055, 153.018, 121.029, 241.050 | −1.973 | kaempferol | [22] | Flavonoid | + | + |
| 77 | 9.153 | C15H10O6 | M−H | 285.04068 | 175.039, 133.029, 151.003 | 0.772 | luteolin | [18] | Flavonoid | + | + |
| 78 | 9.235 | C41H30O26 | M−H | 937.09729 | 301.000, 257.010, 635.090, 785.086 | 2.754 | tellimagrandin II | [11] | Hydrolysable Tannins | − | + |
| 79 | 9.630 | C20H18O11 | M−H | 433.07828 | 300.028, 301.036, 271.025, 151.003 | 2.788 | quercetin pentoside isomer | [18] | Flavonoid | + | − |
| 80 | 9.679 | C20H16O12 | M−H | 447.05780 | 315.015 | 1.231 | methyl ellagic acid pentose | [19] | Phenolic Acid | − | + |
| 81 | 9.742 | C23H22O12 | M−H | 489.10464 | 175.039, 169.014, 271.047, 125.023, 313.057, 229.051 | 1.645 | 3’-O-acetylquercitrin isomer | [18] | Flavonoid | + | + |
| 82 | 9.844 | C23H22O12 | M+H | 491.11835 | 153.018, 201.054, 297.060 | −0.128 | trihydroxynaphthalene-O-(O-trihydroxybenzoyl) glucoside | [18] | Quinones | + | + |
| 83 | 9.910 | C21H20O11 | M−H | 447.09402 | 300.028, 301.036, 271.025, 255.030, 151.003, 243.030, 178.998 | 1.666 | quercitrin | [18] | Flavonoid | + | + |
| 84 | 9.960 | C21H22O10 | M−H | 433.11514 | 271.062, 151.003, 119.049, 177.019 | 3.846 | naringenin-7-O-glucoside isomer | [18] | Flavonoid | + | + |
| 85 | 10.131 | C48H32O31 | M−2H | 551.03961 | 301.000, 169.014, 125.023, 275.020 | 1.437 | calamanin A isomer | [18] | Hydrolysable Tannins | − | + |
| 86 | 10.167 | C9H16O4 | M−H | 187.09717 | 125.096, 97.065, 168.888 | −2.202 | azelaic acid | [18] | Other | + | − |
| 87 | 10.239 | C22H22O12 | M−H | 477.10464 | 169.014, 125.024 | 1.687 | isorhamnetin-3-O-glucoside isomer | [18] | Flavonoid | + | − |
| 88 | 10.423 | C21H22O11 | M−H | 449.10995 | 287.057 | 2.282 | eriodictyol-O-hexoside | [20] | Flavonoid | + | + |
| 89 | 10.526 | C26H18O16 | M−H | 585.05341 | 301.000, 433.040 | 2.061 | ellagic acid galloyl pentose | [19] | Hydrolysable Tannins | − | + |
| 90 | 10.676 | C28H24O14 | M−H | 583.11023 | 300.028, 271.025, 255.030, 301.035, 151.003 | 1.560 | quercetin-O-(p-hydroxy)benzoyl-hexoside | [18] | Flavonoid | + | + |
| 91 | 10.904 | C21H20O12 | M−H | 463.08951 | 301.036, 151.003, 178.998 | 2.846 | quercetin-O-glucoside | [20] | Flavonoid | + | + |
| 92 | 10.909 | C25H26O12 | M−H | 517.13623 | 175.039 | 2.109 | dihydroxynaphthol-O-[O-(dimethoxy-hydroxybenzoyl)] glucopyranoside | [18] | Quinones | + | + |
| 93 | 11.014 | C23H20O12 | M+H | 489.10269 | 327.050, 265.049, 237.055, 309.039 | −0.140 | jugnaphthalenoside A | [18] | Quinones | + | + |
| 94 | 11.015 | C20H16O11 | M−H | 431.09912 | 285.041, 255.030, 284.033, 227.035 | 1.742 | kaempferol-rhamnoside | [11] | Flavonoid | + | + |
| 95 | 11.085 | C37H30O16 | M−H | 729.14972 | 125.023, 169.014, 407.078, 289.072 | 4.964 | (epi)catechin-(4,8’)-3’-O-galloyl-(epi)catechin | [18] | Flavonoid | − | + |
| 96 | 11.231 | C16H20O9 | M−H | 355.10428 | 175.040, 134.036, 160.016 | 2.333 | juglanoside D isomer | [18] | Quinones | + | + |
| 97 | 11.312 | C28H35NO13 | M−H | 592.20520 | 241.108, 403.162, 343.140 | 3.741 | glansreginin A | [23] | Other | − | + |
| 98 | 11.812 | C15H12O6 | M−H | 287.05640 | 135.044, 151.003 | 0.998 | eriodictyol | [20] | Flavonoid | + | + |
| 99 | 11.813 | C21H24O10 | M−H | 435.13080 | 167.034, 125.023, 123.044, 273.078, 119.049 | 2.593 | phlorizin | [18] | Other | + | + |
| 100 | 12.186 | C15H20O10 | M−H | 359.09894 | 197.045 | 1.582 | syringic acid hexoside | [24] | Phenolic Acid | + | + |
| 101 | 12.229 | C15H10O7 | M−H | 301.03577 | 151.003, 107.013, 178.998 | 1.317 | quercetin | [23] | Flavonoid | + | + |
| 102 | 13.474 | C15H12O5 | M−H | 271.06180 | 93.033, 177.019, 119.049, 107.013, 151.003 | 2.243 | naringenin | [18] | Flavonoid | + | + |
| 103 | 14.008 | C15H14O5 | M−H | 273.07748 | 151.003, 189.055, 125.024, 167.034, 123.044, 119.049 | 2.359 | phloretin | [18] | Other | + | + |
| 104 | 14.024 | C9H10O2 | M−H | 149.05992 | 149.009, 105.070 | 3.698 | hydrocinnamic acid | Other | + | + | |
| 105 | 14.582 | C10H12O | M+H | 149.09618 | 149.096, 105.070, 79.055, 65.039 | −3.715 | cuminaldehyde | Other | − | + | |
| 106 | 14.630 | C16H12O6 | M−H | 299.05661 | 256.039, 227.035, 284.033 | 1.672 | kaempferide isomer | [18] | Flavonoid | + | + |
| 107 | 17.493 | C20H20O7 | M+H | 373.12814 | 343.081, 183.029, 271.059, 297.075 | −1.492 | tangeritin | Flavonoid | + | + | |
| 108 | 20.835 | C13H11O9 | M−H | 311.25992 | 149.096 | 2.436 | caftaric acid | [20] | Phenolic Acid | + | + |
| 109 | 21.734 | C27H30O16 | M−H | 609.51074 | 255.233, 271.047 | 1.936 | rutin | [17] | Flavonoid | + | + |
| 110 | 23.493 | C10H6O4 | M−H | 189.01877 | 161.024, 117.034 | −2.972 | dihydroxy-naphthoquinone isomer | [18] | Quinones | + | − |
| Classification | Husk | Pellicle |
|---|---|---|
| Hydrolyzable tannins | digalloyl-glucose, ellagic acid galloyl pentose, galloyl methylgalloyl dexoyhexoside isomer, tetragalloyl-glucose, trigalloyl-glucose, calamanin A isomer, digalloyl-HHDP-glucose, ellagic acid hexoside isomer, ellagic acid pentoside isomer, galloyl-bis-HHDP-glucose, galloyl-HHDP-glucose, heterophylliin E isomer, methyl ellagic acid pentose, Tellimagrandin II, trigalloyl-HHDP-glucose, HHDP-glucose isomer, pedunculagin/casuariin isomer (bis-HHDP-glucose) | |
| Flavonoids | quercetin pentoside isomer, taxifolin, taxifolin-3-O-arabinofuranoside isomer | isorhamnetin-3-O-glucoside isomer, (−)-epicatechin 3-O-gallate |
| Phenolic acid | protocatechualdehyde, 7-hydroxy-methylcoumarin | gallic acid hexoside |
| Quinones | dihydroxy-naphthoquinone isomer | |
| Terpenoids | cuminaldehyde, valoneic acid dilactone | |
| Condensed tannins | B-type procyanidin dimer isomer, procyanidin trimer, (epi)catechin-(4, 8’)-3’-O-galloyl-(epi)catechin |
| Husk | Pellicle | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compounds | FBS | FES | HSS | KFS | MS | FES | HSS | KFS | MS |
| Gallic acid | 16.52 ± 0.49 a | 2.88 ± 0.30 c | 2.72 ± 0.08 c | 4.24 ± 0.08 b c | 1.11 ± 0.01 d | 77.08 ± 6.11 d | 214.28 ± 6.62 c | 353.38 ± 12.26 a | 325.78 ± 1.93 b |
| Neochlorogenic acid | 89.09 ± 3.90 a | 34.61 ± 1.25 b | 23.42 ± 1.50 d | 17.34 ± 0.62 c | 13.65 ± 0.58 e | <LOD a | <LOD a | 374.60 ± 20.48 b | 530.92 ± 18.87 a |
| Catechin | 162.37 ± 3.30 a | 93.97 ± 0.99 b | 68.37 ± 0.29 c | 63.30 ± 2.53 c | 54.71 ± 4.50 c | 587.29 ± 47.49 b | 787.23 ± 44.09 a b | 942.33 ± 53.93 a | 302.39 ± 6.83 c |
| p-Hydroxybenzoic acid | 10.44 ± 0.87 a | 0.30 ± 0.01 c | 0.26 ± 0.01 d | 1.01 ± 0.03 b c | 1.10 ± 0.11 b | 152.98 ± 3.36 b | 174.55 ± 7.91 a | 171.54 ± 18.13 a | 195.56 ± 8.64 a |
| Chlorogenic acid | 2.41 ± 0.14 a | 1.61 ± 0.08 b | 1.18 ± 0.01 c | <LOD a | <LOD a | 15.96 ± 1.32 c | 30.25 ± 0.01 b | 41.36 ± 1.29 a | <LOD a |
| Vanillic acid | 30.25 ± 0.12 a | 0.25 ± 0.04 e | 0.57 ± 0.02 d | 0.94 ± 0.08 c | 1.47 ± 0.04 b | <LOD a | 77.09 ± 6.11 a | 47.24 ± 0.60 b | <LOD a |
| Caffeic acid | <LOD a | <LOD a | <LOD a | <LOD a | 0.66 ± 0.01 a | 13.42 ± 0.86 c | 24.83 ± 0.68 b | 60.12 ± 2.51 a | 80.15 ± 2.75 a |
| Epicatechin | <LOD a | <LOD a | <LOD a | <LOD a | 1.31 ± 0.08 a | <LOD a | <LOD a | <LOD a | <LOD a |
| Syringic acid | 8.23 ± 0.05 a | 2.18 ± 0.15 c | 5.46 ± 0.05 b | 1.61 ± 0.09 c | 2.07 ± 0.22 c | 113.94 ± 5.77 c | 154.54 ± 2.94 b | 210.84 ± 9.07 a | 214.35 ± 8.67 a |
| p-Coumaric acid | 0.76 ± 0.06 a | <LOD a | 0.12 ± 0.00 d | 0.16 ± 0.00 c | 0.29 ± 0.00 b | 2.99 ± 0.26 d | 5.42 ± 0.32 c | 7.42 ± 0.17 b | 12.72 ± 0.02 a |
| Ferulic acid | 2.14 ± 0.10 a | 0.37 ± 0.01 c | 0.46 ± 0.04 b c | 0.30 ± 0.03 d | 0.65 ± 0.03 b | 1.49 ± 0.03 c | 4.70 ± 0.06 a | 1.85 ± 0.12 c | 4.24 ± 0.11 b |
| o-Coumaric acid | 0.37 ± 0.01 a | 0.17 ± 0.01 d | 0.29 ± 0.01 c | 0.19 ± 0.00 d | 0.33 ± 0.01 b | 0.52 ± 0.03 c | 1.50 ± 0.05 b c | 1.52 ± 0.10 b | 1.77 ± 0.06 a |
| Rutin | 54.39 ± 2.53 a | 6.21 ± 0.75 b c | 8.33 ± 0.95 b | 1.52 ± 0.36 d | 2.63 ± 0.04 c | 308.37 ± 10.35 d | 1023.80 ± 7.62 c | 1626.42 ± 7.61 b | 3623.17 ± 32.49 a |
| Myricetin | 326.56 ± 5.48 a | 64.16 ± 7.63 b | 61.21 ± 12.95 b | 34.20 ± 5.14 c | 34.98 ± 2.37 c | 3366.58 ± 9.27 c | 3086.85 ± 37.77 d | 4375.62 ± 83.86 b | 8453.82 ± 383.06 a |
| Quercetin | 23.32 ± 0.79 a | 6.02 ± 0.04 c | 9.99 ± 0.44 b | 5.97 ± 0.44 c | 6.48 ± 0.20 c | 24.52 ± 2.14 b | 34.84 ± 0.75 a | 18.04 ± 1.97 c | 4.54 ± 0.07 d |
| Juglone | 15.42 ± 0.46 a | 5.44 ± 0.20 b | 1.88 ± 0.13 c | 0.79 ± 0.01 d | 0.57 ± 0.01 e | 2.13 ± 0.17 a | 1.37 ± 0.04 b | 2.10 ± 0.08 a | 1.78 ± 0.01 a |
| Husk | Pellicle | |||
|---|---|---|---|---|
| Compounds | TPC | TFC | TPC | TFC |
| Gallic acid | 0.962 ** | 0.969 ** | 0.716 | 0.62 |
| Neochlorogenic acid | 0.996 ** | 0.999 ** | 0.889 | 0.871 |
| Catechin | 0.994 ** | 0.991 ** | −0.632 | −0.721 |
| p-Hydroxybenzoic acid | 0.925 * | 0.959 | 0.946 | 0.895 |
| Chlorogenic acid | 0.884 * | 0.849 | −0.571 | −0.675 |
| Vanillic acid | 0.940 * | 0.971 ** | −0.319 | −0.455 |
| Caffeic acid | −0.48 | −0.36 | 0.921 | 0.886 |
| Epicatechin | −0.48 | −0.36 | - | - |
| Syringic acid | 0.83 | 0.842 | 0.805 | 0.729 |
| p−Coumaric acid | 0.783 | 0.847 | 0.994 ** | 0.969 * |
| Ferulic acid | 0.903 * | 0.949 | 0.512 | 0.448 |
| o−Coumaric acid | 0.439 | 0.532 | 0.766 | 0.661 |
| Rutin | 0.968 ** | 0.987 ** | 0.999 ** | 0.983 * |
| Myricetin | 0.975 ** | 0.992 ** | 0.968 * | 0.993 ** |
| Quercetin | 0.933 * | 0.953 * | −0.84 | −0.884 |
| Juglone | 0.990 ** | 0.993 ** | −0.164 | −0.085 |
| TFC | 0.991 ** | 0.989 * | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, F.; Hu, B.; Jin, Q.; Wang, J.; Wu, C.; Luo, Z. The Analysis of Phenolic Compounds in Walnut Husk and Pellicle by UPLC-Q-Orbitrap HRMS and HPLC. Molecules 2021, 26, 3013. https://doi.org/10.3390/molecules26103013
Sheng F, Hu B, Jin Q, Wang J, Wu C, Luo Z. The Analysis of Phenolic Compounds in Walnut Husk and Pellicle by UPLC-Q-Orbitrap HRMS and HPLC. Molecules. 2021; 26(10):3013. https://doi.org/10.3390/molecules26103013
Chicago/Turabian StyleSheng, Fang, Bangyan Hu, Qiang Jin, Jiangbo Wang, Cuiyun Wu, and Zhengrong Luo. 2021. "The Analysis of Phenolic Compounds in Walnut Husk and Pellicle by UPLC-Q-Orbitrap HRMS and HPLC" Molecules 26, no. 10: 3013. https://doi.org/10.3390/molecules26103013
APA StyleSheng, F., Hu, B., Jin, Q., Wang, J., Wu, C., & Luo, Z. (2021). The Analysis of Phenolic Compounds in Walnut Husk and Pellicle by UPLC-Q-Orbitrap HRMS and HPLC. Molecules, 26(10), 3013. https://doi.org/10.3390/molecules26103013







