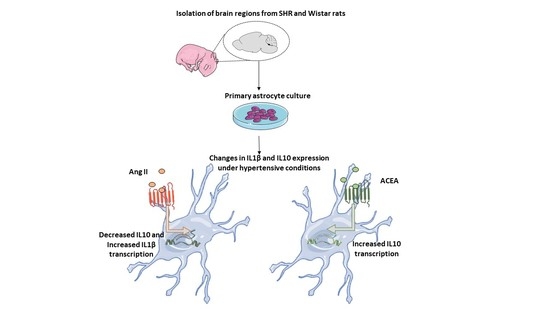
Contrasting Roles of Ang II and ACEA in the Regulation of IL10 and IL1β Gene Expression in Primary SHR Astroglial Cultures
Abstract
1. Introduction
2. Results and Discussion
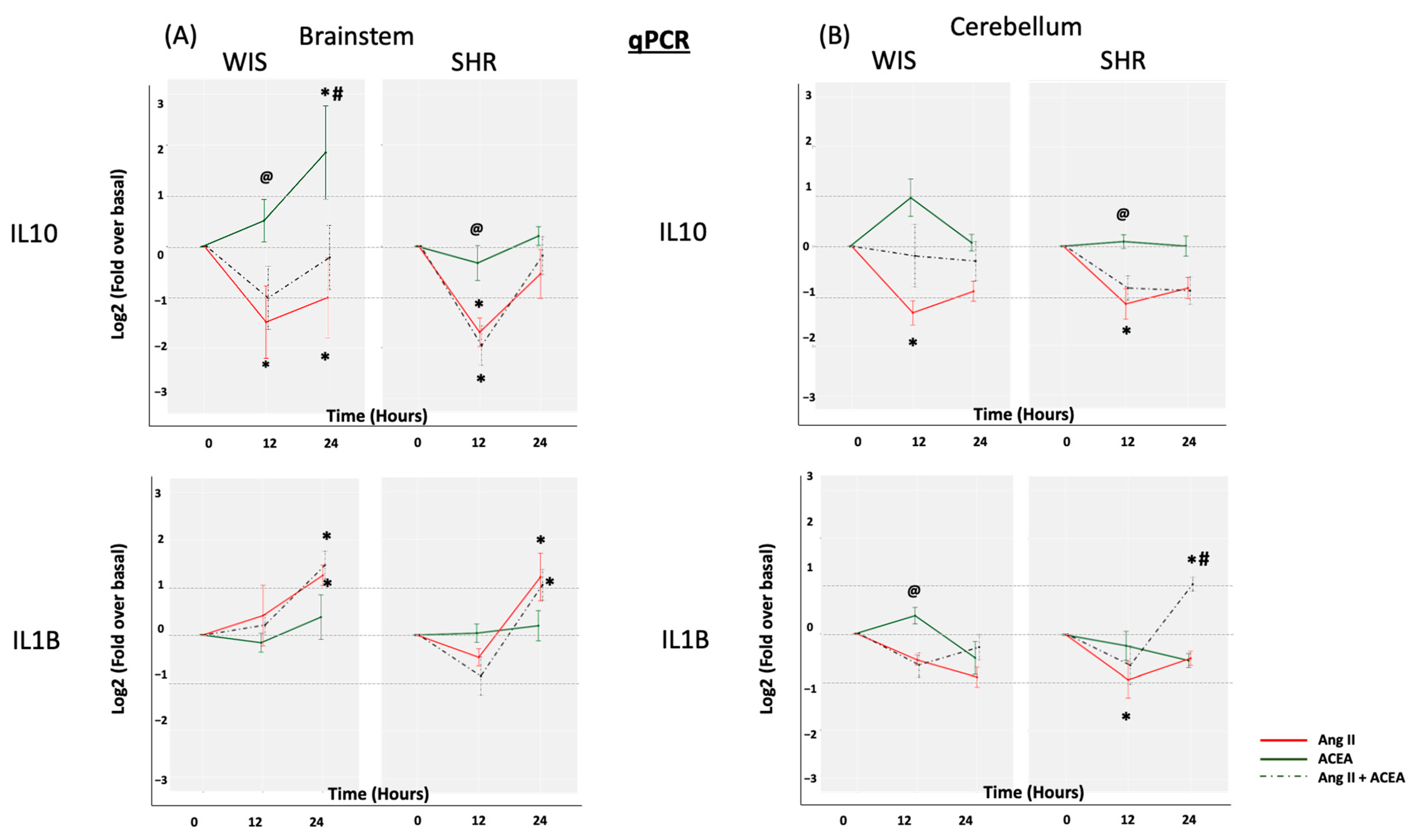
2.1. Elevated Basal IL10 and IL1β mRNA Expression in Brainstem and Cerebellar Astrocytes from SHR When Compared to Wistar Rats
2.2. ACEA-Mediated Increase in IL10 mRNA Expression Is Significantly Greater in Brainstem Astrocytes of Wistar Rats When Compared to SHR
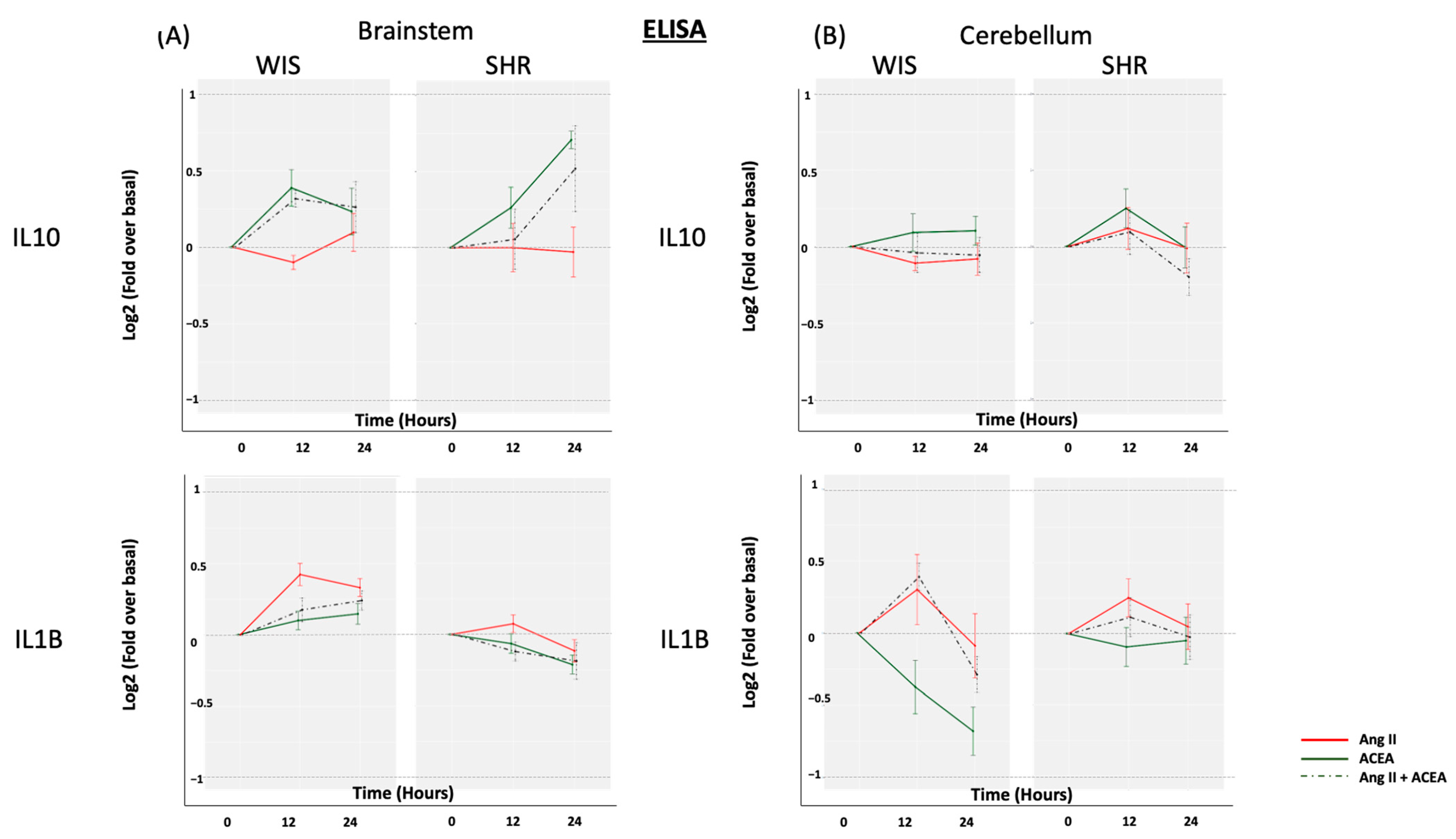
2.3. Neither ACEA nor Ang II Induced Significant Alterations in Secreted IL10 or IL1β from Brainstem and Cerebellar Astrocytes Isolated from Either SHR or Wistar Rats
3. Materials and Methods
3.1. Materials
3.2. Isolation and Culture of Primary Astrocytes
3.3. Cell Treatments
3.4. Total Protein Extraction and Concentration from Conditioned Medium
3.5. Total RNA Extraction and mRNA Expression
3.6. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Doggrell, S.A.; Brown, L. Rat models of hypertension, cardiac hypertrophy and failure. Cardiovasc. Res. 1998, 39, 89–105. [Google Scholar] [CrossRef]
- Haspula, D.; Clark, M.A. Neuroinflammation and sympathetic overactivity: Mechanisms and implications in hypertension. Auton. Neurosci. 2018, 210, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Veerasingham, S.J.; Raizada, M.K. Brain renin-angiotensin system dysfunction in hypertension: Recent advances and perspectives. Br. J. Pharmacol. 2003, 139, 191–202. [Google Scholar] [CrossRef]
- Haspula, D.; Clark, M.A. Molecular Basis of the Brain Renin Angiotensin System in Cardiovascular and Neurologic Disorders: Uncovering a Key Role for the Astroglial Angiotensin Type 1 Receptor AT1R. J. Pharmacol. Exp. Ther. 2018, 366, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Fyhrquist, F.; Metsärinne, K.; Tikkanen, I. Role of angiotensin II in blood pressure regulation and in the pathophysiology of cardiovascular disorders. J. Hum. Hypertens. 1995, 9, 19–24. [Google Scholar]
- Hu, L.; Zhu, D.-N.; Yu, Z.; Wang, J.Q.; Sun, Z.-J.; Yao, T. Expression of angiotensin II type 1 (AT1) receptor in the rostral ventrolateral medulla in rats. J. Appl. Physiol. 2002, 92, 2153–2161. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, T.; Kumagai, H.; Kawai, A.; Onimaru, H.; Imai, M.; Oshima, N.; Sakata, K.; Saruta, T. Rostral ventrolateral medulla neurons of neonatal Wistar-Kyoto and spontaneously hypertensive rats. Hypertension 2002, 40, 560–565. [Google Scholar] [CrossRef]
- Waki, H.; Gouraud, S.S.; Maeda, M.; Paton, J.F. Specific inflammatory condition in nucleus tractus solitarii of the SHR: Novel insight for neurogenic hypertension? Auton. Neurosci. 2008, 142, 25–31. [Google Scholar] [CrossRef]
- Paton, J.F.; Waki, H. Is neurogenic hypertension related to vascular inflammation of the brainstem? Neurosci. Biobehav. Rev. 2009, 33, 89–94. [Google Scholar] [CrossRef]
- Shi, P.; Diez-Freire, C.; Jun, J.Y.; Qi, Y.; Katovich, M.J.; Li, Q.; Sriramula, S.; Francis, J.; Sumners, C.; Raizada, M.K. Brain Microglial Cytokines in Neurogenic Hypertension. Hypertension 2010, 56, 297–303. [Google Scholar] [CrossRef]
- Shi, P.; Raizada, M.K.; Sumners, C. Brain cytokines as neuromodulators in cardiovascular control. Clin. Exp. Pharmacol. Physiol. 2010, 37, e52–e57. [Google Scholar] [CrossRef] [PubMed]
- Winklewski, P.J.; Radkowski, M.; Wszedybyl-Winklewska, M.; Demkow, U. Brain inflammation and hypertension: The chicken or the egg? J. Neuroinflammation 2015, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tayebati, S.K.; Tomassoni, D.; Amenta, F. Neuroinflammatory markers in spontaneously hypertensive rat brain: An immunohistochemical study. CNS Neurol. Disord. Drug Targets 2016, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Gowrisankar, Y.V.; Clark, M.A. Angiotensin II induces interleukin-6 expression in astrocytes: Role of reactive oxygen species and NF-κB. Mol. Cell. Endocrinol. 2016, 437, 130–141. [Google Scholar] [CrossRef]
- Benicky, J.; Sánchez-Lemus, E.; Honda, M.; Pang, T.; Orecna, M.; Wang, J.; Leng, Y.; Chuang, D.-M.; Saavedra, J.M. Angiotensin II AT1 Receptor Blockade Ameliorates Brain Inflammation. Neuropsychopharmacology 2010, 36, 857–870. [Google Scholar] [CrossRef]
- Zangbar, H.S.; Gorji, A.; Ghadiri, T. A Review on the Neurological Manifestations of COVID-19 Infection: A Mechanistic View. Mol. Neurobiol. 2021, 58, 536–549. [Google Scholar] [CrossRef]
- Turu, G.; Simon, A.; Gyombolai, P.; Szidonya, L.; Bagdy, G.; Lenkei, Z.; Hunyady, L. The Role of Diacylglycerol Lipase in Constitutive and Angiotensin AT1 Receptor-stimulated Cannabinoid CB1 Receptor Activity. J. Biol. Chem. 2007, 282, 7753–7757. [Google Scholar] [CrossRef]
- Rozenfeld, R.; Gupta, A.; Gagnidze, K.; Lim, M.P.; Gomes, I.; Lee-Ramos, D.; Nieto, N.; A Devi, L. AT1R-CB1R heteromerization reveals a new mechanism for the pathogenic properties of angiotensin II. EMBO J. 2011, 30, 2350–2363. [Google Scholar] [CrossRef]
- Szekeres, M.; Nádasy, G.L.; Turu, G.; Soltész-Katona, E.; Tóth, Z.E.; Balla, A.; Catt, K.J.; Hunyady, L. Angiotensin II Induces Vascular Endocannabinoid Release, Which Attenuates Its Vasoconstrictor Effect via CB1 Cannabinoid Receptors. J. Biol. Chem. 2012, 287, 31540–31550. [Google Scholar] [CrossRef]
- Haspula, D.; Clark, M.A. Heterologous regulation of the cannabinoid type 1 receptor by angiotensin II in astrocytes of spontaneously hypertensive rats. J. Neurochem. 2016, 139, 523–536. [Google Scholar] [CrossRef]
- Haspula, D.; Clark, M.A. MAPK activation patterns of AT1R and CB1R in SHR versus Wistar astrocytes: Evidence of CB1R hypofunction and crosstalk between AT1R and CB1R. Cell. Signal. 2017, 40, 81–90. [Google Scholar] [CrossRef]
- Molina-Holgado, F.; Pinteaux, E.; Moore, J.D.; Molina-Holgado, E.; Guaza, C.; Gibson, R.M.; Rothwell, N.J. Endogenous in-terleukin-1 receptor antagonist mediates anti-inflammatory and neuroprotective actions of cannabinoids in neurons and glia. J. Neurosci. 2003, 23, 6470–6474. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.S.; Hu, S.; Min, X.; Cabral, G.A.; Lokensgard, J.R.; Peterson, P.K. Synthetic cannabinoid WIN55,212-2 inhibits generation of inflammatory mediators by IL-1β-stimulated human astrocytes. Glia 2004, 49, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Haspula, D.; Clark, M.A. Cannabinoid receptors: An update on cell signaling, pathophysiological roles and therapeutic op-portunities in neurological, cardiovascular, and inflammatory diseases. Int. J. Mol. Sci. 2020, 21, 1–65. [Google Scholar] [CrossRef] [PubMed]
- Paland, N.; Pechkovsky, A.; Aswad, M.; Hamza, H.; Popov, T.; Shahar, E.; Louria-Hayon, I. The Immunopathology of COVID-19 and the Cannabis Paradigm. Front. Immunol. 2021, 12, 631233. [Google Scholar] [CrossRef]
- Agarwal, D.; Welsch, M.A.; Keller, J.N.; Francis, J. Chronic exercise modulates RAS components and improves balance between pro- and anti-inflammatory cytokines in the brain of SHR. Basic Res. Cardiol. 2011, 106, 1069–1085. [Google Scholar] [CrossRef] [PubMed]
- Adriani, W.; Caprioli, A.; Granstrem, O.; Carli, M.; Laviola, G. The spontaneously hypertensive-rat as an animal model of ADHD: Evidence for impulsive and non-impulsive subpopulations. Neurosci. Biobehav. Rev. 2003, 27, 639–651. [Google Scholar] [CrossRef]
- Brozoski, D.T.; Dean, C.; Hopp, F.A.; Hillard, C.J.; Seagard, J.L. Differential endocannabinoid regulation of baroreflex-evoked sympathoinhibition in normotensive versus hypertensive rats. Auton. Neurosci. 2009, 150, 82–93. [Google Scholar] [CrossRef]
- Ma, W.; Lim, W.; Gee, K.; Aucoin, S.; Nandan, D.; Kozlowski, M.; Diaz-Mitoma, F.; Kumar, A. The p38 Mitogen-activated Kinase Pathway Regulates the Human Interleukin-10 Promoter via the Activation of Sp1 Transcription Factor in Lipopolysaccharide-stimulated Human Macrophages. J. Biol. Chem. 2001, 276, 13664–13674. [Google Scholar] [CrossRef]
- Tallant, E.A.; Higson, J.T. Angiotensin II activates distinct signal transduction pathways in astrocytes isolated from neonatal rat brain. Glia 1997, 19, 333–342. [Google Scholar] [CrossRef]
- Chevallet, M.; Diemer, H.; Van Dorssealer, A.; Villiers, C.; Rabilloud, T. Toward a better analysis of secreted proteins: The example of the myeloid cells secretome. Proteomics 2007, 7, 1757–1770. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.S.; Lee, H.J.; Lim, I.; Satoh, J.-I.; Kim, S.U. Human Astrocytes: Secretome Profiles of Cytokines and Chemokines. PLoS ONE 2014, 9, e92325. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, H.A.; Daker, L.I.; Abbass, M.M.; El Fattah, A.A.A. The relationship between the severity of disability and serum IL-8 in acute ischemic stroke patients. Egypt. J. Neurol. Psychiatry Neurosurg. 2018, 54, 1–5. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haspula, D.; Clark, M.A. Contrasting Roles of Ang II and ACEA in the Regulation of IL10 and IL1β Gene Expression in Primary SHR Astroglial Cultures. Molecules 2021, 26, 3012. https://doi.org/10.3390/molecules26103012
Haspula D, Clark MA. Contrasting Roles of Ang II and ACEA in the Regulation of IL10 and IL1β Gene Expression in Primary SHR Astroglial Cultures. Molecules. 2021; 26(10):3012. https://doi.org/10.3390/molecules26103012
Chicago/Turabian StyleHaspula, Dhanush, and Michelle A. Clark. 2021. "Contrasting Roles of Ang II and ACEA in the Regulation of IL10 and IL1β Gene Expression in Primary SHR Astroglial Cultures" Molecules 26, no. 10: 3012. https://doi.org/10.3390/molecules26103012
APA StyleHaspula, D., & Clark, M. A. (2021). Contrasting Roles of Ang II and ACEA in the Regulation of IL10 and IL1β Gene Expression in Primary SHR Astroglial Cultures. Molecules, 26(10), 3012. https://doi.org/10.3390/molecules26103012