Neuroprotective Effect and Antioxidant Potency of Fermented Cultured Wild Ginseng Root Extracts of Panax ginseng C.A. Meyer in Mice
Abstract
1. Introduction
2. Results
2.1. Ginsenoside Content
2.2. In Vitro Experiment
2.2.1. Neuroprotective Effect
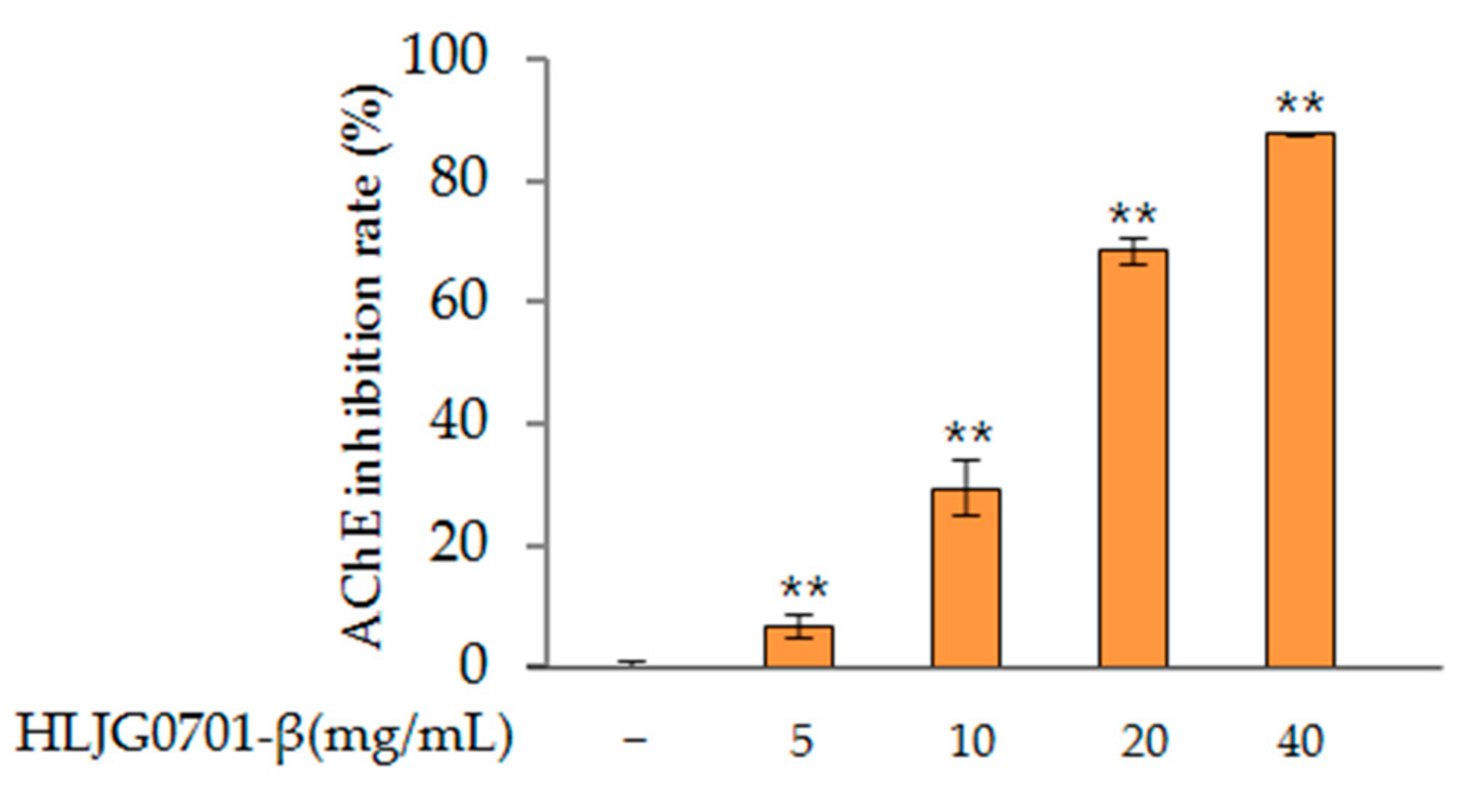
AChE Activity
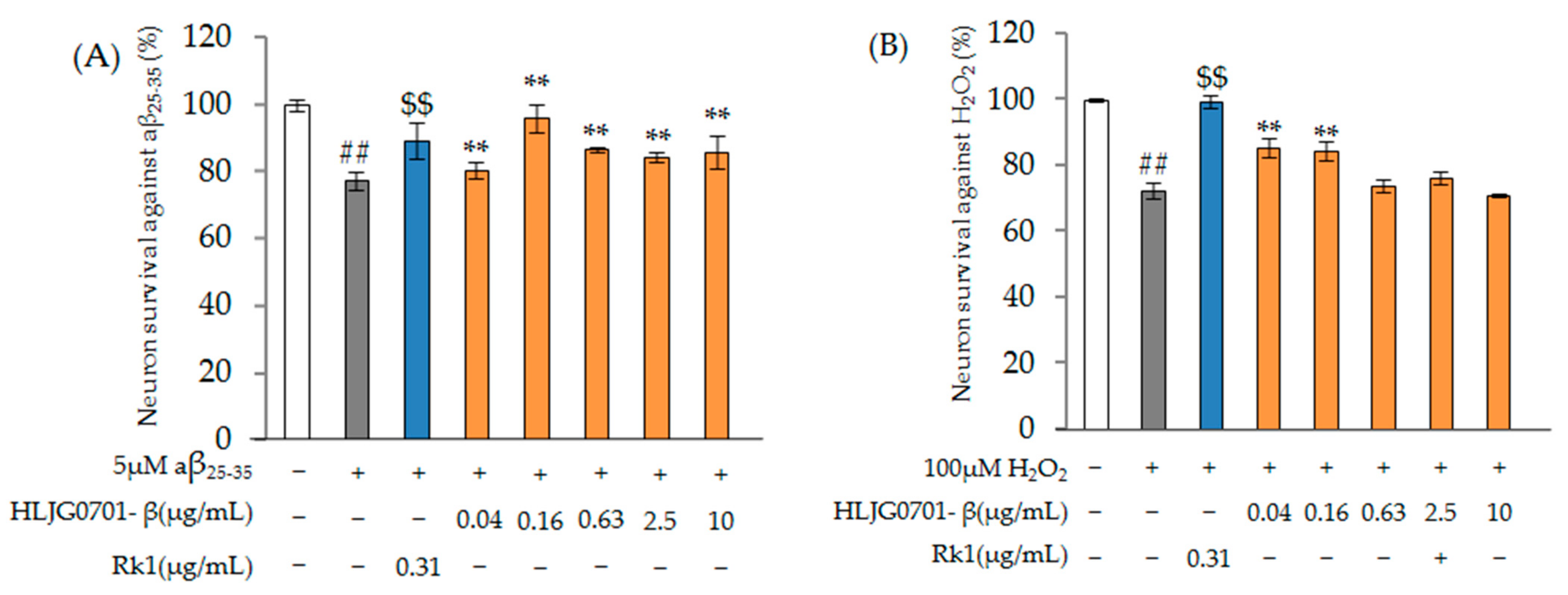
Nerve Cell Damage-Induced Protective Effect
2.2.2. Antioxidant Effect
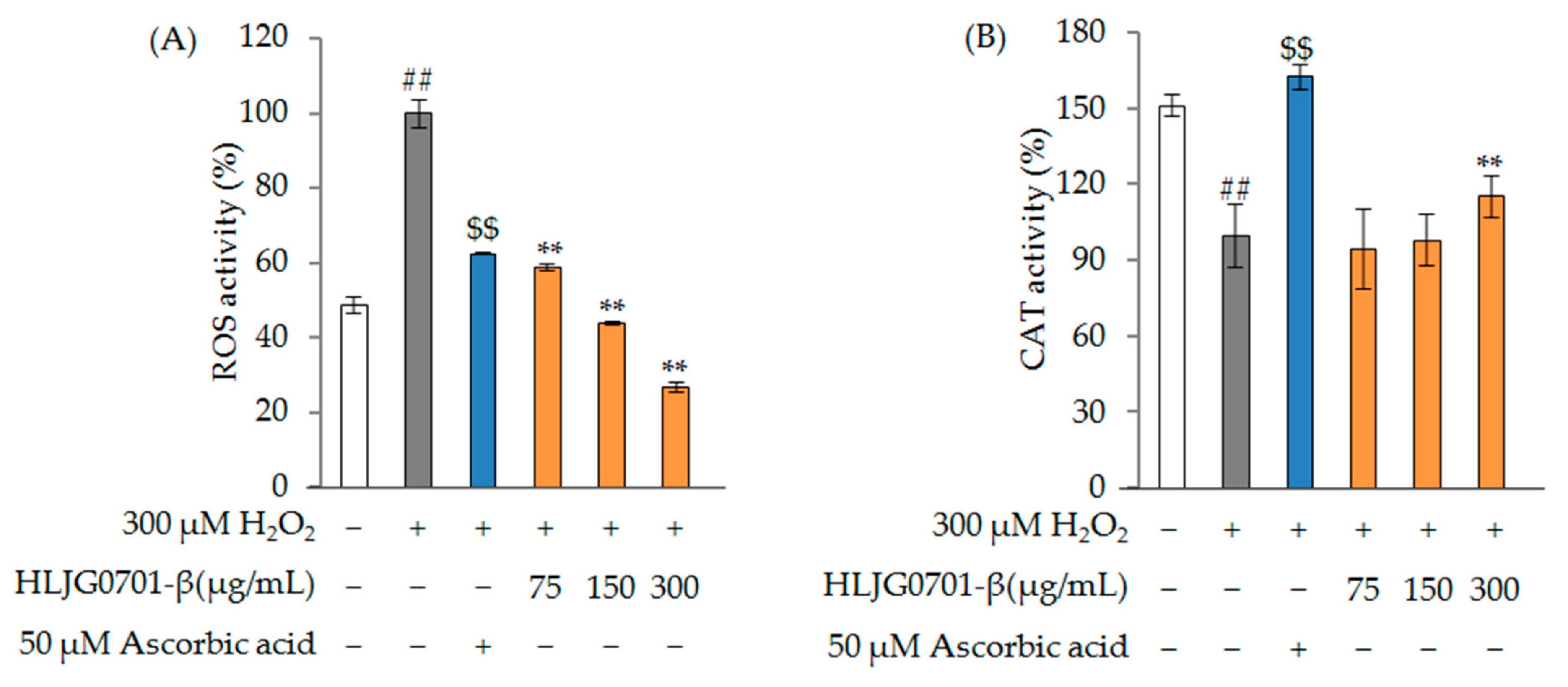
ROS and CAT Activity
2.3. In Vivo Experiment
2.3.1. Scopolamine-Induced Animal Model
Morris Water Maze Task
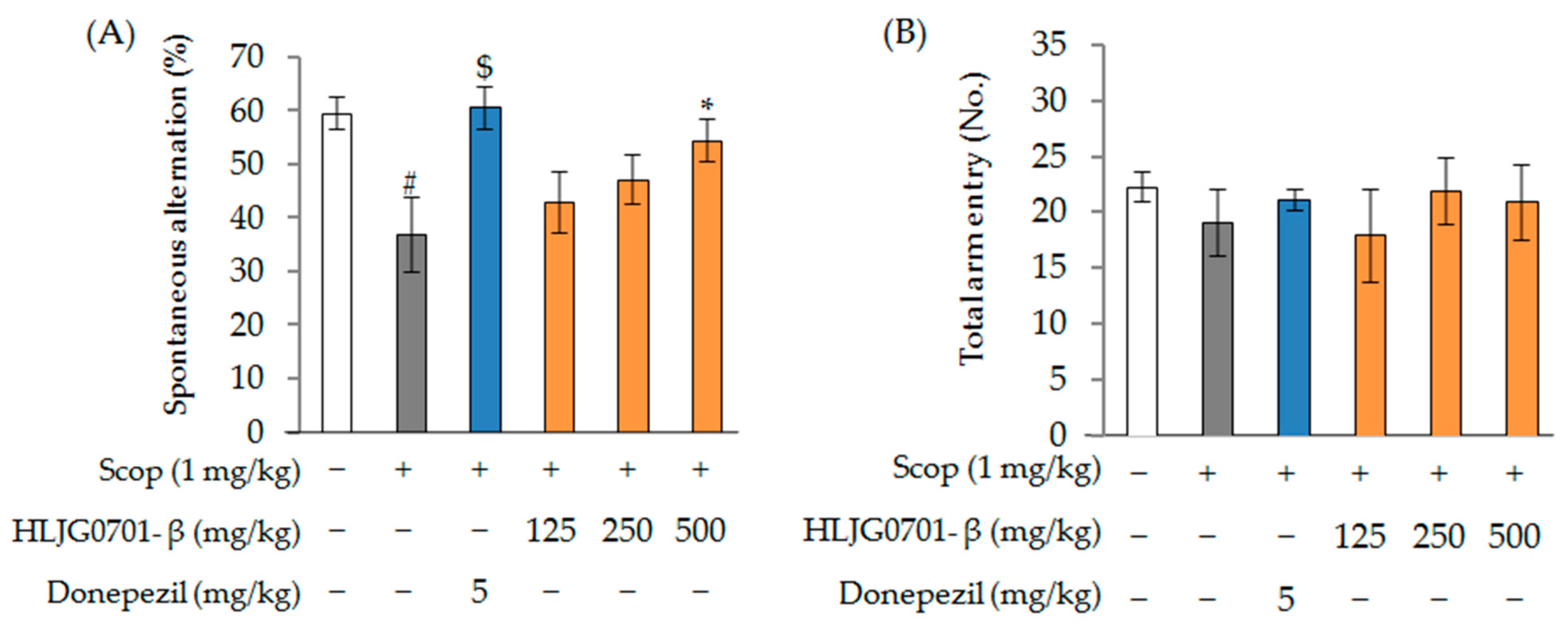
Y-Maze Task
2.3.2. Ovariectomized (OVX) + d-Galactose-Induced Animal Model
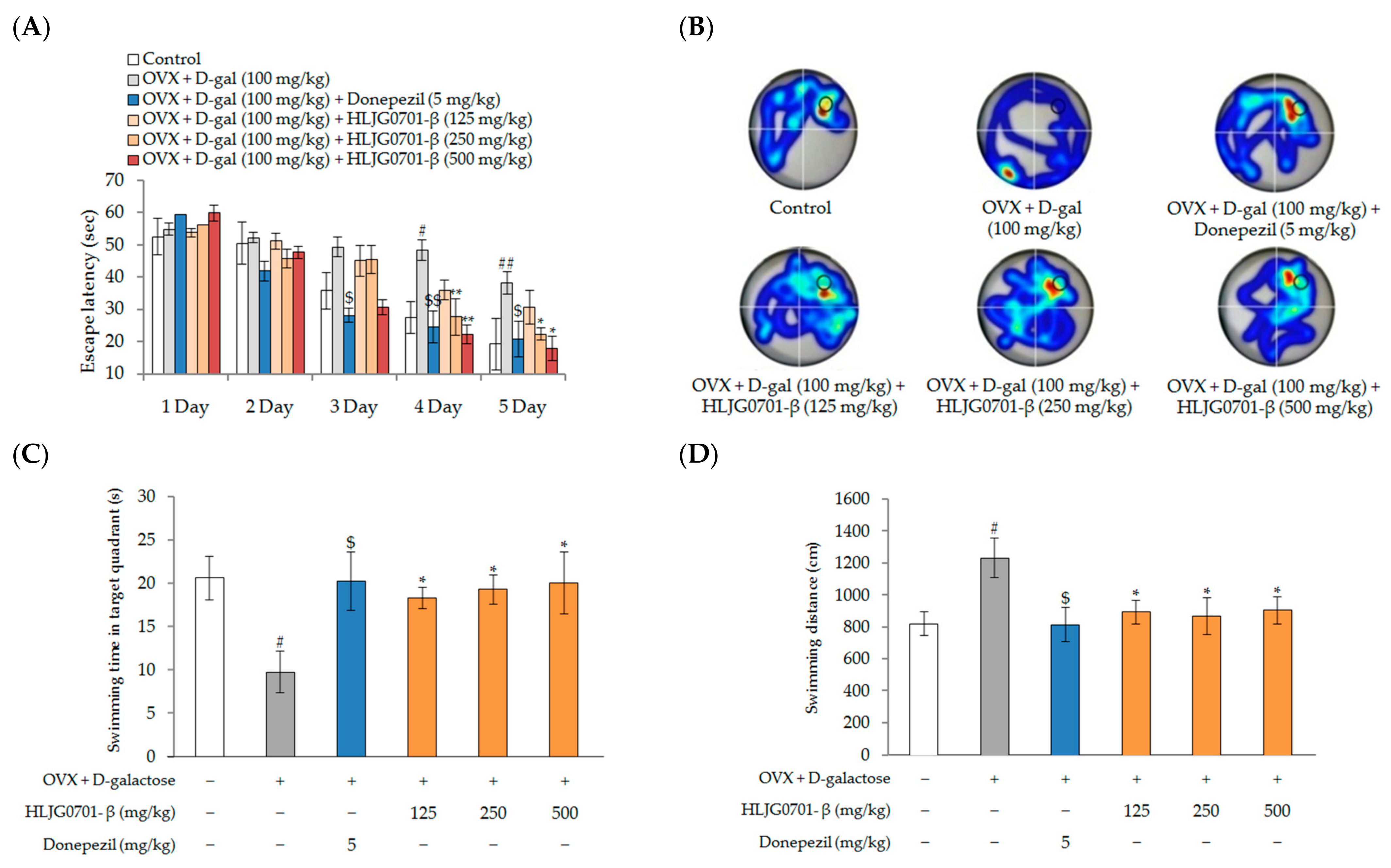
Morris Water Maze Task
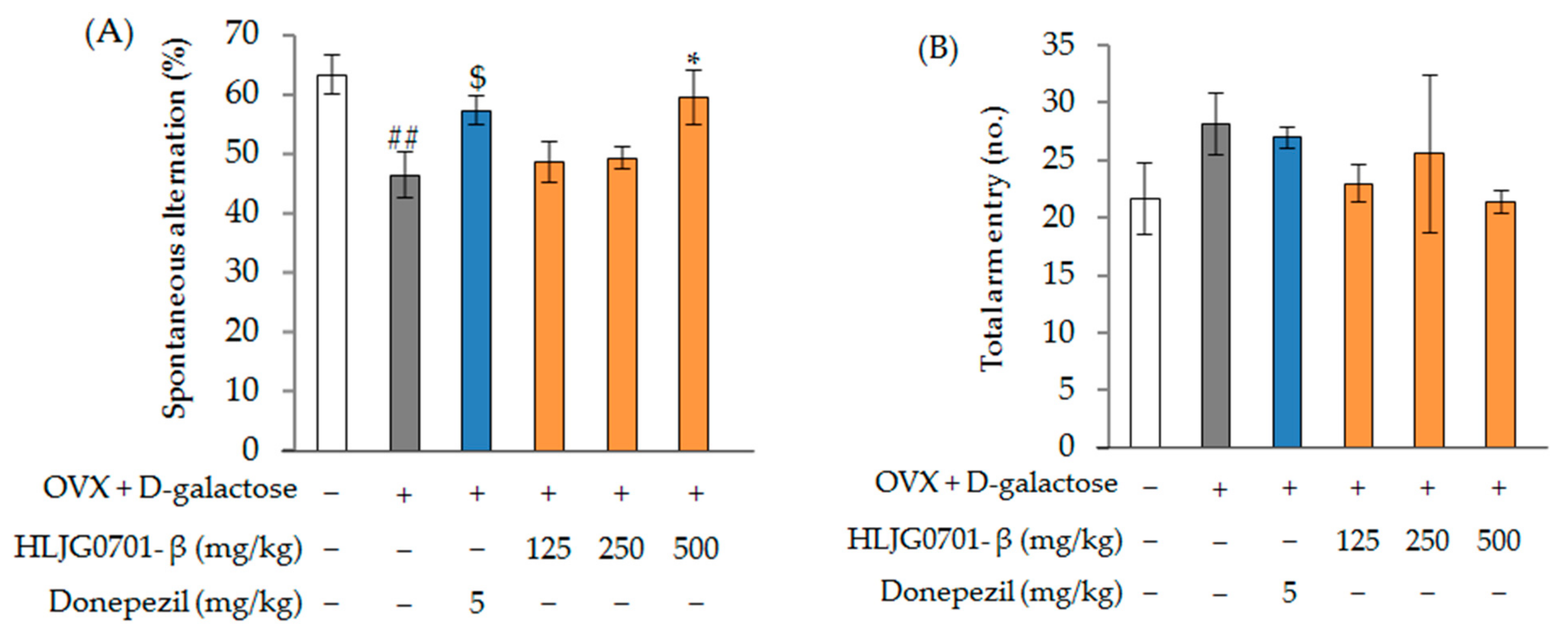
Y-Maze Task
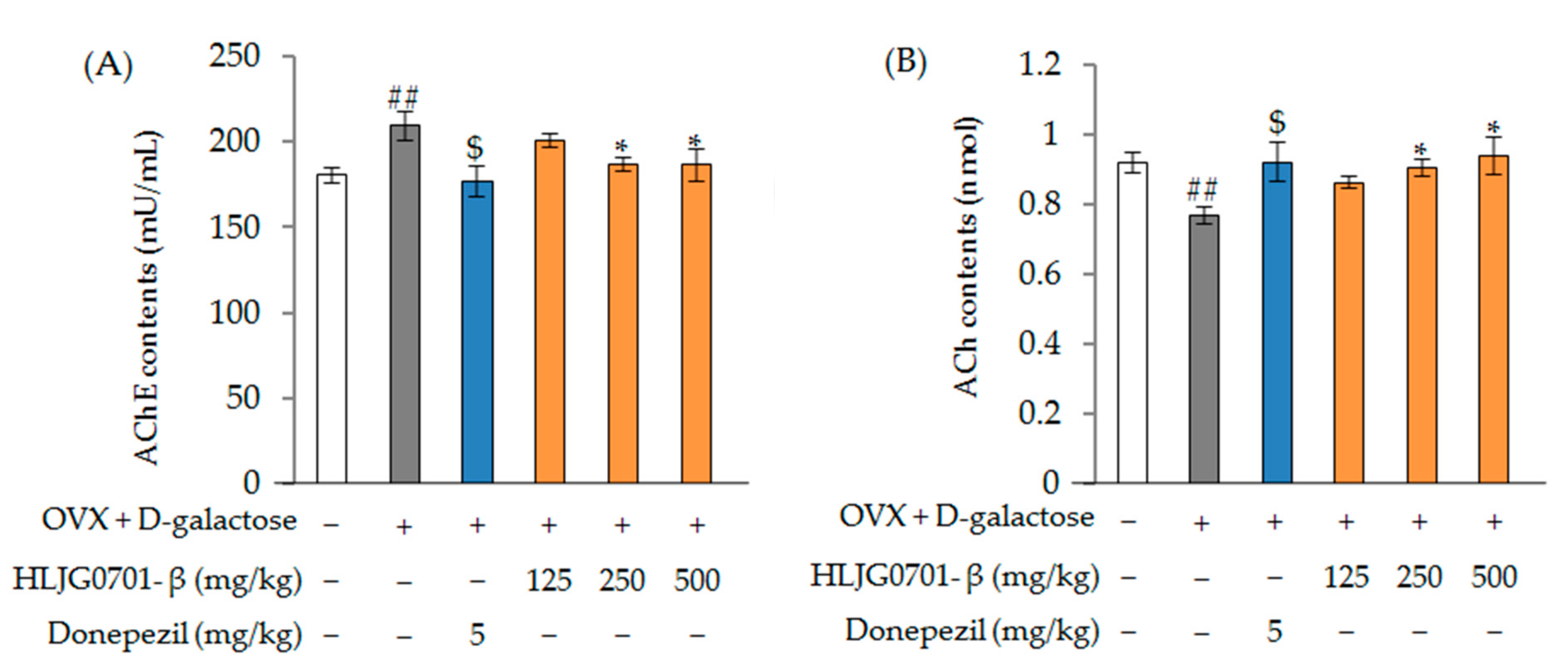
Acetylcholinesterase (AChE) and Acetylcholine (ACh) Contents
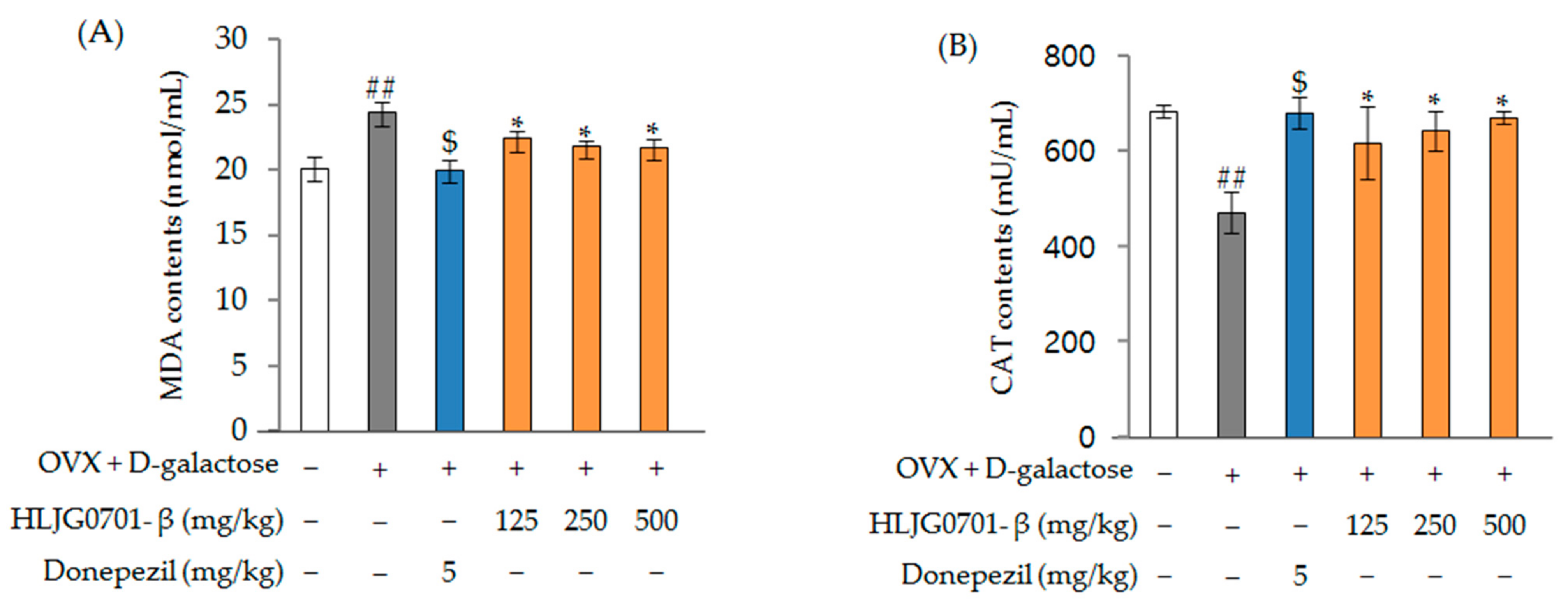
Malondialdehyde (MDA) and Catalase (CAT) Contents
3. Discussion
4. Materials and Methods
4.1. Fermentation Process
4.1.1. Plant and Microbial Materials
4.1.2. Fermentation of Cultured Wild Ginseng Roots
4.2. Analysis of Ginsenosides
4.2.1. Reference Standard, Chemicals and Regents
4.2.2. Sample Preparation and HPLC Analysis
4.3. In Vitro Experiment
4.3.1. Neuroprotective Effect
AChE Activity
Cytotoxicity Assay
Nerve Cell Damage Assay
4.3.2. Antioxidant Effect
ROS and CAT Activity
4.4. In Vivo Experiment
4.4.1. Experimental Animals
4.4.2. Treatments
4.4.3. Morris Water Maze Task
4.4.4. Y-Maze Task
4.4.5. AChE and ACh Contents in Brain Tissue
4.4.6. MDA and CAT Contents in Blood
4.5. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Reitz, C.; Brayne, C.; Mayeux, R. Epidemiology of Alzheimer disease. Nat. Rev. Neurol. 2011, 7, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.M.-Y.; Trojanowski, J.Q. Mechanisms of Parkinson’s Disease linked to pathological α-Synuclein: New targets for drug discovery. Neuron 2006, 52, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Butler, C.; Zeman, A.Z.J. Neurological syndromes which can be mistaken for psychiatric conditions. J. Neuro. Neurol. Psychiatry 2005, 76, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Tönniesa, E.; Trushina, E. Oxidative stress, synaptic dysfunction, and Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef] [PubMed]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef]
- Revett, T.J.; Baker, G.B.; Jhamandas, J.; Kar, S. Glutamate system, amyloid beta peptides and tau protein: Functional interrelationships and relevance to Alzheimer disease pathology. J. Psychiatry Neurosci. 2013, 38, 6–23. [Google Scholar] [CrossRef]
- Fiers, W.; Beyaert, R.; Declercq, W.; Vandenabeele, P. More than one way to die: Apoptosis, necrosis and reactive oxygen damage. Oncogene 1999, 18, 7719–7730. [Google Scholar] [CrossRef]
- Hasselmo, M.E. The role of acetylcholine in learning and memory. Curr. Opin. Neurobiol. 2006, 16, 710–715. [Google Scholar] [CrossRef]
- Musial, A.; Bajda, M.; Malawska, B.; Wieckowska, A. Recent developments in cholinesterases inhibitors for Alzheimer’s disease treatment. Curr. Med. Chem. 2007, 14, 2654–2679. [Google Scholar] [CrossRef]
- Parsons, C.G.; Danysz, W.; Dekundy, A.; Pulte, I. Memantine and cholinesterase inhibitors: Complementary mechanisms in the treatment of Alzheimer’s Disease. Neurotox. Res. 2013, 24, 358–369. [Google Scholar] [CrossRef]
- Čolović, M.B.; Krstić, D.Z.; Lazarević-Pašti, T.D.; Bondžić, A.M.; Vasić, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neurol. 2013, 11, 315–335. [Google Scholar]
- Ferreira-Vieira, T.H.; Gumaraes, I.M.; Silve, F.R.; Ribeiro, F.M. Alzheimer’s Disease: Targeting the cholinergic system. Curr. Neurophamacol. 2016, 14, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Hansen, R.; Gartlehner, G.; Webb, A.P.; Morgan, L.C.; Moore, C.G.; Jonas, D.E. Efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer’s disease: A systematic review and meta-analysis. Clin. Interv. Aging 2008, 3, 211–225. [Google Scholar] [PubMed]
- Mabberley, D.J. The Plant-Book: A Portable Dictionary of the Higher Plants; Cambridge University Press: Cambridge, UK, 1987; p. 706. [Google Scholar]
- Choi, H.K.; Wen, J. A phylogenetic analysis of Panax (Araliaceae): Integrating cpDNA restriction site and nuclear rDNA ITS sequence data. Plant Syst. Evol. 2000, 224, 109–120. [Google Scholar] [CrossRef]
- Nam, K.Y. Clinical applications and efficacy of Korean ginseng (Panax ginseng C.A. Meyer). J. Ginseng Res. 2002, 26, 111–131. [Google Scholar]
- Chang, Y.S.; Chang, Y.H.; Sung, J.H. The effect of ginseng and caffeine products on the antioxidative activities of mouse kidney. J. Ginseng Res. 2006, 30, 15–21. [Google Scholar]
- Kim, M.J.; Jung, N.P. Effect of ginseng saponin on the mouse immune system. J. Ginseng Res. 1987, 20, 130–235. [Google Scholar]
- Shim, M.; Lee, Y. Ginseng as a complementary and alternative medicine for postmenopausal symptoms. J. Ginseng Res. 2009, 33, 89–92. [Google Scholar]
- Tang, W.; Eisenbrand, G. Chinese Drugs of Plant. Origin. In 91 Panax Ginseng C. A. May; Springer: Berlin/Heidelberg, Germany, 1992; pp. 711–737. [Google Scholar]
- Shao, Z.H.; Xie, J.T.; Hoek, T.L.V.; Mehendale, S.; Aung, H.; Li, C.Q.; Qin, Y.; Schumacker, P.T.; Becker, L.B.; Yuan, C.S. Antioxidant effects of American ginseng berry extract in cardiomyocytes exposed to acute oxidant stress. Biochim. Biophys. Acta 2004, 1670, 165–171. [Google Scholar] [CrossRef]
- Xie, J.T.; Shao, Z.H.; Hoek, T.L.V.; Chang, W.T.; Li, J.; Mehendale, S.; Wang, C.Z.; Hsu, C.W.; Becker, L.B.; Yin, J.J.; et al. Antioxidant effects of ginsenoside Re in cardiomyocytes. Eur. J. Pharmacol. 2006, 532, 201–207. [Google Scholar] [CrossRef]
- Kim, C.K.; Cho, D.H.; Lee, K.S.; Lee, D.K.; Park, C.W.; Kim, W.G.; Lee, S.J.; Ha, K.S.; Goo, T.O.; Kwon, Y.G.; et al. Ginseng berry extract prevents atherogenesis via anti-inflammatory action by upregulating phase II gene expression. Evid. Based Complementary Altern. Med. 2012, 2012, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.S.; Seo, E.K.; Gyllenhaal, C.; Block, K.I. Panax ginseng: A role in cancer therapy? Integr. Cancer Sci. Ther. 2003, 2, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, P.; Ko, H.M.; Kim, I.S.; Choi, D.K. Recent trends of nano bioactive compounds from ginseng for its possible preventive role in chronic disease models. RSC Adv. 2015, 5, 98634–98642. [Google Scholar] [CrossRef]
- Chiou, W.F.; Zhang, J.T. Comparison of the pharmacological effects of Panax ginseng and Panax quinquefolium. Acta Pharmacol. Sin. 2008, 29, 1103–1108. [Google Scholar]
- Cheng, Z.K.; Zhang, M.; Ling, C.G.; Zhu, Y.; Ren, H.W.; Hong, C.; Qin, J.; Liu, T.X.; Wang, J.X. Neuroprotective effects of ginsenosides against cerebral ischemia. Molecules 2019, 24, 1102. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Yang, W.; Gao, S.; Lin, J.; Wang, T.; Zhou, K.; Hu, H. Ginsenoside Rb1 inhibit apoptosis in rat model of Alzheimer’s disease induced by Aβ1-40. Am. J. Transl. Res. 2018, 10, 796–805. [Google Scholar]
- Shin, S.J.; Jeon, S.G.; Kim, J.; Jeong, Y.; Kim, S.; Park, Y.H.; Lee, S.K.; Park, H.H.; Hong, S.B.; Oh, S.; et al. Red ginseng attenuates Aβ-Induced mitochondrial dysfunction and Aβ-mediated pathology in an animal model of Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 3030. [Google Scholar] [CrossRef] [PubMed]
- Proctor, J.T.A. Ginseng: Old crop, new directions. In Progress New Crops; Janick, J., Ed.; ASHS Press: Arlington, VA, USA, 1996; pp. 565–577. [Google Scholar]
- Fowler, M.W. Problems in commercial exploitation of plant tissue cultures. In Primary and Secondary Metabolism of Plant Cell Cultures; Neumann, K.H., Barz, W., Reinhardt, E., Eds.; Springer: Berlin/Heidelberg, Germany, 1985; pp. 362–378. [Google Scholar]
- Wu, H.C.; Du Toit, E.S.; Reinhardt, C.F.; Rimando, A.M.; Van der Kooy, F.; Meyer, J.J.M. The phenolic, 3,4-dihydroxybenzoic acid, is an endogenous regulator of rooting in Protea cynaroides. Plant Growth Regul. 2007, 52, 207–215. [Google Scholar] [CrossRef]
- Efferth, T. Biotechnology applications of plant callus cultures. Engineering 2019, 5, 50–59. [Google Scholar] [CrossRef]
- Song, S.W.; Yang, D.C.; Choung, S.Y. Acute oral toxicity of adventitous roots extract derived from wild ginseng in beagle dogs. Toxicol. Res. 2005, 21, 51–55. [Google Scholar]
- Zhou, S.S.; Xu, J.; Zhu, H.; Wu, J.; Xu, J.D.; Yan, R.; Li, X.Y.; Liu, H.H.; Duan, S.M.; Wang, Z.; et al. Gut microbiota-involved mechanisms in enhancing systemic exposure of ginsenosides by coexisting polysaccharides in ginseng decoction. Sci. Rep. 2016, 6, 22474. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.F.; Gao, Y.; Xu, S.Y.; Liu, H.; Xue, X.; Zhang, Y.; Zhang, H.; Liu, M.N.; Xiong, H.; Lin, R.C.; et al. Remarkable impact of steam temperature on ginsenosides transformation from fresh ginseng to red ginseng. J. Ginseng Res. 2018, 42, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Kim, Y.; Kim, M.G. Changes in the ginsenoside content during the fermentation process using microbial strains. J. Ginseng Res. 2015, 39, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H. Gut microbiota-mediated pharmacokinetics of ginseng saponins. J. Ginseng Res. 2018, 42, 255–263. [Google Scholar] [CrossRef]
- Lee, K.H.; Bae, I.Y.; Park, S.I.; Park, J.-D.; Lee, H.G. Antihypertensive effect of Korean Red Ginseng by enrichment of ginsenoside Rg3 and arginine–fructose. J. Ginseng Res. 2016, 40, 237–244. [Google Scholar] [CrossRef][Green Version]
- Park, E.H.; Kim, Y.J.; Yamabe, N.; Park, S.H.; Kim, H.K.; Jang, H.J.; Kim, J.H.; Cheon, G.J.; Ham, J.; Kang, K.S. Stereo specific anticancer effects of ginsenoside Rg3 epimers isolated from heat-processed American ginseng on human gastric cancer cell. J. Ginseng Res. 2014, 38, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Hao, J.; Zhang, J.; Xia, W.; Dong, X.; Hu, X.; Kong, F.; Cui, X. Ginsenoside Rg3 promotes beta-amyloid peptide degradation by enhancing gene expression of neprilysin. J. Med. Food 2009, 61, 375–380. [Google Scholar] [CrossRef]
- Elshafay, A.; Tinh, N.X.; Salman, S.; Shahee, Y.S.; Othman, E.B. Ginsenoside Rk1 bioactivity: A systematic review. PeerJ 2017, 5, e3993. [Google Scholar] [CrossRef]
- Shenghui, C.; Junfei, G.; Liang, F.; Jiping, L.; Minghua, Z.; Xiaobin, J.; Min, L.; Danian, Y. Ginsenoside Rg5 improves cognitive dysfunction and beta-amyloid deposition in STZ-induced memory impaired rats via attenuating neuroinflammatory responses. Int. Immunopharmacol. 2014, 19, 317–326. [Google Scholar]
- Siddiqi, M.H.; Siddiqi, M.Z.; Ahn, S.; Kang, S.; Kim, Y.J.; Veerappan, K.; Yang, D.U.; Yang, D.C. Stimulative effect of ginsenosides Rg5:Rk1 on murine osteoblastic MC3T3-E1 Cells. Phytother. Res. 2014, 28, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Si, M.; Wang, Y.; Liu, L.; Zhang, Y.; Zhou, A.; Wei, W. Ginsenoside metabolite compound K exerts anti-inflammatory and analgesic effects via down regulating COX2. Inflammopharmacology 2018, 27, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Liu, W.J.; Wen, M.L.; Liang, H.; Wu, S.M.; Zhu, Y.Z.; Zhao, J.Y.; Dong, X.Q.; Li, M.G.; Bian, L.; et al. Ameliorative effects of Compound K and ginsenoside Rh1 on non-alcoholic fatty liver disease in rats. Sci. Rep. 2017, 7, 41144. [Google Scholar] [CrossRef] [PubMed]
- Bednarek, P.T.; Orłowska, R.; Koebner, R.M.D.; Zimny, J. Quantification of the tissue-culture induced variation in barley (Hordeum vulgare L.). BMC Plant Biol. 2007, 7, 10. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cheng, J.; Yu, K.; Zhang, M.; Shi, Y.; Duan, C.; Wang, J. The effect of light intensity on the expression of Leucoanthocyanidin reductase in grapevine calluses and analysis of its promoter activity. Gene 2020, 11, 1156. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- An, K.S.; Choi, Y.O.; Lee, S.M.; Ryu, H.Y.; Kang, S.J.; Yeon, Y.; Kim, Y.R.; Lee, J.G.; Kim, C.J.; Lee, Y.J.; et al. Ginsenosides Rg5 and Rk1 enriched cultured wild ginseng root extract bioconversion of Pediococcus pentosaceus HLJG0702: Effect on scopolamine-induced memory dysfunction in mice. Nutrients 2019, 11, 1120. [Google Scholar] [CrossRef] [PubMed]
- Park, J.D. Recent studies on the chemicals of Korean ginseng (Panax ginseng C. A. Meyer). J. Ginseng Res. 1996, 20, 389–415. [Google Scholar]
- Lee, J.G.; Seong, E.S.; Goh, E.J.; Kim, N.Y.; Yu, C.Y. Factors involved in mass propagation of ginseng (Panax ginseng C. A. Meyer) using bioreactor system. Appl. Biol. Chem. 2009, 52, 466–471. [Google Scholar]
- Mohanan, P.; Subramaniyam, S.; Mathiyalagan, R.; Yang, D.C. Molecular signaling of ginsenosides Rb1, Rg1, and Rg3 and their mode of actions. J. Ginseng Res. 2018, 42, 123–132. [Google Scholar] [CrossRef]
- Radad, K.; Moldzio, R.; Rausch, W.D. Ginsenosides and Their CNS Targets. CNS Neurosci. Ther. 2010, 17, 761–768. [Google Scholar] [CrossRef]
- Drachman, D.A. Human memory and the cholinergic system. Arch. Neurol. 1974, 30, 113. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Kim, S.Y.; Oh, Y.S.; Kim, Y.M.; Chin, Y.W.; Cho, J.G. Inhibition of oxidative neurotoxicity and scopolamine-induced memory impairment by γ-mangostin: In vitro and in vivo evidence. Oxidative Med. Cell. Longev. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Ibach, B.; Haen, E. Acetylcholinesterase inhibition in Alzheimers Disease. Curr. Pharm. Des. 2004, 10, 231–251. [Google Scholar] [CrossRef]
- De La Garza, V.W. Pharmacologic treatment of Alzheimer’s disease: An update. Am. Fam. Physician 2003, 68, 1365–1372. [Google Scholar] [PubMed]
- Coyle, J.T.; Price, D.L.; DeLong, M.R. Alzheimer’s disease: A disorder of cortical cholinergic innervation. Science 1983, 219, 1184–1190. [Google Scholar] [CrossRef]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Jung, I.H.; Kim, V.L.T.; Jeong, J.J.; Kim, N.J.; Kim, D.H. Ginsenosides Rg5 and Rh3 protect scopolamine-induced memory deficits in mice. J. Ethnopharmacol. 2013, 146, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.P.; Faraoni, M.B.; Castro, M.J.; Alza, N.P.; Cavallaroa, V. Natural AChE inhibitors from plants and their contribution to Alzheimer’s Disease therapy. Curr. Neuropharmacol. 2013, 11, 388–413. [Google Scholar] [CrossRef] [PubMed]
- Pena, I.D.; Yoon, S.Y.; Kim, H.J.; Park, S.; Hong, E.Y.; Ryu, J.H.; Park, I.H.; Cheong, J.H. Effects of ginseol k-g3, an Rg3-enriched fraction, on scopolamine-induced memory impairment and learning deficit in mice. J. Ginseng Res. 2014, 38, 1–7. [Google Scholar] [CrossRef]
- Benishin, C.G. Actions of ginsenoside Rb1 on choline uptake in central cholinergic nerve endings. Neurochem. Int. 1992, 21, 1–5. [Google Scholar] [CrossRef]
- Benishin, C.G.; Lee, R.L.; Wang, C.; Liu, H.J. Effects of ginsenoside Rb1 on central cholinergic metabolism. Parmacology 1991, 42, 223–229. [Google Scholar]
- Zhang, J.T.; Qu, Z.W.; Liu, Y.; Deng, H.L. Preliminary study on antiamnestic mechanism of ginsenoside Rg1 and Rb1. Chin. Med. J. 1990, 183, 1460–1461. [Google Scholar] [CrossRef]
- Salim, K.N.; McEwen, B.S.; Chao, H.M. Ginsenoside Rb1regulates ChAT, NGF and trkA mRNA expression in the rat brain. Mol. Brain Res. 1997, 47, 177–182. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Q.; Pei, X. Long-term ginsenoside administration prevents memory impairment in aged C57BL/6J mice by up-regulating the synaptic plasticity-related proteins in hippocampus. Behav. Brain Res. 2009, 201, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, Q.; Zhang, Z.; Pei, X.; Wang, J.; Li, Y. Long-term ginsenoside consumption prevents memory loss in agedSAMP8 mice by decreasing oxidative stress and up-regulating the plasticity-related proteins in hippocampus. Brain Res. 2009, 1256, 111–122. [Google Scholar] [CrossRef]
- Kim, J.; Shim, J.; Lee, S.; Cho, W.-H.; Hong, E.; Lee, J.H.; Han, J.-S.; Lee, H.J.; Lee, K.W. Rg3-enriched ginseng extract ameliorates scopolamine-induced learning deficits in mice. BMC Complementary Altern. Med. 2016, 16, 66. [Google Scholar] [CrossRef]
- Bao, H.Y.; Zhang, J.; Yeo, S.J. Memory enhancing and neuroprotective effects of selected ginsenosides. Arch. Pharmacal Res. 2005, 28, 335–342. [Google Scholar] [CrossRef]
- Lee, M.R.; Yun, B.S.; Liu, L.; Zhang, D.L.; Wang, Z.; Wang, C.L.; Gu, L.J.; Wang, C.Y.; Mo, E.K.; Sung, C.K. Effect of black ginseng on memory improvement in the amnesic mice induced by scopolamine. J. Ginseng Res. 2010, 34, 51–58. [Google Scholar] [CrossRef]
- Fang, F.; Chen, X.; Huang, T.; Lue, L.F.; Luddy, J.S.; Yan, S.S. Multi-faced neuroprotective effects of Ginsenoside Rg1in an Alzheimer mouse model. Biochim. Biophys. Acta 2012, 1822, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Shin, E.J.; Shin, T.J. Gintonin, a ginseng derived lysophosphatidic acid receptor ligand, attenuates Alzheimer’s disease-related neuropathies: Involvement of non-amyloidogenic processing. J. Alzheimer’s Dis. 2012, 31, 207–223. [Google Scholar] [CrossRef]
- Karpagam, V.; Sathishkumar, N.; Sathiyamoorthy, S. Identification of BACE1 inhibitors from Panax ginseng saponins-An Insilco approach. Comput. Biol. Med. 2013, 43, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.H.; Ma, J.; Liu, H.P.; Wang, R.R.; Luo, J. The neuroprotective effects of ginsenosides on calcineurin activity and tau phosphorylation in SY5Y cells. Cell. Mol. Neurobiol. 2009, 29, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, J.; Yan, X.; Qin, K.; Shi, M.; Lin, T.; Zhu, Y.; Kang, T.; Zhao, G. Protective effects of ginsenoside Rd against okadaic acid-induced neurotoxicity in vivo and in vitro. J. Ethnopharmacol. 2011, 138, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Yu, J.M.; Kim, H.J.; Kim, H.B.; Kim, S.T.; Choi, Y.W.; Lee, D.I.; Joo, S.S. Ginsenoside Re and Rd enhance the expression of cholinergic markers and neuronal differentiation in Neuro-2a cells. Biol. Pharm. Bull. 2014, 37, 826–833. [Google Scholar] [CrossRef]
- Shi, C.; Zheng, D.D.; Fang, L.; Wu, F.; Kwong, W.H.; Xu, J. Ginsenoside Rg1 promotes nonamyloidgenic cleavage of APP via estrogen receptor signaling to MAPK/ERK and PI3K/Akt. Biochim. Biophys. Acta 2012, 1820, 453–460. [Google Scholar] [CrossRef]
- Chen, X.C.; Zhou, Y.C.; Chen, Y.; Zhu, Y.G.; Fang, F.; Chen, L.M. Ginsenoside Rg1 reduces MPTP-induced substantia nigra neuron loss by suppressing oxidative stress. Acta Pharmacol. Sin. 2005, 26, 56–62. [Google Scholar] [CrossRef]
- Radad, K.; Gille, G.; Moldzio, R.; Saito, H.; Ishige, K.; Rausch, W.D. Ginsenosides Rb1 and Rg1 effects on survival and neurite growth of MPP +-affected mesencephalic dopaminergic cells. J. Neural Transm. 2004, 111, 37–45. [Google Scholar] [CrossRef]
- Chen, X.C.; Chen, Y.; Zhu, Y.G.; Fang, F.; Chen, L.M. Protective effect of ginsenoside Rg1 against MPTP-induced apoptosis in mouse substantia nigra neurons. Acta Pharmacol. Sin. 2002, 23, 829–834. [Google Scholar]
- VanKampen, J.M.; Robertson, H.; Hagg, T.; Drobitch, R. Neuroprotective actions of the ginseng extract G115 in two rodent models of Parkinson’s disease. Exp. Neurol. 2003, 184, 521–529. [Google Scholar] [CrossRef]
- VanKampen, J.M.; Baranowski, D.B.; Shaw, C.A.; Kay, D.G. Panax ginseng is neuroprotective in a novel progressive model of Parkinson’s disease. Exp. Gerontol. 2014, 50, 95–105. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Podgórska, A.; Burian, M.; Szal, B. Extra-cellular but extra-ordinarily important for cells: Apoplastic reactive oxygen species metabolism. Front. Plant Sci. 2017, 8, 1353. [Google Scholar] [CrossRef] [PubMed]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of catalase in oxidative stress- and age-associated degenerative diseases. Oxidative Med. Cell. Longev. 2019, 2019, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Li, X.; Cheng, J.; Hou, L. Drug development for Alzheimer’s Disease: Microglia induced neuroinflammation as a target? Int. J. Mol. Sci. 2019, 20, 588. [Google Scholar] [CrossRef]
- Ong, W.Y.; Farooqui, T.; Koh, H.L.; Farooqui, A.A.; Ling, E.A. Protective effects of ginseng on neurological disorders. Front. Aging Neurosci. 2015, 7, 129. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, M.; Bjørås, M.; Wang, W.; Zhang, G.; Han, J.; Liu, Z.; Zhang, Y.; Wang, B.; Chen, J.; et al. Ginsenoside Rd promotes glutamate clearance by up-regulating glial glutamate transporter GLT-1 via PI3K/AKT and ERK1/2 pathways. Front. Pharmacol. 2013, 4, 152. [Google Scholar] [CrossRef]
- Jeong, E.J.; Lee, K.Y.; Kim, S.H.; Sung, S.H.; Kim, Y.C. Cognitive-enhancing and antioxidant activities of iridoid glycosides from Scrophularia buergeriana in scopolamine-treated mice. Eur. J. Pharm. 2008, 588, 78–84. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef]
- Lorenzini, C.A.; Baldi, E.; Bucherelli, C.; Sacchetti, B.; Tassoni, G. Role of dorsal hippocampus in acquisition, consolidation and retrieval of rat’s passive avoidance response: A tetrodotoxin functional inactivation study. Brain Res. 1996, 730, 32–39. [Google Scholar] [CrossRef]
- Sunita, S.; Sharlene, R.; Holly, B.B. Assessment of spatial memory in mice. Life Sci. 2010, 87, 521–536. [Google Scholar]
- Kim, C.J.; Choi, J.H.; Oh, Y.S.; Seong, E.S.; Lim, J.D.; Yu, C.Y.; Lee, J.G. Enhancement and conversion of ginsenoside contents in cultured wild ginseng adventitious root. Korean J. Med. Crop. Sci. 2020, 28, 445–454. [Google Scholar] [CrossRef]
- Kim, C.J.; Choi, J.H.; Seong, E.S.; Lim, J.D.; Choi, S.K.; Yu, C.Y.; Lee, J.G. The Isoflavonoid constituents and biological active of astragalus radix by fermentation of β-glucosidase strains. Korean J. Med. Crop. 2020, 25, 371–378. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, H.K.; Go, E.J.; Choi, J.H.; Jo, A.R.; Kim, C.J.; Lee, J.G.; Lim, J.D.; Choi, S.K.; Yu, C.Y. Extraction of Low Molecular Weight Ginsenosides from Adventitious Roots Culture of Wild Mountain Ginseng by Steam Processing. Korean J. Med. Crop. 2018, 26, 1–9. [Google Scholar]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Feather-Stone, R.M. A new and rapid colormetric determination of cholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Jia, G.; Yang, H.; Diao, Z.; Liu, Y.; Sun, C. Neural stem cell conditioned medium alleviates Aβ25-35 damage to SH-SY5Y cells through the PCMT1/MST1 pathway. Eur. J. Histochem. 2020, 64, 3190. [Google Scholar] [CrossRef]
- Tan, J.W.; Kim, M.K. Neuroprotective Effects of biochanin a against β-amyloid-induced neurotoxicity in PC12 cells via a mitochondrial-dependent apoptosis pathway. Molecules 2016, 21, 548. [Google Scholar] [CrossRef]
- Park, H.R.; Lee, H.; Park, H.; Jeon, J.W.; Cho, W.K.; Ma, J.Y. Neuroprotective effects of liriope platyphylla extract against hydrogen peroxide-induced cytotoxicity in human neuroblastoma SH-SY5Y cells. BMC Complementary Altern. Med. 2015, 15, 171. [Google Scholar] [CrossRef]
- Bo-Htay, C.; Palee, S.; Apaijai, N.; Chattipakorn, S.C.; Chattipakorn, N. Effects of D-galactose-induced ageing on the heart and its potential interventions. J. Cell. Mol. Med. 2018, 22, 1392–1410. [Google Scholar] [CrossRef]
- Haider, A.; Inam, W.; Khan, S.A.; Mahmood, W.; Abbas, G. β-glucan attenuated scopolamine induced cognitive impairment via hippocampal acetylcholinesterase inhibition in rats. Brain Res. 2016, 1644, 141–148. [Google Scholar] [CrossRef]
- Jo, Y.H.; Lee, H.; Oh, M.H.; Lee, G.H.; Lee, Y.J.; Lee, J.S.; Kim, M.J.; Kim, W.Y.; Kim, J.S.; Yoo, D.S.; et al. Antioxidant and hepatoprotective effects of Korean ginseng extract GS-KG9 in a D-galactosamine-induced liver damage animal model. Nutr. Res. Pract. 2020, 14, 334. [Google Scholar] [CrossRef]
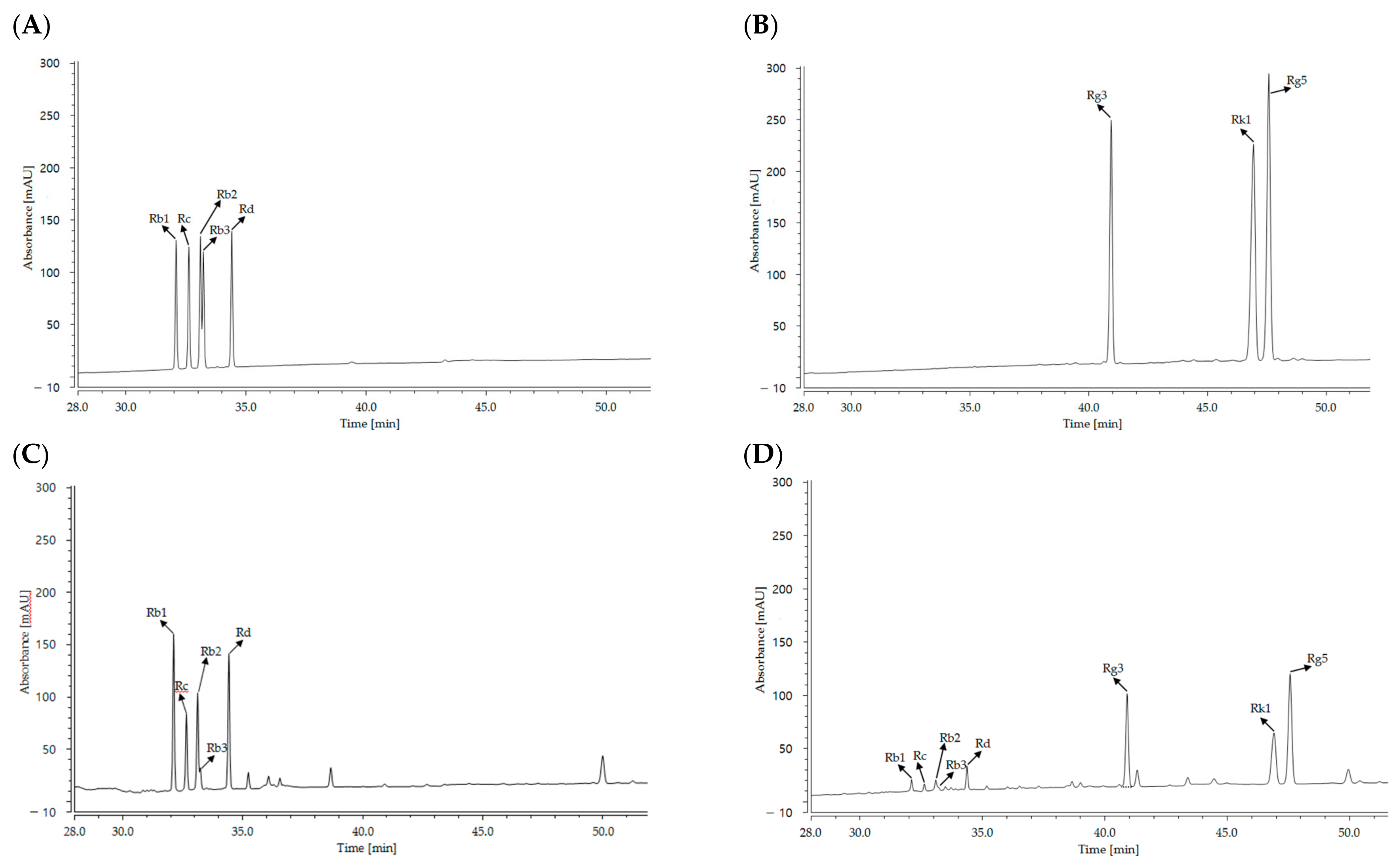

| Treatment | Ginsenoside Contents (mg/g) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| High Molecular Compounds | Small Molecular Compounds | Total | |||||||
| Rb1 | Rc | Rb2 | Rb3 | Rd | Rg3 | Rk1 | Rg5 | ||
| Cultured wild ginseng root | 51.53 ± 1.34 | 38.16 ± 1.10 | 34.36 ± 1.26 | 8.10 ± 0.52 | 55.90 ± 0.85 | N.D.1 | N.D. | N.D. | 188.06 ± 4.98 |
| HLJG0701-β | 9.26 ± 0.28 | 4.93 ± 0.41 | 6.36 ± 0.40 | 2.83 ± 0.35 | 11.26 ± 0.56 | 44.26 ± 1.02 | 15.93 ± 0.32 | 23.10 ± 0.59 | 117.96 ± 3.38 |
| Analyte | Formula | Side Chain | R1 | R2 | R3 | R4 | M.W. | Function | R-Squared |
|---|---|---|---|---|---|---|---|---|---|
| Rb1 | C54H92O23 | A1 | glc(2-1)glc | H | Oglc(6-1)glc | CH3 | 1109.29 | y = 0.0431x + 0.0123 | R2 = 0.9995 |
| Rc | C53H90O22 | A1 | glc(2-1)glc | H | Oglc(6-1)araf | CH3 | 1079.27 | y = 0.0427x + 0.0568 | R2 = 1.000 |
| Rb2 | C53H90O22 | A1 | glc(2-1)glc | H | Oglc(6-1)arap | CH3 | 1079.27 | y = 0.0481x + 0.0197 | R2 = 0.9988 |
| Rb3 | C53H90O22 | A1 | glc(2-1)glc | H | Oglc(6-1)xyl | CH3 | 1079.27 | y = 0.0396x + 0.0875 | R2 = 0.9999 |
| Rd | C48H82O18 | A1 | glc(2-1)glc | H | Oglc(6-1) | CH3 | 947.15 | y = 0.0503x + 0.0605 | R2 = 1.000 |
| Rg3 | C42H72O13 | A1 | glc(2-1)glc | H | OH | CH3 | 785.01 | y = 0.0626x + 0.0333 | R2 = 1.000 |
| Rk1 | C42H70O12 | A2 | glc(2-1)glc | H | - | - | 767.00 | y = 0.1653x + 0.0546 | R2 = 1.000 |
| Rg5 | C42H70O12 | A3 | glc(2-1)glc | H | - | - | 767.00 | y = 0.1911x + 0.1765 | R2 = 0.999 |
 | |||||||||
| Model | Group | Number of Animals | Volume (mg/kg) | Dose (mg/kg) |
|---|---|---|---|---|
| Scopolamine Model (Male, 9 weeks old, 18.37–23.92 g) | Control | M1~10 | 5 | 0 |
| Scopolamine (1 mg/kg) | M11~20 | 5 | 0 | |
| Scop + Donepezil (5 mg/kg) | M21~30 | 5 | 5 | |
| Scop + HLJG0701-β | M31~40 | 5 | 125 | |
| M41~50 | 5 | 250 | ||
| M51~60 | 5 | 500 | ||
| Ovariectomized (OVX) +d-galactose Model (Female, 9 weeks old, 18.40–20.97 g) | Control | F1~10 | 5 | 0 |
| OVX + d-galactose (100 mg/kg) | F11~20 | 5 | 0 | |
| OVX + d-galactose + Donepezil (5 mg/kg) | F21~30 | 5 | 5 | |
| OVX + d-galactose + HLJG0701-β | F31~40 | 5 | 125 | |
| F41~50 | 5 | 250 | ||
| F51~60 | 5 | 500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.-J.; Ryu, H.-Y.; Lee, S.; Lee, H.-J.; Chun, Y.-S.; Kim, J.-K.; Yu, C.-Y.; Ghimire, B.K.; Lee, J.-G. Neuroprotective Effect and Antioxidant Potency of Fermented Cultured Wild Ginseng Root Extracts of Panax ginseng C.A. Meyer in Mice. Molecules 2021, 26, 3001. https://doi.org/10.3390/molecules26103001
Kim C-J, Ryu H-Y, Lee S, Lee H-J, Chun Y-S, Kim J-K, Yu C-Y, Ghimire BK, Lee J-G. Neuroprotective Effect and Antioxidant Potency of Fermented Cultured Wild Ginseng Root Extracts of Panax ginseng C.A. Meyer in Mice. Molecules. 2021; 26(10):3001. https://doi.org/10.3390/molecules26103001
Chicago/Turabian StyleKim, Chul-Joong, Hyeon-Yeol Ryu, Somin Lee, Han-Joo Lee, Yoon-Soek Chun, Jong-Kyu Kim, Chang-Yeon Yu, Bimal Kumar Ghimire, and Jae-Geun Lee. 2021. "Neuroprotective Effect and Antioxidant Potency of Fermented Cultured Wild Ginseng Root Extracts of Panax ginseng C.A. Meyer in Mice" Molecules 26, no. 10: 3001. https://doi.org/10.3390/molecules26103001
APA StyleKim, C.-J., Ryu, H.-Y., Lee, S., Lee, H.-J., Chun, Y.-S., Kim, J.-K., Yu, C.-Y., Ghimire, B. K., & Lee, J.-G. (2021). Neuroprotective Effect and Antioxidant Potency of Fermented Cultured Wild Ginseng Root Extracts of Panax ginseng C.A. Meyer in Mice. Molecules, 26(10), 3001. https://doi.org/10.3390/molecules26103001







