Coordination Behavior of [Cp″2Zr(µ1:1-As4)] towards Lewis Acids
Abstract
1. Introduction
2. Results and Discussion
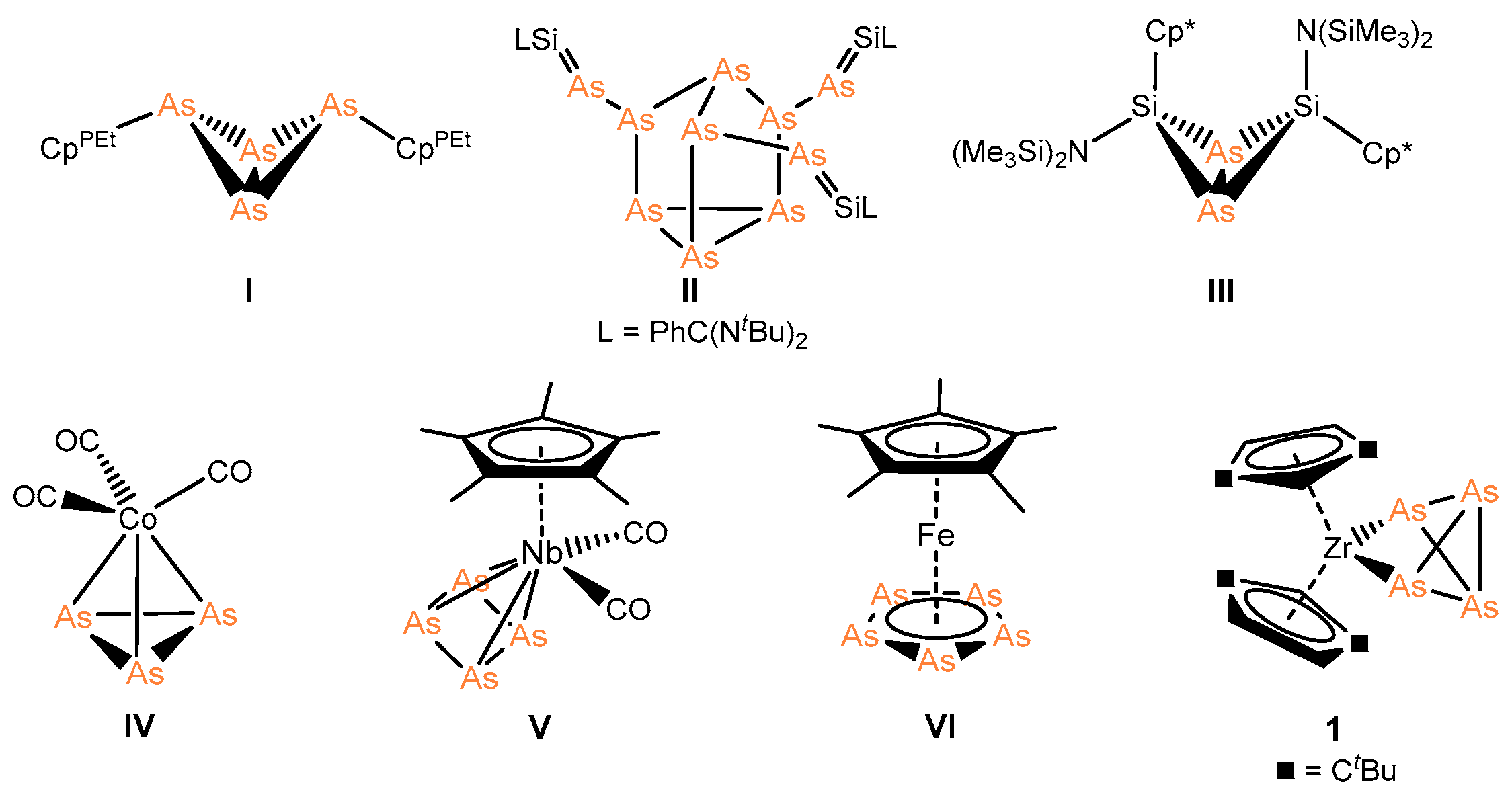
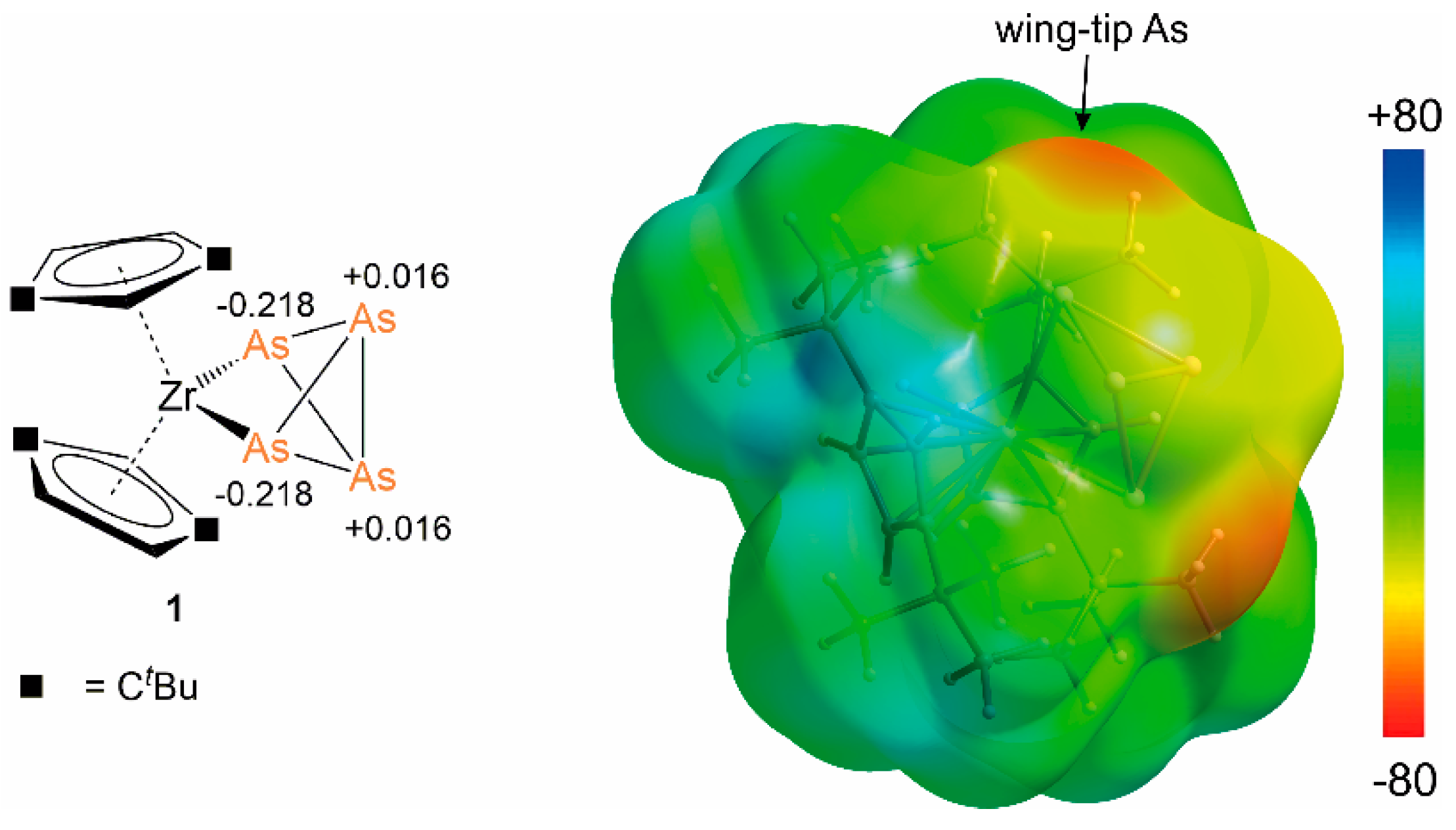
2.1. General Consideration
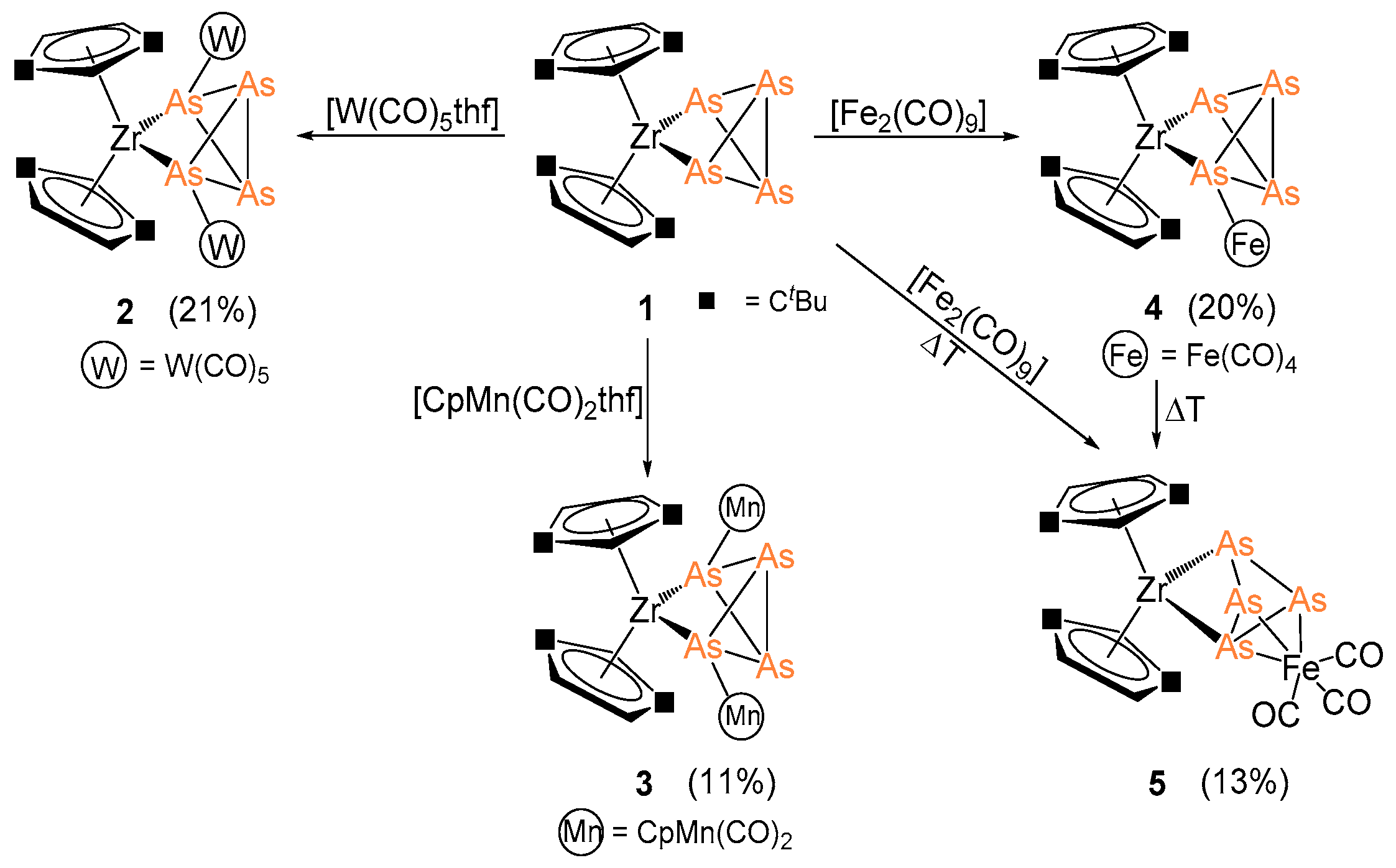
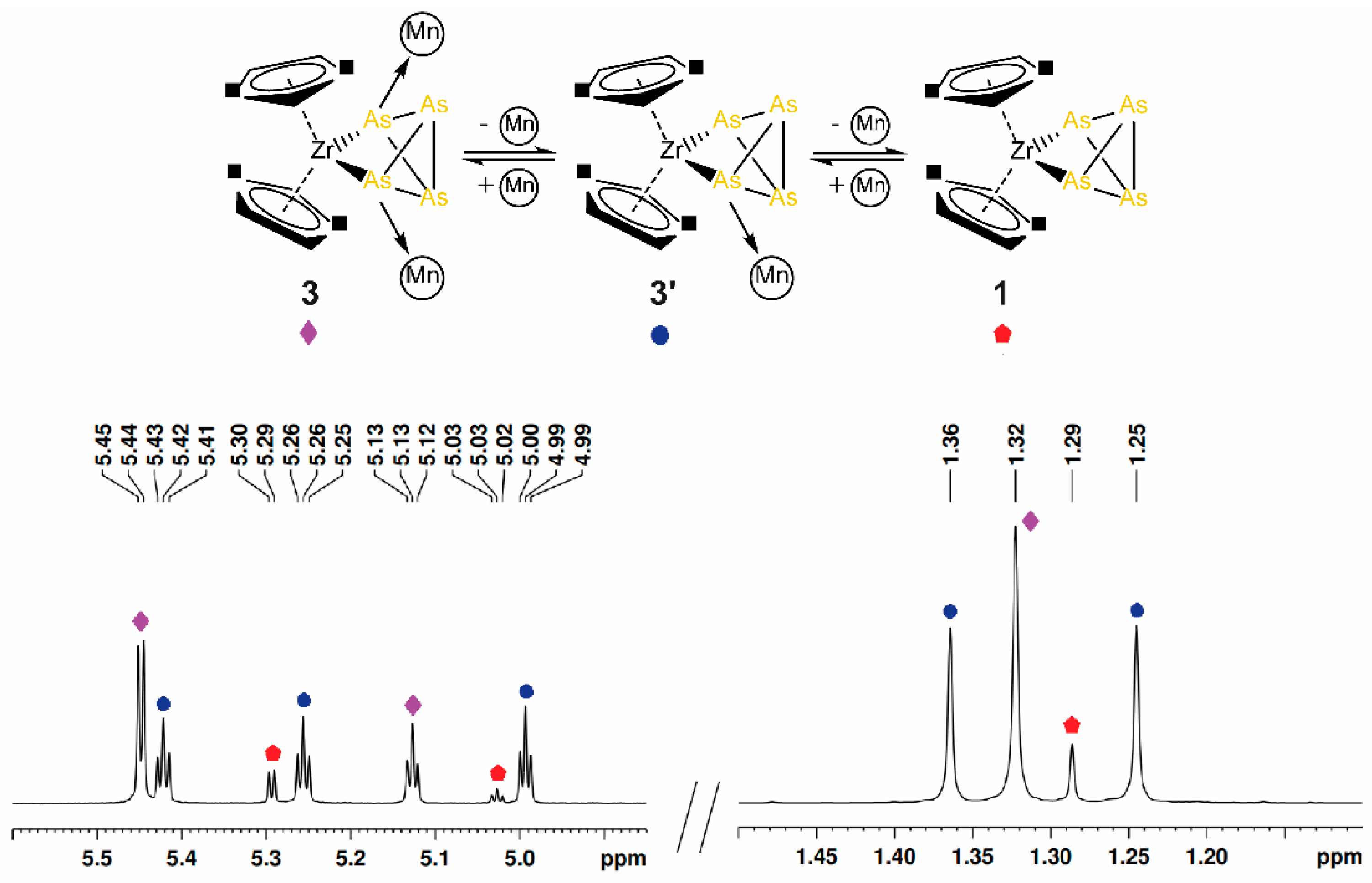
2.2. Coordination of 1 to Transition Metal Complexes
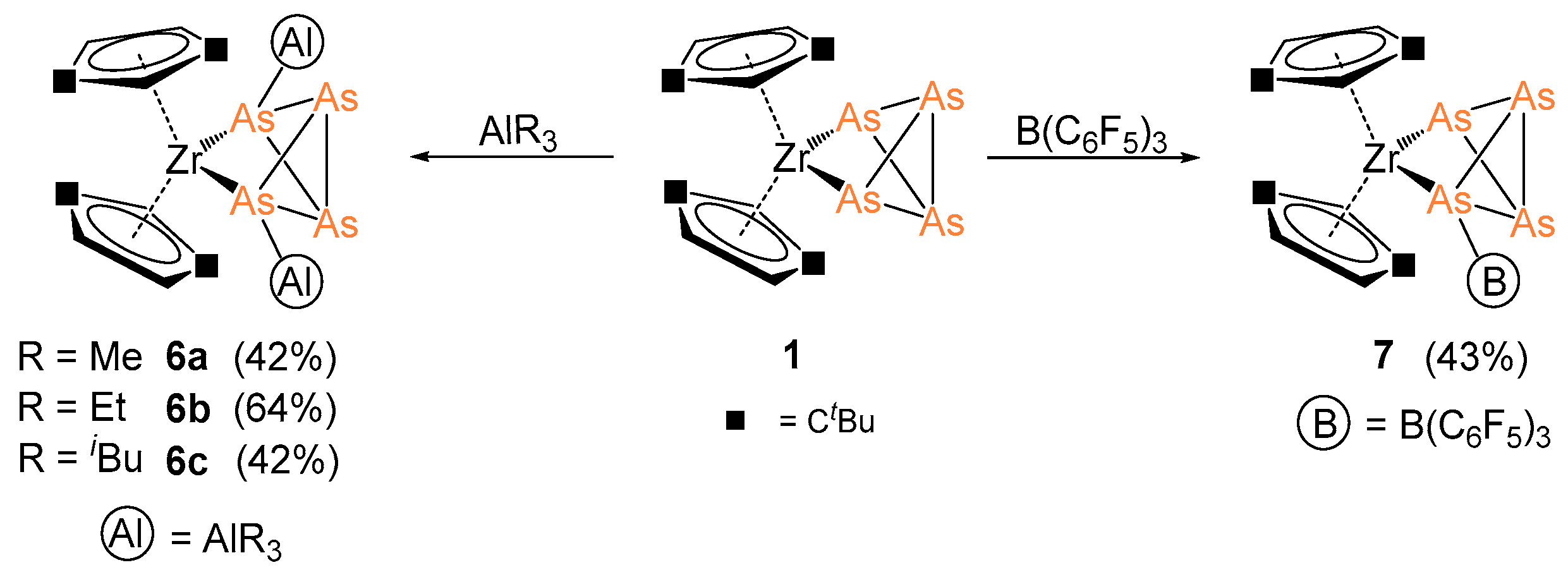
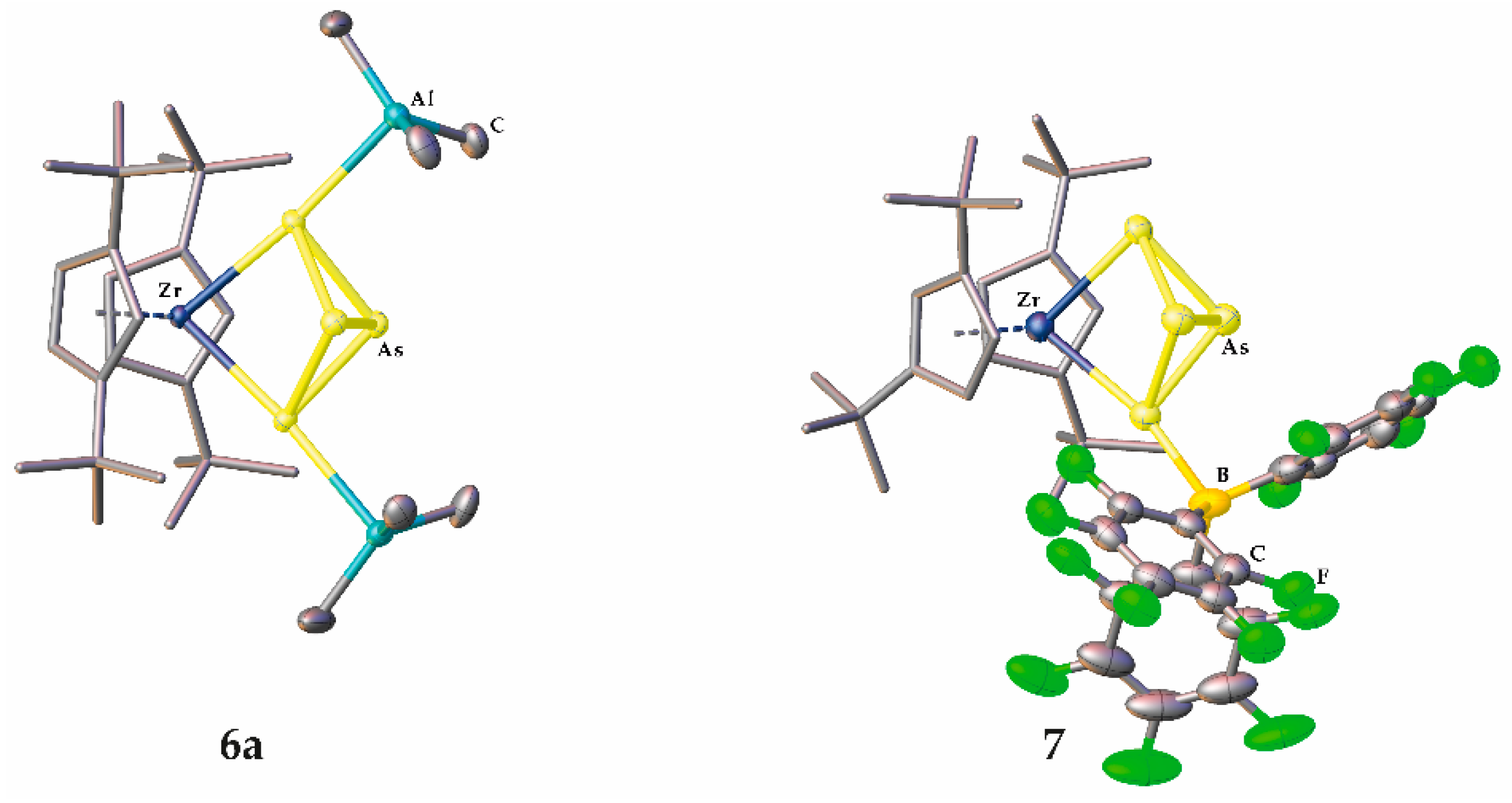
2.3. Coordination towards Main Group Compounds
3. Materials and Methods
3.1. General Informations
3.2. Synthesis and Characterization of the Compounds 2–7
3.2.1. Synthesis and Characterization of [Cp″2Zr(µ3,η1:1:1:1-As4)(W(CO)5)2] (2)
3.2.2. Synthesis and Characterization of [Cp″2Zr(µ3,η1:1:1:1-As4)(CpMn(CO)2)2] (3)
3.2.3. Synthesis and Characterization of [Cp″2Zr(µ,η1:1:1-As4)(Fe(CO)4)] (4)
3.2.4. Synthesis and Characterization of [Cp″2Zr(µ,η3:1:1-As4)(Fe(CO)3)] (5)
3.2.5. Synthesis and Characterization of [Cp″2Zr(µ3,η1:1:1:1-As4){AlR3}2] (R = Me (6a), Et (6b), iBu (6c))
3.2.6. Synthesis and Characterization of [Cp″2Zr(µ,η1:1:1-As4)(B(C6F5)3)] (7)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gambarotta, S. Dinitrogen fixation and activation after 30 years: A puzzle still unsolved. J. Organomet. Chem. 1995, 500, 117. [Google Scholar] [CrossRef]
- Chae, H.K.; Siberio-Pérez, D.Y.; Kim, J.; Go, Y.; Eddaoudi, M.; Matzger, A.J.; O’Keeffe, M.; Yaghi, O.M. A route to high surface area, porosity and inclusion of large molecules in crystals. Nature 2004, 427, 523. [Google Scholar] [CrossRef]
- Chang, C.W.; Okawa, D.; Majumdar, A.; Zettl, A. Solid-state thermal rectifier. Science 2006, 314, 1121. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Weinberger, M.B.; Levine, J.B.; Cumberland, R.W.; Kavner, A.; Yang, J.M.; Tolbert, S.H.; Kaner, R.B. Synthesis of ultra-incompressible superhard rhenium diboride at ambient pressure. Science 2007, 316, 436. [Google Scholar] [CrossRef]
- Ding, K.; Pierpont, A.W.; Brennessel, W.W.; Lukat-Rodgers, G.; Rodgers, K.R.; Cundari, T.R.; Bill, E.; Holland, P.L. Cobalt-dinitrogen complexes with weakened N-N bonds. J. Am. Chem. Soc. 2009, 131, 9471. [Google Scholar] [CrossRef]
- Hidai, M.; Mizobe, Y. Recent Advances in the Chemistry of Dinitrogen Complexes. Chem. Rev. 1995, 95, 1115. [Google Scholar] [CrossRef]
- Stephan, D.W. Frustrated Lewis pairs: A new strategy to small molecule activation and hydrogenation catalysis. Dalton Trans. 2009, 17, 3129. [Google Scholar] [CrossRef] [PubMed]
- Stephan, D.W.; Erker, G. Frustrated Lewis pair chemistry: Development and perspectives. Angew. Chem. Int. Ed. 2015, 54, 6400. [Google Scholar] [CrossRef] [PubMed]
- Caporali, M.; Gonsalvi, L.; Rossin, A.; Peruzzini, M. P4 activation by late-transition metal complexes. Chem. Rev. 2010, 110, 4178. [Google Scholar] [CrossRef] [PubMed]
- Scheer, M.; Balázs, G.; Seitz, A.E. P4 activation by main group elements and compounds. Chem. Rev. 2010, 110, 4236. [Google Scholar] [CrossRef] [PubMed]
- Cossairt, B.M.; Piro, N.A.; Cummins, C.C. Early-transition-metal-mediated activation and transformation of white phosphorus. Chem. Rev. 2010, 110, 4164. [Google Scholar] [CrossRef] [PubMed]
- Seidl, M.; Balázs, G.; Scheer, M. The Chemistry of Yellow Arsenic. Chem. Rev. 2019, 119, 8406. [Google Scholar] [CrossRef]
- Heinl, S.; Balázs, G.; Stauber, A.; Scheer, M. CpPEt2 As4—An Organic-Substituted As4 Butterfly Compound. Angew. Chem. Int. Ed. 2016, 55, 15524. [Google Scholar] [CrossRef] [PubMed]
- Seitz, A.E.; Eckhardt, M.; Sen, S.S.; Erlebach, A.; Peresypkina, E.V.; Roesky, H.W.; Sierka, M.; Scheer, M. Different Reactivity of As4 towards Disilenes and Silylenes. Angew. Chem. Int. Ed. 2017, 56, 6655. [Google Scholar] [CrossRef] [PubMed]
- Foust, A.S.; Foster, M.S.; Dahl, L.F. Organometallic pnicogen complexes. III. Preparation and structural characterization of the triarsenic-cobalt atom cluster system As3Co(CO)3. The first known X3-transition metal analog of group Va tetrahedral X4 molecules. J. Am. Chem. Soc. 1969, 91, 5631–5633. [Google Scholar] [CrossRef]
- Scherer, O.J.; Vondung, J.; Wolmershäuser, G. Tetraarsacyclobutadien als Komplexligand. J. Organomet. Chem. 1989, 376, C35–C38. [Google Scholar] [CrossRef]
- Scherer, O.J.; Blath, C.; Wolmershäuser, G. Ferrocene mit einem Pentaarsacyclopentadienyl-Liganden. J. Organomet. Chem. 1990, 387, C21–C24. [Google Scholar] [CrossRef]
- Piesch, M.; Dielmann, F.; Reichl, S.; Scheer, M. A General Pathway to Heterobimetallic Triple-Decker Complexes. Chem. Eur. J. 2020, 26, 1518. [Google Scholar] [CrossRef]
- Schmidt, M.; Konieczny, D.; Peresypkina, E.V.; Virovets, A.V.; Balázs, G.; Bodensteiner, M.; Riedlberger, F.; Krauss, H.; Scheer, M. Arsenic-Rich Polyarsenides Stabilized by Cp*Fe Fragments. Angew. Chem. Int. Ed. 2017, 56, 7307. [Google Scholar] [CrossRef]
- Schoo, C.; Bestgen, S.; Schmidt, M.; Konchenko, S.N.; Scheer, M.; Roesky, P.W. Sterically induced reductive linkage of iron polypnictides with bulky lanthanide complexes by ring-opening of THF. Chem. Commun. 2016, 52, 13217. [Google Scholar] [CrossRef]
- Krauss, H.; Balázs, G.; Bodensteiner, M.; Scheer, M. The potential of a cyclo-As5 ligand complex in coordination chemistry. Chem. Sci. 2010, 1, 337. [Google Scholar] [CrossRef]
- Fleischmann, M.; Dütsch, L.; Moussa, M.E.; Schindler, A.; Balázs, G.; Lescop, C.; Scheer, M. Organometallic polyphosphorus and -arsenic ligands as linkers between pre-assembled linear Cu(I) fragments. Chem. Commun. 2015, 51, 2893. [Google Scholar] [CrossRef]
- Fleischmann, M.; Welsch, S.; Krauss, H.; Schmidt, M.; Bodensteiner, M.; Peresypkina, E.V.; Sierka, M.; Gröger, C.; Scheer, M. Complexes of monocationic Group 13 elements with pentaphospha- and pentaarsaferrocene. Chem. Eur. J. 2014, 20, 3759. [Google Scholar] [CrossRef]
- Schmidt, M.; Seitz, A.E.; Eckhardt, M.; Balázs, G.; Peresypkina, E.V.; Virovets, A.V.; Riedlberger, F.; Bodensteiner, M.; Zolnhofer, E.M.; Meyer, K.; et al. Transfer Reagent for Bonding Isomers of Iron Complexes. J. Am. Chem. Soc. 2017, 139, 13981. [Google Scholar] [CrossRef]
- Heinl, V.; Schmidt, M.; Eckhardt, M.; Eberl, M.; Seitz, A.E.; Balázs, G.; Seidl, M.; Scheer, M. E4 Transfer (E = P, As) to Ni Complexes. Chem. Eur. J. 2021, 27. [Google Scholar] [CrossRef]
- Seitz, A.E.; Eckhardt, M.; Erlebach, A.; Peresypkina, E.V.; Sierka, M.; Scheer, M. Pnictogen-Silicon Analogues of Benzene. J. Am. Chem. Soc. 2016, 138, 10433. [Google Scholar] [CrossRef]
- Seitz, A.E.; Vogel, U.; Eberl, M.; Eckhardt, M.; Balázs, G.; Peresypkina, E.V.; Bodensteiner, M.; Zabel, M.; Scheer, M. Coordination Behavior of Cp″2Zr(η1:1-P4 ) towards Different Lewis Acids. Chem. Eur. J. 2017, 23, 10319. [Google Scholar] [CrossRef]
- Haiduc, I.; Tiekink, E. Inverse Coordination Chemistry: A Novel Chemical Concept; Sunway University Press: Sunway City, Malaysia, 2020. [Google Scholar]
- Haiduc, I. Inverse Coordination Chemistry: An emerging new chemical concept. Oxygen and other chalcogens as coordination centers. Coord. Chem. Rev. 2017, 338, 1. [Google Scholar] [CrossRef]
- Haiduc, I. Nitrogen centered inverse coordination complexes. A survey of molecular topologies. J. Coord. Chem. 2018, 71, 3139. [Google Scholar] [CrossRef]
- Mulvey, R.E. s-Block metal inverse crowns: Synthetic and structural synergism in mixed alkali metal–magnesium (or zinc) amide chemistry. Chem. Commun. 2001, 12, 1049–1056. [Google Scholar] [CrossRef]
- Clegg, W.; Henderson, K.; Kennedy, A.R.; Mulvey, R.E.; O’Hara, C.T.; Rowlings, R.B.; Tooke, D.M. Regioselective Tetrametalation of Ferrocene in a Single Reaction: Extension of s-Block Inverse Crown Chemistry to the d-Block. Angew. Chem. Int. Ed. 2001, 40, 3902. [Google Scholar] [CrossRef]
- Duer, M.J.; García, F.; Kowenicki, R.A.; Naseri, V.; McPartlin, M.; Stead, M.L.; Stein, R.S.; Wright, D.S. Inverse coordination of an ionic lattice by a metal host. Angew. Chem. Int. Ed. 2005, 44, 5729. [Google Scholar] [CrossRef]
- Pyykkö, P.; Atsumi, M. Molecular single-bond covalent radii for elements 1–118. Chem. Eur. J. 2009, 15, 186. [Google Scholar] [CrossRef]
- Grünbauer, R.; Balázs, G.; Scheer, M. The Butterfly Complex {Cp*Cr(CO)3}2(μ,η1:1-P4) as a Versatile Ligand and Its Unexpected P1/P3 Fragmentation. Chem. Eur. J. 2020, 26, 11722. [Google Scholar] [CrossRef]
- Keeley, D.F.; Johnson, R.E. Über die Bildungsweise von Eisen-nonacarbonyl aus Eisen-pentacarbonyl. J. Inorg. Nucl. Chem. 1959, 11, 33. [Google Scholar] [CrossRef]
- Speyer, E.; Wolf, H. Über die Bildungsweise von Eisen-nonacarbonyl aus Eisen-pentacarbonyl. Ber. Dtsch. Chem. Ges. 1927, 60, 1424. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heinl, V.; Balázs, G.; Koschabek, S.; Eckhardt, M.; Piesch, M.; Seidl, M.; Scheer, M. Coordination Behavior of [Cp″2Zr(µ1:1-As4)] towards Lewis Acids. Molecules 2021, 26, 2966. https://doi.org/10.3390/molecules26102966
Heinl V, Balázs G, Koschabek S, Eckhardt M, Piesch M, Seidl M, Scheer M. Coordination Behavior of [Cp″2Zr(µ1:1-As4)] towards Lewis Acids. Molecules. 2021; 26(10):2966. https://doi.org/10.3390/molecules26102966
Chicago/Turabian StyleHeinl, Veronika, Gábor Balázs, Sarah Koschabek, Maria Eckhardt, Martin Piesch, Michael Seidl, and Manfred Scheer. 2021. "Coordination Behavior of [Cp″2Zr(µ1:1-As4)] towards Lewis Acids" Molecules 26, no. 10: 2966. https://doi.org/10.3390/molecules26102966
APA StyleHeinl, V., Balázs, G., Koschabek, S., Eckhardt, M., Piesch, M., Seidl, M., & Scheer, M. (2021). Coordination Behavior of [Cp″2Zr(µ1:1-As4)] towards Lewis Acids. Molecules, 26(10), 2966. https://doi.org/10.3390/molecules26102966





