Ergosta-7,9(11),22-trien-3β-ol Alleviates Intracerebral Hemorrhage-Induced Brain Injury and BV-2 Microglial Activation
Abstract
1. Introduction
2. Results
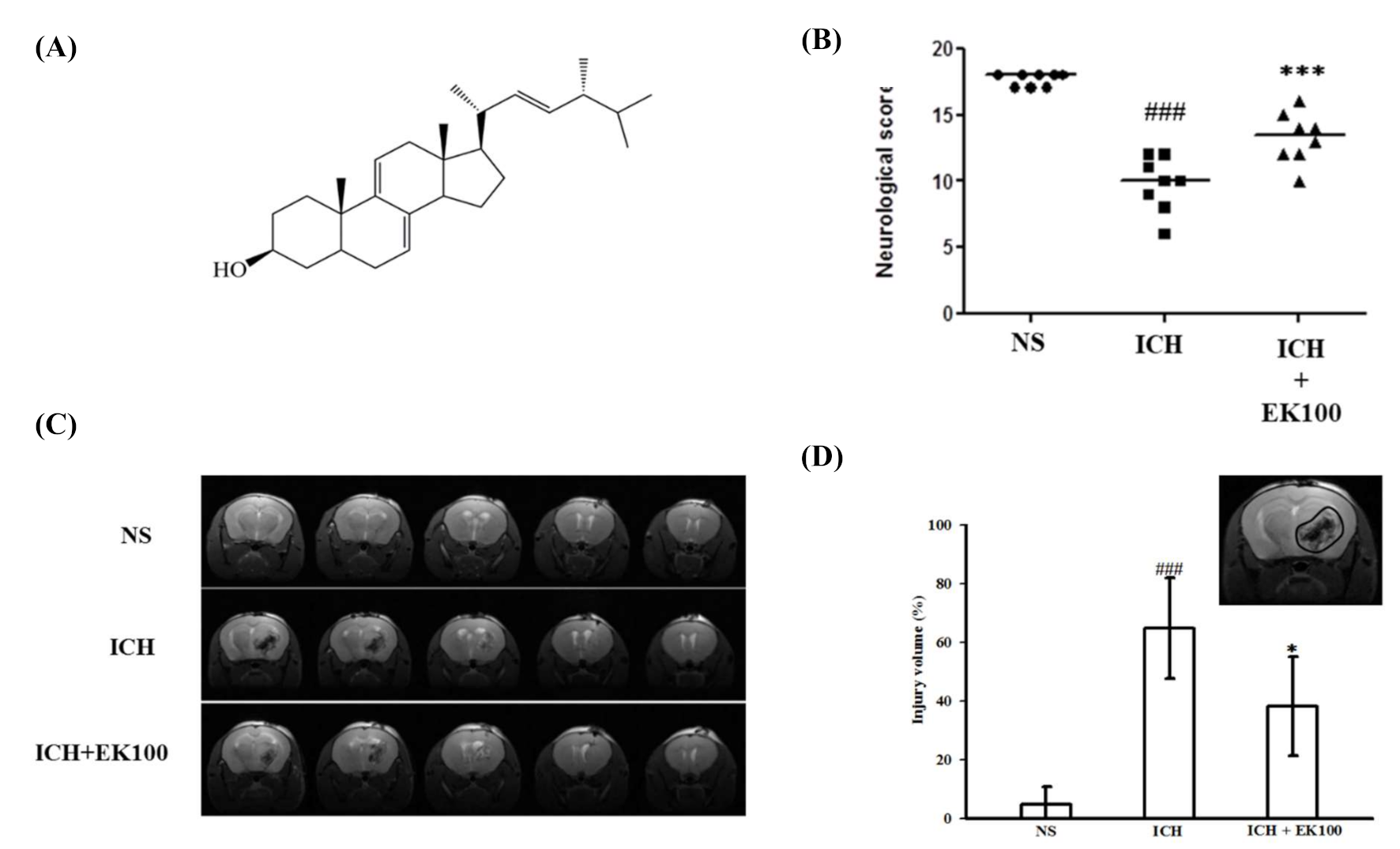
2.1. Effects of Ek100 on Neurobehavioral Deficits and T2 Images of Brain Injurious Lesion After Intracerebral Hemorrhage (ICH)
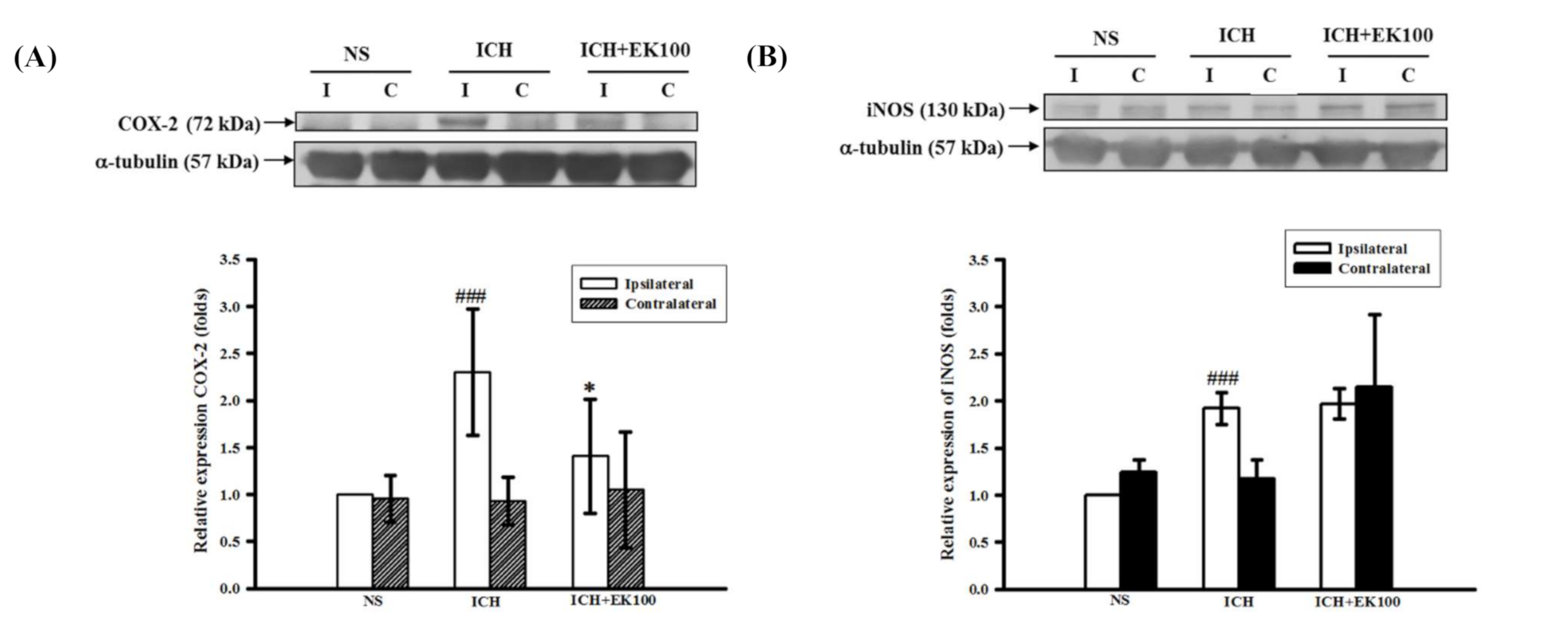
2.2. Effects of EK100 on the Expression of Cerebral COX-2/iNOS Proteins after ICH
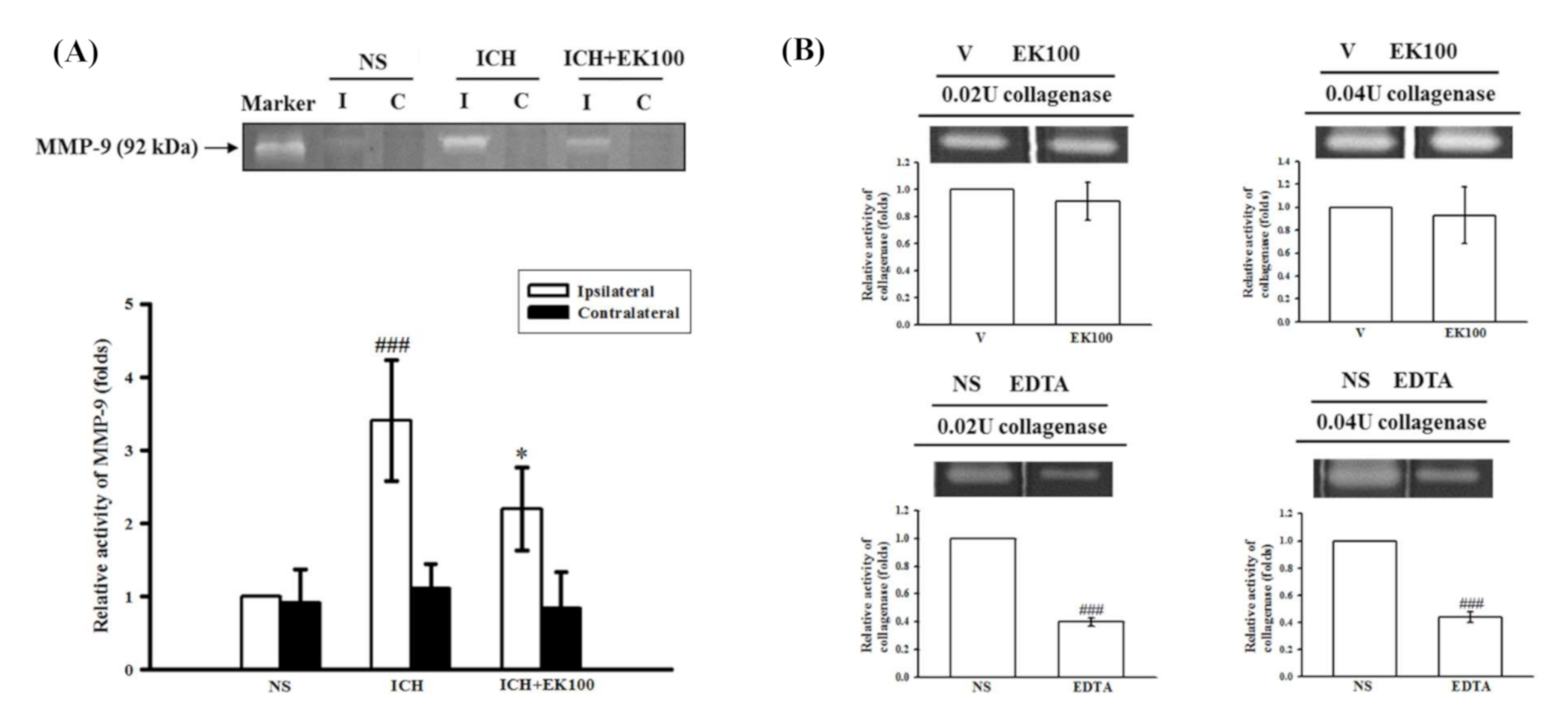
2.3. Effects of EK100 on the Brain Gelatinolytic Activity of MMP-9 after ICH In Vivo and on Collagenase VII-mediated Gelatinolysis In Vitro
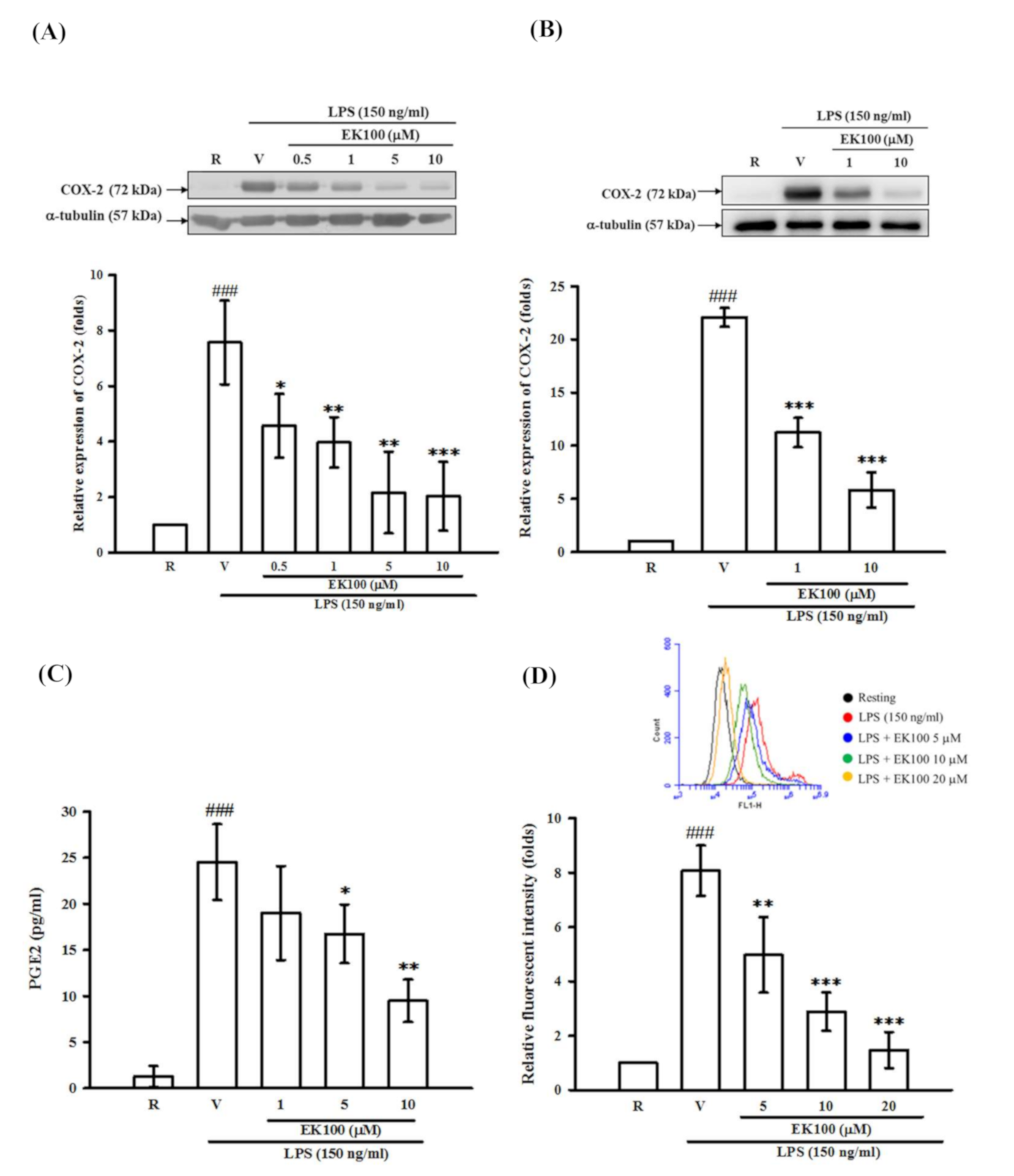
2.4. Effects of EK100 on the Expression of COX-2 Protein in Activated Microglial BV-2 cells, Primary Microglia, Cellular Viability and Productions of PGE2 and ROS
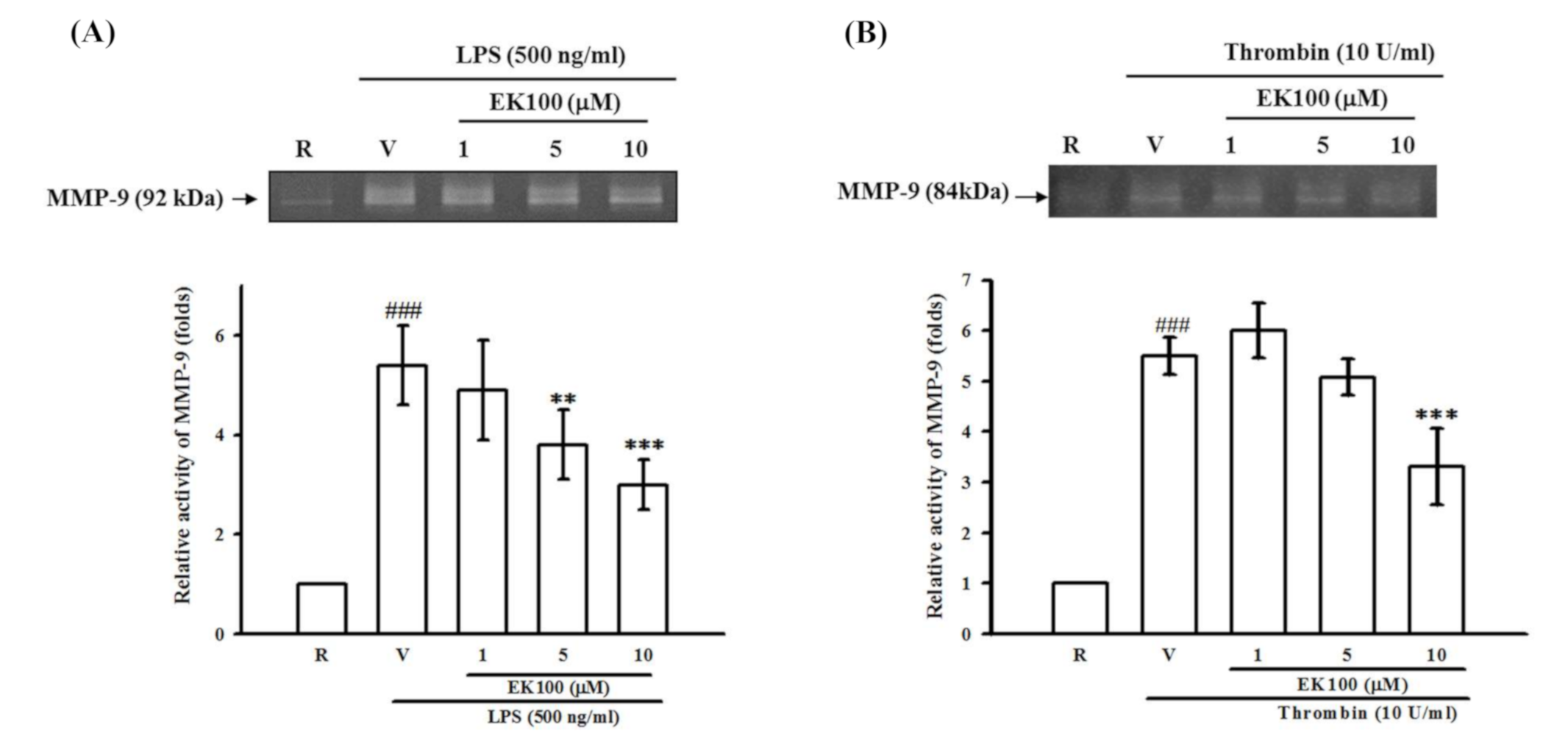
2.5. Effects of EK100 on LPS- and Thrombin-Induced MMP-9 Gelatinolysis in Astrocytes
2.6. Effects of EK100 on IκBα Degradation in Activated Microglial BV-2 Cells
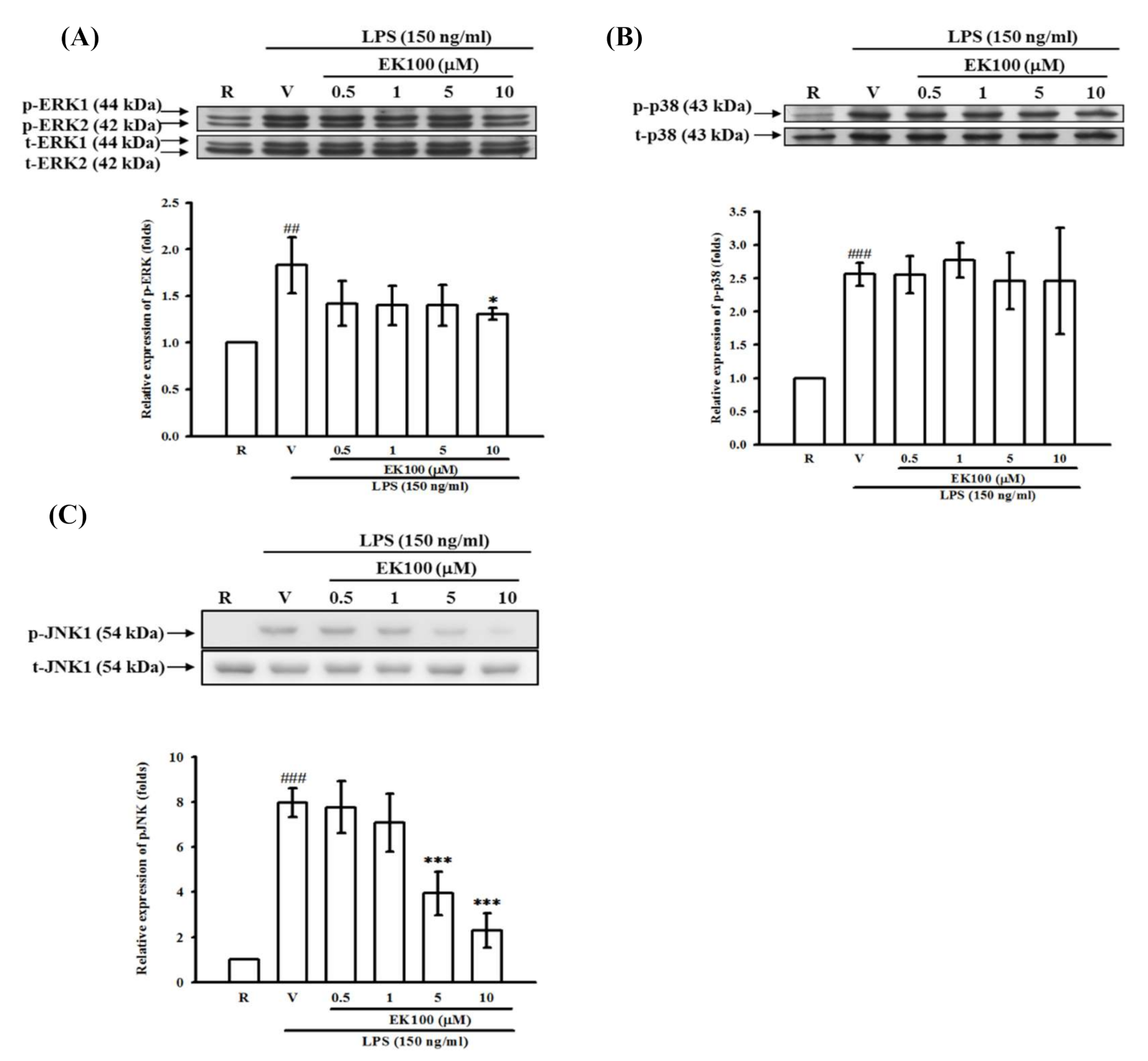
2.7. Effects of EK100 on ERK, p38, and JNK MAPK Activation in Activated Microglial BV-2 Cells
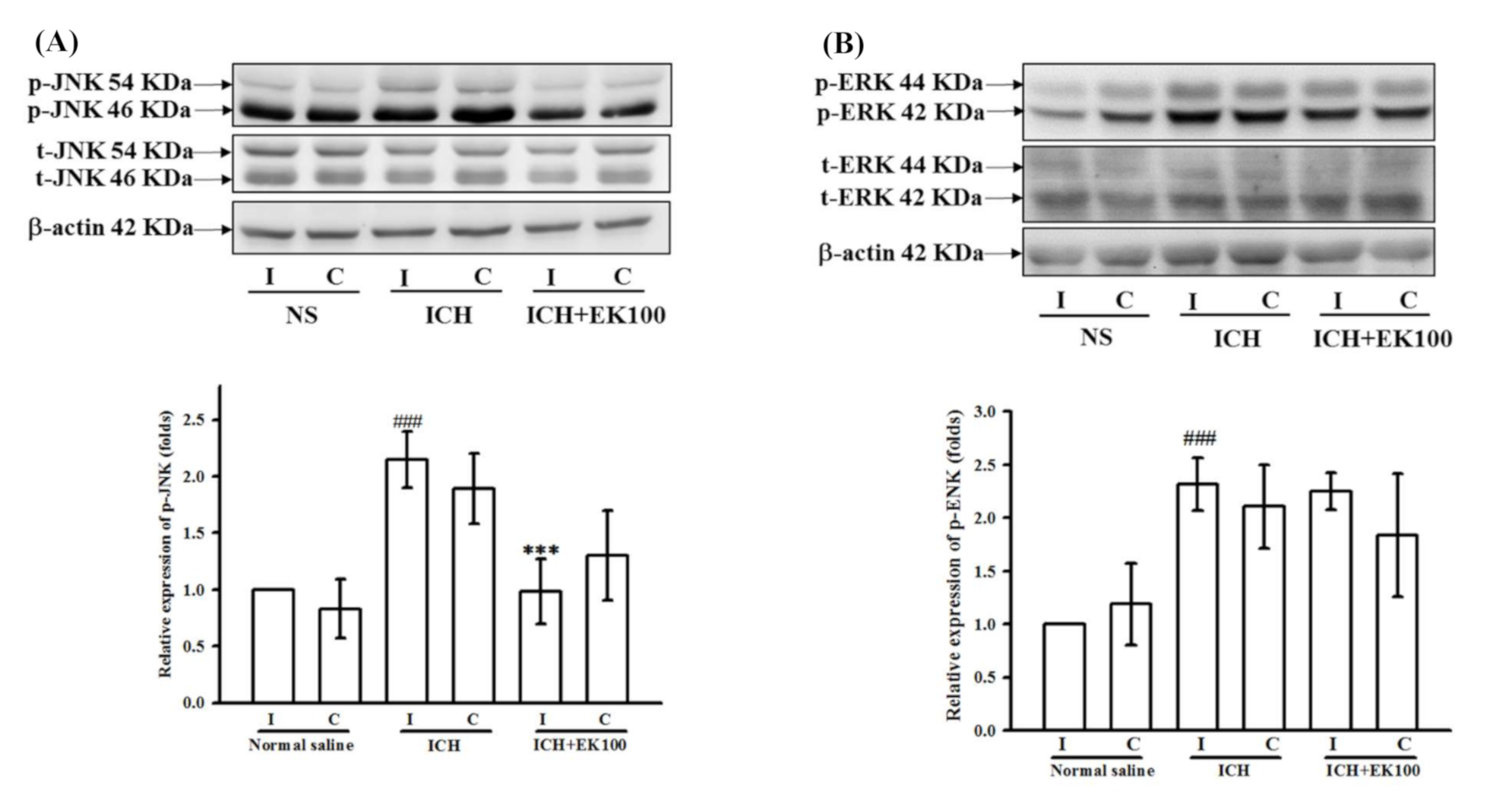
2.8. Effects of EK100 on JNK MAPK Activation in the Ipsilateral Brain after ICH In Vivo
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animal Manipulation and ICH Induction
4.3. Neurobehavioral Deficits
4.4. Magnetic Resonance Imaging (MRI) and Brain Injury Measurement
4.5. Substrate Embedded Zymography
4.6. Cell Cultivation
4.7. Cell Viability
4.8. Determination of the PGE2 Levels by the Enzyme-Linked Immunosorbent Assay (ELISA)
4.9. Detection of Intracellular ROS Levels
4.10. Western Blot Analyses
4.11. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Fayad, P.B.; Awad, I.A. Surgery for intracerebral hemorrhage. Neurology. 1998, 51, S69–73. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Lawes, C.M.; Bennett, D.A.; Barker-Collo, S.L.; Parag, V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: A systematic review. Lancet Neurol. 2009, 8, 355–369. [Google Scholar] [CrossRef]
- Pinho, J.; Costa, A.S.; Araujo, J.M.; Amorim, J.M.; Ferreira, C. Intracerebral hemorrhage outcome: A comprehensive update. J. Neurol. Sci. 2019, 398, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Aronowski, J.; Hall, C.E. New horizons for primary intracerebral hemorrhage treatment: Experience from preclinical studies. Neurol. Res. 2005, 27, 268–279. [Google Scholar] [CrossRef]
- Kim, H.; Edwards, N.J.; Choi, H.A.; Chang, T.R.; Jo, K.W.; Lee, K. Treatment strategies to attenuate perihematomal edema in patients with intracerebral hemorrhage. World Neurosurg. 2016, 94, 32–41. [Google Scholar] [CrossRef]
- Wang, J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog. Neurobiol. 2010, 92, 463–477. [Google Scholar] [CrossRef]
- Xi, G.; Keep, R.F.; Hoff, J.T. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006, 5, 53–63. [Google Scholar] [CrossRef]
- Wang, J.; Dore, S. Inflammation after intracerebral hemorrhage. J. Cereb. Blood Flow Metab. 2007, 27, 894–908. [Google Scholar] [CrossRef]
- Chaturvedi, M.; Kaczmarek, L. MMP-9 inhibition: A therapeutic strategy in ischemic stroke. Mol. Neurobiol. 2014, 49, 563–573. [Google Scholar] [CrossRef]
- Katsuki, H. Exploring neuroprotective drug therapies for intracerebral hemorrhage. J. Pharmacol. Sci. 2010, 114, 366–378. [Google Scholar] [CrossRef]
- Ren, H.; Han, R.; Chen, X.; Liu, X.; Wan, J.; Wang, L.; Yang, X.; Wang, J. Potential therapeutic targets for intracerebral hemorrhage-associated inflammation: An update. J. Cereb. Blood Flow Metab. 2020, 40, 1752–1768. [Google Scholar] [CrossRef]
- Geethangili, M.; Tzeng, Y.M. Review of pharmacological effects of Antrodia camphorata and its bioactive compounds. Evid. Based Complement Alternat. Med. 2011, 2011, 212641. [Google Scholar] [CrossRef]
- Hsiao, G.; Shen, M.Y.; Lin, K.H.; Lan, M.H.; Wu, L.Y.; Chou, D.S.; Lin, C.H.; Su, C.H.; Sheu, J.R. Antioxidative and hepatoprotective effects of Antrodia camphorata extract. J. Agric. Food Chem. 2003, 51, 3302–3308. [Google Scholar] [CrossRef]
- Shen, Y.C.; Chou, C.J.; Wang, Y.H.; Chen, C.F.; Chou, Y.C.; Lu, M.K. Anti-inflammatory activity of the extracts from mycelia of Antrodia camphorata cultured with water-soluble fractions from five different Cinnamomum species. FEMS Microbiol. Lett. 2004, 231, 137–143. [Google Scholar] [CrossRef]
- Wang, L.C.; Wang, S.E.; Wang, J.J.; Tsai, T.Y.; Lin, C.H.; Pan, T.M.; Lee, C.L. In vitro and in vivo comparisons of the effects of the fruiting body and mycelium of Antrodia camphorata against amyloid β-protein-induced neurotoxicity and memory impairment. Appl. Microbiol. Biotechnol. 2012, 94, 1505–1519. [Google Scholar] [CrossRef]
- Phan, C.W.; David, P.; Naidu, M.; Wong, K.H.; Sabaratnam, V. Therapeutic potential of culinary-medicinal mushrooms for the management of neurodegenerative diseases: Diversity, metabolite, and mechanism. Crit. Rev. Biotechnol. 2015, 35, 355–368. [Google Scholar] [CrossRef]
- Huang, G.J.; Huang, S.S.; Lin, S.S.; Shao, Y.Y.; Chen, C.C.; Hou, W.C.; Kuo, Y.H. Analgesic effects and the mechanisms of anti-inflammation of ergostatrien-3beta-ol from Antrodia camphorata submerged whole broth in mice. J. Agric. Food Chem. 2010, 58, 7445–7452. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Lin, C.H.; Shih, C.C. Ergostatrien-3β-ol from Antrodia camphorata inhibits diabetes and hyperlipidemia in high-fat-diet treated mice via regulation of hepatic related genes, glucose transporter 4, and AMP-activated protein kinase phosphorylation. J. Agric. Food Chem. 2015, 63, 2479–2489. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Lin, T.Y.; You, Y.J.; Wen, K.C.; Sung, P.J.; Chiang, H.M. Antiinflammatory and antiphotodamaging effects of ergostatrien-3β-ol, isolated from Antrodia camphorata, on hairless mouse skin. Molecules 2016, 21, E1213. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Chern, C.M.; Liou, K.T.; Kuo, Y.H.; Shen, Y.C. Ergostatrien-7, 9 (11), 22-trien-3β-ol from Antrodia camphorata ameliorates ischemic stroke brain injury via downregulation of p65 NF-κB and caspase 3, and activation of Akt/GSK3/catenin-associated neurogenesis. Food Funct. 2019, 10, 4725–4738. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Wang, J.; Stetler, R.A.; Yang, Q.W. Inflammation in intracerebral hemorrhage: From mechanisms to clinical translation. Prog. Neurobiol. 2014, 115, 25–44. [Google Scholar] [CrossRef] [PubMed]
- MacLellan, C.L.; Silasi, G.; Auriat, A.M.; Colbourne, F. Rodent models of intracerebral hemorrhage. Stroke 2010, 41, S95–S98. [Google Scholar] [CrossRef]
- Lee, J.Y.; Sagher, O.; Keep, R.; Hua, Y.; Xi, G. Comparison of experimental rat models of early brain injury after subarachnoid hemorrhage. Neurosurgery 2009, 65, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, S.; Katsuki, H.; Kume, T.; Akaike, A. Thrombin-induced delayed injury involves multiple and distinct signaling pathways in the cerebral cortex and the striatum in organotypic slice cultures. Neurobiol. Dis. 2006, 22, 130–142. [Google Scholar] [CrossRef]
- Kathirvelu, B.; Carmichael, S.T. Intracerebral hemorrhage in mouse models: Therapeutic interventions and functional recovery. Metab. Brain. Dis. 2015, 30, 449–459. [Google Scholar] [CrossRef]
- Lee, SH.; Park, HK.; Ryu, WS.; Lee, J.S.; Bae, H.J.; Han, M.K.; Lee, Y.S.; Kwon, H.M.; Kim, C.K.; Park, E.S.; et al. Effects of celecoxib on hematoma and edema volumes in primary intracerebral hemorrhage: A multicenter randomized controlled trial. Eur. J. Neurol. 2013, 20, 1161–1169. [Google Scholar] [CrossRef]
- Zeng, Z.; Gong, X.; Hu, Z. L-3-n-butylphthalide attenuates inflammation response and brain edema in rat intracerebral hemorrhage model. Aging 2020, 12, 11768–11780. [Google Scholar] [CrossRef]
- Iadecola, C. Cyclooxygenase-2 and stroke: The long and short of it. Ann. Neurol. 2003, 54, 141–142. [Google Scholar] [CrossRef]
- Mohan, S.; Ahmad, A.S.; Glushakov, A.V.; Chambers, C.; Dore, S. Putative role of prostaglandin receptor in intracerebral hemorrhage. Front. Neurol. 2012, 3, 145. [Google Scholar] [CrossRef]
- Chu, K.; Jeong, S.W.; Jung, K.H.; Han, S.Y.; Lee, S.T.; Kim, M.; Roh, J.K. Celecoxib induces functional recovery after intracerebral hemorrhage with reduction of brain edema and perihematomal cell death. J. Cereb. Blood Flow Metab. 2004, 24, 926–933. [Google Scholar] [CrossRef]
- Wei, F.; Cui, Y.; Guo, X.; Dong, G.; Li, X. Correlations of inflammatory factors, CCCK-18, MMP-9 and D-dimer with APACHE II score and prognosis of patients with acute cerebral hemorrhage. Minerva Med. 2020. [Google Scholar] [CrossRef]
- Tejima, E.; Zhao, B.Q.; Tsuji, K.; Rosell, A.; Leyen, K.V.; Gonzalez, R.G.; Montaner, J.; Wang, X.; Lo, E.H. Astrocytic induction of matrix metalloproteinase-9 and edema in brain hemorrhage. J. Cereb. Blood Flow Metab. 2007, 27, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Hollenberg, M.D.; Yong, V.W. Combination of thrombin and matrix metalloproteinase-9 exacerbates neurotoxicity in cell culture and intracerebral hemorrhage in mice. J. Neurosci. 2006, 26, 10281–10291. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.W.; Poddar, R.; Thompson, J.F.; Rosenberg, G.A.; Yang, Y. Intranuclear matrix metalloproteinases promote DNA damage and apoptosis induced by oxygen-glucose deprivation in neurons. Neuroscience. 2012, 220, 277–290. [Google Scholar] [CrossRef]
- Wang, J.; Tsirka, S.E. Neuroprotection by inhibition of matrix metalloproteinases in a mouse model of intracerebral haemorrhage. Brain 2005, 128, 1622–1633. [Google Scholar] [CrossRef]
- Kim, D.W.; Im, S.H.; Kim, J.Y.; Kim, D.E.; Oh, G.T.; Jeong, S.W. Decreased brain edema after collagenase-induced intracerebral hemorrhage in mice lacking the inducible nitric oxide synthase gene. Laboratory investigation. J. Neurosurg. 2009, 111, 995–1000. [Google Scholar] [CrossRef]
- Szabo, C.; Ischiropoulos, H.; Radi, R. Peroxynitrite: Biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov. 2007, 6, 662–680. [Google Scholar] [CrossRef]
- Xue, M.; Yong, V.W. Matrix metalloproteinases in intracerebral hemorrhage. Neurol. Res. 2008, 30, 775–782. [Google Scholar] [CrossRef]
- Ohnishi, M.; Monda, A.; Takemoto, R.; Matsuoka, Y.; Kitamura, C.; Ohashi, K.; Shibuya, H.; Inoue, A. Sesamin suppresses activation of microglia and p44/42 MAPK pathway, which confers neuroprotection in rat intracerebral hemorrhage. Neuroscience 2012, 232C, 45–52. [Google Scholar] [CrossRef]
- Guadagno, J.; Xu, X.; Karajgikar, M.; Brown, A.; Cregan, S.P. Microglia-derived TNFalpha induces apoptosis in neural precursor cells via transcriptional activation of the Bcl-2 family member Puma. Cell Death Dis. 2013, 4, e538. [Google Scholar] [CrossRef]
- Gong, C.; Ennis, S.R.; Hoff, J.T.; Keep, R.F. Inducible cyclooxygenase-2 expression after experimental intracerebral hemorrhage. Brain Res. 2011, 901, 38–46. [Google Scholar] [CrossRef]
- Lin, S.; Yin, Q.; Zhong, Q.; Lv, F.L.; Zhou, Y.; Li, J.Q.; Wang, J.Z.; Su, B.Y.; Yang, Q.W. Heme activates TLR4-mediated inflammatory injury via MyD88/TRIF signaling pathway in intracerebral hemorrhage. J. Neuroinflammation 2012, 9, 46. [Google Scholar] [CrossRef]
- Wang, Y.C.; Wang, P.F.; Fang, H.; Chen, J.; Xiong, X.Y.; Yang, Q.W. Toll-like receptor 4 antagonist attenuates intracerebral hemorrhage-induced brain injury. Stroke 2013, 44, 2545–2552. [Google Scholar] [CrossRef]
- Henn, A.; Lund, S.; Hedtjärn, M.; Schrattenholz, A.; Pörzgen, P.; Leist, M. The suitability of BV2 cells as alternative model system for primary microglia cultures or for animal experiments examining brain inflammation. ALTEX 2009, 26, 83–94. [Google Scholar] [CrossRef]
- Bani-Hani, M.G.; Greenstein, D.; Mann, B.E.; Green, C.J.; Motterlini, R. Modulation of thrombin-induced neuroinflammation in BV-2 microglia by carbon monoxide-releasing molecule 3. J. Pharmacol. Exp. Ther. 2006, 318, 1315–1322. [Google Scholar] [CrossRef]
- Minghetti, L.; Walsh, D.T.; Levi, G.; Perry, V.H. In vivo expression of cyclooxygenase-2 in rat brain following intraparenchymal injection of bacterial endotoxin and inflammatory cytokines. J. Neuropathol. Exp. Neurol. 1999, 58, 1184–1191. [Google Scholar] [CrossRef]
- Wu, C.H.; Chen, C.C.; Lai, C.Y.; Hung, T.H.; Lin, C.C.; Chao, M.; Chen, S.F. Treatment with TO901317, a synthetic liver X receptor agonist, reduces brain damage and attenuates neuroinflammation in experimental intracerebral hemorrhage. J. Neuroinflamm. 2016, 13, 62. [Google Scholar] [CrossRef]
- James, M.L.; Wang, H.; Cantillana, V.; Lei, B.; Kernagis, D.N.; Dawson, H.N.; Klaman, L.D.; Laskowitz, D.T. TT-301 inhibits microglial activation and improves outcome after central nervous system injury in adult mice. Anesthesiology 2012, 116, 1299–1311. [Google Scholar] [CrossRef]
- Park, H.Y.; Han, M.H.; Park, C.; Jin, C.Y.; Kim, G.Y.; Choi, I.W.; Kim, N.D.; Nam, T.J.; Kwon, T.K.; Choi, Y.H. Anti-inflammatory effects of fucoidan through inhibition of NF-kappaB, MAPK and Akt activation in lipopolysaccharide-induced BV2 microglia cells. Food Chem. Toxicol. 2011, 49, 1745–1752. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Y.; Strong, R.; Zhang, J.; Grotta, J.C.; Aronowski, J. Distinct patterns of intracerebral hemorrhage-induced alterations in NF-kappaB subunit, iNOS, and COX-2 expression. J. Neurochem. 2007, 101, 652–663. [Google Scholar] [CrossRef]
- Jayasooriya, R.G.; Kang, C.H.; Seo, M.J.; Choi, Y.H.; Jeong, Y.K.; Kim, G.Y. Exopolysaccharide of Laetiporus sulphureus var. miniatus downregulates LPS-induced production of NO, PGE(2), and TNF-alpha in BV2 microglia cells via suppression of the NF-kappaB pathway. Food Chem. Toxicol. 2011, 49, 2758–2764. [Google Scholar] [CrossRef]
- Jin, C.Y.; Moon, D.O.; Lee, K.J.; Kim, M.O.; Lee, J.D.; Choi, Y.H.; Park, Y.M.; Kim, G.Y. Piceatannol attenuates lipopolysaccharide-induced NF-kappaB activation and NF-kappaB-related proinflammatory mediators in BV2 microglia. Pharmacol. Res. 2006, 54, 461–467. [Google Scholar] [CrossRef]
- Akundi, R.S.; Candelario-Jalil, E.; Hess, S.; Hull, M.; Lieb, K.; Gebicke-Haerter, P.J.; Fiebich, B.L. Signal transduction pathways regulating cyclooxygenase-2 in lipopolysaccharide-activated primary rat microglia. Glia 2005, 51, 199–208. [Google Scholar] [CrossRef]
- Wu, Y.; Pang, J.; Peng, J.; Cao, F.; Guo, Z.; Jiang, L.; Teng, Z.; Huang, Z.; Cheng, C.; Jiang, Y.; et al. Apolipoprotein E deficiency aggravates neuronal injury by enhancing neuroinflammation via the JNK/c-jun pathway in the early phase of experimental subarachnoid hemorrhage in mice. Oxidative Med. Cell. Longev. 2019, 2019, 3832648. [Google Scholar] [CrossRef]
- Gong, Y.; Xue, B.; Jiao, J.; Jing, L.; Wang, X. Triptolide inhibits COX-2 expression and PGE2 release by suppressing the activity of NF-kappaB and JNK in LPS-treated microglia. J. Neurochem. 2008, 107, 779–788. [Google Scholar] [CrossRef]
- Kim, B.W.; Koppula, S.; Kim, I.S.; Lim, H.W.; Hong, S.M.; Han, S.D.; Hwang, B.Y.; Choi, D.K. Anti-neuroinflammatory activity of Kamebakaurin from Isodon japonicus via inhibition of c-Jun NH(2)-terminal kinase and p38 mitogen-activated protein kinase pathway in activated microglial cells. J. Pharmacol. Sci. 2011, 116, 296–308. [Google Scholar] [CrossRef]
- Han, J.E.; Choi, J.W. Control of JNK for an activation of NADPH oxidase in LPS-stimulated BV2 microglia. Arch. Pharm. Res. 2012, 35, 709–715. [Google Scholar] [CrossRef]
- Michel-Monigadon, D.; Bonny, C.; Hirt, L. c-Jun N-terminal kinase pathway inhibition in intracerebral hemorrhage. Cerebrovasc. Dis. 2010, 29, 564–570. [Google Scholar] [CrossRef]
- Hu, C.Y.; Guo, Y.Q.; Hao, Y.H.; Zheng, L.N.; Qi, Y.H. Research on mechanism of sevoflurane in alleviating cerebral ischemia-reperfusion injury in rats through JNK signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 3907–3914. [Google Scholar]
- Pei, H.; Jiang, T.; Liu, G.; Li, Z.; Luo, K.; An, J.; Li, G.; Guo, Y. The effect of minimally invasive hematoma aspiration on the JNK signal transduction pathway after experimental intracerebral hemorrhage in rats. Int. J. Mol. Sci. 2016, 17, 710. [Google Scholar] [CrossRef]
- Yu, A.; Zhang, T.; Zhong, W.; Duan, H.; Wang, S.; Ye, P.; Wang, J.; Zhong, S.; Yang, Z. miRNA-144 induces microglial autophagy and inflammation following intracerebral hemorrhage. Immunol Lett. 2017, 182, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, H.; Hijioka, M.; Hisatsune, A.; Isohama, Y.; Shudo, K.; Katsuki, H. A retinoic acid receptor agonist Am80 rescues neurons, attenuates inflammatory reactions, and improves behavioral recovery after intracerebral hemorrhage in mice. J. Cereb. Blood Flow Metab. 2011, 31, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.L.; Yen, J.L.; Kuo, Y.C.; Kang, J.J.; Cheng, Y.W.; Huang, W.J.; Hsiao, G. HDAC8 inhibitor WK2-16 therapeutically targets lipopolysaccharide-induced mouse model of neuroinflammation and microglial activation. Int. J. Mol. Sci. 2019, 20, E410. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, G.; Lee, J.J.; Chen, Y.C.; Lin, J.H.; Shen, M.Y.; Lin, K.H.; Chou, D.S.; Sheu, J.R. Neuroprotective effects of PMC, a potent alpha-tocopherol derivative, in brain ischemia-reperfusion: Reduced neutrophil activation and anti-oxidant actions. Biochem. Pharmacol. 2007, 73, 682–693. [Google Scholar] [CrossRef]
- Wu, C.J.; Chou, Y.C.; Cheng, Y.W.; Hsiao, C.J.; Wang, C.H.; Wang, H.Y.; Sheu, J.R.; Hsiao, G. Aristolochic acid downregulates monocytic matrix metalloproteinase-9 by inhibiting nuclear factor-kappaB activation. Chem. Biol. Interact. 2011, 192, 209–219. [Google Scholar] [CrossRef]
- Wang, C.H.; Hsiao, C.J.; Lin, Y.N.; Wu, J.W.; Kuo, Y.C.; Lee, C.K.; Hsiao, G. Carbamazepine attenuates inducible nitric oxide synthase expression through Akt inhibition in activated microglial cells. Pharm. Biol. 2014, 15, 1–9. [Google Scholar] [CrossRef]
- McCarthy, K.D.; Vellis, J.D. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J. Cell Biol. 1980, 85, 890–902. [Google Scholar] [CrossRef]
- Hsiao, G.; Fong, T.H.; Tzu, N.H.; Lin, K.H.; Chou, D.S.; Sheu, J.R. A potent antioxidant, lycopene, affords neuroprotection against microglia activation and focal cerebral ischemia in rats. In Vivo. 2004, 18, 351–356. [Google Scholar]
- Lin, F.L.; Ho, J.D.; Cheng, Y.W.; Chiou, G.C.Y.; Yen, J.L.; Chang, H.M.; Lee, T.H.; Hsiao, G. Theissenolactone C exhibited ocular protection of endotoxin-induced uveitis by attenuating ocular inflammatory responses and glial activation. Front. Pharmacol. 2018, 9, 326. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsueh, P.-J.; Wang, M.-H.; Hsiao, C.-J.; Chen, C.-K.; Lin, F.-L.; Huang, S.-H.; Yen, J.-L.; Tsai, P.-H.; Kuo, Y.-H.; Hsiao, G. Ergosta-7,9(11),22-trien-3β-ol Alleviates Intracerebral Hemorrhage-Induced Brain Injury and BV-2 Microglial Activation. Molecules 2021, 26, 2970. https://doi.org/10.3390/molecules26102970
Hsueh P-J, Wang M-H, Hsiao C-J, Chen C-K, Lin F-L, Huang S-H, Yen J-L, Tsai P-H, Kuo Y-H, Hsiao G. Ergosta-7,9(11),22-trien-3β-ol Alleviates Intracerebral Hemorrhage-Induced Brain Injury and BV-2 Microglial Activation. Molecules. 2021; 26(10):2970. https://doi.org/10.3390/molecules26102970
Chicago/Turabian StyleHsueh, Po-Jen, Mong-Heng Wang, Che-Jen Hsiao, Chih-Kuang Chen, Fan-Li Lin, Shu-Hsien Huang, Jing-Lun Yen, Ping-Huei Tsai, Yueh-Hsiung Kuo, and George Hsiao. 2021. "Ergosta-7,9(11),22-trien-3β-ol Alleviates Intracerebral Hemorrhage-Induced Brain Injury and BV-2 Microglial Activation" Molecules 26, no. 10: 2970. https://doi.org/10.3390/molecules26102970
APA StyleHsueh, P.-J., Wang, M.-H., Hsiao, C.-J., Chen, C.-K., Lin, F.-L., Huang, S.-H., Yen, J.-L., Tsai, P.-H., Kuo, Y.-H., & Hsiao, G. (2021). Ergosta-7,9(11),22-trien-3β-ol Alleviates Intracerebral Hemorrhage-Induced Brain Injury and BV-2 Microglial Activation. Molecules, 26(10), 2970. https://doi.org/10.3390/molecules26102970







