Comparison of CO2 Reduction Performance with NH3 and H2O between Cu/TiO2 and Pd/TiO2
Abstract
1. Introduction
2. Materials and Method
2.1. Preparation of Cu/TiO2 and Pd/TiO2 Photocatalyst
2.2. Characterization of Cu/TiO2 and Pd/TiO2 Film
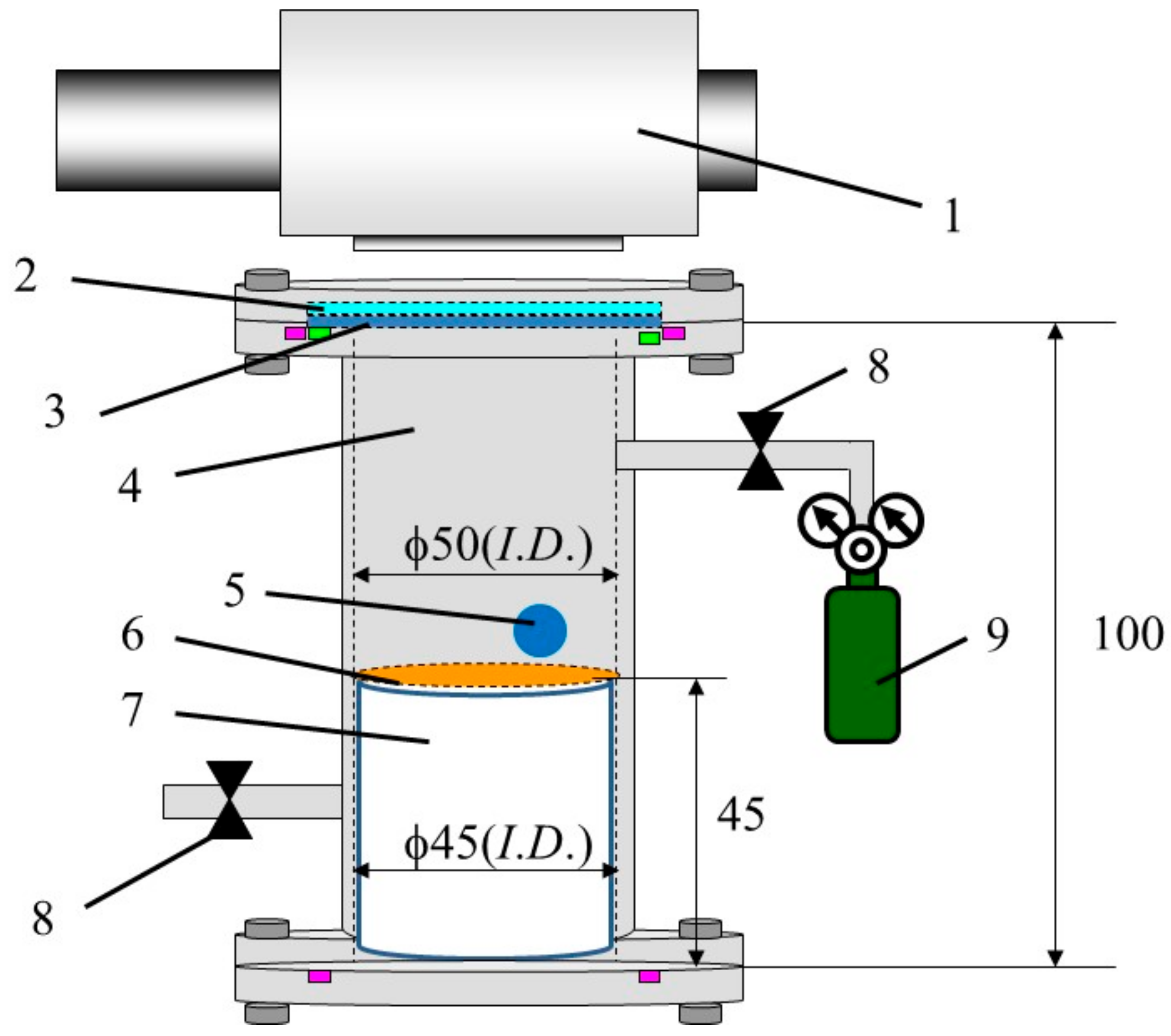
2.3. CO2 Reduction Experiment
3. Results and Discussion
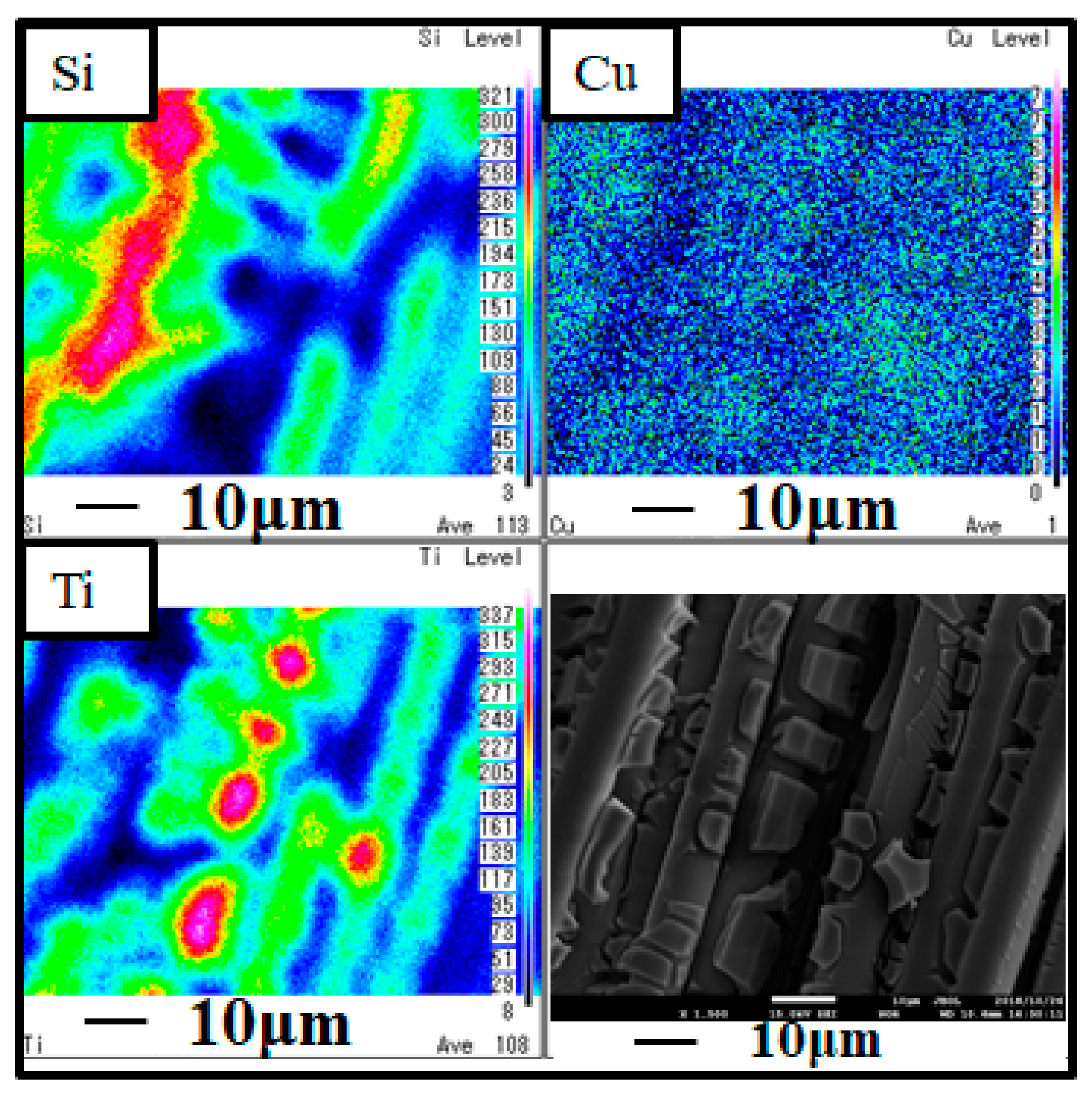
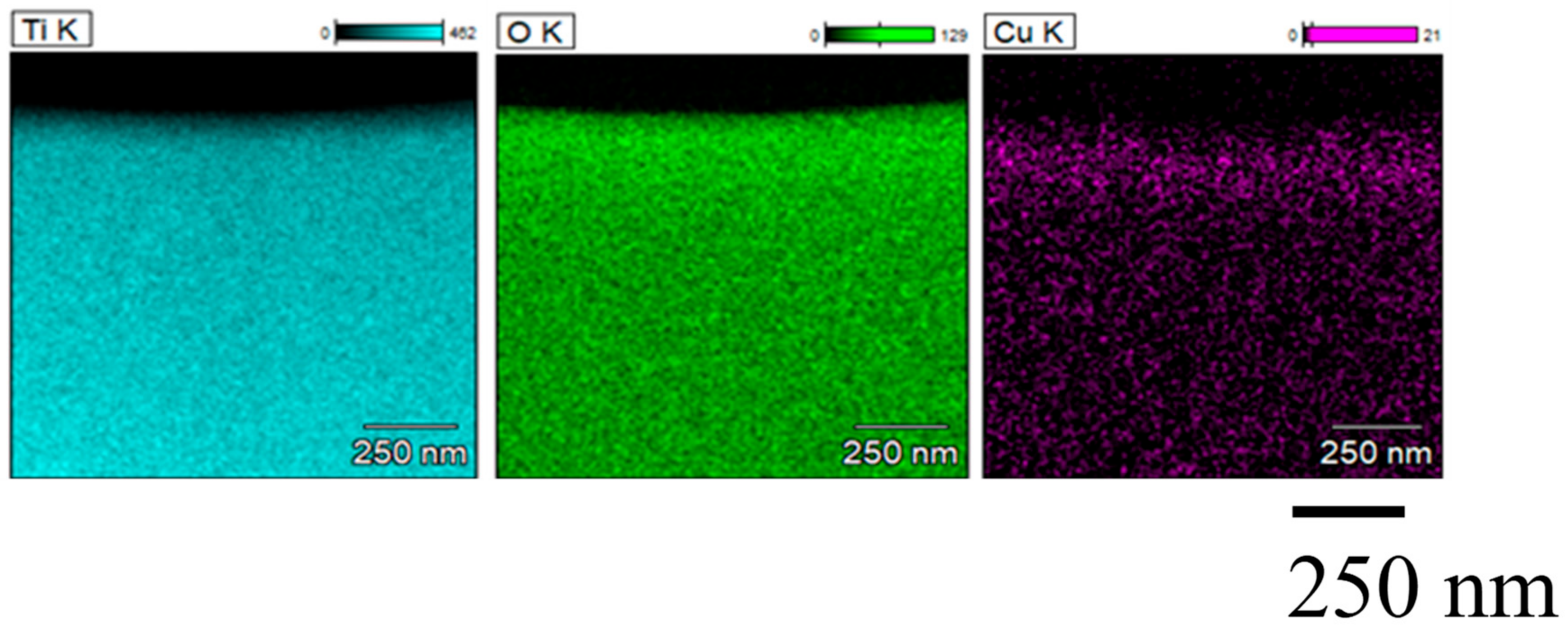
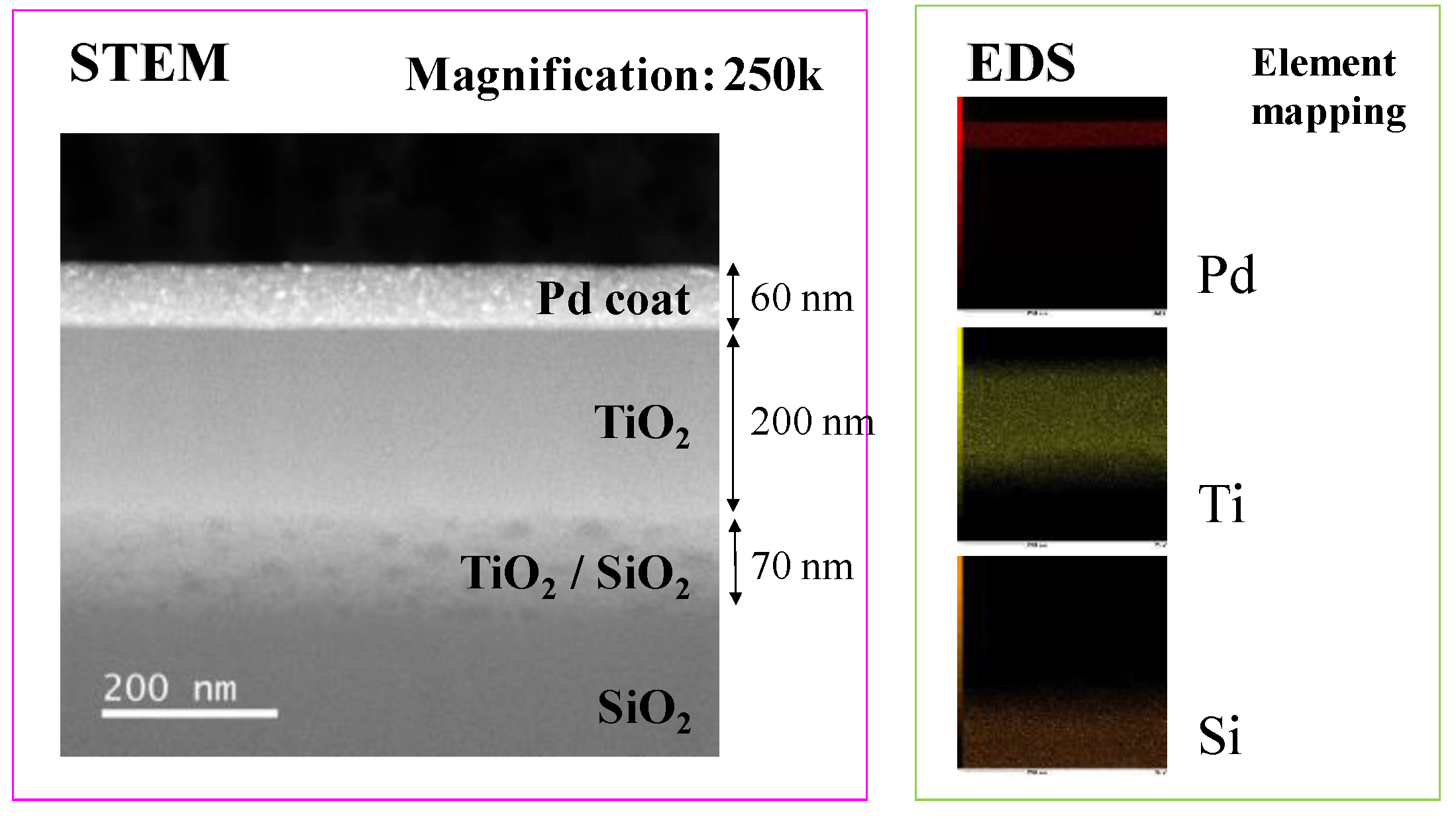
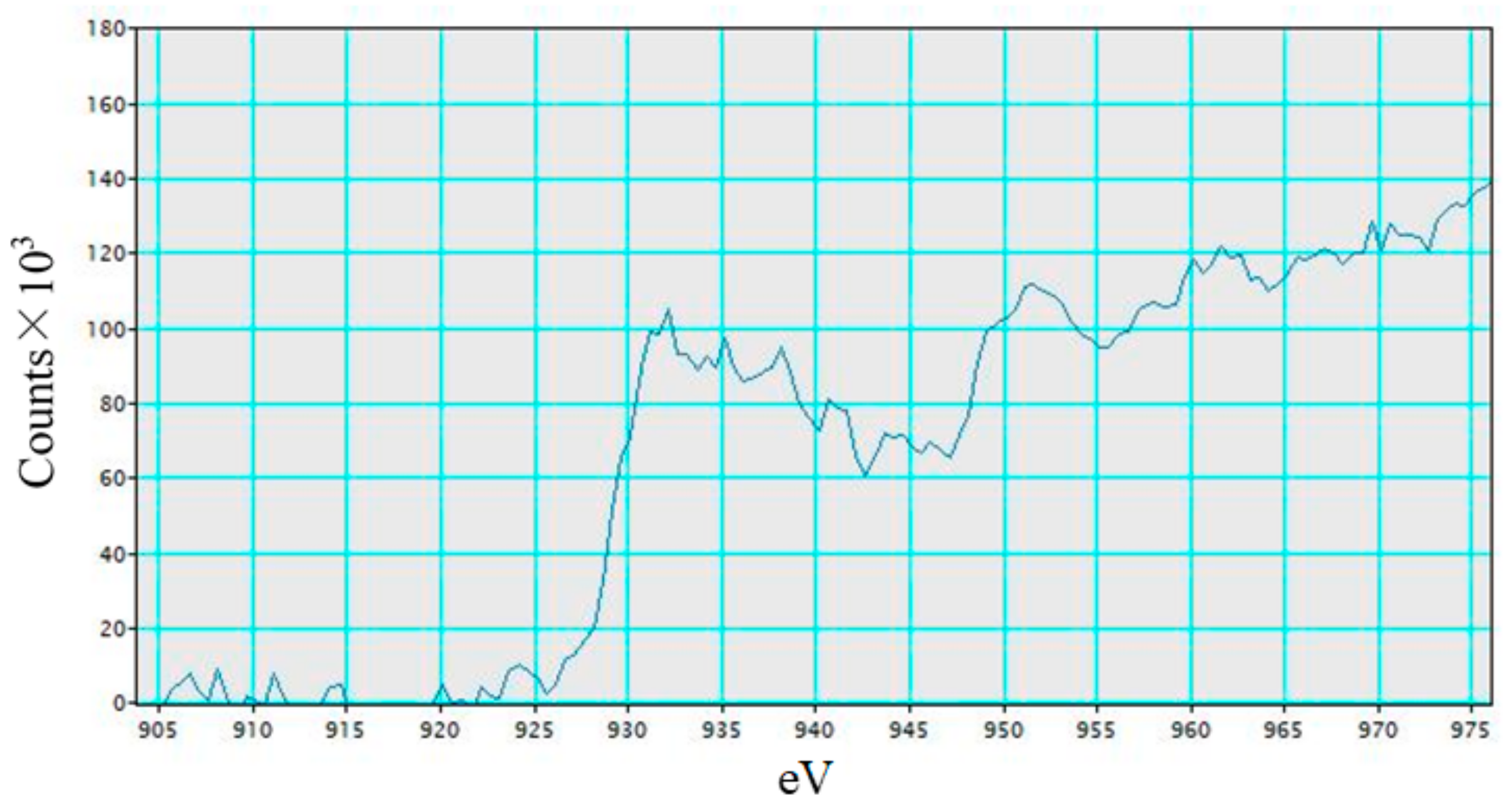
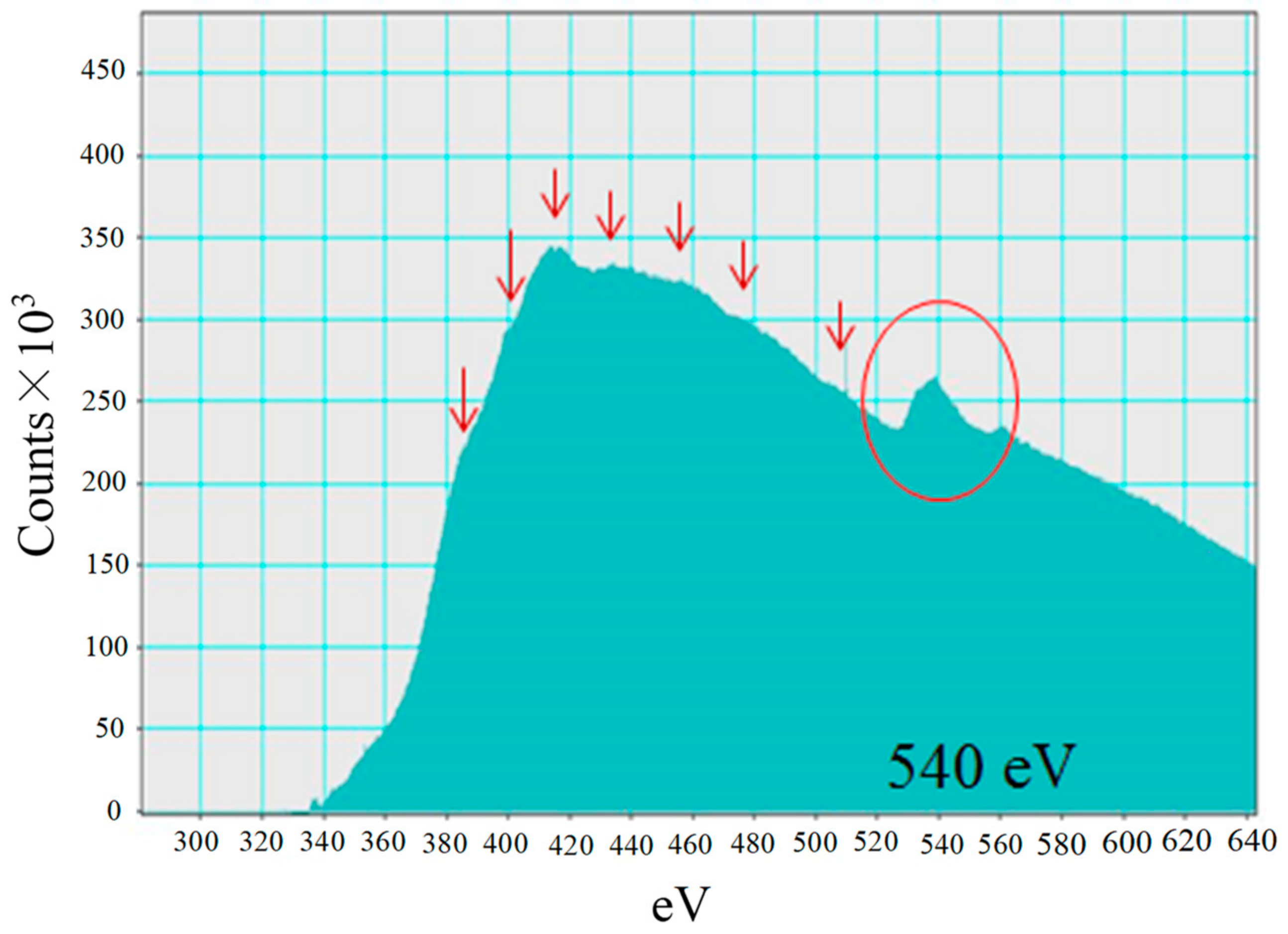
3.1. Characterization Analysis of Cu/TiO2 and Pd/TiO2 Film
3.2. CO2 Reduction Characteristics of Cu/TiO2
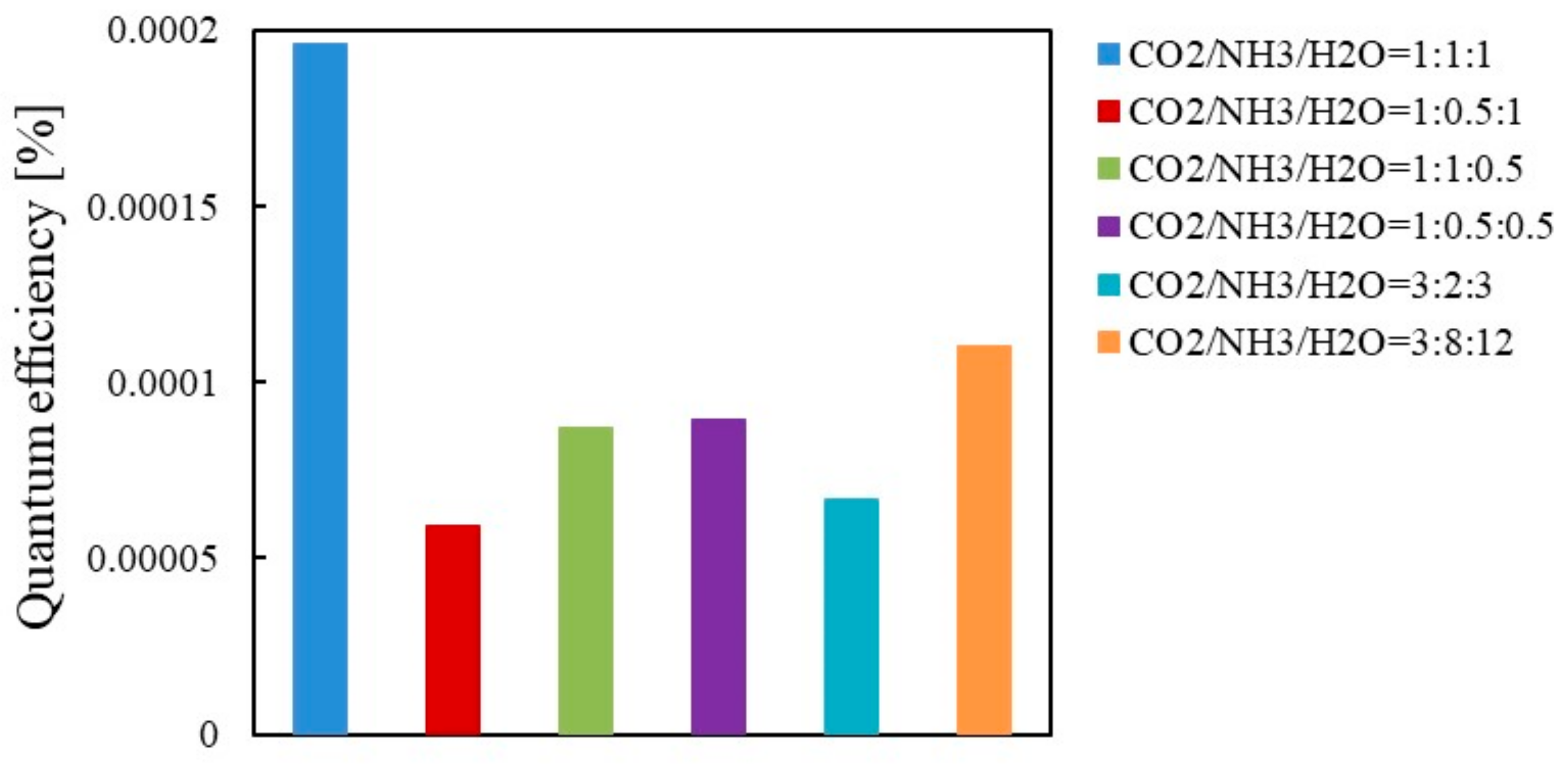
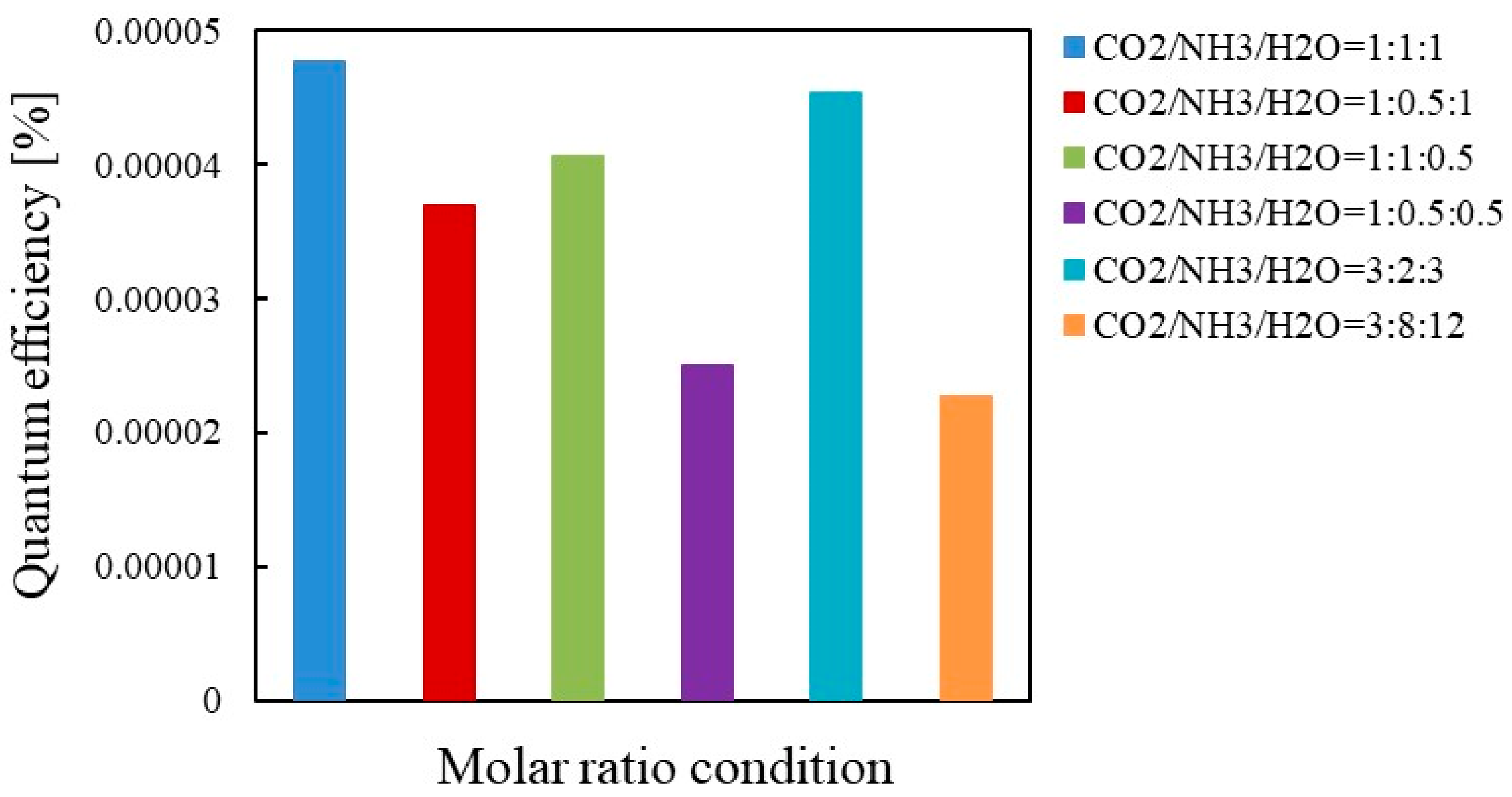
3.3. The Quantum Efficiency Evaluation
4. Conclusions
- (1)
- TiO2 film coated on netlike glass fiber was teeth like. Cu and Pd particles were loaded on TiO2 film uniformly. It is confirmed that the pulse arc plasma gun method can control the amount of metal doped on TiO2 irrespective of metal type.
- (2)
- Cu in Cu/TiO2 prepared in this study exists as Cu+ ion in Cu2O. Pd in Pd/TiO2 prepared in this study exists as Pd metal.
- (3)
- In the case of Cu/TiO2 under the illumination condition of Xe lamp with UV light, the CO2 reduction performance for the molar ratio of CO2/NH3/H2O = 1:1:1 was the highest where the molar quantity of CO per unit weight of photocatalyst was up to 10.2 mol/g.
- (4)
- In the case of Cu/TiO2 under the illumination condition of Xe lamp without UV light, the CO2 reduction performance for the molar ratio of CO2/NH3/H2O = 1:0.5:0.5 was the highest where the molar quantity of CO per unit weight of photocatalyst was 2.5 mol/g.
- (5)
- In the case of Pd/TiO2 under the illumination condition of Xe lamp with UV light, the CO2 reduction performance for the molar ratio of CO2/NH3/H2O = 1:1:1 was the highest where the molar quantity of CO per unit weight of photocatalyst was up to 5.5 mol/g.
- (6)
- In the case of Pd/TiO2 under the illumination condition of Xe lamp without UV light, the CO2 reduction performance for the molar ratio of CO2/NH3/H2O = 1:1:1 was the highest where the molar quantity of CO per unit weight of photocatalyst was up to 3.5 mol/g.
- (7)
- As to Cu/TiO2, the highest quantum efficiency was 1.96 × 10−4 under the illumination condition of Xe lamp with UV light. On the other hand, it was 3.14 × 10−3 if t was set at 6 h when the highest molar quantity of CO per unit weight of photocatalyst was obtained.
- (8)
- As to Pd/TiO2, the highest quantum efficiency was 4.20 × 10−4 under the illumination condition of Xe lamp with UV light. On the other hand, it was 0.53 × 10−4 if t was set at 96 h which was the same illumination time of Xe lamp as Cu/TiO2.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Global Monitoring Laboratory. Available online: https://www/esrl/noaa.gov/gmd/ccgg/trends/global/html (accessed on 15 December 2020).
- Tahir, M.; Amin, N.S. Advances in Visible Light Responsive Titanium Oxide-based Photocatalyts for CO2 Conversion to Hydrocarbon Fuels. Energy Conv. Manag. 2013, 76, 192–214. [Google Scholar] [CrossRef]
- Matavos-Aramyan, S.; Soukhakian, S.; Jazebizadeh, H.M.; Moussavi, M.; Hojjati, M.R. On Engineering Strategies for Photoselective CO2 Reduction—A through Review. Appl. Mater. Today 2020, 18, 100499. [Google Scholar] [CrossRef]
- Remiro-Buenamanana, S.; Garcia, H. Photoassisted CO2 Conversion to Fuels. ChemCatChem Minirev. 2019, 11, 342–356. [Google Scholar] [CrossRef]
- Abdullah, H.; Khan, M.M.R.; Ong, H.R.; Yaakob, Z. Modified TiO2 Photocatalyst for CO2 Photocatalytic Reduction: An Overview. J. CO2 Utilizat. 2017, 22, 15–32. [Google Scholar] [CrossRef]
- Tahir, M.; Amin, N.S. Indium-doped TiO2 Nanoparticles for Photocatalytic CO2 Reduction with H2O Vapors to CH4. Appl. Catal. B Envion. 2015, 162, 98–109. [Google Scholar] [CrossRef]
- Sohn, Y.; Huang, W.; Taghipour, F. Recent Progress and Perspectives in the Photocatalytic CO2 Reduction of Ti-oxide-based Nanomaterials. Appl. Surface Sci. 2017, 396, 1696–1711. [Google Scholar] [CrossRef]
- Hashermizadeh, I.; Golovko, V.B.; Choi, J.; Tsang, D.C.W.; Yip, A.C.K. Photocatalytic Reduction of CO2 to Hydrocarbons Using Bio-templated Porous TiO2 Architectures under UV and Visible Light. Chem. Eng. J. 2018, 347, 64–73. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, P.; Borkar, R.; Bansiwal, A.; Labhsetwar, N.; Jain, S.L. Metal-organic Hybrid: Photoreduction of CO2 Using Graphic Carbon Nitride Supported Heteroleptic Iridium Complex under Visible Light Irradiation. Carbon 2017, 123, 371–379. [Google Scholar] [CrossRef]
- Camarillo, R.; Toston, S.; Martinez, F.; Jimenez, C.; Rincon, J. Preparation of TiO2-based Catalyst with Supercritical Fluid Technology: Characterization and Photocatalytic Activity in CO2 Reduction. J. Chem. Tech. Biotechnol. 2017, 92, 1710–1720. [Google Scholar] [CrossRef]
- Lin, L.Y.; Nie, Y.; Kavadiya, S.; Soundappan, T.; Biswas, P. N-doped Reduced Graphene Oxide Promoted Nano TiO2 as a Bifunctional Adsorbent/Photocatalyst for CO2 Photoreduction: Effect of N Species. Chem. Eng. J. 2017, 316, 449–460. [Google Scholar] [CrossRef]
- Low, J.; Cheng, B.; Yu, J. Surface Modification and Enhanced Photocatalytic CO2 Reduction Performance of TiO2: A Review. Appl. Surf. Sci. 2017, 392, 658–686. [Google Scholar] [CrossRef]
- Rej, S.; Bisetto, M.; Naldoni, A.; Fornasiero, P. Well-defined Cu2O Photocatalysts for Solar Fuels and Chemicals. J. Mater. Chem. A 2021, 9, 5915–5951. [Google Scholar] [CrossRef]
- Goren, Z.; Willner, I.; Nelson, A.J. Selective Photoreduction of CO2/HCO3− to formate by aqueous suspensions and colloids of Pd-TiO2. J. Physic. Chem. 1990, 94, 3784–3790. [Google Scholar] [CrossRef]
- Tseng, I.H.; Chang, W.C.; Wu, J.C.S. Photoreduction of CO2 Using Sol-gel Derived Titania and Titania-supported Copper Catalysts. Appl. Catal. B 2002, 37, 37–38. [Google Scholar] [CrossRef]
- Izumi, Y. Recent Advances in the Photocatalytic Conversion of Carbon Dioxide to Fuels with Water and/or Hydrogen Using Solar Energy and Beyond. Coordin. Chem. Rev. 2013, 257, 171–186. [Google Scholar] [CrossRef]
- Lo, C.C.; Hung, C.H.; Yuan, C.S.; Wu, J.F. Photoreduction of Carbon Dioxide with H2 and H2O over TiO2 and ZrO2 in a Circulated Photocatalytic Reactor. Solar Energy Mater. Sci. 2007, 91, 1765–1774. [Google Scholar] [CrossRef]
- Aguirre, M.E.; Zhou, R.; Eugene, A.J.; Guzman, M.I.; Grela, M.A. Cu2O/TiO2 Heterostructure for CO2 Reduction through a Direct Z-scheme: Protecting Cu2O from Photocorrosion. Appl. Catal. B Environ. 2017, 217, 485–493. [Google Scholar] [CrossRef]
- Kavil, Y.N.; Shaban, Y.A.; Farawati, R.K.A.; Orif, M.I.; Zobidi, M.; Khan, S.U.M. Photocatalytic Conversion of CO2 into Methanol over Cu-C/TiO2 Nanoparticles under UV Light and Natural Sunlight. J. Photochem. Photobiol. A Chem. 2017, 347, 244–253. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, X.; Li, Z.; Ye, X.; Liu, Y.; Chen, Y.; Yang, L.; Chen, M.; Zhang, D.; Li, G.; et al. Cooperation between Inside and Outside of TiO2: Lattice Cu+ Accelerates Carrier Migration to the Surface of Metal Copper for Photocatalytic CO2 Reduction. Appl. Catal. B Environ. 2020, 264, 118515. [Google Scholar] [CrossRef]
- She, H.; Zhao, Z.; Bai, W.; Huang, J.; Wang, L.; Wang, Q. Enhanced Performance of Photocatalytic CO2 Reduction with Synergistic Effect between Chitosan and Cu: TiO2. Mater. Res. Bull. 2020, 124, 110758. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Y.; Shangguan, W. Metal (oxide) modified (M = Pd, Ag, Au and Cu) H2SrTa2O7 for photocatalytic CO2 reduction with H2O: The effect of cocatalysts on promoting activity toward CO and H2 evolution. Int. J. Hydrogen Energy 2019, 44, 4123–4132. [Google Scholar] [CrossRef]
- Yu, Y.; Lan, Z.; Guo, L.; Wang, E.; Yao, J.; Cao, Y. Synergetic effects of Zn and Pd species in TiO2 towards efficient photo-reduction of CO2 into CH4. New J. Chem. 2018, 42, 483–488. [Google Scholar] [CrossRef]
- Singhal, N.; Kumar, U. Noble metal modified TiO2: Selective photoreduction of CO2 to hydrocarbons. Mol. Catal. 2017, 439, 91–99. [Google Scholar] [CrossRef]
- Su, K.Y.; Chen, C.Y.; Wu, R.J. Preparation of Pd/TiO2 Nanowires for the Photoreduction of CO2 into Renewable Hydrocarbon Fuels. J. Taiwan Inst. Chem. Eng. 2019, 996, 409–418. [Google Scholar] [CrossRef]
- Yu, Y.; Zheng, W.; Cao, Y. TiO2-Pd/C Composited Photocatalyst with Improved Photocatalytic Activity for Photoreduction of CO2 into CH4. New J. Chem. 2017, 41, 3204–3210. [Google Scholar] [CrossRef]
- Yui, T.; Kan, A.; Saitoh, C.; Koike, K.; Ibusuki, T.; Ishitani, O. Photochemical Reduction of CO2 Using TiO2: Effects of Organic Absorbates on TiO2 and Deposition of Pd onto TiO2. ACS Appl. Mater. Int. 2011, 3, 2594–2600. [Google Scholar] [CrossRef]
- Nishimura, A.; Ishida, N.; Tatematsu, D.; Hirota, M.; Koshio, A.; Kokai, F.; Hu, E. Effect of Fe Loading Condition and Reductants on CO2 Reduction Performance with Fe/TiO2 Photocatalyst. Int. J. Photoenergy 2017, 2017. [Google Scholar] [CrossRef]
- Nishimura, A.; Sakakibara, Y.; Inoue, T.; Hirota, M.; Koshio, A.; Kokai, F.; Hu, E. Impact of Molar Ratio of NH3 and H2O on CO2 Reduction Performance over Cu/TiO2 Photocatalyst. Phys. Astron. Int. J. 2019, 3, 176–182. [Google Scholar]
- Nemoto, J.; Goken, N.; Ueno, K. Photodecomposition of Ammonia to Dinitrogen and Dihydrogen on Platinized TiO2 Nanoparticles in an Aqueous Solution. J. Photochem. Photobiol. A Chem. 2007, 185, 295–300. [Google Scholar] [CrossRef]
- Kočí, K.; Matějová, L.; Reli, M.; Čapek, L.; Matějka, V.; Lacný, Z.; Kuśtrowski, P.; Obalová, L. Solgel derived Pd supported TiO2-ZrO2 and TiO2 photocatalysts; their examination in photocatalytic reduction of carbon dioxide. Catal. Today 2014, 230, 20–26. [Google Scholar] [CrossRef]
- Nishimura, A.; Toyoda, R.; Tatematsu, D.; Hirota, M.; Koshio, A.; Kokai, F.; Hu, E. Optimum reductants ratio for CO2 reduction by overlapped Cu/TiO2. AIMS Mater. Sci. 2019, 6, 214–233. [Google Scholar] [CrossRef]
- Japan Society of Mechanical Engineering. JSME Heat Transfer Handbook, 1st ed.; Maruzen: Tokyo, Japan, 1993; pp. 366–369. [Google Scholar]
- Nishimura, A.; Inoue, T.; Sakakibara, Y.; Hirota, M.; Koshio, A.; Kokai, F.; Hu, E. Optimum molar ratio of H2 and H2O to reduce CO2 using Pd/TiO2. AIMS Mater. Sci. 2019, 6, 464–483. [Google Scholar] [CrossRef]
- Yang, G.; Cheng, S.; Li, C.; Zhong, J.; Ma, C.; Wang, Z.; Xiang, W. Investigation of the oxidization states of Cu additive in colored borosilicate glasses by electron energy loss spectroscopy. J. Appl. Phys. 2014, 116, 223707. [Google Scholar] [CrossRef]
- Liu, L.; Gao, F.; Zhao, H.; Li, Y. Tailoring Cu valence and oxygen vacancy in Cu/TiO2 catalysts for enhanced CO2 photoreduction efficiency. Appl. Catal. B Environ. 2013, 134–135, 349–358. [Google Scholar] [CrossRef]
- Nishimura, A.; Inoue, T.; Sakakibara, Y.; Hirota, M.; Koshio, A.; Hu, E. Impact of Pd loading on CO2 reduction performance over Pd/TiO2 with H2 and H2O. Molecules 2020, 25, 1468. [Google Scholar] [CrossRef]
- Jensen, J.; Mikkelsen, M.; Krebs, F.C. Flexible substrates as basis for photocatalytic reduction of carbon dioxide. Sol. Energy Mater. Sol. Cells 2011, 95, 2949–2958. [Google Scholar] [CrossRef]
- Paulino, P.N.; Salim, V.M.M.; Resende, N.S. Zu-Cu promoted TiO2 photocatalyst for CO2 reduction with H2O under UV light. Appl. Catal. B Environ. 2016, 185, 362–370. [Google Scholar] [CrossRef]
- Tahir, M.; Amin, N.A.S. Photo-induced CO2 reduction by hydrogen for selective CO evolution in a dynamic monolith photoreactor loaded with Ag-modified TiO2 nanocatalyst. Int. J. Hydrogen Energy 2017, 42, 15507–15522. [Google Scholar] [CrossRef]
- Hoque, M.A.; Guzman, G.J. Photocatalytic activity: Experimental features to report in heterogeneous photocatalysis. Materials 2018, 11, 1990. [Google Scholar] [CrossRef]
- Nahar, S.; Zain, M.F.; Kadhum, A.A.H.; Hasan, H.A.; Hasan, M.R. Advances in photocatalytic CO2 reduction with water: A review. Materials 2017, 10, 629. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.N.; Zhan, Z.; Woo, M.H.; Wu, C.Y.; Biswas, P. Photocatalytic reduction of CO2 with H2O on Mesoporous Silica Supported Cu/TiO2 Catalysts. Appl. Catal. B Environ. 2010, 100, 386–392. [Google Scholar] [CrossRef]
- Razzaq, A.; Ali, S.; Asif, M.; In, S.I. Layered double hydroxide (LDH) based photocatalysts: An outstanding strategy for efficient photocatalytic CO2 conversion. Catalyst 2020, 10, 1185. [Google Scholar] [CrossRef]
- Olowoyo, J.O.; Kumar, M.; Dash, T.; Saran, S.; Bhandari, S.; Kumar, U. Self-organized copper impregnation and doping in TiO2 with enhanced photocatalytic conversion of H2O and CO2 to fuel. Int. J. Hydrogen Energy 2018, 43, 19468–19480. [Google Scholar] [CrossRef]
- Jiao, J.; Wei, Y.; Zhao, Y.; Zhao, Z.; Duan, A.; Liu, J.; Pang, Y.; Li, J.; Jiang, G.; Wang, Y. AuPd/3DOM-TiO2 catalysts for photocatalytic reduction of CO2: High efficient separation of photogenerated charge carriers. Appl. Catal. B Environ. 2017, 209, 228–239. [Google Scholar] [CrossRef]




| Time [h] | 0 | 3 | 6 | 9 | 12 | 15 | 18 | 21 | 24 | 48 | 72 | 96 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CO2:NH3:H2O = 1:1:1 | 0 | 6.3 | 10.2 | 9.5 | 8.5 | 8.4 | 7.4 | 6.8 | 5.7 | 3.8 | 6.6 | 4.5 |
| CO2:NH3:H2O = 1:0.5:1 | 0 | 3.0 | 4.4 | 5.1 | 5.0 | 4.8 | 5.3 | 5.0 | 5.0 | 3.1 | 2.6 | 2.7 |
| CO2:NH3:H2O = 1:1:0.5 | 0 | 4.8 | 6.3 | 6.5 | 7.1 | 6.8 | 6.8 | 6.8 | 7.9 | 4.7 | 5.4 | 3.9 |
| CO2:NH3:H2O = 1:0.5:0.5 | 0 | 5.3 | 7.0 | 5.0 | 6.4 | 7.7 | 8.0 | 6.5 | 5.1 | 3.8 | 3.6 | 4.2 |
| CO2:NH3:H2O = 3:2:3 | 0 | 4.6 | 5.9 | 6.0 | 4.7 | 5.4 | 5.9 | 5.8 | 4.0 | 3.6 | 1.7 | 2.3 |
| CO2:NH3:H2O = 3:8:12 | 0 | 3.4 | 5.7 | 6.3 | 6.6 | 4.7 | 4.3 | 4.6 | 5.1 | 3.5 | 4.8 | 6.2 |
| Time [h] | 0 | 3 | 6 | 9 | 12 | 15 | 18 | 21 | 24 | 48 | 72 | 96 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CO2:NH3:H2O = 1:1:1 | 0 | 0.6 | 0.8 | 0.9 | 0.9 | 1.0 | 1.2 | 1.4 | 2.0 | 1.9 | 1.5 | 1.2 |
| CO2:NH3:H2O = 1:0.5:1 | 0 | 0.9 | 1.0 | 1.3 | 1.1 | 1.4 | 1.6 | 1.1 | 1.0 | 0.7 | 0.9 | 1.1 |
| CO2:NH3:H2O = 1:1:0.5 | 0 | 0.8 | 1.4 | 1.7 | 1.6 | 1.4 | 1.2 | 1.1 | 1.0 | 1.3 | 1.0 | 1.6 |
| CO2:NH3:H2O = 1:0.5:0.5 | 0 | 0.5 | 1.2 | 1.4 | 1.4 | 2.1 | 2.5 | 1.7 | 1.3 | 1.7 | 2.3 | 1.7 |
| CO2:NH3:H2O = 3:2:3 | 0 | 0.5 | 1.1 | 1.6 | 1.4 | 0.9 | 1.3 | 1.5 | 2.2 | 1.4 | 1.1 | 1.3 |
| CO2:NH3:H2O = 3:8:12 | 0 | 0.4 | 1.1 | 1.5 | 1.0 | 0.7 | 0.6 | 0.9 | 0.9 | 1.1 | 1.5 | 1.4 |
| Time [h] | 0 | 3 | 6 | 9 | 12 |
|---|---|---|---|---|---|
| CO2:NH3:H2O = 1:1:1 | 0 | 1.6 | 5.5 | 5.0 | 2.8 |
| CO2:NH3:H2O = 1:0.5:1 | 0 | 1.4 | 1.4 | 0 | 0.1 |
| CO2:NH3:H2O = 1:1:0.5 | 0 | 2.1 | 1.1 | 1.1 | 1.4 |
| CO2:NH3:H2O = 1:0.5:0.5 | 0 | 2.0 | 1.5 | 0.8 | 1.2 |
| CO2:NH3:H2O = 3:2:3 | 0 | 1.5 | 1.6 | 1.4 | 2.4 |
| CO2:NH3:H2O = 3:8:12 | 0 | 1.7 | 1.2 | 1.8 | 1.1 |
| Time [h] | 0 | 24 | 48 | 72 | 96 |
|---|---|---|---|---|---|
| CO2:NH3:H2O = 1:1:1 | 0 | 1.7 | 3.5 | 3.2 | 3.1 |
| CO2:NH3:H2O = 1:0.5:1 | 0 | 1.8 | 2.0 | 2.1 | 2.5 |
| CO2:NH3:H2O = 1:1:0.5 | 0 | 2.5 | 2.3 | 3.1 | 1.6 |
| CO2:NH3:H2O = 1:0.5:0.5 | 0 | 2.5 | 1.4 | 2.0 | 2.0 |
| CO2:NH3:H2O = 3:2:3 | 0 | 1.6 | 1.6 | 3.0 | 2.8 |
| CO2:NH3:H2O = 3:8:12 | 0 | 1.7 | 1.7 | 1.5 | 1.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishimura, A.; Shimada, R.; Sakakibara, Y.; Koshio, A.; Hu, E. Comparison of CO2 Reduction Performance with NH3 and H2O between Cu/TiO2 and Pd/TiO2. Molecules 2021, 26, 2904. https://doi.org/10.3390/molecules26102904
Nishimura A, Shimada R, Sakakibara Y, Koshio A, Hu E. Comparison of CO2 Reduction Performance with NH3 and H2O between Cu/TiO2 and Pd/TiO2. Molecules. 2021; 26(10):2904. https://doi.org/10.3390/molecules26102904
Chicago/Turabian StyleNishimura, Akira, Ryouga Shimada, Yoshito Sakakibara, Akira Koshio, and Eric Hu. 2021. "Comparison of CO2 Reduction Performance with NH3 and H2O between Cu/TiO2 and Pd/TiO2" Molecules 26, no. 10: 2904. https://doi.org/10.3390/molecules26102904
APA StyleNishimura, A., Shimada, R., Sakakibara, Y., Koshio, A., & Hu, E. (2021). Comparison of CO2 Reduction Performance with NH3 and H2O between Cu/TiO2 and Pd/TiO2. Molecules, 26(10), 2904. https://doi.org/10.3390/molecules26102904








