The Effects of CO2 Additional on Flame Characteristics in the CH4/N2/O2 Counterflow Diffusion Flame
Abstract
1. Introduction
2. Numerical Method
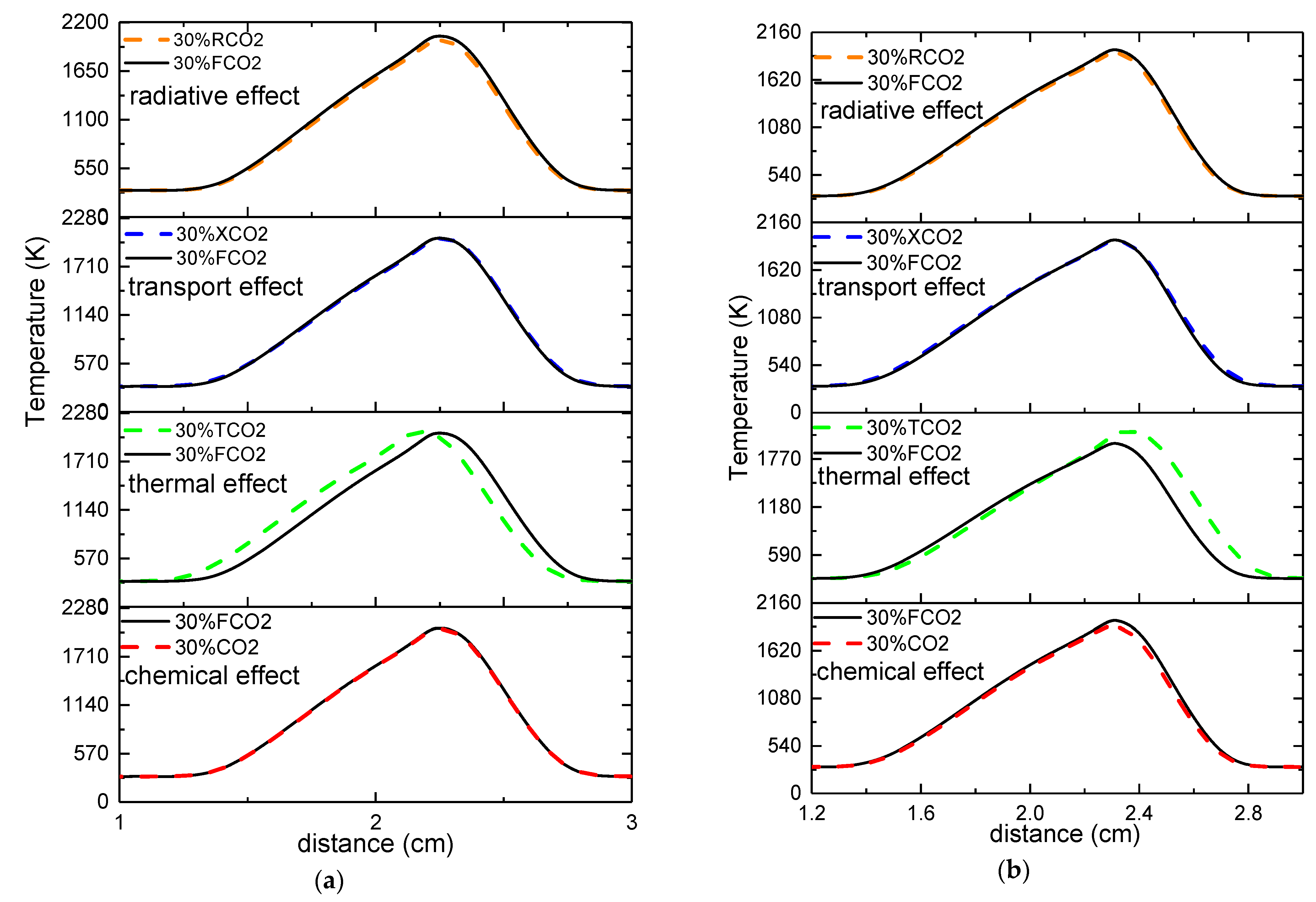
- The chemical effect. FCO2, which has the same thermal, transport, and radiative properties as CO2, but does not participate in the chemical reaction, was introduced to evaluate the chemical effect of CO2. Therefore, any differences in the results between FCO2 and CO2 were entirely contributed by the chemical effect of CO2.
- The thermal effect. TCO2, which has the chemical, transport, and radiative properties as FCO2, but owns the same thermal properties as normal N2, was employed. Thus, any differences in the results between FCO2 and TCO2 were totally caused by the thermal effect of CO2.
- The transport effect. XCO2, which has the same chemical, thermal, and transport properties as FCO2, but owns the same transport properties as N2. The transport effect of CO2 was revealed by the difference in results between FCO2 and XCO2.
- The radiative effect. RCO2, which has the same chemical, thermal, and transport properties as FCO2, but is hot transparent body and cannot contribute to radiative heat transfer. Therefore, the radiative effects of CO2 were determined as a function of the difference in results between FCO2 and RCO2.
3. Results and Discussion
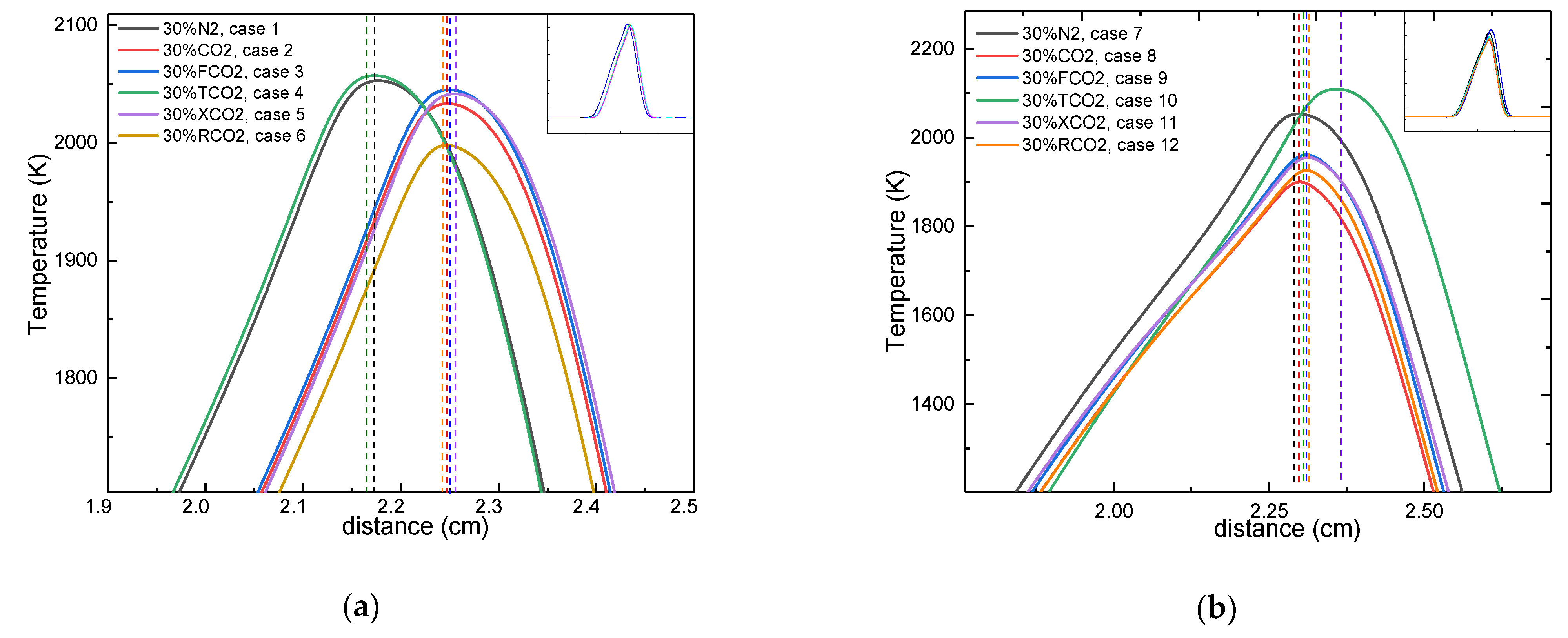
3.1. The CO2 Effect on the Flame Temperature
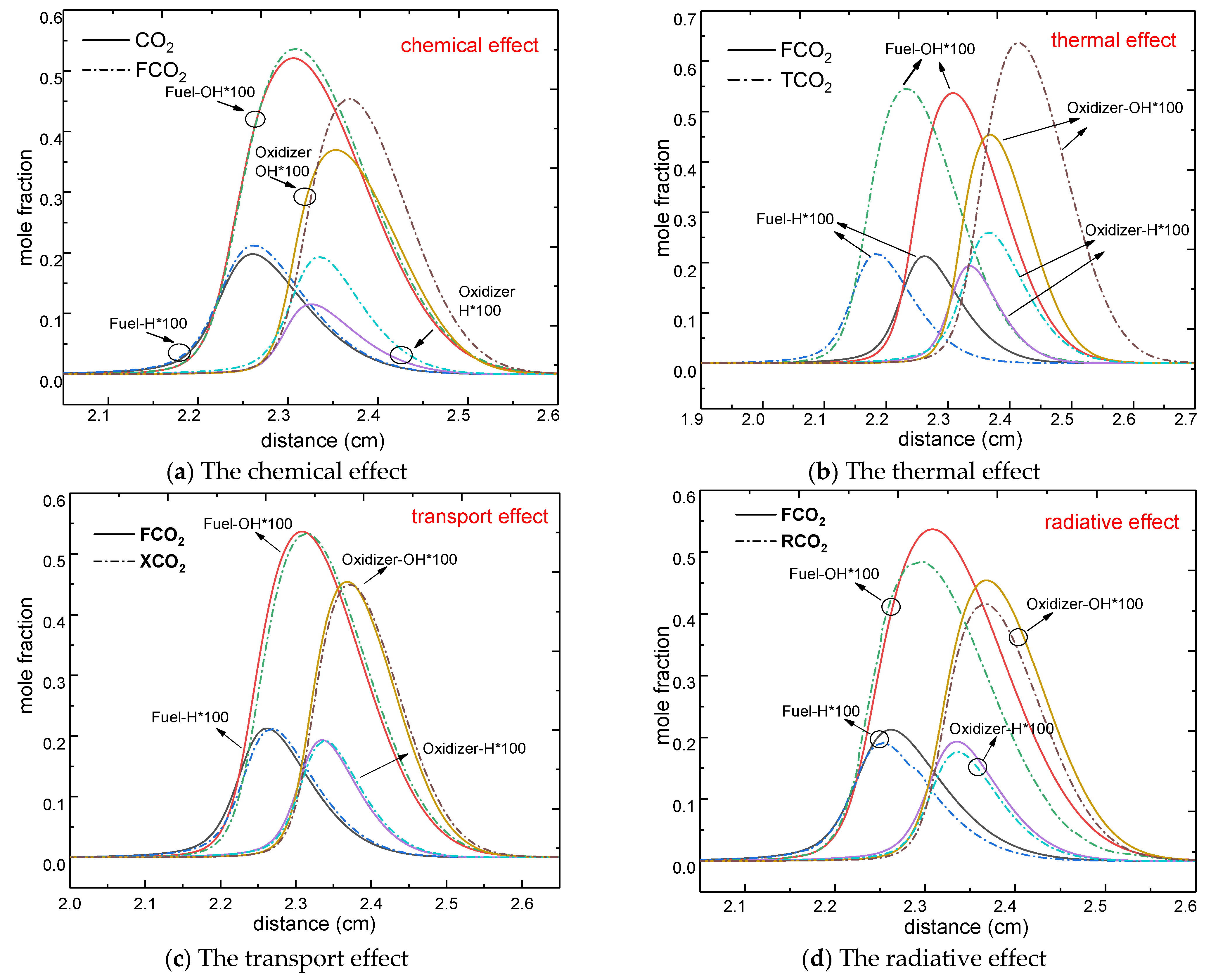
3.2. The CO2 Effect on the H and OH Radicals
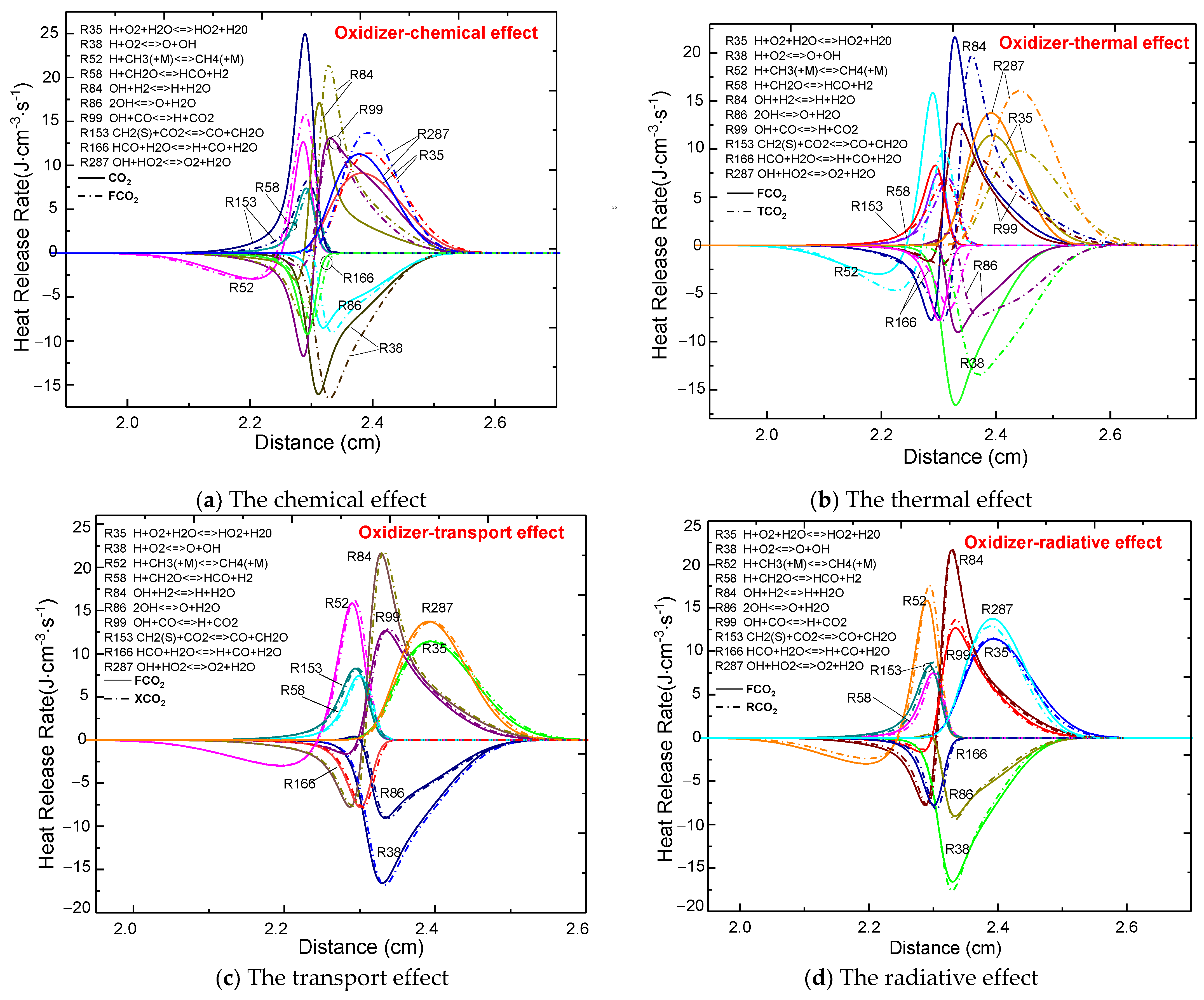
3.3. The CO2 Effect on the Heat Release Rate (HRR)
3.3.1. Comparison of the Four Effects upon Dilution of the Fuel Side
3.3.2. Comparison of the Four Effects upon Dilution of the Oxidizer Side
4. Conclusions
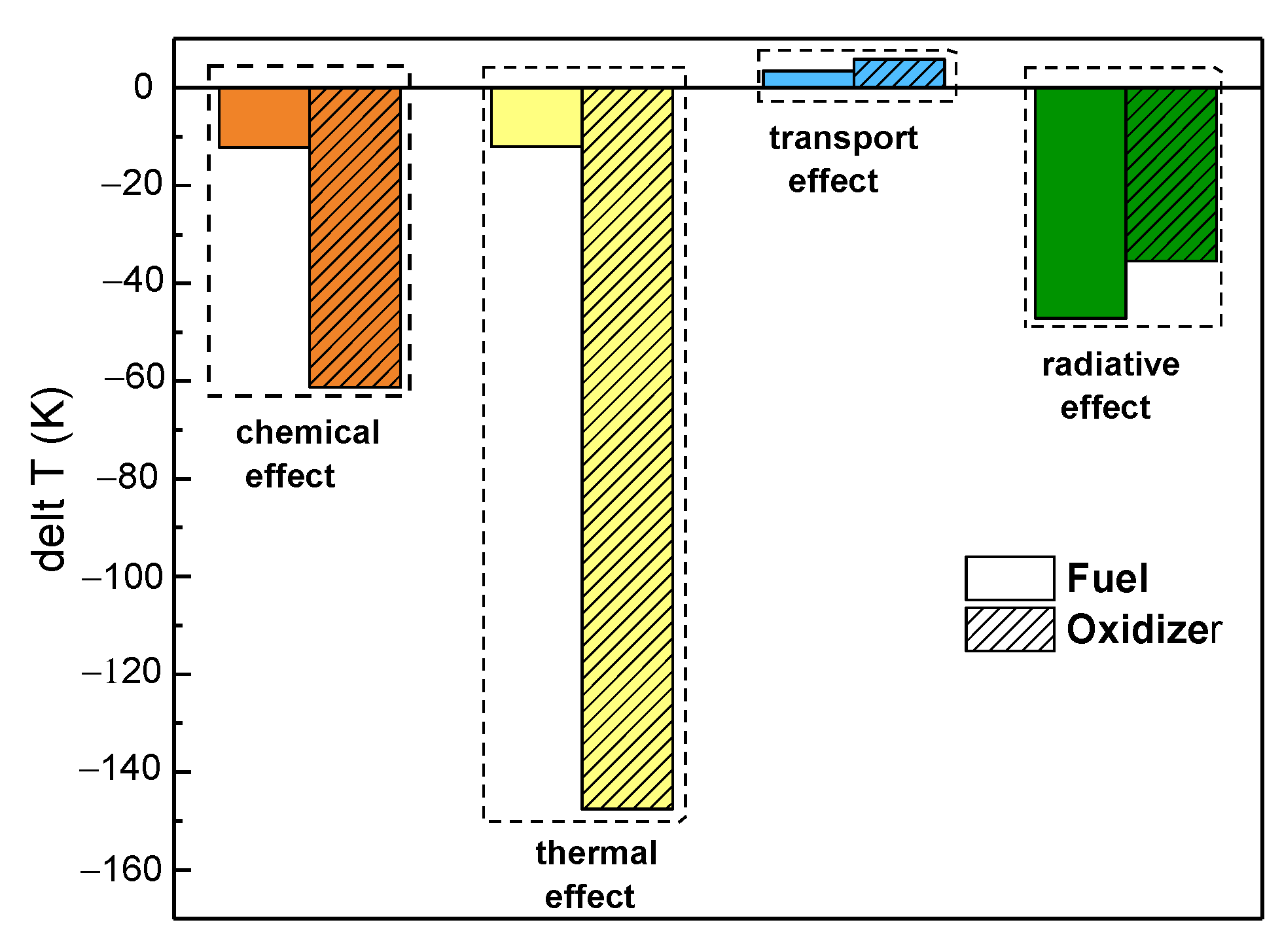
- The synergistic effects of CO2 on the flame temperature were negative regardless of which side was diluted, with a greater effect seen upon dilution of the oxidizer side. The chemical, thermal, and radiative properties led to a decrease in flame temperature, whereas the transport properties increased the flame temperature. Moreover, the thermal properties had a more prominent effect on the flame temperature upon dilution of the fuel side, whereas the radiative properties had a predominant effect on the flame temperature upon dilution of the oxidizer side.
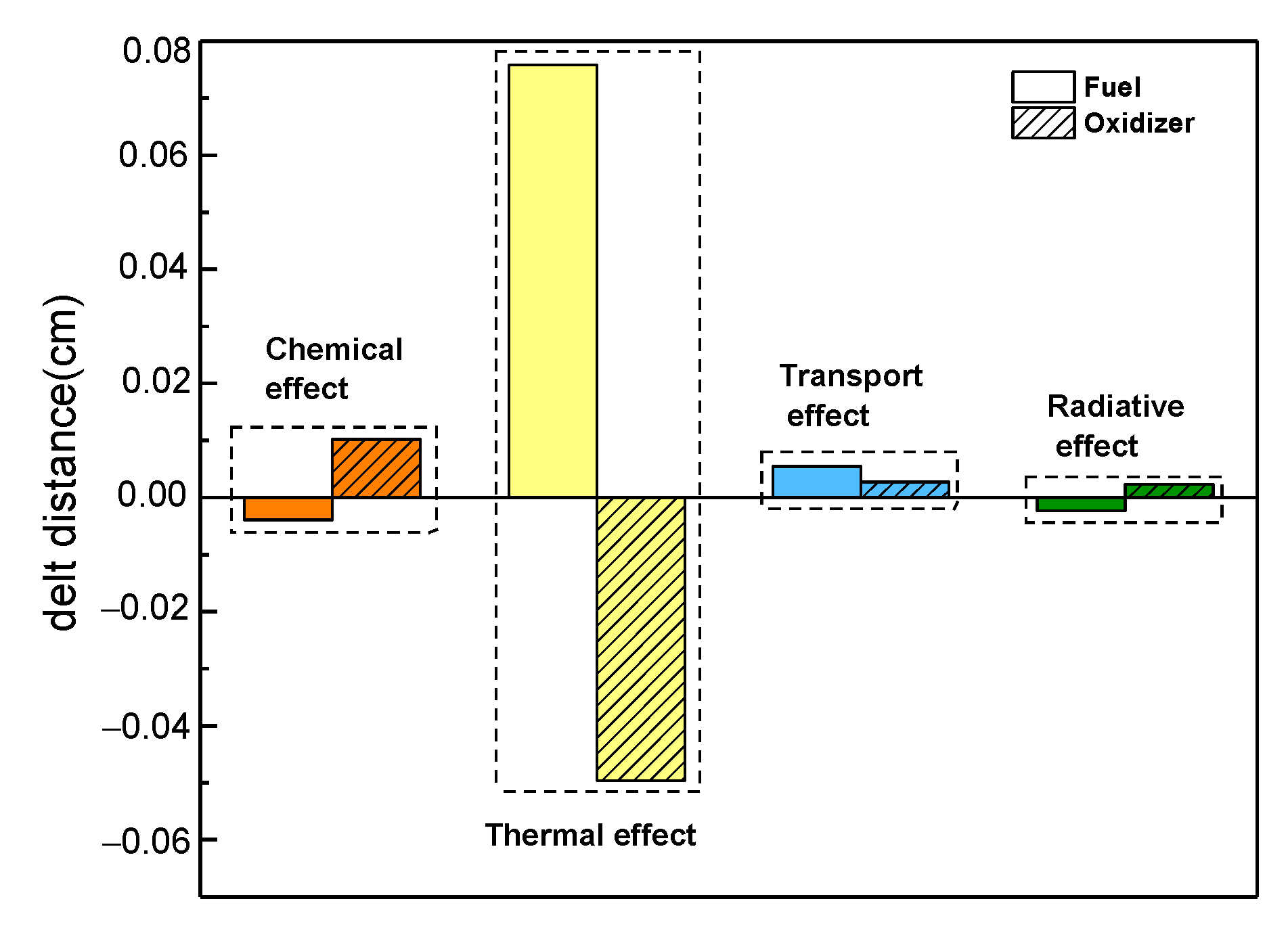
- The effects of CO2 showed great differences with respect to the position of the flame front. When the fuel side was diluted with CO2, the chemical and radiative effects moved the position of the flame front toward the fuel side, whereas the thermal and transport effects moved the position of the flame front toward the oxidizer side. However, when the oxidizer side was diluted with CO2, the chemical, transport, and radiative effects moved the position of the flame front toward the oxidizer side, with the opposite outcome seen due to the thermal effect. Consistently, the thermal effect on the position of the flame front was the most conspicuous regardless of which side was diluted.
- The chemical and radiative effects of CO2 resulted in a slight decrease in the mole fraction of H and OH radicals regardless of which side was diluted. The thermal properties of CO2 only affected the distribution of H and OH radicals upon dilution of the fuel side, with no significant effect on its peak. However, the thermal properties of CO2 not only affected the distribution of OH and H radicals upon dilution of the oxidizer side, but also reduced their maximum mole fraction, whereas the effect of transport properties on OH and H radicals could be neglected.
- The influence of CO2 on the heat release rate was consistent with its influence on temperature and free radical concentration regardless of which side was diluted.
Author Contributions
Funding
Conflicts of Interest
References
- Gu, M.; Chu, H.; Liu, F. Effects of simultaneous hydrogen enrichment and carbon dioxide dilution of fuel on soot formation in an axisymmetric coflow laminar ethylene/air diffusion flame. Combust. Flame 2016, 166, 216–228. [Google Scholar] [CrossRef]
- Baratta, M.; Chiriches, S.; Goel, P.; Misul, D. CFD modelling of natural gas combustion in IC engines under different EGR dilution and H2-doping conditions. Transp. Eng. 2020, 2, 100018. [Google Scholar] [CrossRef]
- Hu, E.; Huang, Z.; Liu, B.; Zheng, J.; Gu, X. Experimental study on combustion characteristics of a spark-ignition engine fueled with natural gas–hydrogen blends combining with EGR. Int. J. Hydrog. Energy 2009, 34, 1035–1044. [Google Scholar] [CrossRef]
- Hu, X.; Yu, Q.; Liu, J. Chemical effect of CO2 on the laminar flame speeds of oxy-methane mixtures in the condition of various equivalence ratios and oxygen concentrations. Int. J. Hydrog. Energy 2016, 41, 15068–15077. [Google Scholar] [CrossRef]
- Ren, F.; Xiang, L.; Chu, H.; Han, W. Effects of strain rate and CO2 on NO formation in CH4/N2/O2 counter-flow diffusion flames. Therm. Sci. 2018, 22, 769–776. [Google Scholar] [CrossRef]
- Shih, H. Computed extinction limits and flame structures of H2/O2 counterflow diffusion flames with CO2 dilution. Int. J. Hydrog. Energy 2009, 34, 4005–4013. [Google Scholar] [CrossRef]
- Jia, H.; Zou, C.; Lu, L.; Zheng, H.; Qian, X.; Yao, H. Ignition of CH4 intensely diluted with N2 and CO2 versus hot air in a counterflow jets. Energy 2018, 165, 315–325. [Google Scholar] [CrossRef]
- Xu, H.; Liu, F.; Sun, S.; Zhao, Y.; Meng, S.; Tang, W. Effects of H2O and CO2 diluted oxidizer on the structure and shape of laminar coflow syngas diffusion flames. Combust. Flame 2017, 177, 67–78. [Google Scholar] [CrossRef]
- Shih, H.; Hsu, J. A computational study of combustion and extinction of opposed-jet syngas diffusion flames. Int. J. Hydrog. Energy 2011, 36, 15868–15879. [Google Scholar] [CrossRef]
- Fotache, C.G.; Kreutz, T.G.; Law, C.K. Ignition of counterflowing methane versus heated air under reduced and elevated pressures. Combust. Flame 1997, 108, 442–470. [Google Scholar] [CrossRef]
- Galmiche, B.; Halter, F.; Foucher, F.; Dagaut, P. Effects of Dilution on Laminar Burning Velocity of Premixed Methane/Air Flames. Energ. Fuels 2011, 25, 948–954. [Google Scholar] [CrossRef]
- Jithin, E.V.; Varghese, R.J.; Velamati, R.K. Experimental and numerical investigation on the effect of hydrogen addition and N2/CO2 dilution on laminar burning velocity of methane/oxygen mixtures. Int. J. Hydrog. Energy 2020, 45, 16838–16850. [Google Scholar] [CrossRef]
- Xie, M.; Fu, J.; Zhang, Y.; Shu, J.; Ma, Y.; Liu, J.; Zeng, D. Numerical analysis on the effects of CO2 dilution on the laminar burning velocity of premixed methane/air flame with elevated initial temperature and pressure. Fuel 2020, 264, 116858. [Google Scholar] [CrossRef]
- Hu, E.; Jiang, X.; Huang, Z.; Iida, N. Numerical Study on the Effects of Diluents on the Laminar Burning Velocity of Methane–Air Mixtures. Energ. Fuels 2012, 26, 4242–4252. [Google Scholar] [CrossRef]
- Yang, J.; Gong, Y.; Guo, Q.; Zhu, H.; Wang, F.; Yu, G. Experimental studies of the effects of global equivalence ratio and CO2 dilution level on the OH* and CH* chemiluminescence in CH4/O2 diffusion flames. Fuel 2020, 278, 118307. [Google Scholar] [CrossRef]
- Gascoin, N.; Yang, Q.; Chetehouna, K. Thermal effects of CO2 on the NOX formation behavior in the CH4 diffusion combustion system. Appl. Therm. Eng. 2017, 110, 144–149. [Google Scholar] [CrossRef]
- Ren, F.; Xiang, L.; Chu, H.; Ya, Y.; Han, W.; Nie, X. Numerical investigation on the effect of CO2 and steam for the H2 intermediate formation and NOX emission in laminar premixed methane/air flames. Int. J. Hydrog. Energy 2020, 45, 3785–3794. [Google Scholar] [CrossRef]
- Wang, J.; Niioka, T. The effect of radiation reabsorption on NO formation in CH4/air counterflow diffusion flames. Combust. Theory Model. 2001, 5, 385–398. [Google Scholar] [CrossRef]
- Wang, J.; Niioka, T. Numerical study of radiation reabsorption effect on NOX formation in CH4/air counterflow premix flame. Proc. Combust. Inst. 2002, 29, 2211–2218. [Google Scholar] [CrossRef]
- Giles, D.; Som, S.; Aggarwal, S. NOx emission characteristics of counterflow syngas diffusion flames with airstream dilution. Fuel 2006, 85, 1729–1742. [Google Scholar] [CrossRef]
- Kim, S.; Park, J.; Keel, S. Thermal and chemical contributions of added H2O and CO2 to major flame structures and NO emission characteristics in H2/N2 laminar diffusion flame. Int. J. Energy Res. 2002, 26, 1073–1086. [Google Scholar] [CrossRef]
- Park, J.; Kim, S.; Lee, K.; Kim, T.K. Chemical effect of diluents on flame structure and NO emission characteristic in methane-air counterflow diffusion flame. Int. J. Energy Res. 2002, 26, 1141–1160. [Google Scholar] [CrossRef]
- Lee, C.; Lee, S.; Han, J.; Park, J. Numerical study on effect of CO2 addition in fame structure and NOX formation of CH4-air counterflow diffusion flames. Int. J. Energy Res. 2001, 25, 343–354. [Google Scholar] [CrossRef]
- Liu, F.; Guo, H.; Smallwood, G.J.; Gülder, Ö.L. The chemical effects of carbon dioxide as an additive in an ethylene diffusion flame: Implications for soot and NOx formation. Combust. Flame 2001, 125, 778–787. [Google Scholar] [CrossRef]
- Song, Y.; Zou, C.; He, Y.; Zheng, C. The chemical mechanism of the effect of CO2 on the temperature in methane oxy-fuel combustion. Int. J. Heat Mass Transf. 2015, 86, 622–628. [Google Scholar] [CrossRef]
- Chen, Z.; Qin, X.; Xu, B.; Ju, Y.; Liu, F. Studies of radiation absorption on flame speed and flammability limit of CO2 diluted methane flames at elevated pressures. Proc. Combust. Inst. 2007, 31, 2693–2700. [Google Scholar] [CrossRef]
- Li, X.; Jia, L.; Onishi, T.; Grajetzki, P.; Nakamura, H.; Tezuka, T.; Hasegawa, S.; Maruta, K. Study on stretch extinction limits of CH4/CO2 versus high temperature O2/CO2 counterflow non-premixed flames. Combust. Flame 2014, 161, 1526–1536. [Google Scholar] [CrossRef]
- Min, J.; Baillot, F. Experimental investigation of the flame extinction processes of nonpremixed methane flames inside an air coflow diluted with CO2, N2, or Ar. Combust. Flame 2012, 159, 3502–3517. [Google Scholar] [CrossRef]
- Zhou, Q.; Cheung, C.S.; Leung, C.W.; Li, X.; Huang, Z. Effects of diluents on laminar burning characteristics of bio-syngas at elevated pressure. Fuel 2019, 248, 8–15. [Google Scholar] [CrossRef]
- Vu, T.M.; Park, J.; Kwon, O.B.; Bae, D.S.; Yun, J.H.; Keel, S.I. Effects of diluents on cellular instabilities in outwardly propagating spherical syngas–air premixed flames. Int. J. Hydrog. Energy 2010, 35, 3868–3880. [Google Scholar] [CrossRef]
- Prathap, C.; Ray, A.; Ravi, M.R. Effects of dilution with carbon dioxide on the laminar burning velocity and flame stability of H2/CO mixtures at atmospheric condition. Combust. Flame 2012, 159, 482–492. [Google Scholar] [CrossRef]
- Ratna Kishore, V.; Ravi, M.R.; Ray, A. Adiabatic burning velocity and cellular flame characteristics of H2/CO/CO2/air mixtures. Combust. Flame 2011, 158, 2149–2164. [Google Scholar] [CrossRef]
- Prathap, C.; Ray, A.; Ravi, M.R. Investigation of nitrogen dilution effects on the laminar burning velocity and flame stability of syngas fuel at atmospheric condition. Combust. Flame 2008, 155, 145–160. [Google Scholar] [CrossRef]
- Matynia, A.; Delfau, J.L.; Pillier, L.; Vovelle, C. Comparative study of the influence of CO2 and H2O on the chemical structure of lean and rich methane-air flames at atmospheric pressure. Combust. Explos. Shock Waves 2009, 45, 635–645. [Google Scholar] [CrossRef]
- Shmakov, A.G.; Knyazkov, D.A.; Bolshova, T.A.; Dmitriev, A.M.; Korobeinichev, O.P. Effect of CO2 Addition on the Structure of Premixed Fuel-Rich CH4/O2/N2 and C3H8/O2/N2 Flames Stabilized on a Flat Burner at Atmospheric Pressure. Energ. Fuels 2015, 30, 2395–2406. [Google Scholar] [CrossRef]
- Glarborg, P.; Bentzen, L.L.B. Chemical Effects of a High CO2 Concentration in Oxy-Fuel Combustion of Methane. Energy Fuels 2008, 22, 291–296. [Google Scholar] [CrossRef]
- Giovangigli, V.; Smooke, M.D. Extinction of strained premixed laminar flames with complex chemistry. Combust. Sci. Technol. 1987, 53, 23. [Google Scholar] [CrossRef]
- Kee, R.J.; Miller, J.A.; Evans, G.H.; Dixon-Lewis, G. A computational model of the structure and extinction of strained, opposed flow, premixed methane-air flames. Proc. Combust. Inst. 1988, 22, 1479–1494. [Google Scholar] [CrossRef]
- Lutz, A.; Kee, R.; Grcar, J.; Rupley, F. A Fortran Program for Computing Opposed-Flow Diffusion Flames; Sandia National Laboratories: Livermore, CA, USA, 1997. [Google Scholar]
- Chelliah, H.; Law, C.; Ueda, T. An experimental and theoretical investigation of the dilution, pressure and flow-field effects on the extinction condition of methane-air-nitrogen diffusion flames. Proc. Combust. Inst. 1990, 503–511. [Google Scholar] [CrossRef]
- Wang, P.; Guo, T.; Xu, H.; Zhao, Y.; Meng, S.; Feng, D.; Sun, S. Study on the effect of H2O on the formation of CO in the counterflow diffusion flame of H2/CO syngas in O2/H2O. Fuel 2018, 234, 516–525. [Google Scholar] [CrossRef]
- Oscar, N.; Eva, G. Structures of Ethanol Spray Flames under CO2 Dilution of the Oxidizer in the Counterflow Configuration under MILD Combustion Conditions. Fluids 2020, 5, 194. [Google Scholar]
- Xiao, H.; Lai, S.; Valera-Medina, A.; Li, J.; Liu, J.; Fu, H. Study on counterflow premixed flames using high concentration ammonia mixed with methane. Fuel 2020, 275, 117902. [Google Scholar] [CrossRef]
- Wei, Z.L.; Leung, C.W.; Cheung, C.S.; Huang, Z.H. Effects of equivalence ratio, H2 and CO2 addition on the heat release characteristics of premixed laminar biogas-hydrogen flame. Int. J. Hydrog. Energy 2016, 41, 6567–6580. [Google Scholar] [CrossRef]


| Chemical | Thermal | Transport | Radiative | |
|---|---|---|---|---|
| CO2 | √ | √ | √ | √ |
| FCO2 | — | √ | √ | √ |
| TCO2 | — | N2 | √ | √ |
| XCO2 | — | √ | N2 | √ |
| RCO2 | — | √ | √ | — |
| Flame Case | Strain Rate (s−1) | Distance (m) | Fuel Composition (Mole Fraction) | Oxidizer Composition (Mole Fraction) | ||
|---|---|---|---|---|---|---|
| 1 | 10 | 0.04 | 70% CH4 + 30% N2 | 79% N2 + 21% O2 | 0.10 | 0.1309 |
| 2 | 10 | 0.04 | 70% CH4 + 30% CO2 | 79% N2 + 21% O2 | 0.10 | 0.1087 |
| 3 | 10 | 0.04 | 70% CH4 + 30% FCO2 | 79% N2 + 21% O2 | 0.10 | 0.1087 |
| 4 | 10 | 0.04 | 70% CH4 + 30% TCO2 | 79% N2 + 21% O2 | 0.10 | 0.1087 |
| 5 | 10 | 0.04 | 70% CH4 + 30% XCO2 | 79% N2 + 21% O2 | 0.10 | 0.1087 |
| 6 | 10 | 0.04 | 70% CH4 + 30% RCO2 | 79% N2 + 21% O2 | 0.10 | 0.1087 |
| 7 | 10 | 0.04 | 100% CH4 | 79% N2 + 21% O2 | 0.10 | 0.1341 |
| 8 | 10 | 0.04 | 100% CH4 | 30% CO2 + 49% N2 + 21% O2 | 0.10 | 0.1449 |
| 9 | 10 | 0.04 | 100% CH4 | 30% FCO2 + 49% N2 + 21% O2 | 0.10 | 0.1449 |
| 10 | 10 | 0.04 | 100% CH4 | 30% TCO2 + 49% N2 + 21% O2 | 0.10 | 0.1449 |
| 11 | 10 | 0.04 | 100% CH4 | 30% XCO2 + 49% N2 + 21% O2 | 0.10 | 0.1449 |
| 12 | 10 | 0.04 | 100% CH4 | 30% RCO2 + 49% N2 + 21% O2 | 0.10 | 0.1449 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Wang, J.; Zhang, X.; Li, C. The Effects of CO2 Additional on Flame Characteristics in the CH4/N2/O2 Counterflow Diffusion Flame. Molecules 2021, 26, 2905. https://doi.org/10.3390/molecules26102905
Chen Y, Wang J, Zhang X, Li C. The Effects of CO2 Additional on Flame Characteristics in the CH4/N2/O2 Counterflow Diffusion Flame. Molecules. 2021; 26(10):2905. https://doi.org/10.3390/molecules26102905
Chicago/Turabian StyleChen, Ying, Jingfu Wang, Xiaolei Zhang, and Conghao Li. 2021. "The Effects of CO2 Additional on Flame Characteristics in the CH4/N2/O2 Counterflow Diffusion Flame" Molecules 26, no. 10: 2905. https://doi.org/10.3390/molecules26102905
APA StyleChen, Y., Wang, J., Zhang, X., & Li, C. (2021). The Effects of CO2 Additional on Flame Characteristics in the CH4/N2/O2 Counterflow Diffusion Flame. Molecules, 26(10), 2905. https://doi.org/10.3390/molecules26102905






