Chemical Composition, Antioxidant and Anticancer Activities of Leptocarpha rivularis DC Flower Extracts
Abstract
1. Introduction
2. Results
2.1. Extract Yields
2.2. Phytochemical Content
2.2.1. Total Antioxidant Activity
2.2.2. Reduced Glutathione Assay
2.2.3. GC-MS Analysis
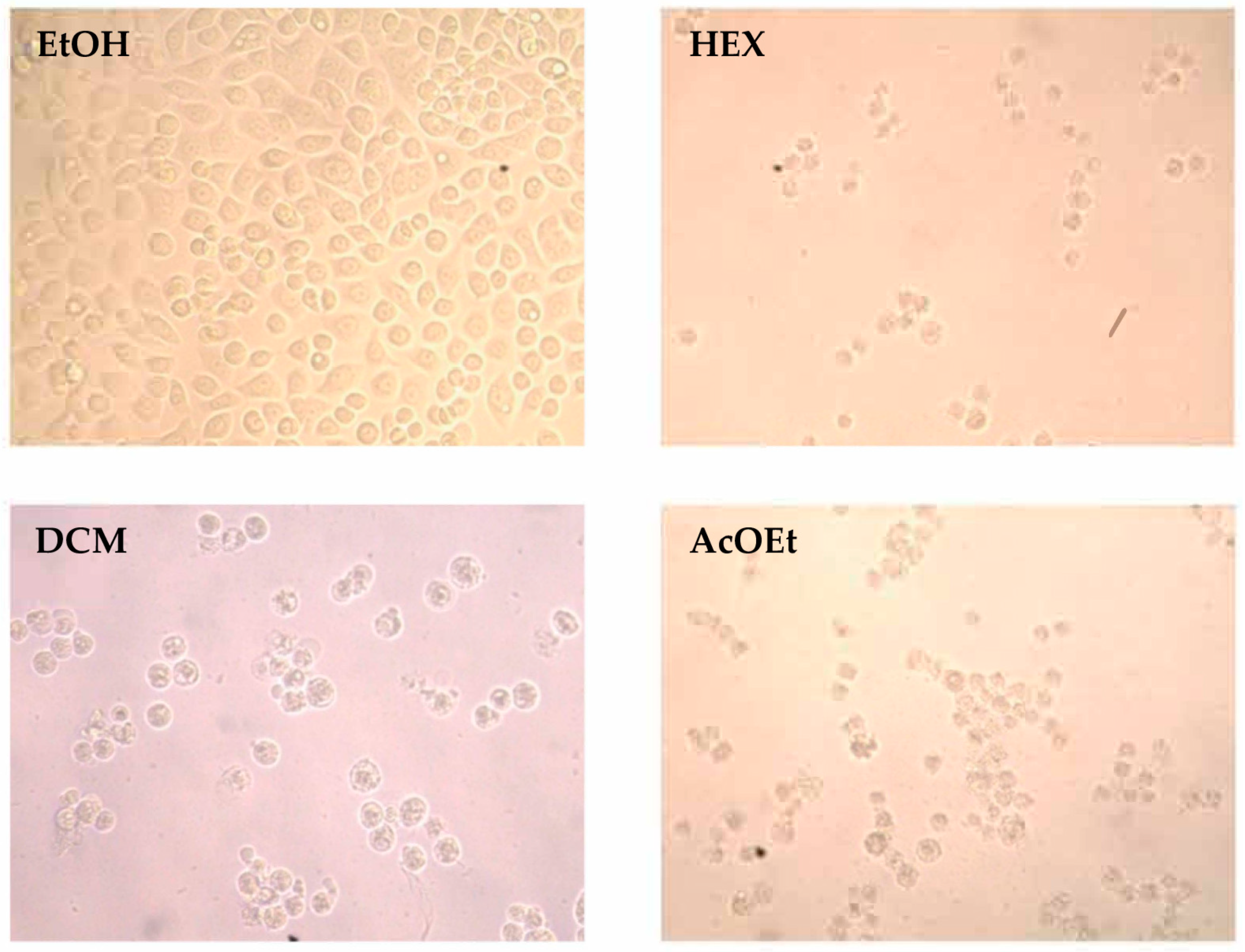
2.3. Cytotoxic Activity
2.4. Selective Index (SI)
3. Discussion
4. Materials and Methods
4.1. General
4.2. Plant Material
4.3. Extraction
4.4. Phytochemical Screening of Flower Extracts
4.4.1. Determination of Total Phenols
4.4.2. Estimation of Total Flavonoid Content (TFC)
4.4.3. Total Anthraquinone Content (TAC)
4.5. Chromatographic Analysis
4.6. Antioxidant Assay
4.6.1. DPPH Radical Scavenging Assay
4.6.2. Ferric Reducing Antioxidant Potential (FRAP) Assay
4.6.3. Total Reactive Antioxidant Properties (TRAP) Assay
4.6.4. Oxygen Radical Absorbance Capacity (ORAC) Assay
4.6.5. Reduced Glutathione Assay
4.7. Cell Viability
4.7.1. Cell Culture
4.7.2. In Vitro Growth Inhibition Assay
4.7.3. Morphological Assessment of Cell Apoptosis
4.7.4. Determination of Mitochondrial Membrane Permeability by Flow Cytometry
4.7.5. Determination of Caspases Activation
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Riedemann, P.; Aldunate, G. Flora Nativa de Valor Ornamental. Identificación y Propagación. Chile—Zona Sur, 2nd ed.; Editorial Andrés Bello: Santiago, Chile, 2011; pp. 202–203. [Google Scholar]
- Urban, O. Botánica de las Plantas Endémicas de Chile, 1st ed.; Editorial Universidad de Concepción: Concepción, Chile, 1934; p. 291. [Google Scholar]
- Hoffman, A. Flora Silvestre de Chile. Zona Austral, 1st ed.; Ediciones Fundación Claudio Gay: Santiago, Chile, 1982; pp. 206–207. [Google Scholar]
- Martinez, R.; Kesternich, V.; Carrasco, H.; Alvarez-Contreras, C.; Montenegro, C.; Ugarte, R.; Gutierrez, E.; Moreno, J.; Garcia, C.; Werner, E.; et al. Synthesis and conformational analysis of leptocarpin derivatives. Influence of modification of the oxirane ring onleptocarpin’s cytotoxic activity. J. Chil. Chem. Soc. 2006, 51, 1010–1014. [Google Scholar] [CrossRef]
- Bosio, C.; Tomasoni, G.; Martínez, R.; Olea, A.F.; Carrasco, H.; Villena, J. Cytotoxic and apoptotic effects of leptocarpin, a plant-derived sesquiterpene lactone, on human cancer cell lines. Chem. Biol. Interact. 2015, 242, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Mena, C. Efecto apoptótico y quimiosensibilizador de leptocarpina en distintos modelos celulares de leucemias, E. A. Y. Q. O: Medicina. Undergraduate Thesis, Universidad Austral de Chile, Valdivia, Chile, 2008. [Google Scholar]
- Alvarez, C. Efecto hipoglucemiante de la infusión de Leptocarpha rivularis en ratas sprague-dawley diabéticas tipo ii por inducción con aloxano. Undergraduate Thesis, Universidad Austral de Chile, Valdivia, Chile, 2005. [Google Scholar]
- Montenegro, I.; Ramirez, I.; Dorta, F.; Madrid, A.; Seeger, M. Micropropagation and determination of the antioxidant capacity of the Leptocarpha rivularis. In Proceedings of the X Plant Biology Meeting, Valdivia, Chile, 1–4 December 2015; p. 153. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Steam, IL, USA, 2007. [Google Scholar]
- Zamzami, N.; Larochette, N.; Kroemer, G. Mitochondrial permeability transition in apoptosis and necrosis. Cell Death Differ. 2005, 12 (Suppl. 2), 1478–1480. [Google Scholar] [CrossRef] [PubMed]
- Obrenovich, M.E.; Li, Y.; Parvathaneni, K.; Yendluri, B.B.; Palacios, H.H.; Leszek, J.; Aliev, G. Antioxidants in health, disease and aging. CNS Neurol. Disord. Drug Targets 2011, 10, 192–207. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Munoz, G.; Miranda, I.L.; Sartori, S.K.; de Rezende, D.C.; Diaz, M.A. Anthraquinones: An Overview. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; Volume 58, pp. 313–338. [Google Scholar]
- Mishra, A.; Sharma, A.K.; Kumar, S.; Saxena, A.K.; Pandey, A.K. Bauhinia variegate leaf extracts exhibit considerable antibacterial, antioxidant, and anticancer activities. Biomed. Res. Int. 2013, 3, 915436. [Google Scholar] [CrossRef]
- Brusselmans, K.; De Schrijver, E.; Heyns, W.; Verhoeven, G.; Swinnen, J.V. Epigallocatechin-3-gallate is a potent natural inhibitor of fatty acid synthase in intact cells and selectively induces apoptosis in prostate cancer cells. Int. J. Cancer 2003, 106, 856–862. [Google Scholar] [CrossRef]
- Brusselmans, K.; Vrolix, R.; Verhoeven, G.; Swinnen, J.V. Induction of cancer cell apoptosis by flavonoids is associated with their ability to inhibit fatty acid synthase activity. J. Biol. Chem. 2005, 280, 5636–5645. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 11–12, 162750. [Google Scholar] [CrossRef]
- Jarial, R.; Thakur, S.; Sakinah, M.; Zularisam, A.W.; Sharad, A.; Kanwar, S.S.; Singh, L. Potent anticancer, antioxidant and antibacterial activities of isolated flavonoids from Asplenium nidus. J. King Saud Univ. Sci. 2018, 30, 185–192. [Google Scholar] [CrossRef]
- Jeong, S.H.; Koo, S.J.; Choi, J.H.; Park, J.H.; Ha, J.; Park, H.J.; Lee, K.T. Intermedeol isolated from the leaves of Ligularia fischeri var. spiciformis induces the differentiation of human acute promyeocytic leukemia HL-60 cells. Planta Med. 2002, 68, 881–885. [Google Scholar] [CrossRef]
- Seo, M.S.; Choi, E.M. The effects of dehydrocostus lactone on osteoblastic MC3T3-E1 cells in redox changes and PI3K/Akt/CREB. Immunopharmacol. Immunotoxicol. 2012, 34, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Perez, E.; Liu, R.; Yan, L.J.; Mallet, R.T.; Yang, S.H. Pyruvate protects mitochondria from oxidative stress in human neuroblastoma SK-N-SH cells. Brain Res. 2007, 1132, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, F.; Zhang, X.; Li, P.; Zhang, X.; Wu, Z.; Li, D. In vitro synergistic antioxidant activity and identification of antioxidant components from Astragalus membranaceus and Paeonia lactiflora. PLoS ONE 2014, 9, e96780. [Google Scholar] [CrossRef] [PubMed]
- Herschbach, C.; Scheerer, U.; Rennenberg, H. Redox states of glutathione and ascorbate in root tips of poplar (Populus tremula×P. alba) depend on phloem transport from the shoot to the roots. J. Exp. Bot. 2010, 61, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Zechmann, B.; Müller, M. Subcellular compartmentation of glutathione in dicotyledonous plants. Protoplasma 2010, 246, 15–24. [Google Scholar] [CrossRef]
- Vanacker, H.; Carver, T.L.W.; Foyer, C.H. Pathogeninduced changes in the antioxidant status of the apoplast in barley leaves. Plant Physiol. 1998, 117, 1103–1114. [Google Scholar] [CrossRef]
- Cairns, N.G.; Pasternak, M.; Wachter, A.; Cobbett, C.S.; Meyer, A.J. Maturation of Arabidopsis seeds is dependent on glutathione biosynthesis within the embryo. Plant Physiol. 2006, 141, 446–455. [Google Scholar] [CrossRef]
- de Sousa, A.; AbdElgawad, H.; Asard, H.; Pinto, A.; Soares, C.; Branco-Neves, S.; Braga, T.; Azenha, M.; Selim, S.; Al Jaouni, S.; et al. Metalaxyl Effects on Antioxidant Defenses in Leaves and Roots of Solanum nigrum L. Front. Plant Sci. 2017, 8, 1967. [Google Scholar] [CrossRef]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Marquez-Garcia, B.; Queval, G.; Foyer, C. Glutathione in plants: An integrated overview. Plant Cell Environ. 2012, 35, 454–484. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of Ascorbate-Glutathione Pathway in Mitigating Oxidative Damage in Plants under Abiotic Stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef]
- Minivayeva, F.; Beckett, R.; Kranner, I. Roles of apoplastic peroxidases in plant response to wounding. Phytochemistry 2015, 112, 122–129. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- De Oliveira, P.; Alves, J.; Damasceno, J.; Oliveira, R.; Dias, H.; Crotti, A.; Tavares, D. Cytotoxicity screening of essential oils in cancer cell lines. Rev. Bras. Farmacogn. 2015, 25, 183–188. [Google Scholar] [CrossRef]
- Martinez, R.; Kesternich, V.; Gutierrez, E.; Dolz, H.; Mansilla, H. Conformational-Analysis and biological activity of leptocarpin and leptocarpin acetate. Planta Med. 1995, 61, 188–189. [Google Scholar] [CrossRef] [PubMed]
- Butturini, E.; di Paola, R.; Suzuki, H.; Paterniti, I.; Ahmad, A.; Mariotto, S.; Cuzzocrea, S. Costunolide and dehydrocostuslactone, two natural sesquiterpene lactones, ameliorate the inflammatory process associated to experimental pleurisy in mice. Eur. J. Pharmacol. 2014, 730, 107–115. [Google Scholar] [CrossRef]
- Eliza, J.; Daisy, P.; Ignacimuthu, S. Antioxidant activity of costunolide and eremanthin isolated from Costus speciosus (Koen ex. Retz) Sm. Chem. Biol. Interact. 2010, 188, 467–472. [Google Scholar] [CrossRef]
- Kuo, P.L.; Ni, W.C.; Tsai, E.M.; Hsu, Y.L. Dehydrocostuslactone disrupts signal transducers and activators of transcription 3 through up-regulation of suppressor of cytokine signaling in breast cancer cells. Mol. Cancer Ther. 2009, 8, 1328–1339. [Google Scholar] [CrossRef]
- Choi, E.J.; Ahn, W.S. Antiproliferative effects of dehydrocostuslactone through cell cycle arrest and apoptosis in human ovarian cancer SK-OV-3 cells. Int. J. Mol. Med. 2009, 23, 211–216. [Google Scholar]
- Hao, L.J.; Zhao, F.; Gao, Z.T.; Xu, H.; Liu, K. Inhibitory efects of sesquiterpenes from Saussurea lappa on the vascular endothelial growth factor. Nat. Prod. Res. Dev. 2010, 22, 687–691. [Google Scholar]
- Long, H.Y.; Huang, Q.X.; Yu, Y.Y.; Zhang, Z.B.; Yao, Z.W.; Chen, H.B.; Feng, J.W. Dehydrocostus lactone inhibits in vitro gastrinoma cancer cell growth through apoptosis induction, sub-G1 cell cycle arrest, DNA damage and loss of mitochondrial membrane potential. Arch. Med. Sci. 2019, 15, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Hong, J.E.; Lim, S.S.; Kwon, G.T.; Kim, J.; Kim, J.S.; Lee, K.W.; Park, J.H. The hexane extract of Saussurea lappa and its active principle, dehydrocostus lactone, inhibit prostate cancer cell migration. J. Med. Food 2012, 15, 24–32. [Google Scholar] [CrossRef]
- Kim, E.J.; Lim, S.S.; Park, S.Y.; Shin, H.K.; Kim, J.S.; Yoon Park, J.H. Apoptosis of DU145 human prostate cancer cells induced by dehydrocostus lactone isolated from the root of Saussurea lappa. Food Chem. Toxicol. 2008, 46, 3651–3658. [Google Scholar] [CrossRef]
- Oh, G.S.; Pae, H.O.; Chung, H.T.; Kwon, J.W.; Lee, J.H.; Kwon, T.O.; Kwon, S.Y.; Chon, B.H.; Yun, Y.G. Dehydrocostus lactone enhances tumor necrosis Factor-a-Induced apoptosis of human leukemia HL-60 cells. Immunopharmacol. Immunotoxicol. 2004, 26, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Jun, N.J.; Mosaddik, A.; Moon, J.Y.; Jang, K.C.; Lee, D.S.; Ahn, K.S.; Cho, S.K. Cytotoxic activity of β-caryophyllene oxide isolated from Jeju Guava (Psidium cattleianum Sabine) leaf. Rec. Nat. Prod. 2011, 5, 242–246. [Google Scholar]
- Park, K.R.; Nam, D.; Yun, H.M.; Lee, S.G.; Jang, H.J.; Sethi, G.; Cho, S.K.; Ahn, K.S. β-Caryophyllene oxide inhibits growth and induces apoptosis through the suppression of PI3K/AKT/mTOR/S6K1 pathways and ROS-mediated MAPKs activation. Cancer Lett. 2011, 312, 178–188. [Google Scholar] [CrossRef]
- Naser, B.; Bodinet, C.; Tegtmeier, M.; Lindequist, U. Thuja occidentalis (Arbor vitae): A review of its pharmaceutical, pharmacological and clinical properties. Evid. Based Complement. Alternat. Med. 2005, 2, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.K.; Batra, A. Antioxidant activity of Thuja occidentalis linn. Asian J. Pharm. Clin. Res. 2009, 2, 73–76. [Google Scholar]
- Mukherjee, A.; Sikdar, S.; Bishayee, K.; Paul, A.; Ghosh, S.; Boujedaini, N.; Khuda-Bukhsh, A.R. Ethanolic extract of Thuja occidentalis blocks proliferation of A549 cells and induces apoptosis in vitro. J. Chin. Integr. Med. 2012, 10, 1451–1459. [Google Scholar] [CrossRef]
- Biswas, R.; Mandal, S.K.; Dutta, S.; Bhattacharyya, S.S.; Boujedaini, N.; Khuda-Bukhsh, A.R. Thujone-rich fraction of Thuja occidentalis demonstrates major anti-cancer potentials: Evidences from in vitro studies on A375 cells. Evid. Based Complement. Alternat. Med. 2011, 2011, 568148. [Google Scholar] [CrossRef]
- Torres, A.; Vargas, Y.; Uribe, D.; Carrasco, C.; Torres, C.; Rocha, R.; Oyarzún, C.; San Martín, R.; Quezada, C. Pro-apoptotic and Anti-Angiogenic Properties of the α/β-Thujone Fraction from Thuja Occidentalis on Glioblastoma Cells. J. Neurooncol. 2016, 128, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Lee, S.; Nam, K.; Kim, K.; Mar, W. Hepatoprotective effects of reynosin against thioacetamide-induced apoptosis in primary hepatocytes and mouse liver. Arch. Pharm. Res. 2013, 36, 485–494. [Google Scholar] [CrossRef]
- Ham, A.; Kim, D.; Kim, K.; Lee, S.; Oh, K.; Shin, J.; Mar, W. Reynosin protects against neuronal toxicity in dopamine-induced SH-SY5Y cells and 6-hydroxydopamine-lesioned rats as models of Parkinson’s disease: Reciprocal up-regulation of E6-AP and down-regulation of α-synuclein. Brain Res. 2013, 1524, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Moon, P.D.; Lee, B.H.; Jeong, H.J.; An, H.J.; Park, S.J.; Kim, H.R.; Ko, S.G.; Um, J.Y.; Hong, S.H.; Kim, H.M. Use of scopoletin to inhibit the production of inflammatory cytokines through inhibition of the IκB/NF-κB signal cascade in the human mast cell line HMC-1. Eur. J. Pharmacol. 2007, 555, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Kwon, K.; Shin, B.; Seo, E.; Lee, Y.; Kim, J.; Ryu, D. Scopoletin induces apoptosis in human promyeloleukemic cells, accompanied by activations of nuclear factor κB and caspase-3. Life Sci. 2005, 77, 824–836. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, K.; Jayakumar, D.; Velusamy, P.; Srinivasan, A.; Mohan, T.; Ravi, D.B.; Uthamaraman, S.; Sathyamoorthy, Y.K.; Rajasekaran, N.S.; Periandavan, K. Morinda citrifolia and Its Active Principle Scopoletin Mitigate Protein Aggregation and Neuronal Apoptosis through Augmenting the DJ-1/Nrf2/ARE Signaling Pathway. Oxidative Med. Cell. Longev. 2019, 2019, 2761041. [Google Scholar] [CrossRef] [PubMed]
- Peron, G.; Marzaro, G.; Dall’Acqua, S. Known triterpenes and their derivatives as scaffolds for the development of new therapeutic agents for cancer. Curr. Med. Chem. 2018, 25, 1259–1269. [Google Scholar] [CrossRef] [PubMed]
- Kangsamaksin, T.; Chaithongyot, S.; Wootthichairangsan, C.; Hanchaina, R.; Tangshewinsirikul, C.; Svasti, J. Lupeol and stigmasterol suppress tumor angiogenesis and inhibit cholangiocarcinoma growth in mice via downregulation of tumor necrosis factor-α. PLoS ONE 2017, 12, e0189628. [Google Scholar] [CrossRef]
- Nagaraj, M.; Sunitha, S.; Varalakshmi, P. Effect of Lupeol, a pentacyclic triterpene, on the lipid peroxidation and antioxidant status in rat kidney after chronic cadmiumexposure. J. Appl. Toxicol. 2000, 20, 413–417. [Google Scholar] [CrossRef]
- Laszczyk, M.; Jäger, S.; Simon-Haarhaus, B.; Scheffler, A.; Schempp, C.M. Physical, chemical and pharmacological characterization of a new oleogel-forming triterpene extract from the outer bark of birch (Betulae Cortex). Planta Med. 2006, 72, 1389–1395. [Google Scholar] [CrossRef]
- Lee, T.K.; Poon, R.T.; Wo, J.Y.; Ma, S.; Guan, X.Y.; Myers, J.N.; Altevogt, P.; Yuen, A.P. Lupeol suppresses cisplatin-induced nuclear factor- KB activation in head and neck squamous cell carcinoma and inhibits local invasion and nodal metastasis in an orthotopic nude mouse model. Cancer Res. 2007, 67, 8800–8809. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Kweon, M.-H.; Yun, J.-M.; Adhami, V.M.; Khan, N.; Syed, D.N.; Mukhtar, H. A novel dietary triterpene Lupeol induces fas-mediated apoptotic death of androgen-sensitive prostate cancer cells and inhibits tumor growth in a xenograft model. Cancer Res. 2005, 65, 11203–11213. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Afaq, F.; Adhami, V.M.; Mukhtar, H. Lupeol modulates NF-κB and PI3K/Akt pathways and inhibits skin cancer in CD-1 mice. Oncogene 2004, 23, 5203–5214. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Hong, D.; Lou, Z.; Tu, X.; Jin, L. Lupeol inhibits migration and invasion of colorectal cancer cells by suppressing RhoA-ROCK1 signaling pathway. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 2185–2196. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M. Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett. 2009, 285, 109–115. [Google Scholar] [CrossRef]
- Wang, W.H.; Chuang, H.Y.; Chen, C.H.; Chen, W.K.; Hwang, J.J. Lupeol acetate ameliorates collagen-induced arthritis and osteoclastogenesis of mice through improvement of microenvironment. Biomed. Pharmacother. 2016, 79, 231–240. [Google Scholar] [CrossRef]
- Endrini, S.; Rahmat, A.; Ismail, P.; Taufiq-Yap, Y.H. Cytotoxic effect of γ-sitosterol from Kejibeling (Strobilanthes crispus) and its mechanism of action towards c-myc gene expression and apoptotic pathway. Med. J. Indones. 2014, 23, 203–208. [Google Scholar] [CrossRef]
- Gao, Q.-H.; Wu, C.-S.; Wang, M. The jujube (Ziziphus Jujuba Mill.) fruit: A review of current knowledge of fruit composition and health benefits. J. Agric. Food Chem. 2013, 61, 3351–3363. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, R.; Ni, D.; Luo, X.; Chen, S.; Luo, C.; Xiao, W. Discovery of betulinaldehyde as a natural RORγt agonist. Fitoterapia 2019, 137, 104200. [Google Scholar] [CrossRef]
- Park, J.S.; Moon, S.J.; Lim, M.; Byun, J.K.; Hwang, S.H.; Yang, S.; Kim, E.K.; Kim, S.M.; Lee, J.; Kwok, S.; et al. Retinoic Acid Receptor-Related Receptor Alpha Ameliorates Autoimmune Arthritis via Inhibiting of Th17 Cells and Osteoclastogenesis. Front. Immunol. 2019, 10, 2270. [Google Scholar] [CrossRef]
- Fabiyi, O.A.; Atolani, O.; Adeyemi, O.S.; Olatunji, G.A. Antioxidant and cytotoxicity of β-amyrin acetate fraction from Bridelia ferruginea leaves. Asian Pac. J. Trop. Biomed. 2012, 2, S981–S984. [Google Scholar] [CrossRef]
- Zengin, H.; Baysal, A.H. Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage-forming bacteria and cell structure-activity relationships evaluated by SEM microscopy. Molecules 2014, 19, 17773–17798. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, K.; Zheng, Y.W.; Murata, S.; Ito, H.; Nakayama, K.; Kurokawa, T.; Sano, N.; Nowatari, T.; Villareal, M.O.; Nagano, Y.N.; et al. Anticancer effect of linalool via cancer-specific hydroxyl radical generation in human colon cancer. World J. Gastroenterol. 2016, 22, 9765–9774. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.Y.; Shieh, D.E.; Chen, C.C.; Yeh, C.S.; Dong, H.P. Linalool induces cell cycle arrest and apoptosis in leukemia cells and cervical cancer cells through CDKIs. Int. J. Mol. Sci. 2015, 16, 28169–28179. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Cheng, X.; Wang, G.; Liao, Y.; Qing, C. Linalool inhibits 22Rv1 prostate cancer cell proliferation and induces apoptosis. Oncol. Lett. 2020, 20, 289. [Google Scholar] [CrossRef]
- Mittal, N.; Malpani, S.; Dyson, M.; Ono, M.; Coon, J.S.; Kim, J.J.; Schink, J.C.; Bulun, S.E.; Pavone, M.E. Fenretinide: A novel treatment for endometrial cancer. PLoS ONE 2014, 9, e110410. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.; Bancheva, S.; Rosselli, S.; Maggio, A. Sesquiterpenoids in subtribe Centaureinae (Cass.) Dumort (tribe Cardueae, Asteraceae): Distribution, 13C NMR spectral data and biological properties. Phytochemistry 2013, 95, 19–93. [Google Scholar] [CrossRef]
- Picman, A. Biological activities of sesquiterpene lactones. Biochem. Syst. Ecol. 1986, 14, 255–281. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Kirpotina, L.N.; Mitchell, P.T.; Kishkentaeva, A.S.; Shaimerdenova, Z.R.; Atazhanova, G.A.; Adekenov, S.M.; Quinn, M.T. The natural natural sesquiterpene lactones arglabin, grosheimin, agracin, parthenolide, and estafiatin inhibit T cell receptor (TCR) activation. Phytochemistry 2018, 146, 36–46. [Google Scholar] [CrossRef]
- Shpatov, A.V.; Popov, S.A.; Salnikova, O.I.; Kukina, T.P.; Shmidt, E.N.; Um, B.H. Composition and Bioactivity of Lipophilic Metabolites from Needles and Twigs of Korean and Siberian Pines (Pinus koraiensis Siebold & Zucc. and Pinus sibirica Du Tour). Chem. Biodivers. 2017, 14, e1600203. [Google Scholar] [CrossRef]
- De Castro, M.L.; Garcıa-Ayuso, L.E. Soxhlet extraction of solid materials: An outdated technique with a promising innovative future. Anal. Chim. Acta 1998, 369, 1–10. [Google Scholar] [CrossRef]
- Mohammad, N.; Sajid, A.; Muhammad, Q. Preliminary phytochemical screening of flowers, leaves, bark, stem and roots of Rhododendron arboreum. Middle East J. Sci. Res. 2011, 10, 472–476. [Google Scholar]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- Madaan, R.; Bansal, G.; Kumar, S.; Sharma, A. Estimation of total phenols and flavonoids in extracts of Actaea spicata roots and antioxidant activity studies. Indian J. Pharm. Sci. 2011, 73, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Mellado, M.; Madrid, A.; Pena-Cortes, H.; López, R.; Jara, C.; Espinoza, L. Antioxidant activity of anthraquinones isolated from leaves of Muehlenbeckia hastulata (je sm.) johnst.(polygonaceae). J. Chil. Chem. Soc. 2013, 58, 1767–1770. [Google Scholar] [CrossRef]
- Kurkin, V.A.; Shmygareva, A.A.; Ryazanova, T.K.; San’kov, A.N. Quantitative Determination of Total Anthraquinone Glycosides in Cassia Syrup Preparation. Pharm. Chem. J. 2017, 50, 691–694. [Google Scholar] [CrossRef]
- Montenegro, I.; Madrid, A.; Zaror, L.; Martínez, R.; Werner, E.; Carrasco, H.; Cuellar, M.; Palma, H. Antimicrobial activity of ethyl acetate extract and essential oil from bark of Laurelia sempervirens against multiresistant bacteria. Bol. Latinoam. Caribe Plant. Med. Aromat. 2012, 11, 306–315. [Google Scholar]
- Canales, N.; Montenegro, I.; Párraga, M.; Olguín, Y.; Godoy, P.; Werner, E.; Madrid, A. In vitro antimicrobial activity of embothrium coccineum used as traditional medicine in patagonia against multiresistant bacteria. Molecules 2016, 21, 1441. [Google Scholar] [CrossRef]
- NIST/EPA/NIH Mass Spectral Library with Search Program (Data Version: NIST 11, Software Version 2.0 g. Available online: http://webbook.nist.gov/chemistry/name-ser.html (accessed on 15 May 2016).
- Santander, R.; Creixell, W.; Sánchez, E.; Tomic, G.; Silva, J.R.; Acevedo, C.A. Recognizing Age at Slaughter of Cattle from Beef Samples Using GC/MS-SPME Chromatographic Method. Food Bioprocess Technol. 2013, 6, 3345–3352. [Google Scholar] [CrossRef]
- Leyton, M.; Mellado, M.; Jara, C.; Montenegro, I.; González, S.; Madrid, A. Free radical-scavenging activity of sequential leaf extracts of Embothrium coccineum. Open Life Sci. 2015. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Romay, C.; Pascual, C.; Lissi, E.A. The reaction between ABTS radical cation and antioxidants and its use to evaluate the antioxidant status of serum samples. Braz. J. Med. Biol. Res. 1996, 29, 175–183. [Google Scholar] [PubMed]
- Alarcón, E.; Campos, A.M.; Edwards, A.M.; Lissi, E.; López-Alarcón, C. Antioxidant capacity of herbal infusions and tea extracts: A comparison of ORAC-fluorescein and ORAC-pyrogallol red methodologies. Food Chem. 2008, 107, 1114–1119. [Google Scholar] [CrossRef]
- Lopez-Alarcon, C.; Lissi, E. A novel and simple ORAC methodology based on the interaction of Pyrogallol red with peroxyl radicals. Free Radic. Res. 2006, 40, 979–985. [Google Scholar] [CrossRef]
- Griffith, O.W. Determination of glutathione disulphide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Lutts, S.; Lefevre, I.; Delperee, C.; Kivits, S.; Dechamps, C.; Robledo, A.; Correal, E. Heavy metal accumulation by the halophyte species Mediterranean saltbush. J. Environ. Qual. 2004, 33, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]



| Extract | Total Phenols (GAE mg·L−1) | S.D. | Total Flavonoids (QE mg·L−1) | S.D. | Total Anthraquinones (EE mg·L−1) | S.D. |
|---|---|---|---|---|---|---|
| Hex | 27.8423 a | 1.7642 | 70.9333 a | 1.8986 | 37.8368 a | 0.8442 |
| DCM | 41.8810 b | 1.4569 | 26.4295 b | 0.4815 | 10.4092 b | 0.2330 |
| AcOEt | 47.8899 b | 0.0449 | 33.8589 b | 1.0233 | 12.9897 b | 0.3568 |
| EtOH | 82.6696 c | 5.8292 | 103.6202 c | 3.9844 | 75.2023 c | 1.7563 |
| Extract | DPPH (IC50 mg·mL−1) | S.D. | FRAP (TEAC mM) | S.D. | TRAP (TEAC mM) | S.D. | ORAC (TEAC µM) | S.D |
|---|---|---|---|---|---|---|---|---|
| Hex | 4.1863 a | 0.1834 | 0.3141 a | 0.0131 | 0.0074 a | 0.0022 | 442.57 a | 12.21 |
| DCM | 1.8953 b | 0.0437 | 0.2656 a | 0.0145 | 0.0961 b | 0.0047 | 932.40 b | 38.55 |
| AcOEt | 2.4088 c | 0.0758 | 0.3063 a | 0.0081 | 0.1288 b | 0.0057 | 1878.17 c | 37.35 |
| EtOH | 2.8300 c | 0.0626 | 0.3687 b | 0.0277 | 0.0729 b | 0.0204 | 744.20 d | 26.61 |
| T | 0.1060 d | 0.0050 | n.a. | n.a. | n.a. | n.a. | n.a | n.a |
| GA | n.a. | n.a. | 1.7200 c | 0.0200 | 1.1300 c | 0.0100 | 696.22 e | 63.84 |
| BHT | 0.0600 d | 0.0010 | 1.5200 d | 0.0700 | 1.0600 c | 0.0200 | 593.87 f | 41.27 |
| Extract | GSH | GSSG | GSH/GSSG |
|---|---|---|---|
| µmol/gDW | |||
| Hex | 1.179 ± 0.909 d | 16.432 ± 3.756 c | 0.062 ± 0.042 b |
| DCM | 28.185 ± 3.385 b | 22.110 ± 5.684 b | 1.376 ± 0.204 a |
| AcOEt | 11.461 ± 1.924 c | 50.427 ± 5.686 b | 0.224 ± 0.013 b |
| EtOH | 86.456 ± 1.755 a | 267.863 ± 9.157 a | 0.323 ± 0.009 b |
| N° Peak | RT (min) | Main Components | RI a | RIref b | %Area c | Match | Identification |
|---|---|---|---|---|---|---|---|
| 1 | 8.94 | Caryophyllene oxide | 1534 | 1534 | 0.37 | 940 | RI, MS |
| 2 | 10.01 | Elemol | 1539 | 1537 | 0.11 | 900 | RI, MS |
| 3 | 10.41 | Isoaromadendrene epoxide | 1580 | 1579 | 0.04 | 890 | RI, MS |
| 4 | 11.86 | 7-Hydroxyfarnesen | 1582 | 1584 | 2.81 | 870 | RI, MS |
| 5 | 12.15 | Paradisol | 1624 | 1627 | 1.33 | 940 | RI, MS |
| 6 | 12.31 | trans-Z-a-Bisabolene epoxide | 1823 | 1820 | 0.31 | 850 | RI, MS |
| 7 | 13.64 | Dehydrocostus lactone | 1865 | 1866 | 0.06 | 870 | RI, MS |
| 8 | 16.86 | 2-methyl-1-hexadecanol | 1888 | 1890 | 0.29 | 880 | RI, MS |
| 9 | 17.51 | Scopoletin | 1924 | 1924 | 0.90 | 870 | RI, MS, Co |
| 10 | 18.32 | Tetradecanoic acid | 1940 | 1940 | 0.04 | 920 | RI, MS |
| 11 | 22.07 | Eicosane | 2000 | 2000 | 0.63 | 900 | RI, MS |
| 12 | 22.24 | α-Linolenic acid | 2100 | 2102 | 0.27 | 920 | RI, MS |
| 13 | 22.81 | Grosheimin | 2143 | 2143 | 1.58 | 850 | RI, MS, Co |
| 14 | 23.28 | (Z)-18-Octadec-9-enolide | 2155 | 2158 | 0.52 | 950 | RI, MS |
| 15 | 25.67 | Glyceryl linolenate | 2157 | 2161 | 0.05 | 880 | RI, MS |
| 16 | 26.46 | Reynosin | 2266 | 2266 | 1.35 | 900 | RI, MS, Co |
| 17 | 31.66 | 4,8,13-Duvatriene-1,3-diol | 2397 | 2400 | 25.70 | 860 | RI, MS |
| 18 | 34.62 | 3-Ethyl-5-(2-ethylbutyl)octadecane | 2410 | 2413 | 3.89 | 930 | RI, MS |
| 19 | 34.81 | Tetraneurin D | 2490 | 2494 | 0.69 | 970 | RI, MS |
| 20 | 35.62 | 2-Methylenecholestan-3-ol | 2650 | 2652 | 0.41 | 970 | RI, MS |
| 21 | 35.86 | α-Tocopherol | 3149 | 3149 | 0.35 | 950 | RI, MS, Co |
| 22 | 37.69 | Stigmasterol | 3170 | 3170 | 0.70 | 940 | RI, MS, Co |
| 23 | 40.50 | γ-Sitosterol | 3290 | 3290 | 0.33 | 930 | RI, MS, Co |
| 24 | 42.69 | ß-Amyrin | 3330 | 3337 | 0.35 | 850 | RI, MS |
| 25 | 43.79 | ß -Amyrin acetate | 3333 | 3339 | 4.78 | 910 | RI, MS |
| 26 | 44.57 | Lupeyl acetate | 3380 | 3380 | 10.96 | 850 | RI, MS, Co |
| 27 | 46.06 | Betulinaldehyde | 3630 | 3629 | 0.13 | 820 | RI, MS |
| 28 | 46.62 | 1-Heptatriacotanol | 3941 | 3942 | 0.46 | 900 | RI, MS |
| Total identified | 59.41 | ||||||
| Unknown | 40.59 |
| N° Peak | RT (min) | Main Components | RI a | RIref b | %Area c | Match | Identification |
|---|---|---|---|---|---|---|---|
| 1 | 8.92 | pyrulic acid methyl ester | 1210 | 1217 | 2.50 | 880 | RI, MS |
| 2 | 9.98 | 10-epi-Elemol | 1549 | 1550 | 0.53 | 900 | RI, MS |
| 3 | 10.39 | Cariophyllene oxide | 1600 | 1606 | 0.24 | 910 | RI, MS |
| 4 | 12.13 | Intermedeol | 1660 | 1675 | 2.94 | 810 | RI, MS |
| 5 | 12.66 | Tetradecanoic acid | 1765 | 1770 | 0.07 | 890 | RI, MS |
| 6 | 13.63 | Tetradecanoic acid, ethyl ester | 1783 | 1790 | 0.55 | 930 | RI MS |
| 7 | 14.09 | Deoxyserecialactone | 1825 | NF | 0.30 | 900 | RI, MS |
| 8 | 14.25 | Neophytadiene | 1835 | 1836 | 0.15 | 870 | RI, MS |
| 9 | 15.91 | 6,10,14-Trimethyl-2-pentadecanone | 1852 | 1855 | 0.20 | 900 | RI, MS |
| 10 | 16.03 | Dehydrocostus lactone | 1860 | 1866 | 2.76 | 890 | RI, MS, Co |
| 11 | 22.01 | 9-trans-Methyl Trisporate C | 2109 | 2100 | 0.51 | 890 | RI, MS, Co |
| 12 | 22.51 | Glyceryl linolenate | 2174 | 2160 | 0.06 | 860 | RI, MS |
| 13 | 23.25 | 5α,6α-Epoxy-3β,17-dihydroxy-16α-methylpregnan-20-one | 2189 | 2189 | 0.71 | 850 | RI, MS |
| 14 | 25.66 | (Z)-18-Octadec-9-enolide | 2190 | 2208 | 2.57 | 980 | RI, MS |
| 15 | 26.48 | Reynosin | 2260 | 2266 | 1.05 | 860 | RI, MS, Co |
| 16 | 29.10 | 9-Tricosene, (Z) | 2270 | 2299 | 0.80 | 870 | RI, MS |
| 17 | 29.90 | Tricosane | 2330 | 2300 | 3.85 | 970 | RI, MS |
| 18 | 31.68 | 2-[(Z)-9-Octadecenyloxy]etanol | 2330 | 2336 | 0.36 | 870 | RI, MS |
| 19 | 32.67 | 3-Ethyl-5-(2’-ethylbutyl)octadecane | 2410 | 2413 | 0.34 | 940 | RI, MS |
| 20 | 33.95 | Thunbergol | 2500 | 2498 | 8.82 | 940 | RI, MS |
| 21 | 23.18 | 2-Methylenecholestan-3-ol | 2650 | 2652 | 1.15 | 920 | RI, MS |
| 22 | 36.05 | Triacontane | 3000 | 3000 | 4.36 | 870 | RI, MS |
| 23 | 36.34 | ß-Amyrin | 3315 | 3337 | 0.43 | 940 | RI, MS |
| 24 | 36.96 | α-Amyrin | 3360 | 3376 | 0.98 | 940 | RI, MS |
| 25 | 37.59 | Tetratriacontane | 3400 | 3400 | 6.39 | 860 | RI, MS, Co |
| 27 | 44.48 | ß -amyrin acetate | 3430 | 3437 | 6.43 | 910 | RI, MS |
| 28 | 44.59 | Lupeol | 3494 | 3486 | 0.25 | 930 | RI, MS, Co |
| 29 | 45.53 | lupeyl acetate | 3530 | 3525 | 14.96 | 900 | RI, MS |
| 30 | 45.67 | Betulinaldehyde | 3743 | 3760. | 0.70 | 900 | RI, MS |
| 31 | 46.62 | 1-Heptatriacotanol | 3940 | 3942 | 6.71 | 880 | RI, MS, Co |
| 32 | 50.52 | Oleic acid 3-(octadecyloxy)propyl ester | 4100 | 4149 | 0.76 | 850 | RI, MS, Co |
| Total identified | 72.43 | ||||||
| Unknown | 27.57 |
| No. | RT (min) | Main Components | RI a | RIref b | % Area c | Match | Identification |
|---|---|---|---|---|---|---|---|
| 1 | 9.575 | Thujone | 1095 | 1089 | 6.46 | 927 | RI, MS, Co |
| 2 | 12.750 | Isopentenyl acetate | 1196 | 1200 | 2.87 | 950 | RI, MS |
| 3 | 13.215 | trans-Sabinyl acetate | 1265 | 1264 | 4.01 | 934 | RI, MS |
| 4 | 18.110 | Intermedeol | 1660 | 1667 | 3.44 | 949 | RI, MS |
| 5 | 18.234 | 6-epi-Shyobunol | 1883 | 1881 | 3.59 | 953 | RI, MS |
| 6 | 18.450 | Eicosane | 2000 | 2000 | 2.87 | 989 | RI, MS |
| 7 | 18.610 | Geranyllinalool | 2040 | 2046 | 2.08 | 910 | RI, MS |
| 8 | 19.85 | Leptocarpin | 2050 | 2050 | 3.46 | 967 | RI, Co |
| 9 | 20.120 | 4,8,13-duvatriene-1,3-diol | 2395 | 2400 | 6.20 | 960 | RI, MS |
| 10 | 21.250 | Linalool | 2486 | 2491 | 2.26 | 899 | RI, MS, Co |
| 11 | 21.590 | 2-Methylenecholestan-3-ol | 2651 | 2652 | 2.32 | 956 | RI, MS |
| 12 | 42.06 | Stigmasterol | 3140 | 3142 | 24.02 | 940 | RI, MS, Co |
| 13 | 42.70 | Fenretinide | 3290 | 3289 | 13.76 | 930 | RI, MS, Co |
| 14 | 43.86 | 1-Heptatriacotanol | 3938 | 3942 | 8.49 | 900 | RI, MS |
| Total identified | 85.83 | ||||||
| Unknown | 14.17 |
| Extracts | PC-3 | HT-29 | MCF-7 | HEK-293 |
|---|---|---|---|---|
| Hex | 4.49 ± 0.54 * | 3.94 ± 0.52 * | 4.71 ± 0.60 * | 82.94 ± 9.59 |
| DCM | 8.46 ± 4.53 * | 3.04 ± 0.79 * | 4.61 ± 1.47 * | >100 |
| AcOEt | 8.80 ± 3.04 * | 3.69 ± 0.38 * | 4.46 ± 0.98 * | >100 |
| EtOH | 90.95 ± 31.5 | 179.5 ± 18.3 | 214.2 ± 54.7 | >100 |
| Leptocarpin | 1.80 ± 0.31 | 1.43 ± 0.25 | 1.28 ± 0.21 | 6.28 ± 0.53 |
| Extracts | PC-3 | HT-29 | MCF-7 |
|---|---|---|---|
| Hex | 21.05 | 18.47 | 17.60 |
| DCM | >32.89 | >11.82 | >21.61 |
| AcOEt | >27.10 | >11.36 | >22.42 |
| EtOH | >0.55 | >1.01 | >0.47 |
| Leptocarpin | 3.48 | 4.39 | 4.90 |
| Extracts | Concentration (µg/mL) | HT-29 | MCF-7 | PC-3 | HEK-293 |
|---|---|---|---|---|---|
| Hex | 6.25 | 81.3 ± 7.3 | 74.6 ± 8.2 | 82.3 ± 6.9 | 78.3 ± 5.8 |
| 37.5 | 35.7 ± 5.4 * | 44.9 ± 6.1 * | 57.5 ± 6.7 * | 73.5 ± 7.2 | |
| DCM | 6.25 | 36.3 ± 5.9 * | 44.6 ± 5.1 * | 53.2 ± 5.4 * | 67.2 ± 10.4 * |
| 37.5 | 9.3 ± 1.6 * | 11.9 ± 5.0 * | 17.4 ± 6.2 * | 61.4 ± 8.2 * | |
| AcOEt | 6.25 | 81.7 ± 8.7 | 76.3 ± 9.1 | 69.9 ± 6.3 * | 77.9 ± 8.3 |
| 37.5 | 13.3 ± 1.4 * | 23.5 ± 5.0 * | 26.2 ± 4.3 * | 69.2 ± 6.4 * | |
| Leptocarpin | 9.0 (25 µM) | 53.3 ± 5.6 * | 21.5 ± 4.2 * | 46.5 ± 7.9 * | 76.9 ± 8.7 |
| Control (ethanol) | - | 92.0 ± 10.2 | 81.4 ± 8.3 | 84.5 ± 7.5 | 80.8 ± 8.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montenegro, I.; Moreira, J.; Ramírez, I.; Dorta, F.; Sánchez, E.; Alfaro, J.F.; Valenzuela, M.; Jara-Gutiérrez, C.; Muñoz, O.; Alvear, M.; et al. Chemical Composition, Antioxidant and Anticancer Activities of Leptocarpha rivularis DC Flower Extracts. Molecules 2021, 26, 67. https://doi.org/10.3390/molecules26010067
Montenegro I, Moreira J, Ramírez I, Dorta F, Sánchez E, Alfaro JF, Valenzuela M, Jara-Gutiérrez C, Muñoz O, Alvear M, et al. Chemical Composition, Antioxidant and Anticancer Activities of Leptocarpha rivularis DC Flower Extracts. Molecules. 2021; 26(1):67. https://doi.org/10.3390/molecules26010067
Chicago/Turabian StyleMontenegro, Iván, Jorge Moreira, Ingrid Ramírez, Fernando Dorta, Elizabeth Sánchez, Juan Felipe Alfaro, Manuel Valenzuela, Carlos Jara-Gutiérrez, Ociel Muñoz, Matias Alvear, and et al. 2021. "Chemical Composition, Antioxidant and Anticancer Activities of Leptocarpha rivularis DC Flower Extracts" Molecules 26, no. 1: 67. https://doi.org/10.3390/molecules26010067
APA StyleMontenegro, I., Moreira, J., Ramírez, I., Dorta, F., Sánchez, E., Alfaro, J. F., Valenzuela, M., Jara-Gutiérrez, C., Muñoz, O., Alvear, M., Werner, E., Madrid, A., Villena, J., & Seeger, M. (2021). Chemical Composition, Antioxidant and Anticancer Activities of Leptocarpha rivularis DC Flower Extracts. Molecules, 26(1), 67. https://doi.org/10.3390/molecules26010067








