Abstract
The aim of this study was to determine, first, the chemical composition of Aloysia polystachya (Griseb) Moldenke essential oil, from leaves harvested in central Chile; and second, its antioxidant and cytotoxic activity. Eight compounds were identified via gas chromatography–mass spectrometry (GC–MS) analyses, with the most representative being R-carvone (91.03%), R-limonene (4.10%), and dihydrocarvone (1.07%). For Aloysia polystachya essential oil, antioxidant assays (2,2-diphenyl-1-picrylhydrazyl (DPPH), H2O2, ferric reducing antioxidant power (FRAP), and total reactive antioxidant potential (TRAP)) showed good antioxidant activity compared to commercial antioxidant controls; and anti-proliferative assays against three human cancer cell lines (colon, HT-29; prostate, PC-3; and breast, MCF-7) determined an IC50 of 5.85, 6.74, and 9.53 µg/mL, and selectivity indices of 4.75, 4.12, and 2.92 for HT-29, PC-3, and MCF-7, respectively. We also report on assays with CCD 841 CoN (colon epithelial). Overall, results from this study may represent, in the near future, developments for natural-based cancer treatments.
1. Introduction
The family Verbenaceae, which is comprised of some 50 species native to the American continent, distributed mainly in temperate climates and some subtropical and desert climates [1], includes Aloysia spp., a genus of flowering plants. Of these, Aloysia polystachya (Griseb) Moldenke (Verbenaceae) is an aromatic shrub that grows throughout South America and whose leaves enjoy widespread use to aromatize “mate” or “tereré” [2] (popularly known as “Té del Burrito” [3], a denomination originating in the Argentinean mountains), or as folk medicine to treat nausea, vomiting, dyspepsia, gastritis, and anxiety disorders [4]. Data from the literature show that alcoholic extracts of this plant exhibit biological effects, including antioxidant [5], antitumor [6], antispasmodic [7], anxiolytic [8], and antidepressant properties [9].
Nevertheless, there are few reports in the literature concerning the biological properties of essential oil from this plant. There are four reports from Argentina and one from Brazil where the chemical composition of the oils has been determined. Of the plants that grow in Argentina, the main constituents of the oils of the leaves of the species collected in the province of Cordova have been described as the monoterpenes α-tujone (83.56%), sabinene (4.61%), and limonene (1.62%) [10,11], while in species collected in the cities of Buenos Aires and Corrientes the predominant monoterpenes are carvone (83.5–84.4%), limonene (14.2–16.5%), and verbenone (1.58%) [12,13]. The essential oil from A. polystachya leaves grown in the southeast region of Brazil revealed a high content of carvone (80.71%) and limonene (14.65%) [14]. In addition, these studies validated the antimicrobial activity of A. polystachya oils against different human pathogens [10,11,12,13,14].
However, to the best of our knowledge, no studies have determined the chemical composition and/or biological activities of the essential oil of the leaves of A. polystachya growing in Chile. Thus, the aim of the present study was to determine the chemical constituents of the essential oil extracted from fresh leaves of A. polystachya growing in Chile and evaluate its antioxidant and cytotoxic activities.
2. Results and Discussion
The hydrodistillation of the fresh leaves of A. polystachya gave light yellow oil with a yield of 1.21% (v/w). The essential oil of A. polystachya fresh leaves is composed mainly by oxygenated monoterpenes (93.61%), followed by hydrocarbon monoterpenes (4.10%) and hydrocarbon sesquiterpenes (1.28%) (Table 1). Eight compounds were identified in the essential oil of A. polystachya, which corresponded to 98.99% of the total oil analyzed, and the main components were: R-carvone (91.03%), R-limonene (4.10%), and dihydrocarvone (1.07%) (Figure 1).

Table 1.
Essential oil composition of A. polystachya.
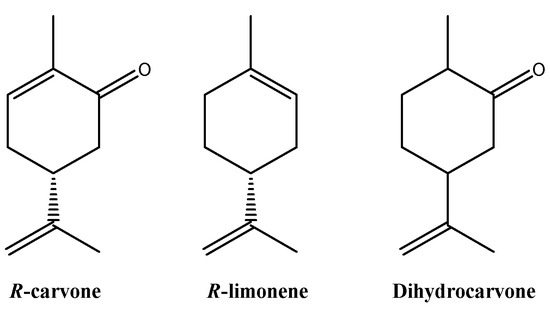
Figure 1.
Structures of the main compounds present in EO of A. polystachya.
Our results on A. polystachya essential oil from fresh leaves collected in central Chile are consistent with other studies, identifying carvone and limonene as the most abundant constituents [13]; however, our report is the first in which carvone constitutes over 90 percent of the composition. This variation in essential oil content and chemical composition is influenced by many factors, including location, plant age, climate, cultivar, distillation method, and type of distillation apparatus used [16].
The antioxidant activity of the fresh leaf essential oil of A. polystachya, examined using four different assays, is shown in Table 2.

Table 2.
Antioxidant activity of essential oil of the fresh leaves of A. polystachya and of three standards, determined by DPPH, H2O2, FRAP, and TRAP assays.
While 2,2-diphenyl-1-picrylhydrazyl (DPPH) values indicate significant antioxidant activity of A. polystachya oil on aromatic herbal essential oils, such as rosemary (Rosmarinus officinalis L.), cedar (Cedrus libani), and lemon balm (Melissa officinalis L.) [17,18], it still presents low radical scavenging activity compared to reference compounds. That said, A. polystachya essential oil was shown to have greater redox properties than commercial standards under ferric reducing antioxidant power (FRAP) values and similar redox under total reactive antioxidant potential (TRAP) values.
The antioxidant activity of A. polystachya essential oil is likely due to the high oxygenated monoterpene content [19], under which carvone is most representative [20]. Carvone is an oxygenated monoterpene with a double bond conjugated with a ketone group, which gives it greater capacity to capture free radicals and better reducing power [20,21,22,23]. Indeed, high carvone content has been linked to antioxidant activity in Mentha spicata and M. gracilis essential oils [24], as well as in essential oils of different species of the Verbenaceae family like Lippia alba and Phyla nodiflora [22,25,26,27]. The antioxidant activity of A. polystachya essential oil is further enhanced by the presence of unsaturated terpenes, like limonene, a monocyclic monoterpene capable of inhibiting free radicals and lipid peroxidation that prevents cell damage by reducing blood pressure and cardiovascular response to stress [28,29]; and other monoterpenes with hydroxyl substitutes, such as terpineol and linalool [30,31].
The inhibitory effects of the essential oil (IC50 values) are presented in Table 3. The tested A. polystachya oil showed prominent cytotoxic activity against all cancer cell lines used in the study.

Table 3.
IC50 a (µg/mL) and selectivity index (SI) of A. polystachya essential oil and main components tested on MCF-7, PC-3, and HT-29 cancer cell lines, and on CCD 841 CoN normal cell line.
Based on criteria established by the National Cancer Institute (NCI) Plant Screening Program, a crude extract of a medicinal plant is considered to have potential if the in vitro cytotoxicity studies reported an IC50 value of less than 20 µg/mL following incubation of 48–72 h [32]. In the present study, the oil showed pronounced effects against HT-29 (colon), PC-3 (prostate), and MCF-7 (breast), with IC50 values of 5.85 ± 0.39, 6.74 ± 0.03, and 9.53 ± 0.45 µg/mL, respectively, results which are consistent with NCI guidelines. Notably, these were significantly lower than the IC50 value obtained for normal cell line, 27.81 ± 0.21 µg/mL. Moreover, A. polystachya oil cytotoxic activity was much higher than 5-fluorouracil (5-FU) in all cell lines tested, although lower than daunorubicin against breast and prostate cell lines. Nevertheless, these results—which are supported by previous in vivo assays of alcohol extracts of this plant [6]—demonstrate the anti-proliferative potential of this species, and particularly so against the colon tumor cell line. Furthermore, essential oils with IC50 values under 30 µg/mL are typically classified as promising anti-cancer agents [33]. This report is therefore indicative that A. polystachya oil may be a potential substrate for the development of new drugs against this disease.
According to recent studies [34,35,36], a selectivity index (SI) value of more than three was considered highly selective against cancer cells. In vitro activity of tested samples against the CCD 841 CoN cell line were used to calculate the selectivity indices (Equation (3)), shown in Table 3. The A. polystachya essential oil presented the best selectivity indices against the HT-29 and PC-3 cell lines (4.75 and 4.13 respectively), and 1.6 times less selectivity against the MCF-7 cell line. Based on these results (and selectivity criteria reported in the literature), the essential oil can be considered a selective agent for the MCF-7 cell line, at values above 2, and for PC-3 and HT-29 cells, at values greater than 3 [32,33,34,35]. Finally, the selective activity of the essential oil against the HT-29 cell line is comparable to that of 5-FU, and superior to daunorubicin.
The activity is most likely a synergistic effect among the major monoterpenes found in the A. polystachya essential oil [37]. Among the identified terpenes, d-limonene has a demonstrated ability to inhibit cell proliferation, e.g., by inducing apoptosis in lung, stomach, and gastric liver cells [38]. Furthermore, carvone has been shown to be cytotoxic in some tumor cell lines [39,40]; for example, M. spicata oil—with similar carvone content (65.33%)—showed similar cytotoxic activity against the HeLa cell line [41], with IC50 values below 10 µg/mL. Besides, carvone is bioactive compound that contribute to the pharmacological activity of the various essential oils in which they are found [42]. Previous studies showed that the use of carvone and limonene mixtures enhanced the cytotoxic activity of each of the monoterpenes separately [43]. In turn, reports suggest that oxygenated monoterpenes, such as terpineol and linalool present in the oil, contribute to the cytotoxic potential of vegetable oils [44,45]. In addition, hydrocarbon sesquiterpenes, such as E-caryophyllene and α-curcumene, would contribute to the synergistic effect of A. polystachya essential oil, since both compounds possess significant anticancer activities, affecting the growth and proliferation of numerous cancer cells [46,47]. However, the individual compounds did not exhibit cytotoxic activity against the tumor lines tested. Despite the fact that carvone and limonene did not show cytotoxicity in this study as in other works [20,48,49], previous studies showed cytotoxic activity against prostate and breast cancer cell lines [50,51,52]. These monoterpenes are present in low concentrations in food and have applications in industry and agriculture, increasing the human exposure to these compounds [53,54]. Finally, our data may represent, in the near future, developments for natural-based cancer treatments or a potential preservative agent.
3. Materials and Methods
3.1. General Data
All reagents, R-carvone, R-limonene, and dihydrocarvone were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA), GIBCO BRL Life Technologies (Grand Island, NY, USA), and Santa Cruz Biotechnology (Santa Cruz, CA, USA).
3.2. Equipment
The analysis of the essential oil was carried out by gas chromatography–mass spectrometry (GC–MS) using a Hewlett-Packard GC/MS 6890 coupled to a Hewlett-Packard 5973 mass-selective detector (electron ionization, 70 eV, Palo Alto, CA, USA) and equipped with a capillary HP-5 MS column. Antioxidant assays were determined in a UV–Vis spectrophotometer (Jenway 6320D, Bibby Scientific Limited, Beacon Road, Stone, Staffordshire ST15 0SA, UK). Anti-proliferative assay was determined in a microplate reader (SpectraMax, Winooski, VT, USA).
3.3. Plant Material
Plant samples were collected from Villa Alemana, Valparaiso Region, Central Chile (S: 33.0497°, W: −71.3927°) at an altitude of approximately 133 m during the spring in October 2019. Botanical identification and authentication was verified by Mr. Patricio Novoa, and a voucher specimen (AP-1019) was deposited at the Natural Products and Organic Synthesis Laboratory of Universidad de Playa Ancha, Valparaíso, Chile.
3.4. Preparation of Essential Oil
The essential oil was extracted from fresh leaves of A. polystachya (500 g) ground in a knife mill by steam distillation carried out using a Clevenger-type apparatus for 4 h [55]. Thereafter, the hydrolate was subjected to liquid-liquid partition in a separatory funnel and three washes with three 10 mL portions of dichloromethane. The essential oil sample was stored at −4 °C until further chemical and biological tests.
3.5. Chemical Analysis
The essential oil of A. polystachya was analyzed by GC–MS. The working conditions were as follows: injector temperature, 250 °C; detector temperature, 280 °C; carrier gas, He at 1.25 mL/min; and oven temperature program: 35 °C for 5 min, increase to 260 °C at 5 °C/min, and then 260 °C for 5 min. Compounds in the chromatogram (see Figure S1 Supplementary Material) were identified by comparison of their mass spectra with those in the NIST 2014 library database, and by comparison of their retention index with those reported in the literature [15], for the same type of column or those of commercial standards, when available. The retention indices were determined under the same operating conditions in relation to a homologous n-alkanes series (C8–C36) by Equation (1):
where n = the number of carbon atoms in the smaller n-alkane; N = the number of carbon atoms in the larger n-alkane; and Tr = the retention time. The components’ relative concentrations were obtained by peak area normalization.
RI = 100 × (n + Tr(unknown) − Tr(n)/Tr(N) − Tr(n))
3.6. Antioxidant Assays
3.6.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Activity
The DPPH free radical scavenging activity was estimated by assay based on the method described in the literature [56]. Briefly, 2.0 mL of 0.5 mmol/L DPPH in ethanol was mixed with 100 μL of essential oil of A. polystachya (0.001, 0.01, 0.1, and 1.0 mg/mL). After 20 min incubation, the absorbance was measured at 517 nm. Trolox and gallic acid were used as positive controls. The percentage of free radical-scavenging capacity was calculated by Equation (2):
where Asample is the absorbance of DPPH mixed with essential oil and Acontrol is the absorbance of DPPH in which sample has been replaced with ethanol. All measurements were performed in triplicate and reported as the average value. The IC50 value was determined by linear regression analysis from the obtained radical scavenging capacity (RSC) values.
RSC% = 100% × (Acontrol − Asample)/Acontrol
3.6.2. H2O2 Scavenging Activity
The H2O2 scavenging activity was determined according to a previously described method [57]. A solution of hydrogen peroxide (40 mM) was prepared in phosphate buffer (pH 7.4). The samples (from 2.5 to 10 μL of the 0.1% essential oil) were added to a hydrogen peroxide solution (0.6 mL, 40 mM). Absorbance of hydrogen peroxide at 230 nm was determined after 10 min. Butylated hydroxytoluene (BHT) and Trolox were used as positive controls. The percentage of scavenging of hydrogen peroxide by the essential oil was calculated using Equation (3):
where A0 represents the absorbance of the control and A1 represents the absorbance in the presence of the essential oil and standards. IC50 was the effective concentration at which 50% of hydrogen peroxide was scavenged.
H2O2% = 100% × (A0 − A1)/A0
3.6.3. Ferric Reducing Antioxidant Power (FRAP) Assay
FRAP assay was carried out using the above method [58]. FRAP test solution was prepared using FeCl3·6H2O in distilled water (final concentration of Fe(III) in the solution was 20 mM), 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ) in 40 mM HCl (final concentration of TPTZ was 10 mM), and 0.3 M CH3COOH/CH3COONa buffer solution at pH = 3.6. The FRAP reagent was prepared daily as follows: acetic acid buffer, TPTZ solution, and FRAP test solution were mixed in this order at the volume ratio of 10:1:1. A 3.0 mL aliquot of FRAP reagent was mixed with 300 μL of deionized water and 100 µL of methanolic essential oil solution (1.0 mg/mL). The mixture was vigorously shaken for 30 s and left in the dark at 37 °C for 30 min. Subsequently the absorbance was measured at 593 nm using ethanol as the blank solution. The obtained absorbance values were interpolated in a Trolox calibrate curve (0–200 mg/L) and the FRAP values were expressed in mM Trolox equivalent antioxidant capacity (mM TEAC). BHT and gallic acid were used as positive controls. All of the measurements were performed in triplicate.
3.6.4. The Total Reactive Antioxidant Potential (TRAP) Assay
The TRAP of the essential oil was determined by ABTS+ (2,2′-azinobi(3-ethylbenzothiazoline-6-sulfonic acid)) assay [57]. The ABTS+ (2,2′-azinobi(3-ethylbenzothiazoline-6-sulfonic acid)) radical solution (150 µM) was mixed with 2,2′-azo-bis(2-amidino propane) (ABAP) solution (10 mM) in phosphate-buffered saline (PBS), pH 7.4 solution (100 mM). The mixture was incubated at 45 °C for 30 min. 10 µL of sample solution was added to 990 µL of the resulting blue-green ABTS radical solution. The decrease of absorbance of TRAP solutions and ABTS as blank were recorded after 30 s at room temperature. Then, the absorbance of the samples was measured at 734 nm. The total antioxidant capacity (TRAP) of the essential oil was expressed in mM Trolox equivalents (TEAC), using a standard curve of Trolox (0–120 mg/L). BHT and gallic acid were used as positive controls. All measurements were replicated three times.
3.7. Cell Lines and Culture Conditions
In this study, we used three different tumor cell lines: human breast adenocarcinoma (MCF-7), human prostate adenocarcinoma (PC-3), and human colorectal adenocarcinoma (HT-29). A normal human cell line (colon epithelials, CCD 841 CoN) was included to evaluate the possible selective activity of the essential oil. The different cell lines were maintained as monolayers in a plastic culture medium (HAM-F10+DMEM, 1:1) supplemented with 10% fetal bovine serum, as well as antibiotics (0.01 mg/mL streptomycin and 0.005 mg/mL penicillin). The cells were incubated at 37 °C in a humidified 5% CO2 atmosphere.
3.8. Anti-Proliferative Assay
The panel of cancer cells were seeded at a density of 2 × 104 cells/well into 96-well plates and assayed as described previously [59]. Test compounds were solubilized just prior to the experiment in 0.1% DMSO. Briefly, cells were treated with increasing concentrations of essential oil (0.625–100 µg/mL) for 72 h at 37 °C in 5% CO2. The cells which received only the medium containing 0.1% DMSO served as the control group. At the end of essential oil exposure, cells were fixed with 50% trichloroacetic acid at 4 °C (TCA final concentration 10%). After washing with distilled water, cells were stained with 0.1% sulforhodamine B (Sigma-Aldrich, St. Louis, MO, USA), dissolved in 1% acetic acid (50 µL/well) for 30 min, and subsequently washed with 1% acetic acid to remove unbound stain. Protein-bound stain was solubilized with 100 µL of 10 mM unbuffered Tris base. The cell density was determined using a fluorescence plate reader (wavelength 540 nm). Daunorubicin and 5-fluorouracil (5-FU) were used as positive controls. Values shown are the mean + SD of three independent experiments in triplicate. Finally, Sigma Plot software (Systat Software, San Jose, CA, USA) was used to calculate the IC50 value.
3.9. Selective Index
The selectivity index (SI) is the ratio between the IC50 value of the A. polystachya essential oil obtained for CCD 841 CoN cells and the value found for the cancer cell line (Equation (4)).
where a SI > 3 was considered to belong to a selective sample [33,34,35,36].
SI = IC50 (CCD 841 CoN)/IC50(cancer cell)
3.10. Statistical Analysis
The data were reported as the mean values ± standard deviation (SD). Due to non-parametric data, a Kruskal–Wallis ANOVA was used with a confidence level of 95% with the STATISTICA 7.0 program.
4. Conclusions
Based on the results of this study, A. polystachya essential oil is an accessible and natural source of carvone. Furthermore, given the potent and promising anti-proliferative activity of the essential oil, novel anti-cancer formulations should be explored. In addition, due to its antioxidant power and the high percentage of carvone added to the presence of limonene and a series of compounds of terpenic origin present in A. polystachya essential oil, it can potentially be used as a food or drug preservative.
Supplementary Materials
The following are available online, Figure S1. GC–MS chromatogram of the essential oil of Aloysia polystachya from Chile.
Author Contributions
A.M. supervised the whole study; B.S. collected the plant; S.F. performed the isolation of the essential oil; A.R. and J.V. conceived and designed the biologic experiments; C.P. and N.C. performed the antioxidant assay; A.C.M. performed the cytotoxic assay; A.M., E.W., and I.M. collaborated in the discussion and interpretation of the results; A.M. wrote the manuscript. All authors read and approved the final manuscript.
Funding
The authors thank FONDECYT (grant No. 1190424) and Programa de Desarrollo Disciplinario de la Facultad de Ciencias Naturales y Exactas 2017, UPLA.
Data Availability Statement
All data are available for scientific community.
Acknowledgments
To the staff of the laboratory LPNSO, UPLA.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the essential oil and compounds are available from the authors.
References
- Siedo, S.J. Four new species of the genus Aloysia (Verbenaceae). Lundellia 2012, 15, 35–46. [Google Scholar] [CrossRef]
- Botta, S.M. Las especies argentinas del género Aloysia (Verbenaceae). Darwiniana 1979, 22, 67–108. [Google Scholar]
- Umansk, S.I.; Schroeder, M.A.; Dirchwolf, P.M. Vegetative propagation protocol of Aloysia polystachya (Griseb.) Moldenke (burrito). Rev. Cubana Plant. Medic. 2018, 23, 2. [Google Scholar]
- Campos, R.; Scarpa, G.F. The cultural-bound disease “empacho” in Argentina. A comprehensive botanico-historical and ethnopharmacological review. J. Ethnopharmacol. 2013, 148, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Aguado, M.I.; Nuñez, M.B.; Bela, A.J.; Okulik, N.B.; Bregni, C. Caracterización fisicoquímica y actividad antioxidante de un extracto etanólico de Aloysia polystachya (Griseb.) Mold. (Verbenaceae). Rev. Mex. Cienc. Farm. 2013, 44, 46–51. [Google Scholar]
- Soares, M.; Palma, A.; Panelo, L.C.; Paz, L.A.; Rosa, F.; Lira, M.C.; Azurmendi, P.; Rubio, M.F.; Lenz, G.; Urtreger, A.J.; et al. Extract from Aloysia polystachya induces the cell death of colorectal cancer stem cells. Nutr. Cancer 2019, 72, 1004–1007. [Google Scholar] [CrossRef]
- Consolini, A.; Berardi, A.B.; Rosella, M.; Volonté, M. Antispasmodic effects of Aloysia polystachya and A. gratissima tinctures and extracts are due to non-competitive inhibition of intestinal contractility inoluced by acethyleholine and calcium. Rev. Bras. Farmacogn. 2011, 21, 889–900. [Google Scholar] [CrossRef]
- Carmona, F.; Saraiva, F.; Alves, P.; Casale, D.; Angelucci, M.A.; Zangiacomi, E.; Soares, A.M. Aloysia polystachya (Griseb.) Moldenke (Verbenaceae) powdered leaves are effective in treating anxiety symptoms: A phase-2, randomized, placebo-controlled clinical trial. J. Ethnopharmacol. 2019, 242, 112060. [Google Scholar] [CrossRef]
- Mora, S.; Díaz, G.; Millán, R.; Lungenstrass, H.; Quirós, S.; Coto, T.; Hellión, M.C. Anxiolytic and antidepressant-like effects of the hydroalcoholic extract from Aloysia polystachya in rats. Pharmacol. Biochem. Behav. 2005, 82, 373–378. [Google Scholar] [CrossRef]
- Cabanillas, C.M.; Lopez, M.L.; Daniele, G.; Zygadlo, J.A. Essential oil composition of Aloysia polystachya (Griseb.) Moldenke under rust disease. Flavour Fragr. J. 2003, 18, 446–448. [Google Scholar] [CrossRef]
- Arena, J.S.; Merlo, C.; Defagó, M.T.; Zygadlo, J.A. Insecticidal and antibacterial effects of some essential oils against the poultry pest Alphitobius diaperinus and its associated microorganisms. J. Pest. Sci. 2020, 93, 403–414. [Google Scholar] [CrossRef]
- Werdin, J.O.; Gutiérrez, M.M.; Murray, A.P.; Ferrero, A.A. Biological Activity of Essential Oils from Aloysia polystachya and Aloysia citriodora (Verbenaceae) against the Soybean Pest Nezara viridula (Hemiptera: Pentatomidae). Nat. Prod. Commun. 2010, 5, 301–306. [Google Scholar] [CrossRef]
- Pérez, C.M.; Torres, C.A.; Aguado, M.I.; Bela, A.J.; Nuñez, M.B.; Bregni, C. Antibacterial activity of essential oils of Aloysia polystachya and Lippia turbinata (Verbenaceae). Boletín Latinoam. Caribe Plantas Med. Aromáticas 2016, 15, 199–205. [Google Scholar]
- Pina, E.; Coppede, J.; Sartoratto, A.; Fachin, A.L.; Bertoni, B.W.; França, S.; Pereira, A.M. Antimicrobial activity and chemical composition of essential oils from Aloysia polystachya (Griseb.) Moldenke grown in Brazil. J. Med. Plants Res. 2012, 6, 5412–5416. [Google Scholar] [CrossRef]
- Adams, P.R. Identification of Essential Oil Components by Gas. Chromatography/Mass Spectromety, 4th ed.; Allured Publishing Corp.: Carol Stream, IL, USA, 2007; pp. 1–8. [Google Scholar]
- Izuegbuna, O.; Otunola, G.; Bradley, G. Chemical composition, antioxidant, antiinflammatory, and cytotoxic activities of Opuntia stricta cladodes. PLoS ONE 2019, 14, e0209682. [Google Scholar] [CrossRef]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant Activity of Essential Oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef]
- Olszowy, M.; Dawidowicz, A.L. Essential oils as antioxidants: Their evaluation by DPPH, ABTS, FRAP, CUPRAC, and b-carotene bleaching methods. Mon. Chem.-Chem. Mon. 2016, 147, 2083–2091. [Google Scholar] [CrossRef]
- Martins, M.; Arantes, S.; Candeias, F.; Tinoco, M.T.; Cruz, J. Antioxidant, antimicrobial and toxicological properties of Schinus molle L. essential oils. J. Ethnopharmacol. 2014, 151, 485–492. [Google Scholar] [CrossRef]
- Bicas, J.L.; Neri, I.A.; Ruiz, A.L.; De Carvalho, J.E.; Pastore, G.M. Evaluation of the antioxidant and antiproliferative potential of bioflavors. Food Chem. Toxicol. 2011, 49, 1610–1615. [Google Scholar] [CrossRef]
- Elmastaş, M.; Dermirtas, I.; Isildak, O.; Aboul-Enein, H.Y. Antioxidant Activity of S-Carvone Isolated from Spearmint (Mentha Spicata L. Fam Lamiaceae). J. Liq. Chromatogr. Relat. Technol. 2006, 29, 1465–1475. [Google Scholar] [CrossRef]
- Sabir, S.M.; Singh, D.; Rocha, J.B. In vitro antioxidant activity of s-carvone isolated from Zanthoxylum alatum. Pharm. Chem. J. 2015, 49, 187–191. [Google Scholar] [CrossRef]
- Pombal, S.; Hernández, Y.; Diez, D.; Mondolis, E.; Mero, A.; Morán-Pinzón, J.; Guerrero, E.I.; Rodilla, J.M. Antioxidant activity of carvone and derivatives against superoxide ion. Nat. Prod. Commun. 2017, 12, 653–655. [Google Scholar] [CrossRef] [PubMed]
- Peña, O.A.; Villarreal, M.L.; Álvarez, L.; Meneses, A.; Rodríguez, V. Cytotoxicity, Post-treatment recovery, and selectivity analysis of naturally occurring podophyllotoxins from Bursera fagaroides var. fagaroides on breast cancer cell lines. Molecules 2016, 21, 1013. [Google Scholar] [CrossRef] [PubMed]
- Trifan, A.; Aprotosoaie, A.C.; Cioanca, O.; Hacianu, M.; Jitareanu, A.; Gille, E. Antioxidant Activity of Essential Oil From Carum carvi L. cultivated in NorthEastern Romania. Med.-Surg. J. 2016, 120, 732–736. [Google Scholar]
- Shailendra, S.; Prakash, S.O.; Vivekanand, P.R.; Pant, A.K.; Mathela, S. Chemical diversity in Mentha spicata: Antioxidant and potato sprout inhibition activity of its essential oils. Nat. Prod. Commun. 2011, 6, 1373–1378. [Google Scholar] [CrossRef]
- Pérez Zamora, C.M.; Torres, C.A.; Nuñez, M.B. Antimicrobial Activity and Chemical Composition of Essential Oils from Verbenaceae Species Growing in South America. Molecules 2018, 23, 544. [Google Scholar] [CrossRef]
- Jing, L.; Zhang, Y.; Fan, S.; Gu, M.; Guan, Y.; Lu, X.; Huang, C.; Zhou, Z. Preventive and ameliorating effects of citrus D-limonene on dyslipidemia and hyperglycemia in mice with high-fat diet-induced obesity. Eur. J. Pharmacol. 2013, 715, 46–55. [Google Scholar] [CrossRef]
- Bai, J.; Zheng, Y.; Wang, G.; Liu, P. Protective effect of D-limonene against oxidative stress-induced cell damage in human lens epithelial cells via the p38 pathway. Oxid. Med. Cell Longev. 2016, 2016, 5962832. [Google Scholar] [CrossRef]
- Jabir, M.S.; Taha, A.A.; Sahib, S.I. Antioxidant activity of linalool. Eng. Technol. J. 2018, 36, 64–67. [Google Scholar] [CrossRef]
- Ruberto, G.; Baratta, M.T. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000, 69, 167–174. [Google Scholar] [CrossRef]
- Boik, J. Natural Compounds in Cancer Therapy, 1st ed.; Silvine, F., Ed.; Oregon Medical Press: Princeton, NJ, USA, 2001. [Google Scholar]
- Oliveira, P.F.; Alves, J.M.; Damasceno, J.L.; Oliveira, R.A.M.; Dias, H.J.; Crotti, A.E.M.; Tavares, D.C. Cytotoxicity screening of essential oils in cancer cell lines. Rev. Bras. Farmacogn. 2015, 25, 183–188. [Google Scholar] [CrossRef]
- Alabsi, A.M.; Lim, K.L.; Paterson, I.C.; Ali-Saeed, R.; Muharram, B.A. Cell Cycle Arrest and Apoptosis Induction via Modulation of Mitochondrial Integrity by Bcl-2 Family Members and Caspase Dependence in Dracaena cinnabari-Treated H400 Human Oral Squamous Cell Carcinoma. Biomed. Res. Int. 2016, 2016, 4904016. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Yang, C.H.; Huang, T.Y.; Tai, M.; Sie, R.H.; Shaw, J.F. Cytotoxic Effects of Chlorophyllides in Ethanol Crude Extracts from Plant Leaves. Evid.-Based Complement. Altern. Med. 2019, 2019, 9494328. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Saiful Yazan, L.; Kassim, N.K.; Che Abdullah, C.A.; Esa, N.; Lim, P.C.; Tan, D.C. Cytotoxic Activity of Christia vespertilionis Root and Leaf Extracts and Fractions against Breast Cancer Cell Lines. Molecules 2020, 25, 2610. [Google Scholar] [CrossRef] [PubMed]
- Sobral, M.V.; Xavier, A.; Lima, T.C.; de Sousa, D.P. Antitumor activity of monoterpenes found in essential oils. Sci. World J. 2014, 2014, 953451. [Google Scholar] [CrossRef] [PubMed]
- Hajizadeh, M.R.; Maleki, H.; Barani, M.; Fahmidehkar, M.A.; Mahmoodi, M.; Torkzadeh-Mahani, M. In vitro cytotoxicity assay of D-limonene niosomes: An efficient nano-carrier for enhancing solubility of plant-extracted agents. Res. Pharm. Sci. 2019, 14, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Alasmari, E.A.; Mehanna, A.S. Cytotoxic effects of S-(+)-Carvone on selected human cancer cell lines. J. Anal. Pharm. Res. 2019, 8, 149–158. [Google Scholar] [CrossRef]
- Al-Sheddi, E.S.; Al-Zaid, N.A.; Al-Oqail, M.M.; Al-Massarani, S.M.; El-Gamal, A.A.; Farshori, N.N. Evaluation of cytotoxicity, cell cycle arrest and apoptosis induced by Anethum graveolens L. essential oil in human hepatocellular carcinoma cell line. Saudi Pharm. J. 2019, 27, 1053–1060. [Google Scholar] [CrossRef]
- Liu, K.; Zhu, Q.; Zhang, J.; Xu, J.; Wang, X. Chemical composition and biological activities of the essential oil of Mentha spicata Lamiaceae. Adv. Mat. Res. 2012, 524–527, 2269–2272. [Google Scholar] [CrossRef]
- De Sousa, D.P. Medicinal Essential Oils: Chemical, Pharmacological and Therapeutic Aspects; Nova Science Publishers: New York, NY, USA, 2012; p. 236. [Google Scholar]
- Paduch, R.; Trytek, M.; Król, S.K.; Kud, J.; Frant, M.; Kandefer-Szerszeń, M.; Fiedurek, J. Biological activity of terpene compounds produced by biotechnological methods. Pharm. Biol. 2016, 54, 1096–1107. [Google Scholar] [CrossRef]
- Hassine, M.; Zardi-Berguaoui, A.; Znati, M.; Flamini, G.; Jannet, H.B.; Hamza, M.A. Chemical composition, antibacterial and cytotoxic activities of the essential oil from the flowers of Tunisian Convolvulus althaeoides L. Nat. Prod. Res. 2014, 28, 769–775. [Google Scholar] [CrossRef]
- Erdogan, A.; Ozkan, A. Investigatıon of antioxıdative, cytotoxic, membrane-damaging and membrane-protective effects of the essentıal oil of Origanum majorana and its oxygenated monoterpene component linalool in human-derived hep g2 cell line. Iran. J. Pharm. Res. 2017, 16, 24–34. [Google Scholar] [PubMed]
- Fidyt, K.; Fiedorowicz, A.; Strzadała, L.; Szumny, A. β-caryophyllene and β-caryophyllene oxide-natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef] [PubMed]
- Dosoky, N.S.; Setzer, W.N. Chemical Composition and Biological Activities of Essential Oils of Curcuma Species. Nutrients 2018, 10, 1196. [Google Scholar] [CrossRef] [PubMed]
- Moro, I.J.; Gondo, G.; Pierri, E.; Pietro, R.; Soares, C.; Sousa, D.; Santos, A. Evaluation of antimicrobial, cytotoxic and chemopreventive activities of carvone and its derivatives. Braz. J. Pharm. Sci. 2017, 53, e00076. [Google Scholar] [CrossRef]
- Mitropoulou, G.; Fitsiou, E.; Spyridopoulou, K.; Tiptiri-Kourpeti, A.; Bardouki, H.; Vamvakias, M.; Panas, P.; Chlichlia, K.; Pappa, A.; Kourkoutas, Y. Citrus medica essential oil exhibits significant antimicrobial and antiproliferative activity. LWT-Food Sci. Technol. 2017, 84, 344–352. [Google Scholar] [CrossRef]
- Chen, J.; Lu, M.; Jing, Y.; Dong, J. The synthesis of L-carvone and limonene derivatives with increased antiproliferative effect and activation of erk pathway in prostate cancer cells. Bioorg Med. Chem. 2006, 14, 6539–6547. [Google Scholar] [CrossRef]
- Jaafari, A.; Mounir, T.; Mouse, H.A.; M’bark, L.A.; Aboufatima, R.; Chait, A. Comparative study of the antitumor effect of natural monoterpenes: Relationship to cell cycle analysis. Rev. Bras. Farmacog. 2012, 22, 534–540. [Google Scholar] [CrossRef]
- Abbas, M.M.; Kandil, Y.İ.; Abbas, M.A. R-(-)-carvone Attenuated Doxorubicin Induced Cardiotoxicity In Vivo and Potentiated Its Anticancer Toxicity In Vitro. Balk. Med. J. 2020, 37, 98–103. [Google Scholar] [CrossRef]
- Carvalho, C.C.R.; Fonseca, M.R. Carvone: Why and how should one bother to produce this terpene. Food Chem. 2006, 95, 413–422. [Google Scholar] [CrossRef]
- Ibáñez, M.D.; Sanchez-Ballester, N.M.; Blázquez, M.A. Encapsulated Limonene: A Pleasant Lemon-Like Aroma with Promising Application in the Agri-Food Industry. A Review. Molecules 2020, 25, 2598. [Google Scholar] [CrossRef] [PubMed]
- Madrid, A.; Godoy, P.; González, S.; Zaror, L.; Moller, A.; Werner, E.; Cuellar, M.; Villena, J.; Montenegro, I. Chemical Characterization and Anti-Oomycete Activity of Laureliopsis philippianna Essential Oils against Saprolegnia parasitica and S. australis. Molecules 2015, 20, 8033–8047. [Google Scholar] [CrossRef] [PubMed]
- Arvouet-Grand, A.; Vennat, B.; Pourrat, A.; Legret, P. Standardization d’une extrait de propolis et identification des principaux constituents. Pharm. Belg. 1994, 49, 462–468. [Google Scholar]
- Leyton, M.; Mellado, M.; Jara, C.; Montenegro, I.; González, S.; Madrid, A. Free radical-scavenging activity of sequential leaf extracts of Embothrium coccineum. Open Life Sci. 2015, 10, 256–264. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Madrid Villegas, A.; Espinoza Catalán, L.; Montenegro Venegas, I.; Villena García, J.; Carrasco Altamirano, H. New Catechol Derivatives of Safrole and Their Antiproliferative Activity towards Breast Cancer Cells. Molecules 2011, 16, 4632–4641. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).