The Maillard Reaction as Source of Meat Flavor Compounds in Dry Cured Meat Model Systems under Mild Temperature Conditions
Abstract
1. Introduction
2. Results
2.1. Analysis of Physicochemical Changes in the Model Systems
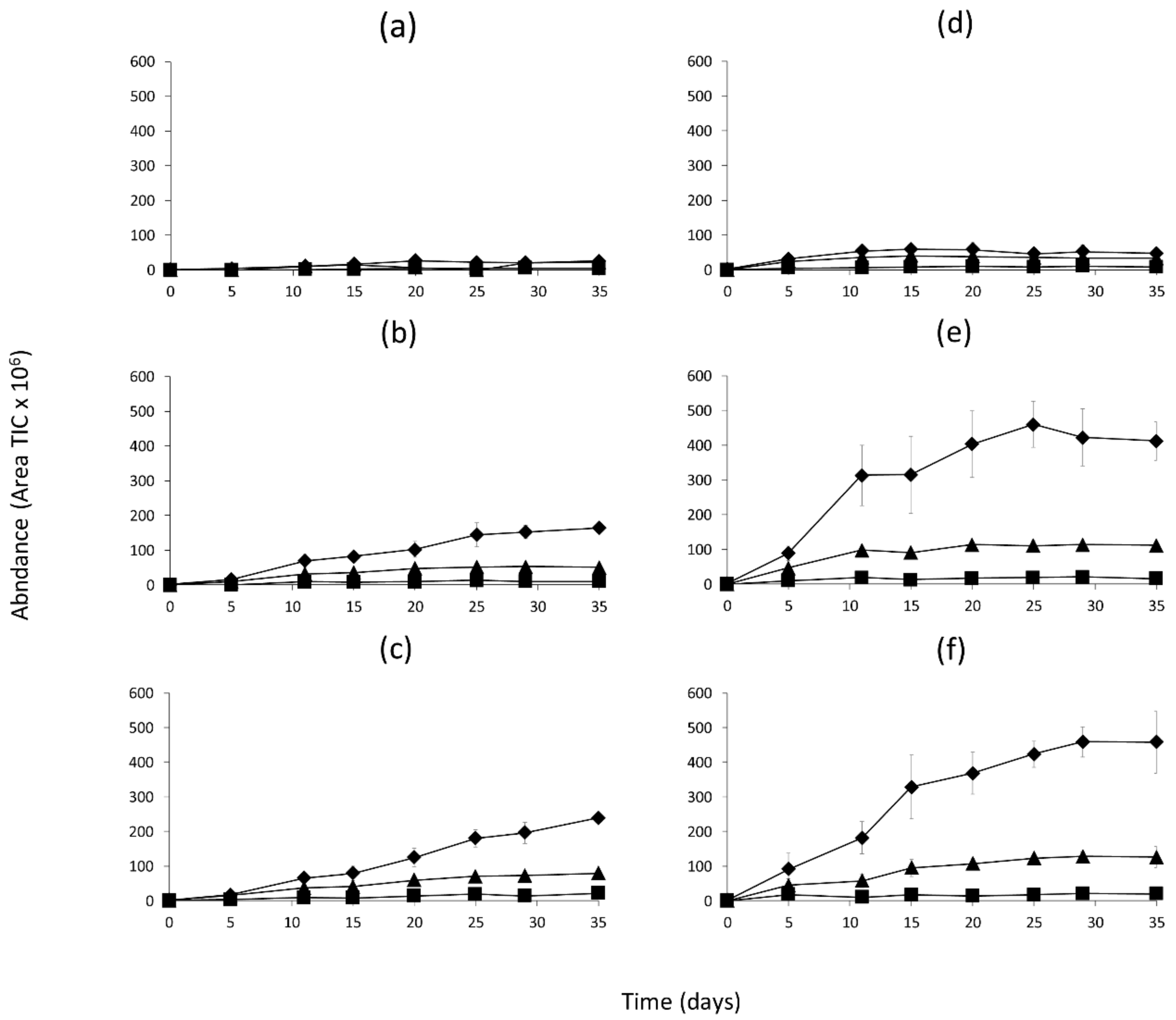
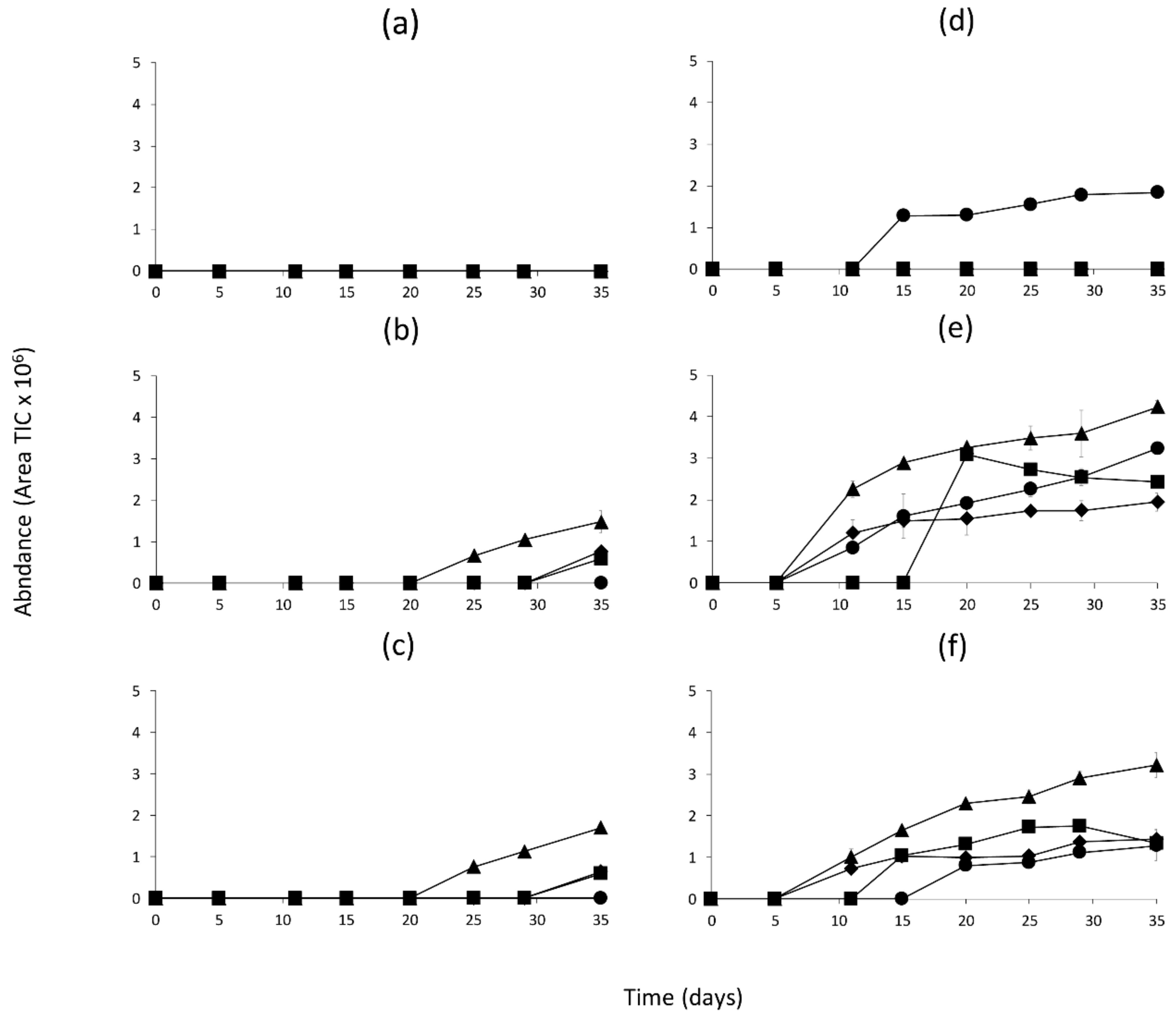
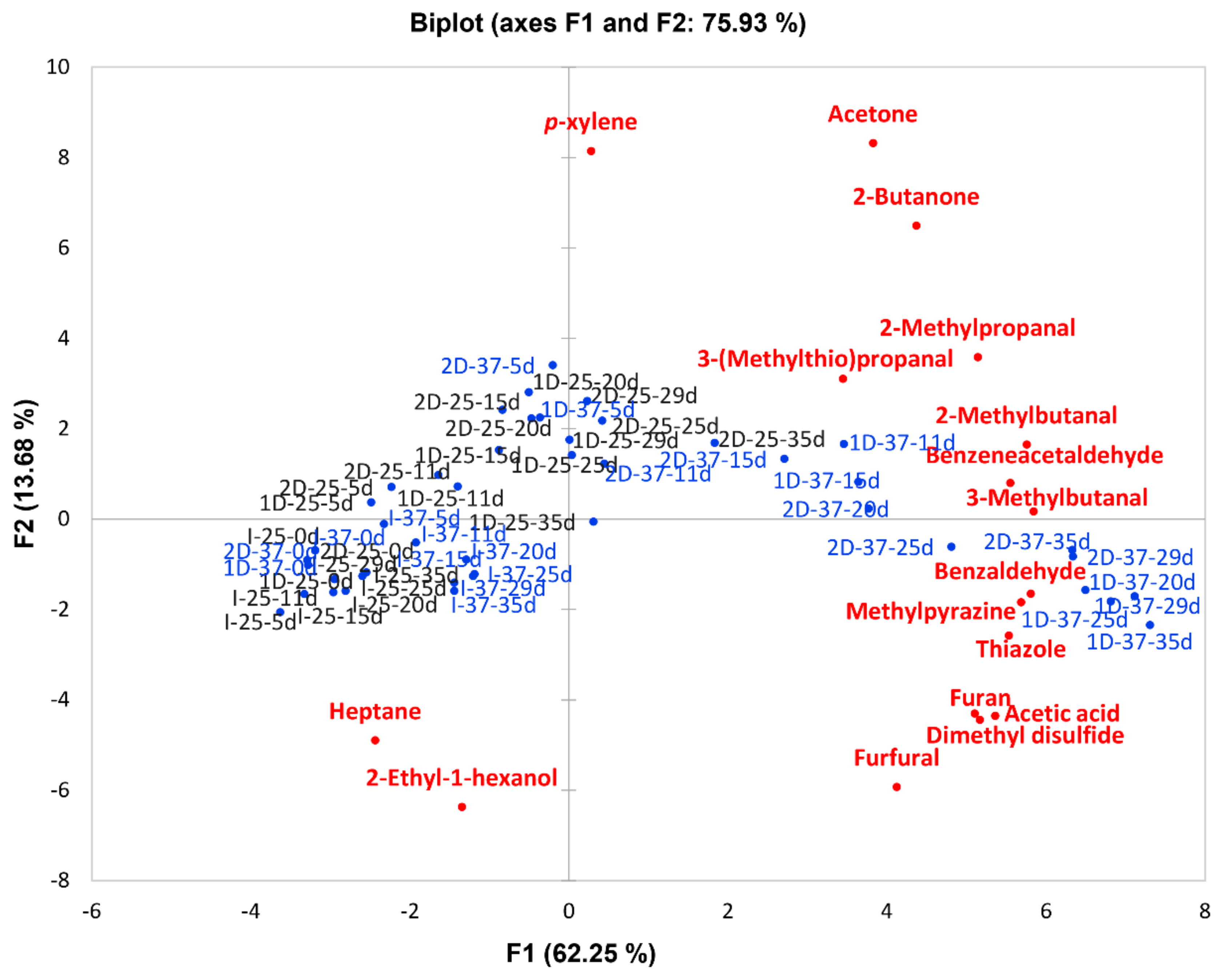
2.2. Identification of Volatile Compounds Generated in the Model Systems
2.3. Quantitation of Volatile Compounds Generated in the Model Systems
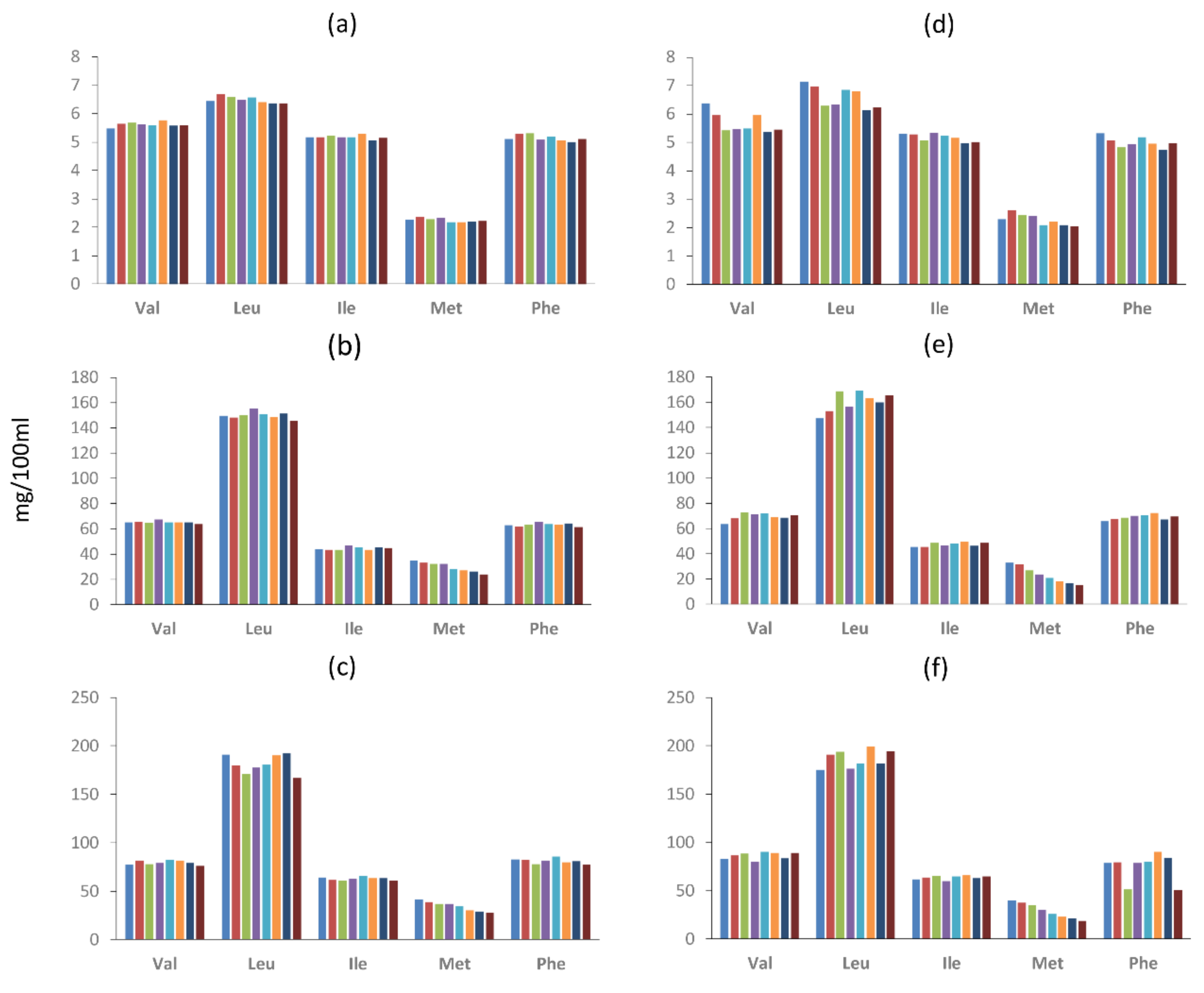
2.4. Amino Acid Concentration in the Model Systems
3. Discussion
4. Materials and Methods
4.1. Analysis of Water Activity and pH
4.2. Analysis of Volatile Compounds
4.3. Analysis of Amino Acids
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Flores, M. Understanding the implications of current health trends on the aroma of wet and dry cured meat products. Meat Sci. 2018, 144, 53–61. [Google Scholar] [CrossRef]
- Toldrá, F.; Flores, M. Section XIII. Meat and meat products: Processed pork meat flavors. In Handbook of Food Products Manufacturing; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; Volume 2, pp. 281–301. ISBN 9780470049648. [Google Scholar]
- Resconi, V.C.; Escudero, A.; Campo, M.M. The development of aromas in ruminant meat. Molecules 2013, 18, 6748–6781. [Google Scholar] [CrossRef]
- Chiang, J.H.; Eyres, G.T.; Silcock, P.J.; Hardacre, A.K.; Parker, M.E. Changes in the physicochemical properties and flavour compounds of beef bone hydrolysates after Maillard reaction. Food Res. Int. 2019. [Google Scholar] [CrossRef]
- Van Boekel, M.A.J.S. Formation of flavour compounds in the Maillard reaction. Biotechnol. Adv. 2006, 24, 230–233. [Google Scholar] [CrossRef]
- Mottram, D.S. Flavour formation in meat and meat products: A review. Food Chem. 1998. [Google Scholar] [CrossRef]
- Elmore, J.S.; Campo, M.M.; Enser, M.; Mottram, D.S. Effect of lipid composition on meat-like model systems containing cysteine, ribose, and polyunsaturated fatty acids. J. Agric. Food Chem. 2002. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, T.; Xie, J.; Xiao, Q.; Du, W.; Wang, Y.; Cheng, J.; Wang, S. Meat flavor generation from different composition patterns of initial Maillard stage intermediates formed in heated cysteine-xylose-glycine reaction systems. Food Chem. 2019. [Google Scholar] [CrossRef]
- Hou, L.; Xie, J.; Zhao, J.; Zhao, M.; Fan, M.; Xiao, Q.; Liang, J.; Chen, F. Roles of different initial Maillard intermediates and pathways in meat flavor formation for cysteine-xylose-glycine model reaction systems. Food Chem. 2017. [Google Scholar] [CrossRef]
- Koutsidis, G.; Elmore, J.S.; Oruna-Concha, M.J.; Campo, M.M.; Wood, J.D.; Mottram, D.S. Water-soluble precursors of beef flavour. Part II: Effect of post-mortem conditioning. Meat Sci. 2008. [Google Scholar] [CrossRef]
- Toldrá, F. Chapter 9–The storage and preservation of meat: III—Meat processing. In Lawrie’s Meat Science, 8th ed.; Toldra, F., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2017; pp. 265–296. ISBN 978-0-08-100694-8. [Google Scholar]
- Toldrá, F. Characterization of Proteolysis. In Dry-Cured Meat Products; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; pp. 113–134. ISBN 9780470385111. [Google Scholar]
- Modi, V.K.; Linforth, R.; Taylor, A.J. Effect of pH and water activity in generation of selected meaty aroma compounds in a meat model system. Am. J. Food Technol. 2008, 3, 68–78. [Google Scholar] [CrossRef]
- Flores, M.; Olivares, A. Flavor. In Handbook of Fermented Meat and Poultry, 2nd ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 217–225. ISBN 9781118522653. [Google Scholar]
- Cano-García, L.; Rivera-Jiménez, S.; Belloch, C.; Flores, M. Generation of aroma compounds in a fermented sausage meat model system by Debaryomyces hansenii strains. Food Chem. 2014, 151. [Google Scholar] [CrossRef]
- Marco, A.; Navarro, J.L.; Flores, M. The influence of nitrite and nitrate on microbial, chemical and sensory parameters of slow dry fermented sausage. Meat Sci. 2006. [Google Scholar] [CrossRef]
- Martins, S.I.F.S.; Jongen, W.M.F.; Van Boekel, M.A.J.S. A review of Maillard reaction in food and implications to kinetic modelling. Trends Food Sci. Technol. 2000, 11, 364–373. [Google Scholar] [CrossRef]
- Limacher, A.; Kerler, J.; Davidek, T.; Schmalzried, F.; Blank, I. Formation of furan and methylfuran by maillard-type reactions in model systems and food. J. Agric. Food Chem. 2008, 56. [Google Scholar] [CrossRef]
- Beck, H.C.; Hansen, A.M.; Lauritsen, F.R. Metabolite production and kinetics of branched-chain aldehyde oxidation in Staphylococcus xylosus. Enzyme Microb. Technol. 2002, 31, 94–101. [Google Scholar] [CrossRef]
- Wolle, D.D.; Banavara, D.S.; Rankin, S.A. Short communication: Empirical and mechanistic evidence for the role of pyridoxal-5′-phosphate in the generation of methanethiol from methionine. J. Dairy Sci. 2006, 89, 4545–4550. [Google Scholar] [CrossRef]
- De Revel, G.; Marchand, S.; Bertrad, A. Identification of Maillard–Type Aroma Compounds in Winelike Model Systems of Cysteine—Carbonyls: Occurrence in Wine; American Chemical Society: Washington, DC, USA, 2003; pp. 353–364. [Google Scholar]
- Meynier, A.; Mottram, D.S. The effect of pH on the formation of volatile compounds in meat-related model systems. Food Chem. 1995, 52, 361–366. [Google Scholar] [CrossRef]
- Van Lancker, F.; Adams, A.N.; De Kimpe, N. Formation of pyrazines in maillard model systems of lysine-containing dipeptides. J. Agric. Food Chem. 2010. [Google Scholar] [CrossRef]
- Mandin, O.; Duckham, S.C.; Ames, J.M. Volatile compounds from potato-like model systems. J. Agric. Food Chem. 1999, 47, 2355–2359. [Google Scholar] [CrossRef]
- Hidalgo, F.J.; Zamora, R. Formation of phenylacetic acid and benzaldehyde by degradation of phenylalanine in the presence of lipid hydroperoxides: New routes in the amino acid degradation pathways initiated by lipid oxidation products. Food Chem. X 2019, 2. [Google Scholar] [CrossRef]
- Tabanelli, G.; Coloretti, F.; Chiavari, C.; Grazia, L.; Lanciotti, R.; Gardini, F. Effects of starter cultures and fermentation climate on the properties of two types of typical Italian dry fermented sausages produced under industrial conditions. Food Control 2012. [Google Scholar] [CrossRef]
- Zamora, R.; Hidalgo, F.J. The Maillard reaction and lipid oxidation. Lipid Technol. 2011, 23, 59–62. [Google Scholar] [CrossRef]
- Petersen, C.D.V.; Beck, H.C.; Lauritsen, F.R. On-line monitoring of important organoleptic methyl-branched aldehydes during batch fermentation of starter culture staphylococcus xylosus reveal new insight into their production in a model fermentation. Biotechnol. Bioeng. 2004, 85, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Balagiannis, D.P.; Parker, J.K.; Pyle, D.L.; Desforges, N.; Wedzicha, B.L.; Mottram, D.S. Kinetic modeling of the generation of 2- and 3-methylbutanal in a heated extract of beef liver. J. Agric. Food Chem. 2009. [Google Scholar] [CrossRef] [PubMed]
- Eichner, K.; Karel, M. The influence of water content and water activity on the sugar-amino browning reaction in model systems under various conditions. J. Agric. Food Chem. 1972, 20, 218–223. [Google Scholar] [CrossRef]
- Majcher, M. Saccharides-derived flavor compounds. In Food Flavors: Chemical, Sensory and Technological Properties; CRC Press: Boca Raton, FL, USA, 2011; pp. 95–120. ISBN 9781439814925. [Google Scholar]
- Toldrá, F.; Flores, M. Dry and semidry. In Encyclopedia of Meat Sciences; Academic Press: Cambridge, MA, USA, 2014; ISBN 9780123847317. [Google Scholar]
- Perea-Sanz, L.; Peris, D.; Belloch, C.; Flores, M. Debaryomyces hansenii metabolism of sulfur amino acids as precursors of volatile sulfur compounds of interest in meat products. J. Agric. Food Chem. 2019. [Google Scholar] [CrossRef]
- Martins, S.I.F.S.; Van Boekel, M.A.J.S. A kinetic model for the glucose/glycine Maillard reaction pathways. Food Chem. 2005, 90, 257–269. [Google Scholar] [CrossRef]
- Pripi-Nicolau, L.; De Revel, G.; Bertrand, A.; Maujean, A. Formation of flavor components by the reaction of amino acid and carbonyl compounds in mild conditions. J. Agric. Food Chem. 2000. [Google Scholar] [CrossRef]
- Zhu, C.Z.; Zhao, J.L.; Tian, W.; Liu, Y.X.; Li, M.Y.; Zhao, G.M. Contribution of histidine and lysine to the generation of volatile compounds in Jinhua ham exposed to ripening conditions via Maillard reaction. J. Food Sci. 2018, 83, 46–52. [Google Scholar] [CrossRef]
- Olivares, A.; Navarro, J.L.; Flores, M. Distribution of volatile compounds in lean and subcutaneous fat tissues during processing of dry fermented sausages. Food Res. Int. 2009. [Google Scholar] [CrossRef]
- Marco, A.; Navarro, J.L.; Flores, M. Volatile compounds of dry-fermented sausages as affected by solid-phase microextraction (SPME). Food Chem. 2004. [Google Scholar] [CrossRef]
- Olivares, A.; Navarro, J.L.; Flores, M. Characterization of volatile compounds responsible for the aroma in naturally fermented sausages by gas chromatography-olfactometry. Food Sci. Technol. Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Olivares, A.; Navarro, J.L.; Salvador, A.; Flores, M. Sensory acceptability of slow fermented sausages based on fat content and ripening time. Meat Sci. 2010, 86, 251–257. [Google Scholar] [CrossRef]
- Corral, S.; Leitner, E.; Siegmund, B.; Flores, M. Determination of sulfur and nitrogen compounds during the processing of dry fermented sausages and their relation to amino acid generation. Food Chem. 2016, 190, 657–664. [Google Scholar] [CrossRef]
- Corral, S.; Salvador, A.; Belloch, C.; Flores, M. Improvement the aroma of reduced fat and salt fermented sausages by Debaromyces hansenii inoculation. Food Control 2015, 47, 526–535. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]

| Compound | LRI 1 | LRI std 2 | RI 3 | Initial Stage | 1st Drying Stage | 2nd Drying Stage | Aac Precursor 5 | Odor 6 | Threshold in Oil (mg/kg) 7 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I-25° | I-37° | 1D-25° | 1D-37° | 2D-25° | 2D-37° | |||||||
| Furan | 514 | b | + 4 | + | + | + | Ala, Thr, Ser [18] | ethereal | 4.5–6 a | |||
| Acetone | 530 | 527 | a | + | + | + | + | + | + | ethereal | 100–1000 | |
| 2-Methylpropanal | 594 | 590 | a | + | + | + | + | + | + | Val [19] | pungent, floral | 0.043 |
| 2-Butanone | 632 | 629 | a | + | + | + | + | + | woody, yogurt | 10–100 | ||
| 3-Methylbutanal | 692 | 687 | a | + | + | + | + | + | + | Leu [19] | fruity green cocoa | 0.0054–0.013 |
| Heptane | 700 | 700 | a | + | + | + | + | + | + | - | ||
| 2-Methylbutanal | 702 | 698 | a | + | + | + | + | + | + | Ile [19] | musty chocolate, malty | 0.0022–0.152 |
| Acetic acid | 715 | 714 | a | + | + | + | + | + | + | pungent vinegar | 0.12–0.75 | |
| Dimethyl disulfide | 773 | 774 | a | + | + | + | + | Met [20] | onion, cabbage | 0.012 | ||
| Thiazole | 776 | 776 | a | + | + | + | + | Cys [21] | nutty, meaty | 0.038–3.1 a | ||
| Methylpyrazine | 860 | 860 | a | + | + | + | + | Gly, Lys, Cys [22,23] | nutty, cocoa, roasted, | 27 | ||
| p-xylene | 892 | 893 | a | + | + | + | + | + | + | - | ||
| Furfural | 898 | 898 | a | + | + | + | brown, bready, nutty, caramel | 155 b | ||||
| 3-(Methylthio)propanal | 968 | 968 | a | + | + | + | + | + | + | Met [24] | cooked potato, onion, | 0.0002 |
| Benzaldehyde | 1020 | 1013 | a | + | + | + | + | + | + | Phe [25] | bitter almond, cherry | 0.06 |
| 2-Ethyl-1-hexanol | 1083 | 1083 | a | + | + | + | + | + | + | sweet, fatty, fruity | 0.27–25 a | |
| Benzeneacetaldehyde | 1110 | 1104 | a | + | + | + | + | + | + | Phe [26] | floral, chocolate | 0.002–0.03 a |
| Amino Acids | Initial Stage | 1st Drying Stage | 2nd Drying Stage |
|---|---|---|---|
| Ala | 29.91 | 91.85 | 111.80 |
| Gly | 10.94 | 33.06 | 42.58 |
| Val | 6.01 | 72.05 | 90.35 |
| β-Ala | 3.48 | 4.35 | 4.54 |
| Leu | 5.96 | 151.80 | 176.80 |
| Ile | 4.17 | 45.49 | 62.08 |
| Thr | 4.55 | 35.15 | 54.99 |
| Ser | 4.72 | 31.96 | 19.24 |
| Pro | 4.26 | 83.60 | 89.05 |
| Asn | 1.40 | 14.74 | 21.71 |
| Asp | 16.67 | 106.70 | 100.10 |
| Met | 2.00 | 33.99 | 36.27 |
| Glu | 10.53 | 74.25 | 73.45 |
| Phe | 3.82 | 70.95 | 83.85 |
| Gln | 36.47 | 64.90 | 65.00 |
| Orn | 0.23 | 23.10 | 31.01 |
| Lys | 6.82 | 77.55 | 120.90 |
| His | 2.76 | 27.34 | 38.48 |
| Tyr | 4.47 | 29.21 | 26.98 |
| Trp | 1.34 | 16.89 | 17.03 |
| C-C | 2.53 | 3.22 | 5.36 |
| Arg | 8.30 | 5.29 | 9.49 |
| Cys | 4.03 | 10.40 | 11.12 |
| Dry Curing Stages | Initial Stage | 1st Drying Stage | 2nd Drying Stage |
|---|---|---|---|
| pH | 5.45 | 4.38 | 4.60 |
| Glycerol (ml/100 mL) | 0 | 9 | 23 |
| aw | 0.968 | 0.944 | 0.895 |
| NaCl (mg/mL) | 27 | 27 | 27 |
| NaNO2 (mg/mL) | 0.15 | 0 | 0 |
| NaNO3 (mg/mL) | 0.15 | 0.10 | 0.075 |
| Sodium ascorbate (mg/mL) | 0.5 | 0.5 | 0.5 |
| Glucose (mg/mL) | 20 | 5 | 5 |
| Amino acids (mg/100 mL) | Table 2 (Initial stage) | Table 2 (1st drying stage) | Table 2 (2nd drying stage) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Belloch, C.; Flores, M. The Maillard Reaction as Source of Meat Flavor Compounds in Dry Cured Meat Model Systems under Mild Temperature Conditions. Molecules 2021, 26, 223. https://doi.org/10.3390/molecules26010223
Li L, Belloch C, Flores M. The Maillard Reaction as Source of Meat Flavor Compounds in Dry Cured Meat Model Systems under Mild Temperature Conditions. Molecules. 2021; 26(1):223. https://doi.org/10.3390/molecules26010223
Chicago/Turabian StyleLi, Lei, Carmela Belloch, and Mónica Flores. 2021. "The Maillard Reaction as Source of Meat Flavor Compounds in Dry Cured Meat Model Systems under Mild Temperature Conditions" Molecules 26, no. 1: 223. https://doi.org/10.3390/molecules26010223
APA StyleLi, L., Belloch, C., & Flores, M. (2021). The Maillard Reaction as Source of Meat Flavor Compounds in Dry Cured Meat Model Systems under Mild Temperature Conditions. Molecules, 26(1), 223. https://doi.org/10.3390/molecules26010223








