Anti-Inflammatory and Neuromodulatory Effects Induced by Tanacetum parthenium Water Extract: Results from In Silico, In Vitro and Ex Vivo Studies
Abstract
1. Introduction
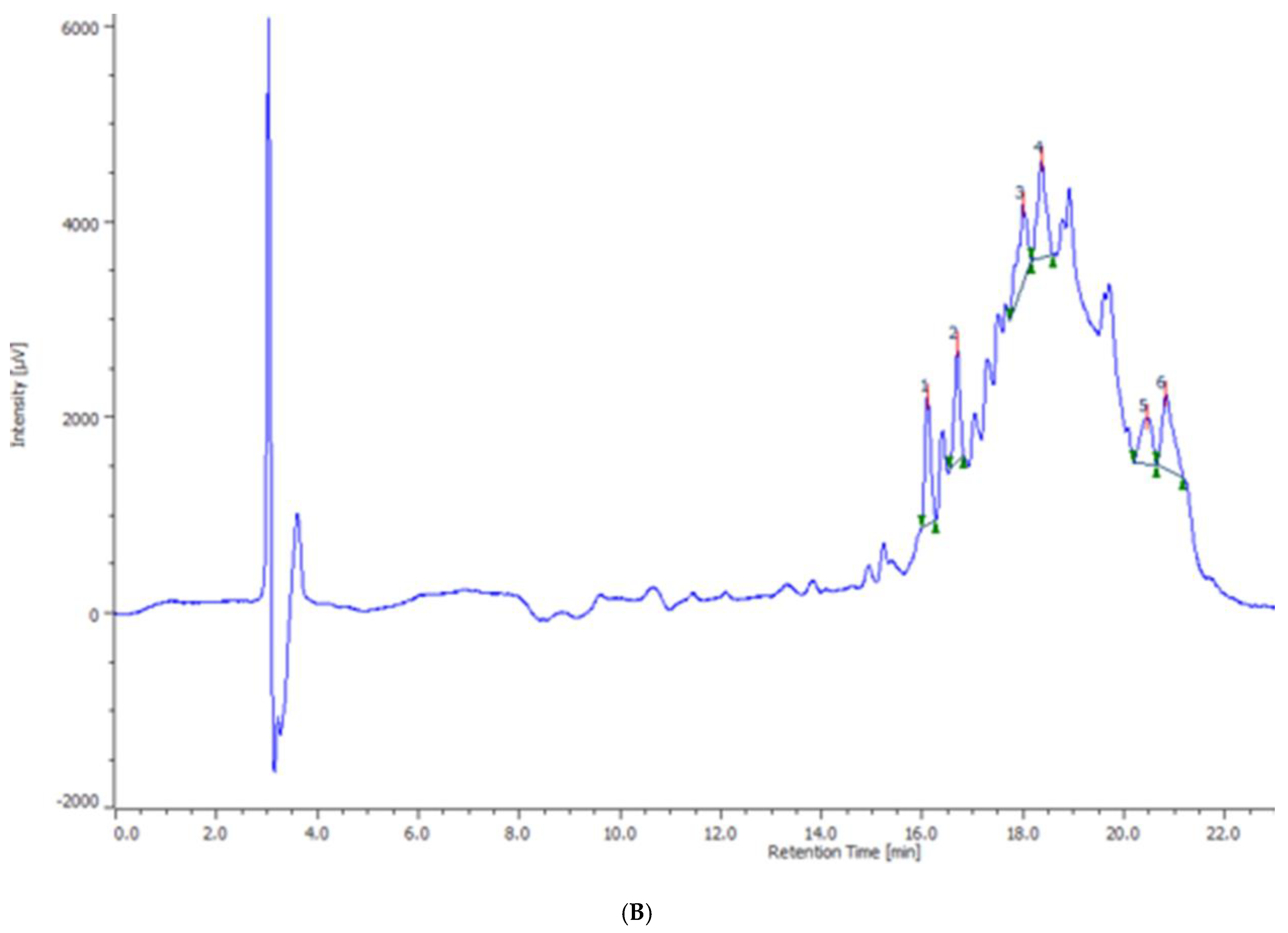
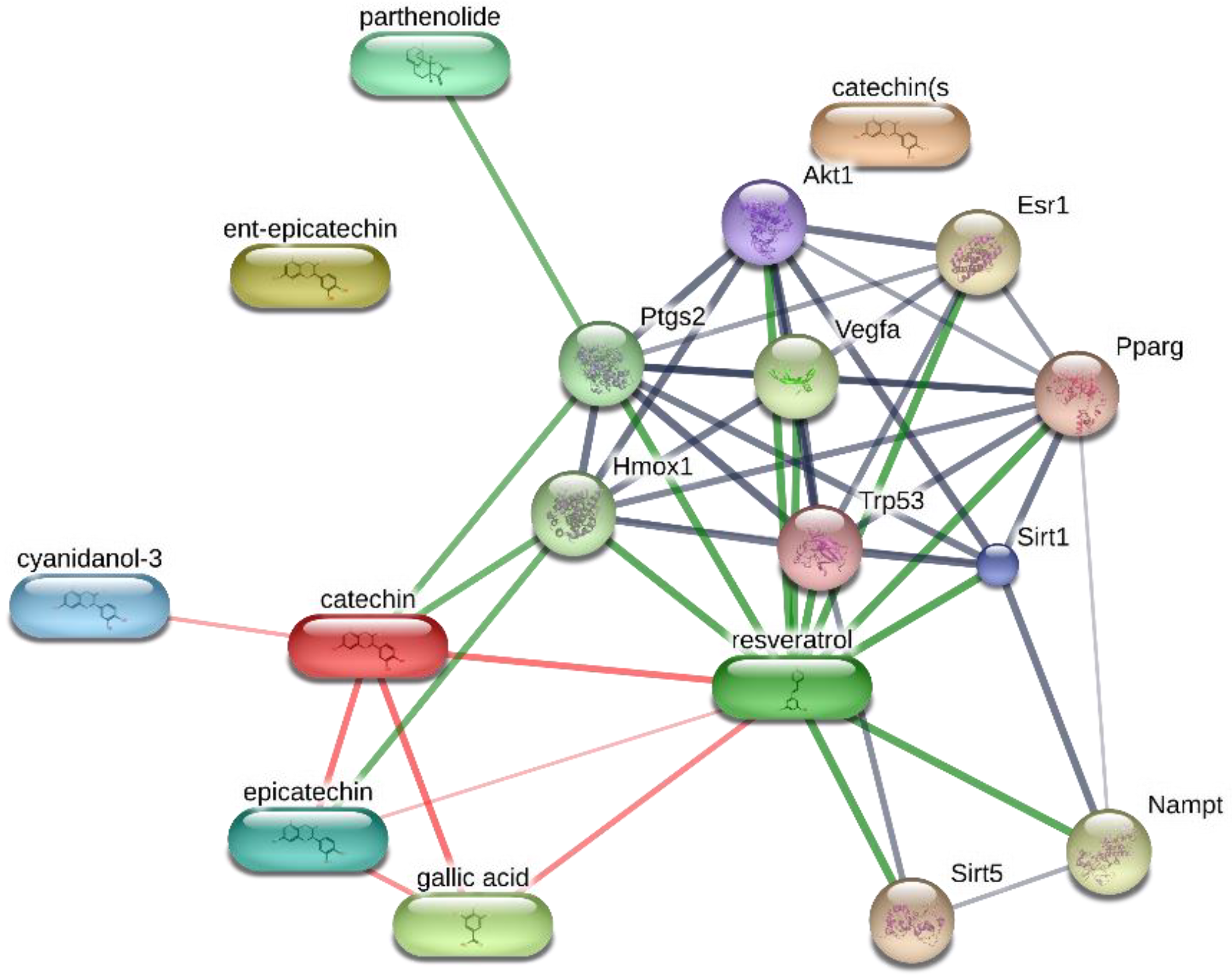
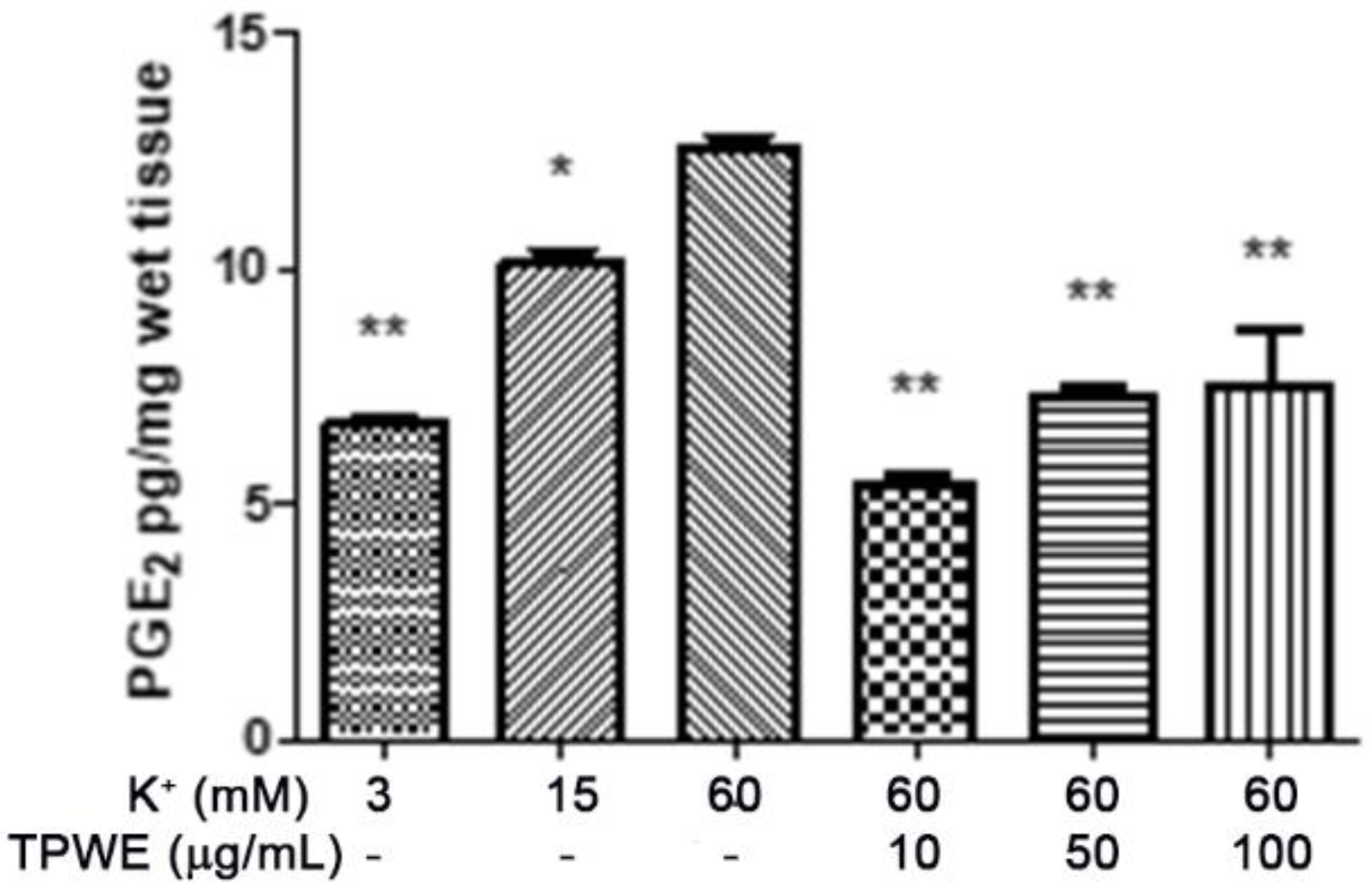
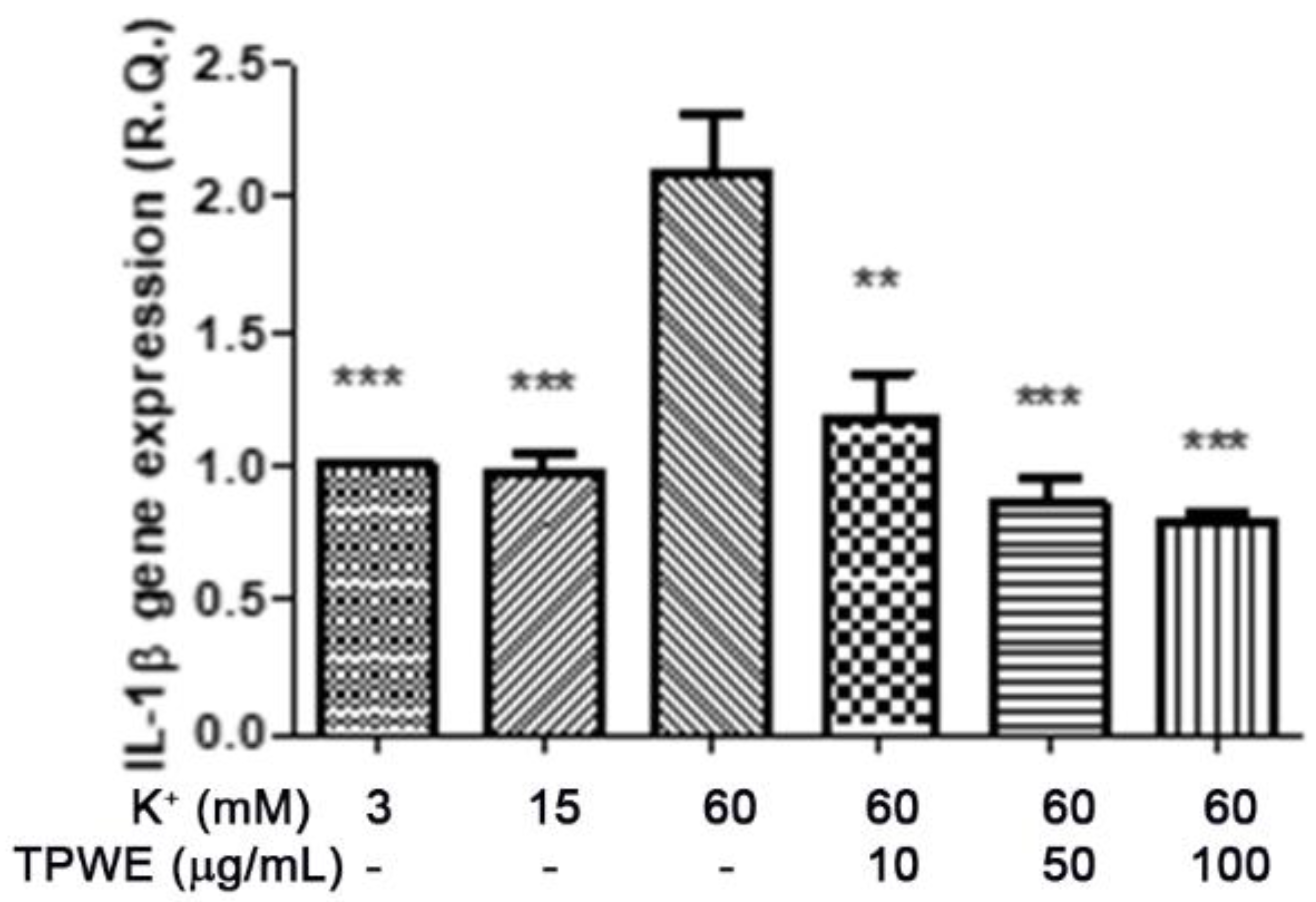
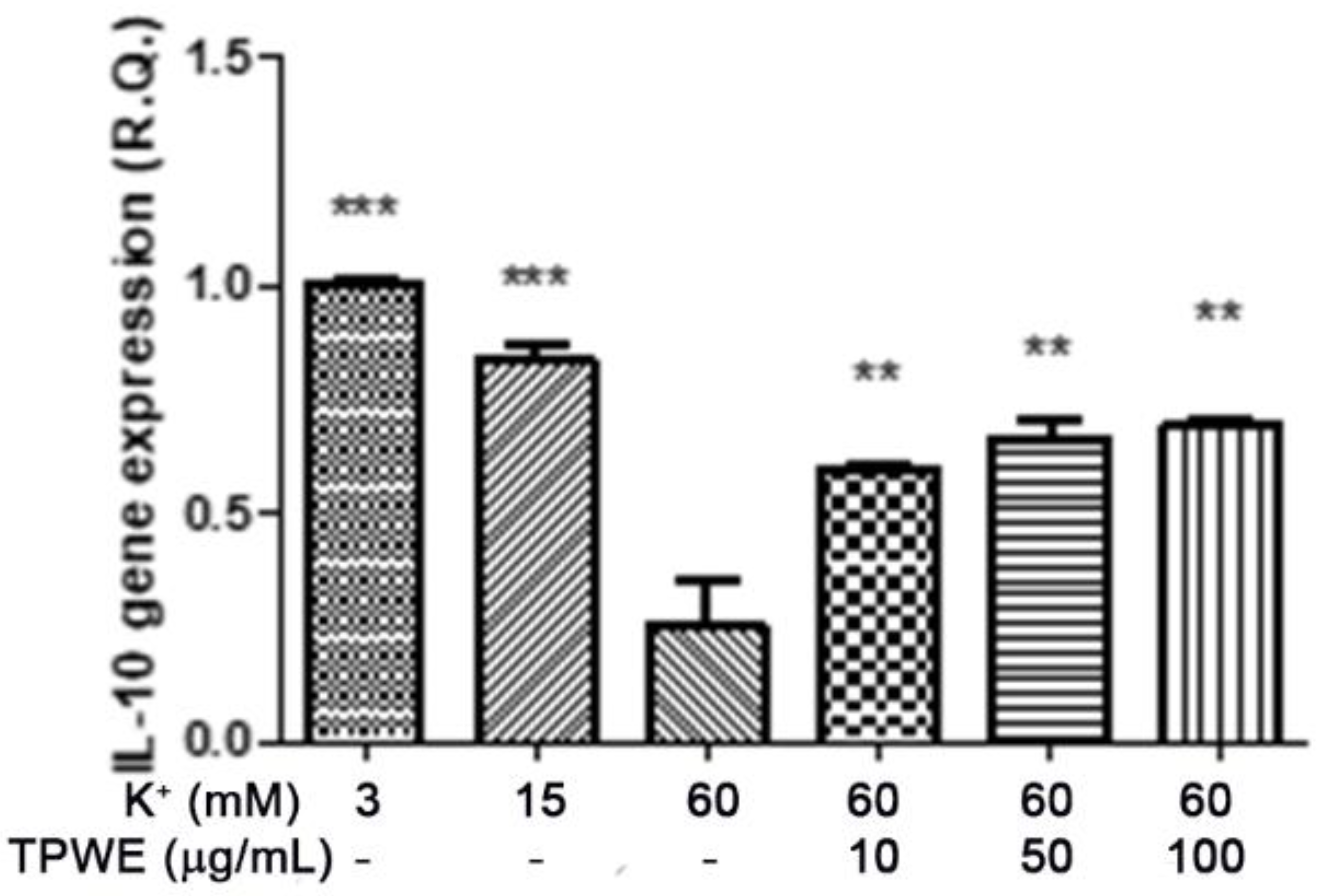
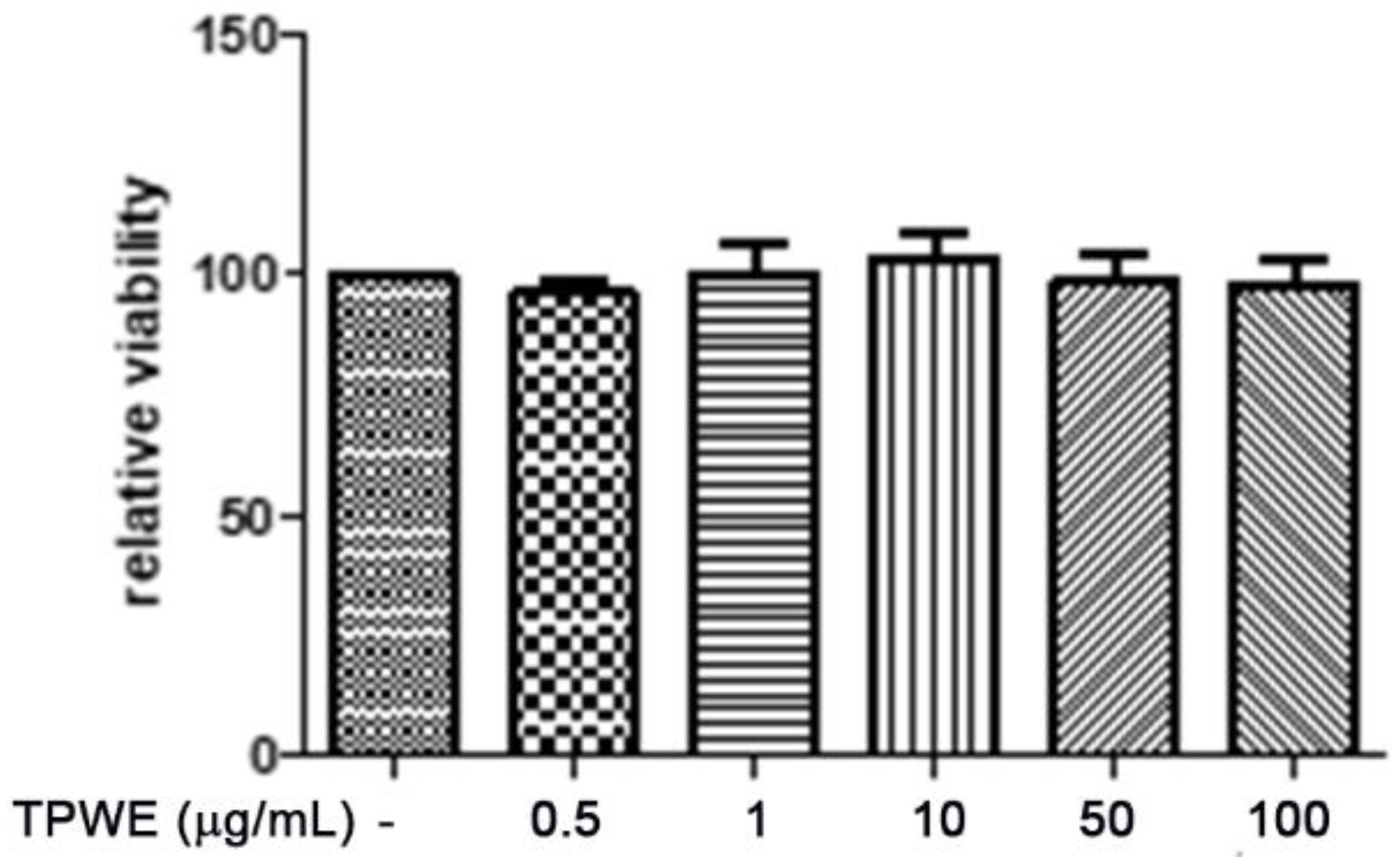
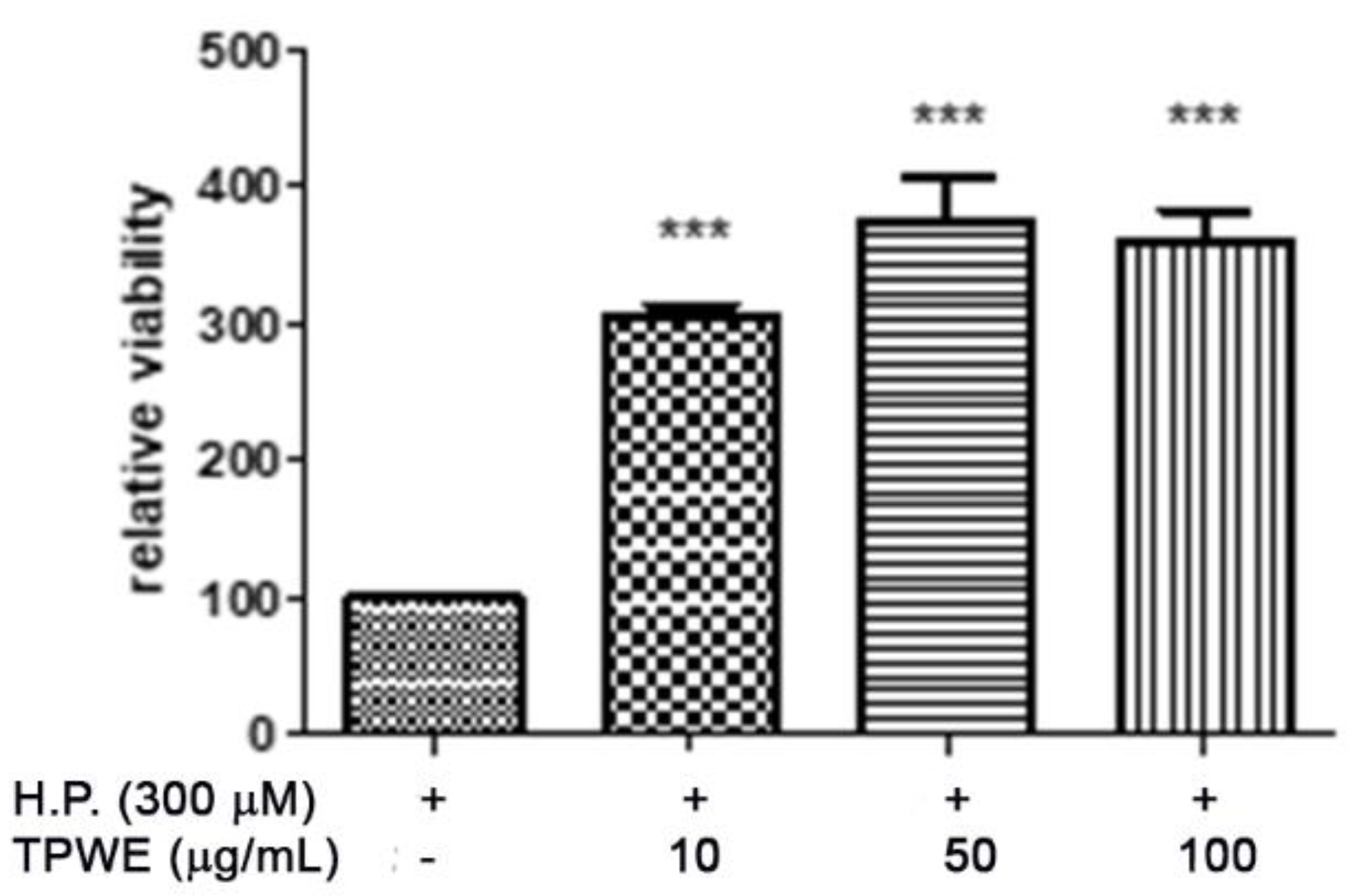
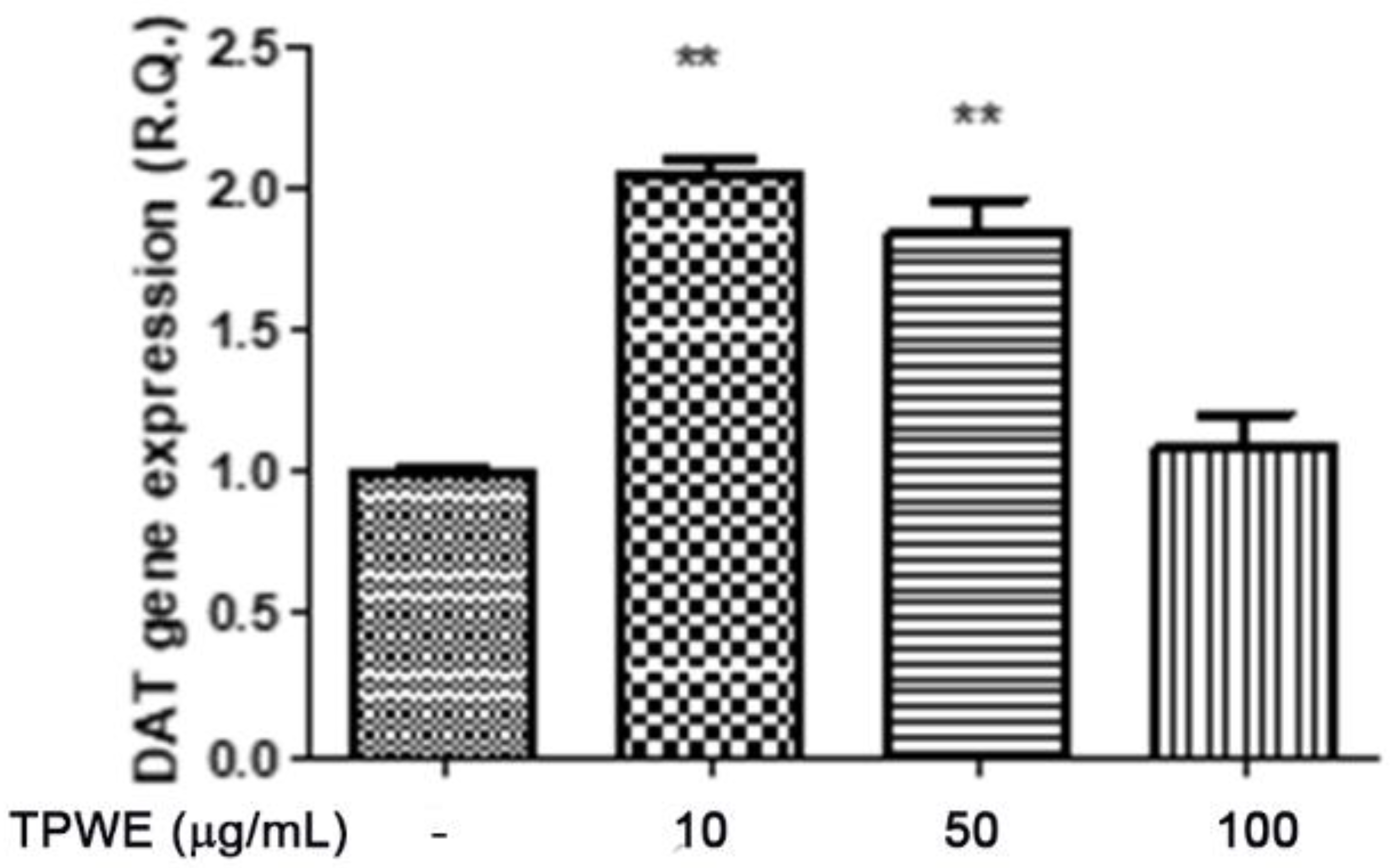
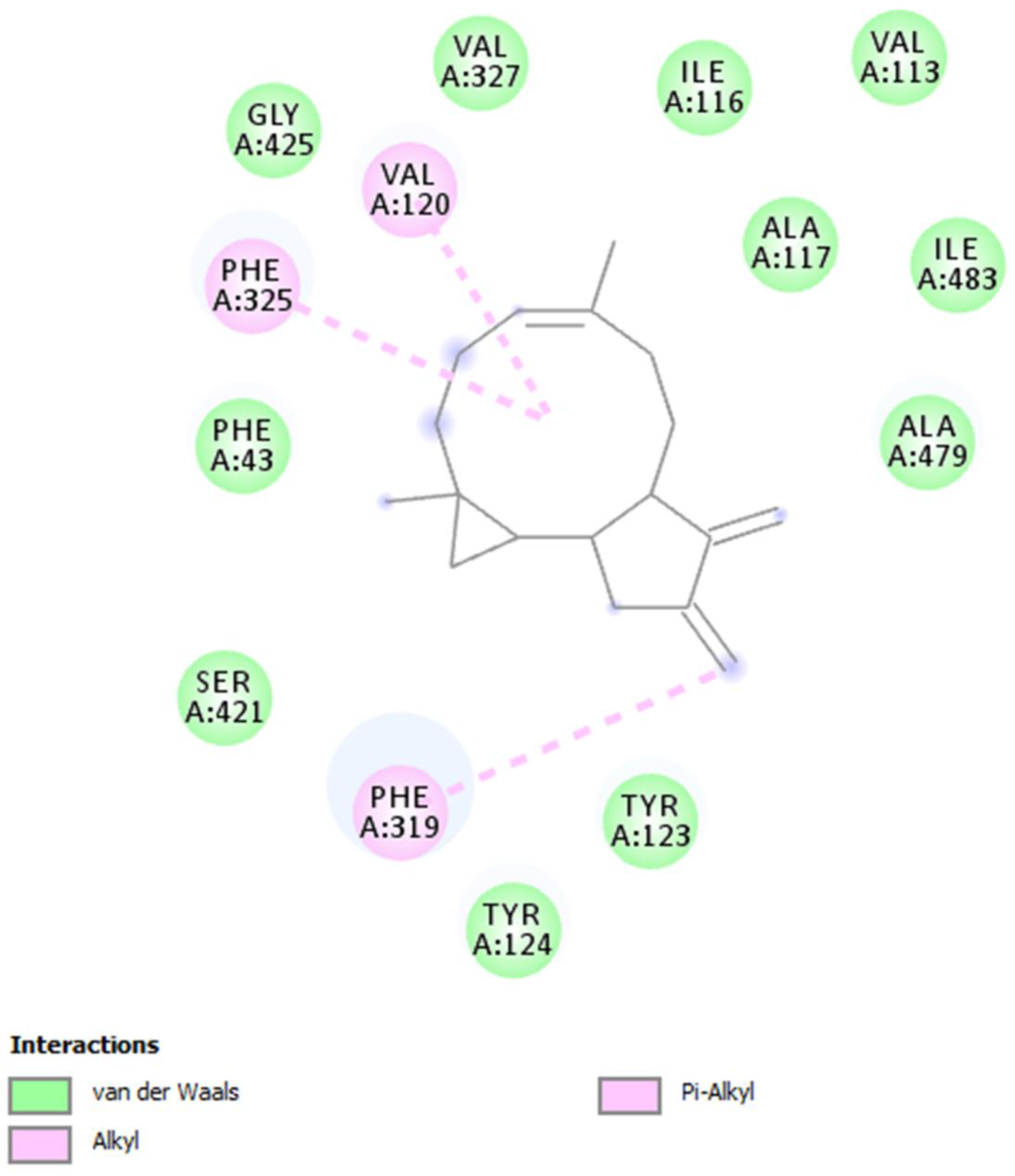
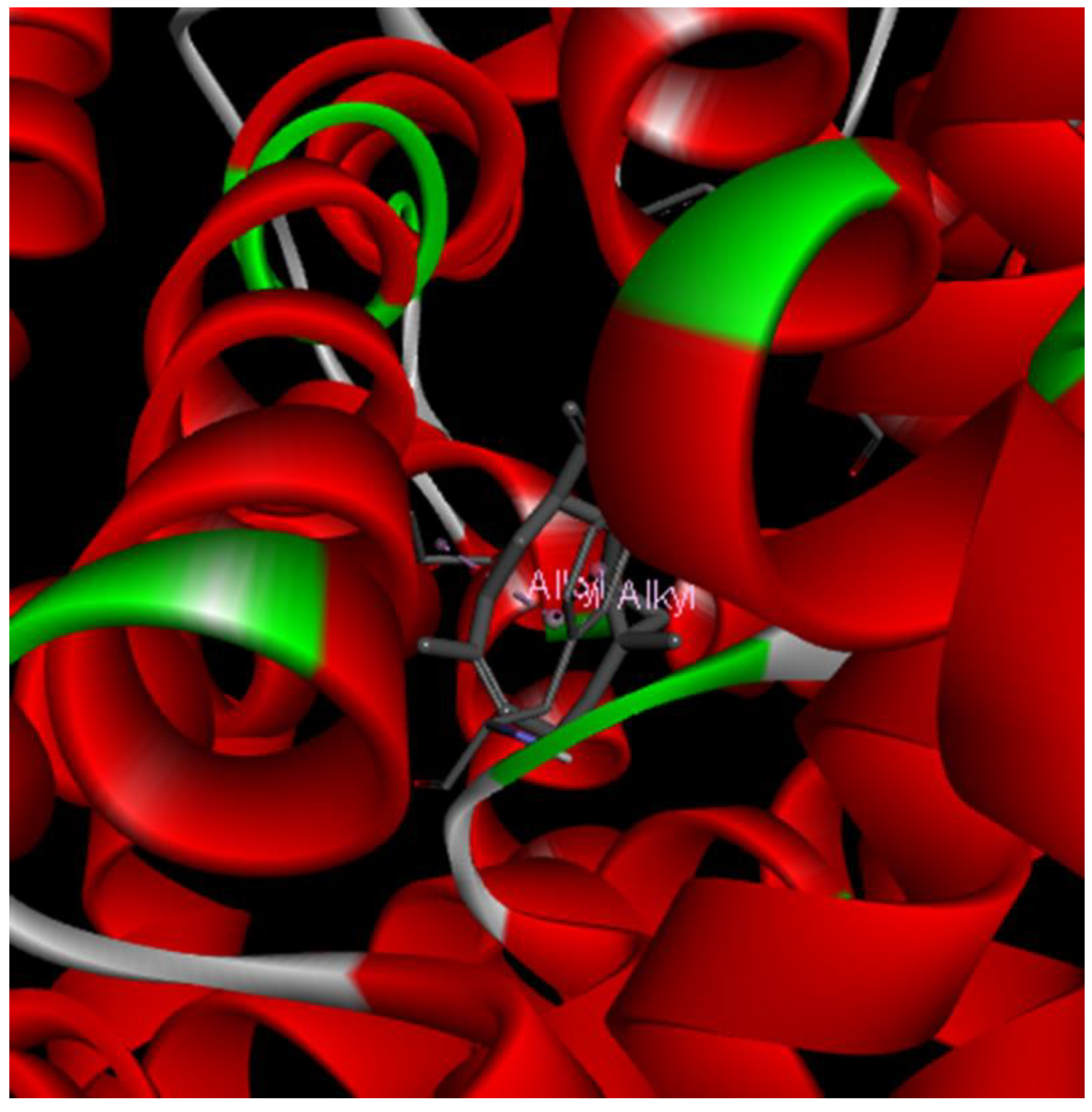
2. Results and Discussion
3. Materials and Methods
3.1. Extract Preparation
3.2. Phytochemical Analysis
3.3. In Vitro Studies
3.4. Ex Vivo CSD Paradigm
- K+ 3 mM, corresponding to the basal condition;
- K+ 15 mM, corresponding to the physiologic depolarizing stimulus;
- K+ 60 mM, corresponding to the excitotoxic depolarizing stimulus.
3.5. RNA Extraction, Reverse Transcription, and Real-Time Reverse Transcription Polymerase Chain Reaction (Real-Time RT PCR)
3.6. High-Performance Liquid Chromatography (HPLC) Determination of DA
3.7. Bioinformatics
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Rajapakse, T.; Davenport, W.J. Phytomedicines in the Treatment of Migraine. CNS Drugs 2019, 33, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Hering, R.; Glover, V.; Pattichis, K.; Catarci, T.; Steiner, T.J. 5HT in migraine patients with medication-induced headache. Cephalalgia 1993, 13, 410–412. [Google Scholar] [CrossRef] [PubMed]
- Lance, J.W. 5-Hydroxytryptamine and its role in migraine. Eur. Neurol. 1991, 31, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Massiou, H.; Bousser, M.G. Prophylactic drug treatment of migraine. Rev. Neurol. (Paris) 2005, 161, 681–684. [Google Scholar] [CrossRef]
- Noseda, R.; Burstein, R. Migraine pathophysiology: Anatomy of the trigeminovascular pathway and associated neurological symptoms, cortical spreading depression, sensitization, and modulation of pain. Pain 2013, 154 (Suppl. 1), S44–S53. [Google Scholar] [CrossRef] [PubMed]
- Edvinsson, L.; Haanes, K.A.; Warfvinge, K.; Krause, D.N. CGRP as the target of new migraine therapies-successful translation from bench to clinic. Nat. Rev. Neurol. 2018, 14, 338–350. [Google Scholar] [CrossRef]
- Supornsilpchai, W.; Sanguanrangsirikul, S.; Maneesri, S.; Srikiatkhachorn, A. Serotonin depletion, cortical spreading depression, and trigeminal nociception. Headache 2006, 46, 34–39. [Google Scholar] [CrossRef]
- May, A.; Burstein, R. Hypothalamic regulation of headache and migraine. Cephalalgia 2019, 39, 1710–1719. [Google Scholar] [CrossRef]
- Kalra, S.P.; Dube, M.G.; Pu, S.; Xu, B.; Horvath, T.L.; Kalra, P.S. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr. Rev. 1999, 20, 68–100. [Google Scholar] [CrossRef]
- Hoffmann, J.; Supronsinchai, W.; Akerman, S.; Andreou, A.P.; Winrow, C.J.; Renger, J.; Hargreaves, R.; Goadsby, P.J. Evidence for orexinergic mechanisms in migraine. Neurobiol. Dis. 2015, 74, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Akerman, S.; Goadsby, P.J. Dopamine and migraine: Biology and clinical implications. Cephalalgia 2007, 27, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Charbit, A.R.; Akerman, S.; Goadsby, P.J. Dopamine: What’s new in migraine? Curr. Opin. Neurol. 2010, 23, 275–281. [Google Scholar] [CrossRef]
- Eken, C. Critical reappraisal of intravenous metoclopramide in migraine attack: A systematic review and meta-analysis. Am. J. Emerg. Med. 2015, 33, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Lupi, C.; Benemei, S.; Guerzoni, S.; Pellesi, L.; Negro, A. Pharmacokinetics and pharmacodynamics of new acute treatments for migraine. Expert Opin. Drug Metab. Toxicol. 2019, 15, 189–198. [Google Scholar] [CrossRef]
- Loder, E.; Rizzoli, P. Pharmacologic Prevention of Migraine: A Narrative Review of the State of the Art in 2018. Headache 2018, 58 (Suppl. 3), 218–229. [Google Scholar] [CrossRef] [PubMed]
- Frediani, F.; D’Arrigo, G.; Galli, A.; Altavilla, R.; Di Fiore, P. Exploring new strategy in erenumab therapy for migraine patients. Neurol. Sci. 2020, 41 (Suppl. 2), 507–508. [Google Scholar] [CrossRef]
- Wider, B.; Pittler, M.H.; Ernst, E. Feverfew for preventing migraine. Cochrane Database Syst. Rev. 2015, 4, CD002286. [Google Scholar] [CrossRef]
- Sangermani, R.; Boncimino, A. The use of nutraceutics in children’s and adolescent’s headache. Neurol. Sci. 2017, 38 (Suppl. 1), 121–124. [Google Scholar] [CrossRef]
- Guilbot, A.; Bangratz, M.; Ait Abdellah, S.; Lucas, C. A combination of coenzyme Q10, feverfew and magnesium for migraine prophylaxis: A prospective observational study. BMC Complement. Altern. Med. 2017, 17, 433. [Google Scholar] [CrossRef]
- Moscano, F.; Guiducci, M.; Maltoni, L.; Striano, P.; Ledda, M.G.; Zoroddu, F.; Raucci, U.; Villa, M.P.; Parisi, P. An observational study of fixed-dose Tanacetum parthenium nutraceutical preparation for prophylaxis of pediatric headache. Ital. J. Pediatr. 2019, 45, 36. [Google Scholar] [CrossRef]
- Pareek, A.; Suthar, M.; Rathore, G.S.; Bansal, V. Feverfew (Tanacetum parthenium L.): A systematic review. Pharmacogn. Rev. 2011, 5, 103–110. [Google Scholar] [CrossRef]
- Materazzi, S.; Benemei, S.; Fusi, C.; Gualdani, R.; De Siena, G.; Vastani, N.; Andersson, D.A.; Trevisan, G.; Moncelli, M.R.; Wei, X.; et al. Parthenolide inhibits nociception and neurogenic vasodilatation in the trigeminovascular system by targeting the TRPA1 channel. Pain 2013, 154, 2750–2758. [Google Scholar] [CrossRef]
- Di Giacomo, V.; Ferrante, C.; Ronci, M.; Cataldi, A.; Di Valerio, V.; Rapino, M.; Recinella, L.; Chiavaroli, A.; Leone, S.; Vladimir-Knežević, S.; et al. Multiple pharmacological and toxicological investigations on Tanacetum parthenium and Salix alba extracts: Focus on potential application as anti-migraine agents. Food Chem. Toxicol. 2019, 133, 110783. [Google Scholar] [CrossRef]
- Schwarz, D.; Bloom, D.; Castro, R.; Pagán, O.R.; Jiménez-Rivera, C.A. Parthenolide Blocks Cocaine’s Effect on Spontaneous Firing Activity of Dopaminergic Neurons in the Ventral Tegmental Area. Curr. Neuropharmacol. 2011, 9, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, C.; Angelini, P.; Venanzoni, R.; Angeles Flores, G.; Tirillini, B.; Recinella, L.; Chiavaroli, A.; Brunetti, L.; Leone, S.; Di Simone, S.C.; et al. Antimicrobial, Antioxidant, and Antiproliferative Effects of Coronilla minima: An Unexplored Botanical Species. Antibiotics 2020, 9, 611. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, C.; Chiavaroli, A.; Angelini, P.; Venanzoni, R.; Angeles Flores, G.; Brunetti, L.; Petrucci, M.; Politi, M.; Menghini, L.; Leone, S.; et al. Phenolic Content and Antimicrobial and Anti-Inflammatory Effects of Solidago virga-aurea, Phyllanthus niruri, Epilobium angustifolium, Peumus boldus, and Ononis spinosa Extracts. Antibiotics 2020, 9, 783. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.D.; Song, J.Y.; Lee, S.J.; Chung, I.M.; Kim, M.J.; Heo, K.; Yu, C.Y. Comparison of resveratrol contents in medicinal plants. Korean J. Med. Crop Sci. 2004, 12, 163–170. [Google Scholar]
- Bertelli, A.A.E. Modulatory effect of resveratrol, a natural phytoalexin, on endothelial adhsion molecules and intracellular signal transduction. Pharm. Biol. 1998, 36, 44–52. [Google Scholar] [CrossRef]
- Chiavaroli, A.; Brunetti, L.; Orlando, G.; Recinella, L.; Ferrante, C.; Leone, S.; Di Michele, P.; Di Nisio, C.; Vacca, M. Resveratrol inhibits isoprostane production in young and aged rat brain. J. Biol. Regul. Homeost. Agents 2010, 24, 441. [Google Scholar] [PubMed]
- Yokota, C.; Kuge, Y.; Inoue, H.; Tamaki, N.; Minematsu, K. Bilateral induction of the S-100A9 gene in response to spreading depression is modulated by the cyclooxygenase-2 activity. J. Neurol. Sci. 2005, 234, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Jander, S.; Schroeter, M.; Peters, O.; Witte, O.W.; Stoll, G. Cortical spreading depression induces proinflammatory cytokine gene expression in the rat brain. J. Cereb. Blood Flow Metab. 2001, 21, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Dutta, M.; Paul, G. Gallic acid protects rat liver mitochondria ex vivo from bisphenol a induced oxidative stress mediated damages. Toxicol. Rep. 2019, 6, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, E.M.; Souza, L.K.; Araújo, T.S.; Nogueira, K.M.; Sousa, F.B.; Araújo, A.R.; Martins, C.S.; Pacífico, D.M.; de C Brito, G.A.; Souza, E.P.; et al. Carvacrol reduces irinotecan-induced intestinal mucositis through inhibition of inflammation and oxidative damage via TRPA1 receptor activation. Chem. Biol. Interact. 2016, 260, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.B.; Duarte, H.; Ferreira, A.V.; Rocha, N.P.; Teixeira, A.L.; Domingues, R.B. Migraine is associated with altered levels of neurotrophins. Neurosci. Lett. 2015, 587, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Juang, K.D.; Yang, C.Y. Psychiatric comorbidity of chronic daily headache: Focus on traumatic experiences in childhood, post-traumatic stress disorder and suicidality. Curr. Pain Headache Rep. 2014, 18, 405. [Google Scholar] [CrossRef] [PubMed]
- Noseda, R.; Jakubowski, M.; Kainz, V.; Borsook, D.; Burstein, R. Cortical projections of functionally identified thalamic trigeminovascular neurons: Implications for migraine headache and its associated symptoms. J. Neurosci. 2011, 31, 14204–14217. [Google Scholar] [CrossRef]
- Noseda, R.; Kainz, V.; Borsook, D.; Burstein, R. Neurochemical pathways that converge on thalamic trigeminovascular neurons: Potential substrate for modulation of migraine by sleep, food intake, stress and anxiety. PLoS ONE 2014, 9, e103929. [Google Scholar] [CrossRef]
- Menghini, L.; Leporini, L.; Vecchiotti, G.; Locatelli, M.; Carradori, S.; Ferrante, C.; Zengin, G.; Recinella, L.; Chiavaroli, A.; Leone, S.; et al. Crocus sativus L. stigmas and byproducts: Qualitative fingerprint, antioxidant potentials and enzyme inhibitory activities. Food Res. Int. 2018, 109, 91–98. [Google Scholar] [CrossRef]
- Ferrante, C.; Recinella, L.; Ronci, M.; Menghini, L.; Brunetti, L.; Chiavaroli, A.; Leone, S.; Di Iorio, L.; Carradori, S.; Tirillini, B.; et al. Multiple pharmacognostic characterization on hemp commercial cultivars: Focus on inflorescence water extract activity. Food Chem. Toxicol. 2019, 125, 452–461. [Google Scholar] [CrossRef]
- Di Giacomo, V.; Chiavaroli, A.; Orlando, G.; Cataldi, A.; Rapino, M.; Di Valerio, V.; Leone, S.; Brunetti, L.; Menghini, L.; Recinella, L.; et al. Neuroprotective and Neuromodulatory Effects Induced by Cannabidiol and Cannabigerol in Rat Hypo-E22 cells and Isolated Hypothalamus. Antioxidants 2020, 9, 71. [Google Scholar] [CrossRef]
- Raiteri, L.; Stigliani, S.; Zedda, L.; Raiteri, M.; Bonanno, G. Multiple mechanisms of transmitter release evoked by “pathologically” elevated extracellular [K+]: Involvement of transporter reversal and mitochondrial calcium. J. Neurochem. 2002, 80, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, M.; Ferrante, C.; Carradori, S.; Secci, D.; Leporini, L.; Chiavaroli, A.; Leone, S.; Recinella, L.; Orlando, G.; Martinotti, S.; et al. Optimization of Aqueous Extraction and Biological Activity of Harpagophytum procumbens Root on Ex Vivo Rat Colon Inflammatory Model. Phytother. Res. 2017, 31, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, C.; Orlando, G.; Recinella, L.; Leone, S.; Chiavaroli, A.; Di Nisio, C.; Shohreh, R.; Manippa, F.; Ricciuti, A.; Vacca, M.; et al. Central apelin-13 administration modulates hypothalamic control of feeding. J. Biol. Regul. Homeost. Agents 2016, 30, 883–888. [Google Scholar] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Iodice, P.; Ferrante, C.; Brunetti, L.; Cabib, S.; Protasi, F.; Walton, M.E.; Pezzulo, G. Fatigue modulates dopamine availability and promotes flexible choice reversals during decision making. Sci. Rep. 2017, 7, 535. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Recinella, L.; Chiavaroli, A.; di Giacomo, V.; Antolini, M.D.; Acquaviva, A.; Leone, S.; Brunetti, L.; Menghini, L.; Ak, G.; Zengin, G.; et al. Anti-Inflammatory and Neuromodulatory Effects Induced by Tanacetum parthenium Water Extract: Results from In Silico, In Vitro and Ex Vivo Studies. Molecules 2021, 26, 22. https://doi.org/10.3390/molecules26010022
Recinella L, Chiavaroli A, di Giacomo V, Antolini MD, Acquaviva A, Leone S, Brunetti L, Menghini L, Ak G, Zengin G, et al. Anti-Inflammatory and Neuromodulatory Effects Induced by Tanacetum parthenium Water Extract: Results from In Silico, In Vitro and Ex Vivo Studies. Molecules. 2021; 26(1):22. https://doi.org/10.3390/molecules26010022
Chicago/Turabian StyleRecinella, Lucia, Annalisa Chiavaroli, Viviana di Giacomo, Marco Daniel Antolini, Alessandra Acquaviva, Sheila Leone, Luigi Brunetti, Luigi Menghini, Gunes Ak, Gokhan Zengin, and et al. 2021. "Anti-Inflammatory and Neuromodulatory Effects Induced by Tanacetum parthenium Water Extract: Results from In Silico, In Vitro and Ex Vivo Studies" Molecules 26, no. 1: 22. https://doi.org/10.3390/molecules26010022
APA StyleRecinella, L., Chiavaroli, A., di Giacomo, V., Antolini, M. D., Acquaviva, A., Leone, S., Brunetti, L., Menghini, L., Ak, G., Zengin, G., Di Simone, S. C., Ferrante, C., & Orlando, G. (2021). Anti-Inflammatory and Neuromodulatory Effects Induced by Tanacetum parthenium Water Extract: Results from In Silico, In Vitro and Ex Vivo Studies. Molecules, 26(1), 22. https://doi.org/10.3390/molecules26010022














