Ab Initio Molecular Dynamics Simulations of the Interaction between Organic Phosphates and Goethite
Abstract
1. Introduction
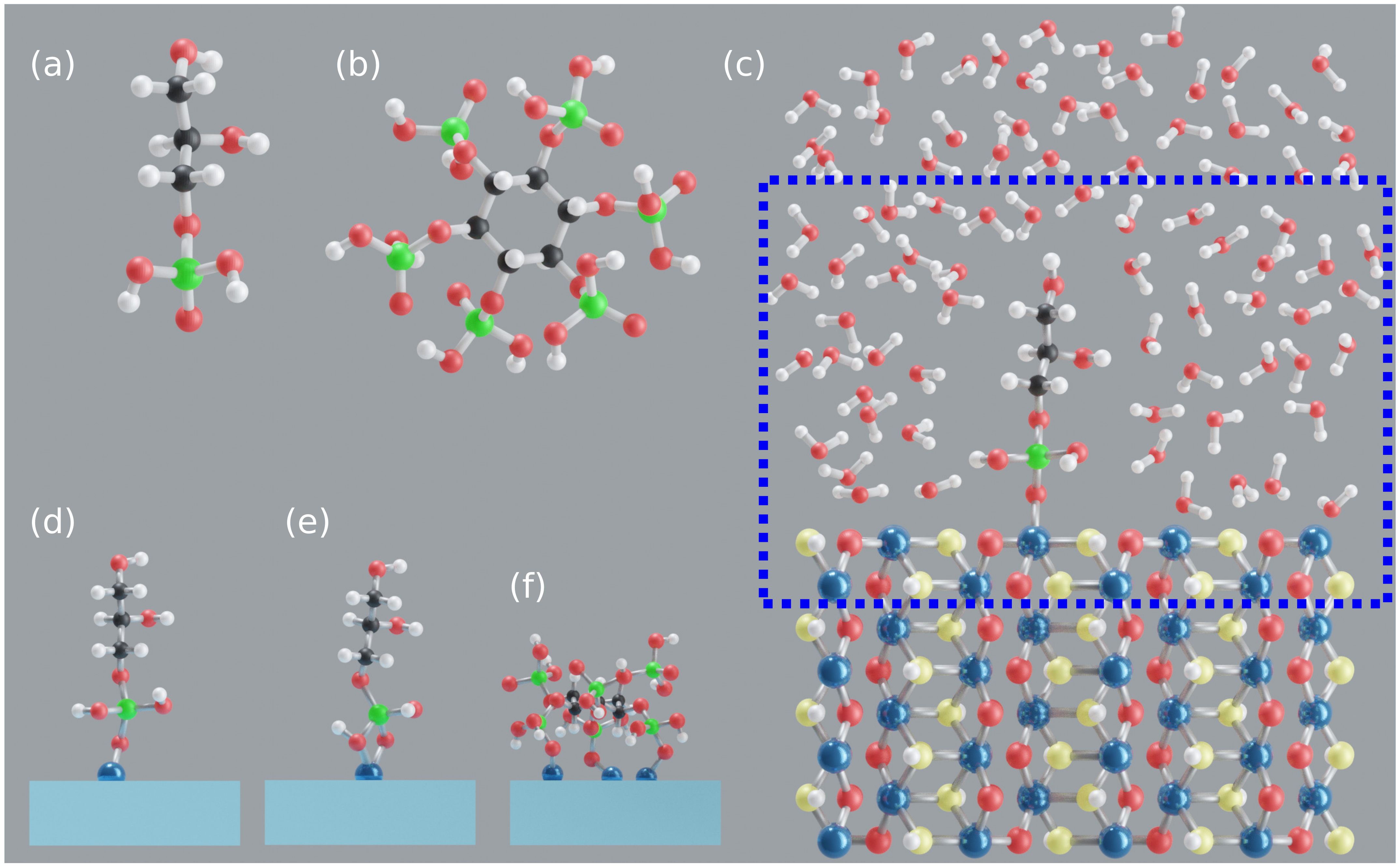
2. Modeling and Computations
2.1. Goethite Surface
2.2. Model Systems
2.3. Computational Details
3. Results and Discussion
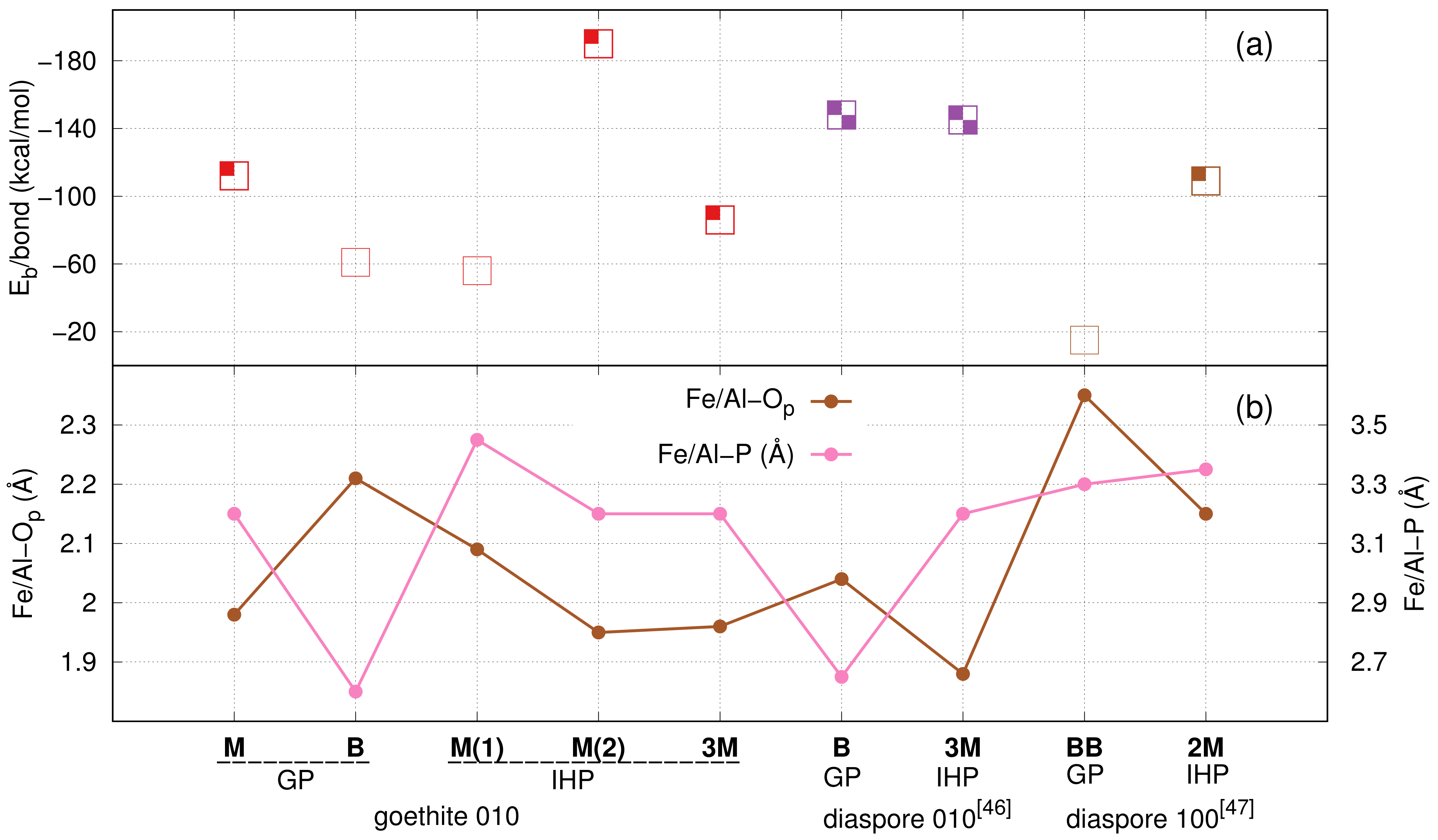
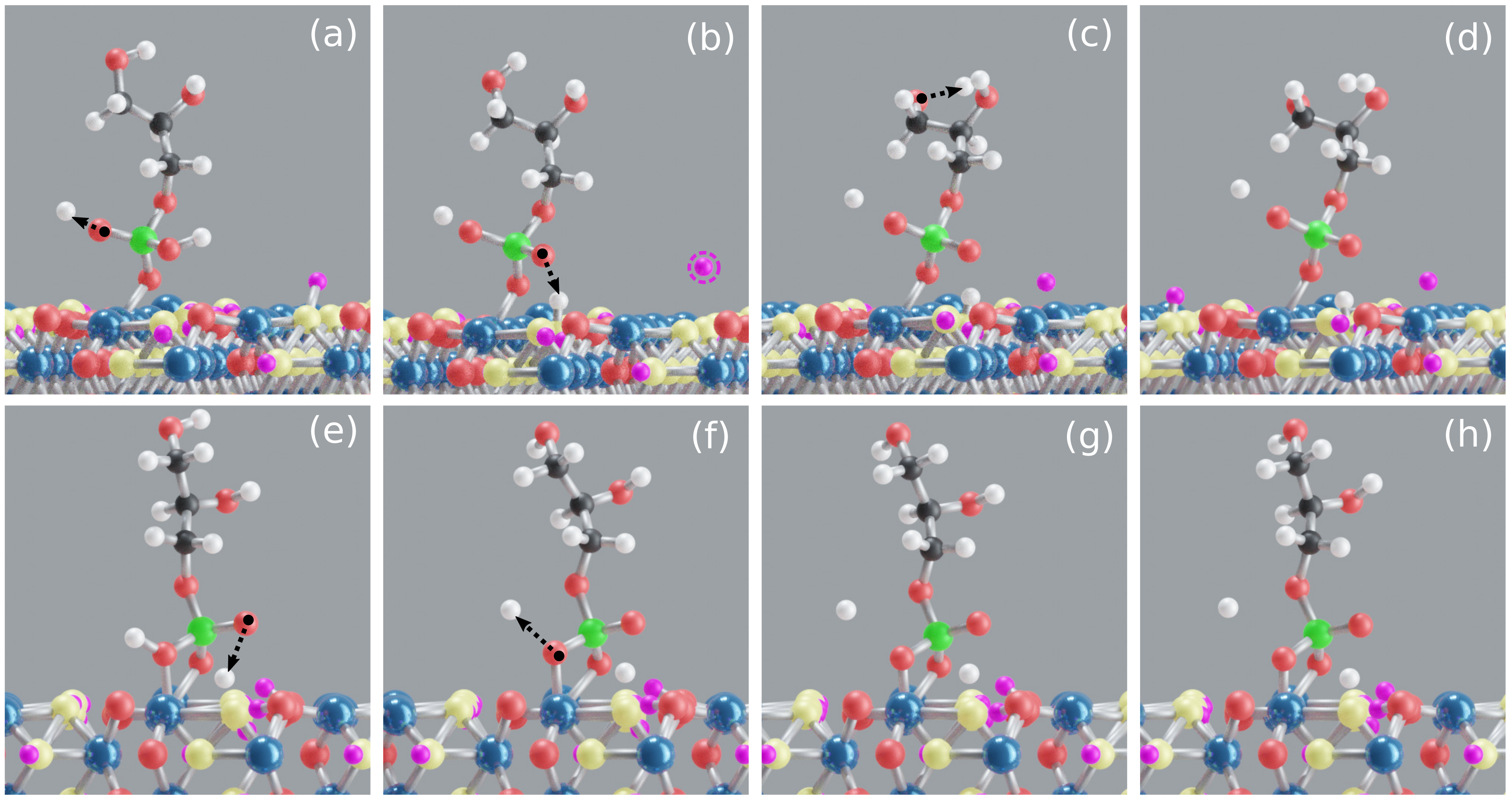
3.1. Goethite–GP–Water Interactions
3.1.1. GP M Motif
3.1.2. GP B Motif
3.1.3. Discussion of Goethite–GP–Water Interactions
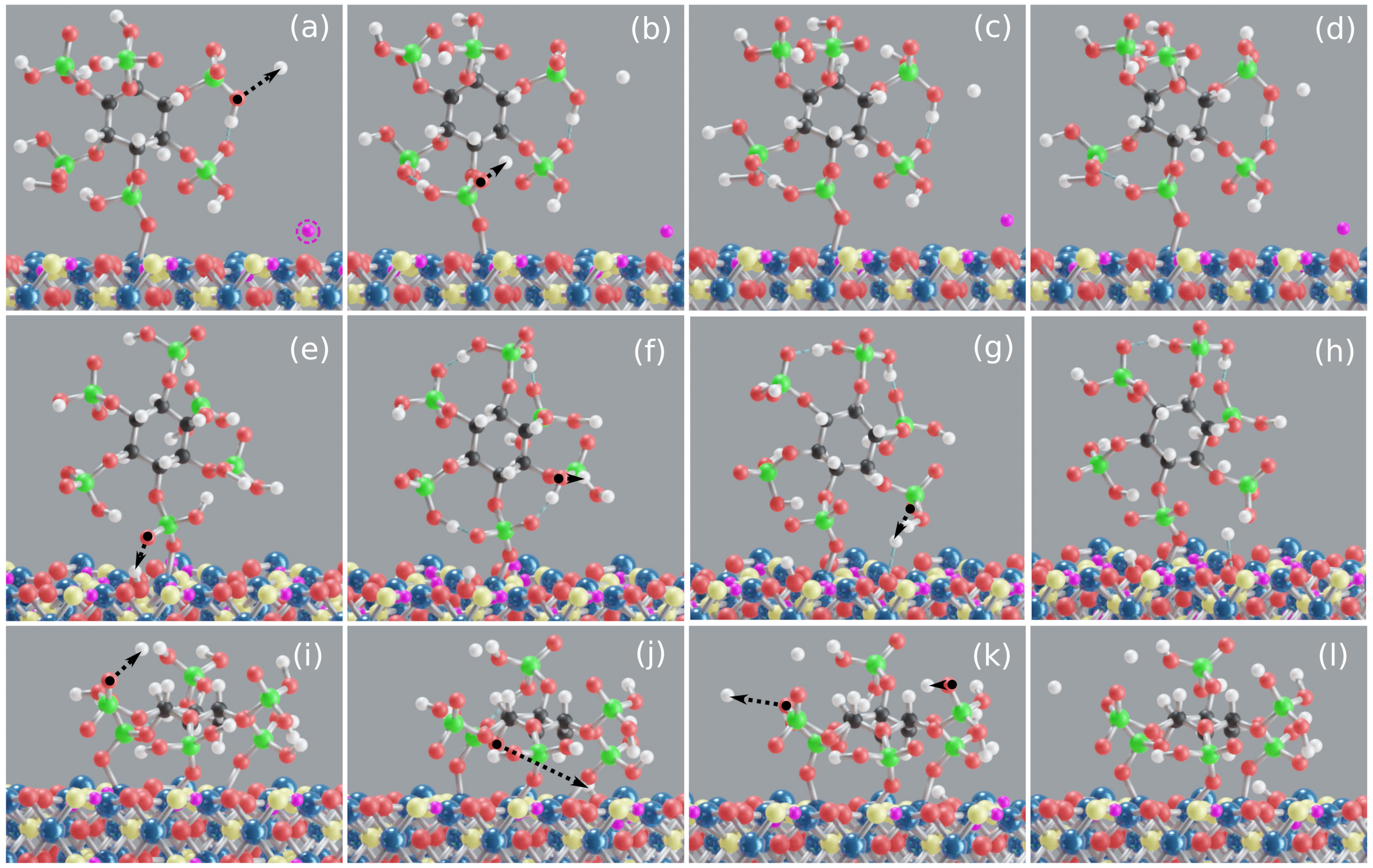
3.2. Goethite–IHP–Water Interaction
3.2.1. IHP M Motifs
3.2.2. IHP 3M Motif
3.2.3. Discussion of Goethite–IHP–Water Interactions
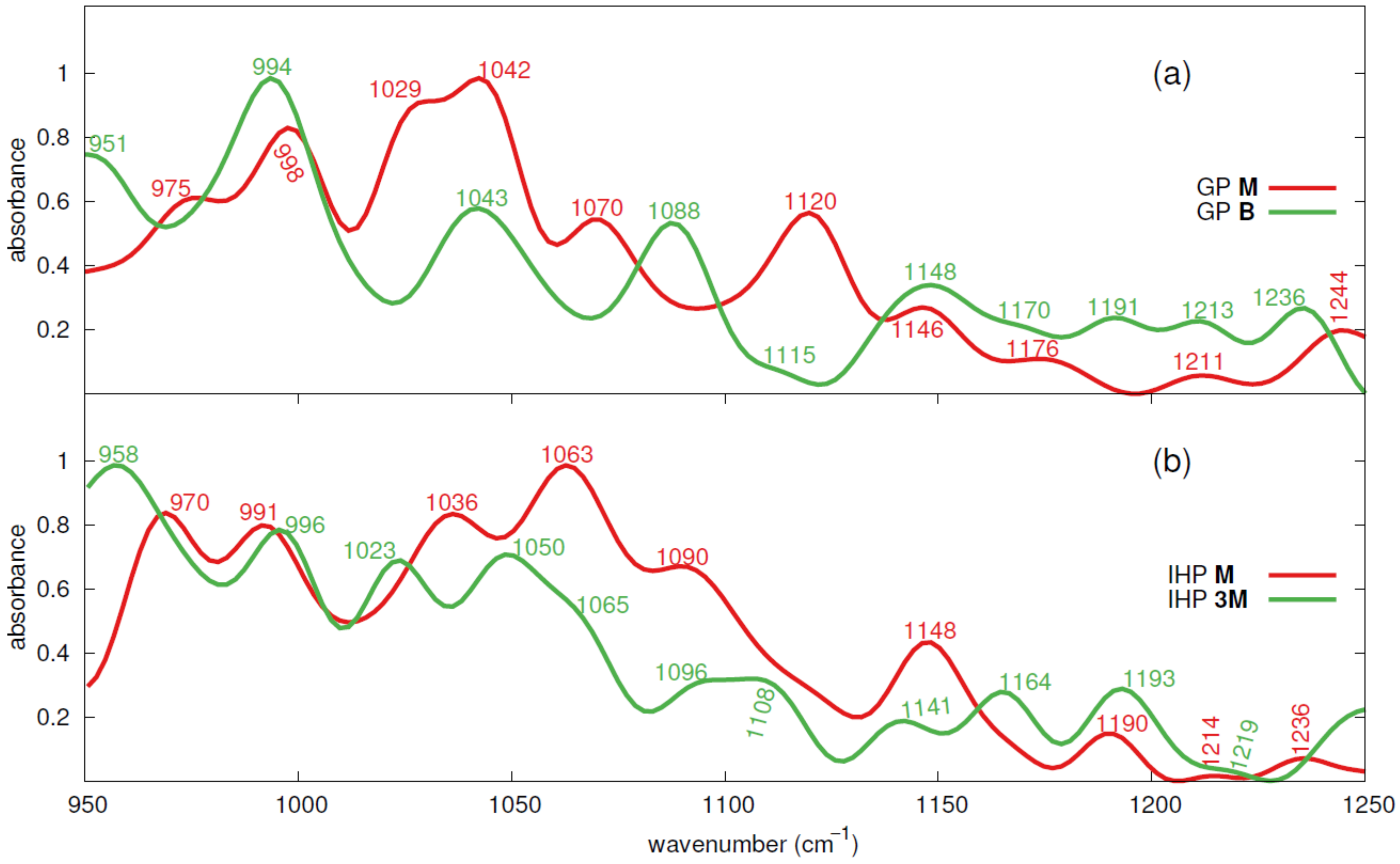
3.3. Theoretical IR Spectra of GP and IHP Adsorbed onto Goethite
3.3.1. GP IR Spectra
3.3.2. IHP IR Spectra
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cordell, D.; Drangert, J.O.; White, S. The Story of Phosphorus: Global Food Security and Food for Thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Cordell, D.; Neset, T.S.S. Phosphorus Vulnerability: A Qualitative Framework for Assessing the Vulnerability of National and Regional Food Systems to the Multi-Dimensional Stressors of Phosphorus Scarcity. Glob. Environ. Chang. 2014, 24, 108–122. [Google Scholar] [CrossRef]
- Reitzel, K.; Bennett, W.W.; Berger, N.; Brownlie, W.J.; Bruun, S.; Christensen, M.L.; Cordell, D.; van Dijk, K.; Egemose, S.; Eigner, H.; et al. New Training to Meet the Global Phosphorus Challenge. Environ. Sci. Technol. 2019, 53, 8479–8481. [Google Scholar] [CrossRef]
- Steen, I. Phosphorus Availability in the 21st Century: Management of a Non-Renewable Resource. Phosphorus Potassium 1998, 217, 25–31. [Google Scholar]
- Smil, V. Phosphorus in the Environment: Natural Flows and Human Interferences. Annu. Rev. Environ. Resour. 2000, 25, 53–88. [Google Scholar] [CrossRef]
- Rosmarin, A. The Precarious Geopolitics of Phosphorous. Down Earth Sci. Environ. Fortn. 2004, 30, 27–31. [Google Scholar]
- Withers, P. Closing the Phosphorus Cycle. Nat. Sustain. 2019, 1001–1002. [Google Scholar] [CrossRef]
- Withers, P.; Forber, K.; Lyon, C.; Rothwell, S.; Doody, D.; Jarvie, H.; Martin-Ortega, J.; Jacobs, B.; Cordell, D.; Patton, M.; et al. Towards Resolving the Phosphorus Chaos Created by Food Systems. Ambio A J. Hum. Environ. 2019. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Gros, P.; Kühn, O.; Leinweber, P. Molecular Level Investigation of the Role of Peptide Interactions in the Glyphosate Analytics. Chemosphere 2018, 196, 129–134. [Google Scholar] [CrossRef]
- Gros, P.; Ahmed, A.; Kühn, O.; Leinweber, P. Glyphosate Binding in Soil as Revealed by Sorption Experiments and Quantum-Chemical Modeling. Sci. Total Environ. 2017, 586, 527–535. [Google Scholar] [CrossRef]
- Gros, P.; Ahmed, A.A.; Kühn, O.; Leinweber, P. Influence of Metal Ions on Glyphosate Detection by FMOC-Cl. Environ. Model. Assess. 2019, 191, 244. [Google Scholar] [CrossRef] [PubMed]
- Hens, M.; Merckx, R. Functional Characterization of Colloidal Phosphorus Species in the Soil Solution of Sandy Soils. Environ. Sci. Technol. 2001, 35, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Kruse, J.; Abraham, M.; Amelung, W.; Baum, C.; Bol, R.; Kühn, O.; Lewandowski, H.; Niederberger, J.; Oelmann, Y.; Rüger, C.; et al. Innovative Methods in Soil Phosphorus Research: A Review. J. Soil Sci. Plant Nutr. 2015, 178, 43–88. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Bol, R.; Nischwitz, V.; Siebers, N.; Willbold, S.; Vereecken, H.; Amelung, W.; Klumpp, E. Phosphorus Containing Water Dispersible Nanoparticles in Arable Soil. J. Environ. Qual. 2015, 44, 1772–1781. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.A.; Gypser, S.; Leinweber, P.; Freese, D.; Kühn, O. Infrared Spectroscopic Characterization of Phosphate Binding at the Goethite-Water Interface. Phys. Chem. Chem. Phys. 2019, 21, 4421–4434. [Google Scholar] [CrossRef] [PubMed]
- Gypser, S.; Hirsch, F.; Schleicher, A.M.; Freese, D. Impact of Crystalline and Amorphous Iron- and Aluminum Hydroxides on Mechanisms of Phosphate Adsorption and Desorption. J. Environ. Sci. 2018, 70, 175–189. [Google Scholar] [CrossRef]
- Sharpley, A.N.; Chapra, S.C.; Wedepohl, R.; Sims, J.T.; Daniel, T.C.; Reddy, K.R. Managing Agricultural Phosphorus for Protection of Surface Waters: Issues and Options. J. Environ. Qual. 1994, 23, 437–451. [Google Scholar] [CrossRef]
- Holzmann, S.; Missong, A.; Puhlmann, H.; Siemens, J.; Bol, R.; Klumpp, E.; von Wilpert, K. Impact of Anthropogenic Induced Nitrogen Input and Liming on Phosphorus Leaching in Forest Soils. J. Soil Sci. Plant Nutr. 2015, 179, 443–453. [Google Scholar] [CrossRef]
- Bol, R.; Julich, D.; Brödlin, D.; Siemens, J.; Kaiser, K.; Dippold, M.A.; Spielvogel, S.; Zilla, T.; Mewes, D.; von Blanckenburg, F.; et al. Dissolved and Colloidal Phosphorus Fluxes in Forest Ecosystems—An Almost Blind Spot in Ecosystem Research. J. Soil Sci. Plant Nutr. 2016, 179, 425–438. [Google Scholar] [CrossRef]
- Boy, J.; Valarezo, C.; Wilcke, W. Water Flow Paths in Soil Control Element Exports in an Andean Tropical Montane Forest. Eur. J. Soil Sci. 2008, 59, 1209–1227. [Google Scholar] [CrossRef]
- Newman, R.H.; Tate, K.R. Soil Phosphorus Characterisation by 31 P Nuclear Magnetic Resonance. Commun. Soil Sci. Plant Anal. 1980, 11, 835–842. [Google Scholar] [CrossRef]
- Tejedor-Tejedor, M.I.; Anderson, M.A. The Protonation of Phosphate on the Surface of Goethite as Studied by CIR-FTIR and Electrophoretic Mobility. Langmuir 1990, 6, 602–611. [Google Scholar] [CrossRef]
- Turner, B.L.; Papházy, M.J.; Haygarth, P.M.; Mckelvie, I.D. Inositol Phosphates in the Environment. Philos. Trans. R. Soc. B 2002, 357, 449–469. [Google Scholar] [CrossRef] [PubMed]
- Doolette, A.; Smernik, R.; Dougherty, W. Spiking Improved Solution Phosphorus31 Nuclear Magnetic Resonance Identification of Soil Phosphorus Compounds. Soil Sci. Soc. Am. J. 2009, 73. [Google Scholar] [CrossRef]
- Gerke, J. Phytate (Inositol Hexakisphosphate) in Soil and Phosphate Acquisition from Inositol Phosphates by Higher Plants: A Review. Plants 2015, 4, 253–266. [Google Scholar] [CrossRef]
- Pant, H.K.; Warman, P.R.; Nowak, J. Identification of Soil Organic Phosphorus by 31 P Nuclear Magnetic Resonance Spectroscopy. Commun. Soil. Sci. Plant Anal. 1999, 30, 757–772. [Google Scholar] [CrossRef]
- Vincent, A.G.; Vestergren, J.; Gröbner, G.; Persson, P.; Schleucher, J.; Giesler, R. Soil Organic Phosphorus Transformations in a Boreal Forest Chronosequence. Plant Soil 2013, 367, 149–162. [Google Scholar] [CrossRef]
- Missong, A.; Bol, R.; Willbold, S.; Siemens, J.; Klumpp, E. Phosphorous Forms in Forest Soil Colloids as Revealed by Liquid-State P-NMR. J. Soil Sci. Plant Nutr. 2016, 179, 159–167. [Google Scholar] [CrossRef]
- Li, H.; Wan, B.; Yan, Y.; Zhang, Y.; Cheng, W.; Feng, X. Adsorption of Glycerophosphate on Goethite: A Macroscopic and Infrared Spectroscopic Study. J. Soil Sci. Plant Nutr. 2017, 181, 557–565. [Google Scholar] [CrossRef]
- Johnson, B.B.; Quill, E.; Angove, M.J. An Investigation of the Mode of Sorption of Inositol Hexaphosphate to Goethite. J. Colloid Interface Sci. 2012, 367, 436–442. [Google Scholar] [CrossRef]
- Celi, L.; M, P.; Marsan, F.A.; Barberis, E. Effects of pH and Electrolytes on Inositol Hexaphosphate Interaction with Goethite. Soil Sci. Soc. Am. J. 2001, 65, 753–760. [Google Scholar] [CrossRef]
- Tsao, T.M.; Chen, Y.M.; Wang, M.K. Origin, Separation and Identification of Environmental Nanoparticles: A Review. J. Environ. Monit. 2011, 13, 1156–1163. [Google Scholar] [CrossRef]
- Tan, K. Principles of Soil Chemistry, 4th ed.; Books in Soils, Plants and the Environment; Taylor & Francis: Abingdon, UK, 2011. [Google Scholar]
- Brady, N. The Nature and Properties of Soils, 15th ed.; Pearson Education Ltd.: Pearson, Harlow, UK, 2017. [Google Scholar]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties Reactions Occurrence and Uses; Wiley-VCH Verlag GmbH and Co. KGaA: Weinheim, Germany, 2003. [Google Scholar] [CrossRef]
- Parfitt, R.L.; Atkinson, R.J. Phosphate Adsorption on Goethite (α-FeOOOH). Nature 1976, 264, 740–742. [Google Scholar] [CrossRef]
- Torrent, J.; Schwertmann, U.; Barron, V. Fast and Slow Phosphate Sorption by Goethite-Rich Natural Materials. Clays Clay Miner. 1992, 40, 14–21. [Google Scholar] [CrossRef]
- Chitrakar, R.; Tezuka, S.; Sonoda, A.; Sakane, K.; Ooi, K.; Hirotsu, T. Phosphate Adsorption on Synthetic Goethite and Akaganeite. J. Colloid Interface Sci. 2006, 298, 602–608. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Gypser, S.; Freese, D.; Leinweber, P.; Kuehn, O. Molecular Level Picture of the Interplay between pH and Phosphate Binding at the Goethite–Water Interface. Phys. Chem. Chem. Phys. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ognalaga, M.; Frossard, E.; Thomas, F. Glucose-1-Phosphate and Myo-Inositol Hexaphosphate Adsorption Mechanisms on Goethite. Soil Sci. Soc. Am. J. 1994, 332–337. [Google Scholar] [CrossRef]
- Yan, Y.; Koopal, L.K.; Liu, F.; Huang, Q.; Feng, X. Desorption of Myo-Inositol Hexakisphosphate and Phosphate from Goethite by Different Reagents. J. Plant. Nutr. Soil Sci. 2015, 178, 878–887. [Google Scholar] [CrossRef]
- Kubicki, J.D. (Ed.) Molecular Modeling of Geochemical Reactions; Wiley and Sons: Chichester, UK, 2016. [Google Scholar]
- Persson, P.; Andersson, T.; Nelson, H.; Sjöberg, S.; Giesler, R.; Lövgren, L. Surface Complexes of Monomethyl Phosphate Stabilized by Hydrogen Bonding on Goethite (Alpha-FeOOH) Nanoparticles. J. Colloid Interface Sci. 2012, 386, 350–358. [Google Scholar] [CrossRef]
- Kubicki, J.D.; Paul, K.W.; Kabalan, L.; Zhu, Q.; Mrozik, M.K.; Aryanpour, M.; Pierre-Louis, A.M.; Strongin, D.R. ATR–FTIR and Density Functional Theory Study of the Structures, Energetics, and Vibrational Spectra of Phosphate Adsorbed onto Goethite. Langmuir 2012, 28, 14573–14587. [Google Scholar] [CrossRef]
- Guan, X.H.; Shang, C.; Zhu, J.; Chen, G.H. ATR-FTIR Investigation on the Complexation of Myo-Inositol Hexaphosphate with Aluminum Hydroxide. J. Colloid Interface Sci. 2006, 293, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.A.; Leinweber, P.; Kühn, O. Unravelling the Nature of Glyphosate Binding to Goethite Surfaces by Ab Initio Molecular Dynamics Simulations. Phys. Chem. Chem. Phys. 2018, 20, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Ganta, P.B.; Kühn, O.; Ahmed, A.A. QM/MM Simulations of Organic Phosphorus Adsorption at the Diaspore–Water Interface. Phys. Chem. Chem. Phys. 2019, 21, 24316–24325. [Google Scholar] [CrossRef] [PubMed]
- Ganta, P.B.; Kühn, O.; Ahmed, A.A. QM/MM Molecular Dynamics Investigation of the Binding of Organic Phosphates to the 100 Diaspore Surface. Front. For. Glob. Chang. 2020, 3, 71. [Google Scholar] [CrossRef]
- Gerzabek, M.; Aquino, A.; Haberhauer, G.; Tunega, D.; Lischka, H. Molecular Modelling—Opportunities for Soil Research. Bodenkultur 2001, 52, 133–146. [Google Scholar]
- Kwon, K.D.; Kubicki, J.D. Molecular Orbital Theory Study on Surface Complex Structures of Phosphates to Iron Hydroxides: Calculation of Vibrational Frequencies and Adsorption Energies. Langmuir 2004, 20, 9249–9254. [Google Scholar] [CrossRef]
- Aquino, A.J.; Tunega, D.; Haberhauer, G.; Gerzabek, M.H.; Lischka, H. Acid–Base Properties of a Goethite Surface Model: A Theoretical View. Geochim. Cosmochim. Acta 2008, 72, 3587–3602. [Google Scholar] [CrossRef]
- Strauss, R.; BrÜmmer, G.; BARROW, N. Effects of Crystallinity of Goethite: II. Rates of Sorption and Desorption of Phosphate. Eur. J. Soil Sci. 1997, 48, 101–114. [Google Scholar] [CrossRef]
- Kosmulski, M. pH-Dependent Surface Charging and Points of Zero Charge. IV. Update and New Approach. J. Colloid Interface Sci. 2009, 337, 439–448. [Google Scholar] [CrossRef]
- Senn, H.M.; Thiel, W. QM/MM Methods for Biomolecular Systems. Angew. Chem. 2009, 48, 1198–1229. [Google Scholar] [CrossRef]
- Xiu, F.; Zhou, L.; Xia, S.; Yu, L. Adsorption Mechanism of Water Molecule on Goethite (010) Surface. J. Ocean Univ. China 2016, 15, 1021–1026. [Google Scholar] [CrossRef]
- Guo, H.; Barnard, A.S. Thermodynamic Modelling of Nanomorphologies of Hematite and Goethite. J. Mater. Chem. 2011, 21, 11566–11577. [Google Scholar] [CrossRef]
- Persson, P.; Nilsson, N.; Sjöberg, S. Structure and Bonding of Orthophosphate Ions at the Iron Oxide–Aqueous Interface. J. Colloid Interface Sci. 1996, 177, 263–275. [Google Scholar] [CrossRef]
- Abdala, D.B.; Northrup, P.A.; Arai, Y.; Sparks, D.L. Surface Loading Effects on Orthophosphate Surface Complexation at the Goethite/Water Interface as Examined by Extended X-Ray Absorption Fine Structure (EXAFS) Spectroscopy. J. Colloid Interface Sci. 2015, 437, 297–303. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD—Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Groenhof, G. Introduction to QM/MM Simulations. In Biomolecular Simulations: Methods and Protocols; Monticelli, L., Salonen, E., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 43–66. [Google Scholar] [CrossRef]
- Ozboyaci, M.; Kokh, D.B.; Corni, S.; Wade, R.C. Modeling and Simulation of Protein–Surface Interactions: Achievements and Challenges. Q. Rev. Biophys. 2016, 49, e4. [Google Scholar] [CrossRef]
- VandeVondele, J.; Krack, M.; Mohamed, F.; Parrinello, M.; Chassaing, T.; Hutter, J. Quickstep: Fast and Accurate Density Functional Calculations Using a Mixed Gaussian and Plane Waves Approach. Comput. Phys. Commun. 2005, 167, 103–128. [Google Scholar] [CrossRef]
- Krack, M. Pseudopotentials for H to Kr Optimized for Gradient-Corrected Exchange-Correlation Functionals. Theor. Chem. Acc. 2005, 114, 145–152. [Google Scholar] [CrossRef]
- VandeVondele, J.; Hutter, J. Gaussian Basis Sets for Accurate Calculations on Molecular Systems in Gas and Condensed Phases. J. Chem. Phys. 2007, 127. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Phys. Chem. 2010, 132. [Google Scholar] [CrossRef] [PubMed]
- Mundy, C.J.; Balasubramanian, S.; Bagchi, K.; Hutter, J.; Kuo, A.S.I.; Laino, T.; VandeVondele, J. Frontiers in Simulation Technology (FIST). (accessed by CP2K, version 5.1). 2017. Available online: www.cp2k.org (accessed on 12 November 2020).
- CP2K. Open Source Molecular Dynamics Code. version 5.1, Released October 24, 2017 (r18096). Available online: https://www.cp2k.org/ (accessed on 12 November 2020).
- Kühne, T.D.; Iannuzzi, M.; Del Ben, M.; Rybkin, V.V.; Seewald, P.; Stein, F.; Laino, T.; Khaliullin, R.Z.; Schütt, O.; Schiffmann, F.; et al. CP2K: An electronic structure and molecular dynamics software package—Quickstep: Efficient and accurate electronic structure calculations. J. Chem. Phys. 2020, 152, 194103. [Google Scholar] [CrossRef]
- Cygan, R.T.; Liang, J.J.; Kalinichev, A.G. Molecular Models of Hydroxide, Oxyhydroxide, and Clay Phases and the Development of a General Force Field. J. Phys. Chem. B 2004, 108, 1255–1266. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Grigera, J.R.; Straatsma, T.P. The Missing Term in Effective Pair Potentials. J. Phys. Chem. 1987, 91, 6269–6271. [Google Scholar] [CrossRef]
- Zoete, V.; Cuendet, M.A.; Grosdidier, A.; Michielin, O. SwissParam: A Fast Force Field Generation Tool for Small Organic Molecules. J. Comput. Chem. 2011, 32, 2359–2368. [Google Scholar] [CrossRef] [PubMed]
- Laino, T.; Mohamed, F.; Laio, A.; Parrinello, M. An Efficient Linear-Scaling Electrostatic Coupling for Treating Periodic Boundary Conditions in QM/MM Simulations. J. Chem. Theory Comput. 2006, 2, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical Sampling through Velocity Rescaling. J. Chem. Phys. 2007. [Google Scholar] [CrossRef]
- Boys, S.F.; Bernardi, F. The Calculation of Small Molecular Interactions by the Differences of Separate Total Energies: Some Procedures with Reduced Errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Brehm, M.; Kirchner, B. TRAVIS—A Free Analyzer and Visualizer for Monte Carlo and Molecular Dynamics Trajectories. J. Chem. Inf. Model. 2011, 51, 2007–2023. [Google Scholar] [CrossRef]
- Thomas, M.; Brehm, M.; Fligg, R.; Vöhringer, P.; Kirchner, B. Computing Vibrational Spectra from Ab Initio Molecular Dynamics. Phys. Chem. Chem. Phys. 2013, 15, 6608–6622. [Google Scholar] [CrossRef]
- Thomas, M.; Brehm, M.; Kirchner, B. Voronoi Dipole Moments for the Simulation of Bulk Phase Vibrational Spectra. Phys. Chem. Chem. Phys. 2015, 17, 3207–3213. [Google Scholar] [CrossRef] [PubMed]
- Brehm, M.; Thomas, M. An Efficient Lossless Compression Algorithm for Trajectories of Atom Positions and Volumetric Data. J. Chem. Inf. Model. 2018, 58, 2092–2107. [Google Scholar] [CrossRef] [PubMed]
- Lü, C.; Yan, D.; He, J.; Zhou, B.; Li, L.; Zheng, Q. Environmental Geochemistry Significance of Organic Phosphorus: An Insight from Its Adsorption on Iron Oxides. J. Appl. Geochem. 2017, 84, 52–60. [Google Scholar] [CrossRef]
- Sheals, J.; Sjoberg, S.; Persson, P. Adsorption of Glyphosate on Goethite: Molecular Characterization of Surface Complexes. Environ. Sci. Technol. 2002, 36, 3090–3095. [Google Scholar] [CrossRef]
- Rose, J.; Flank, A.M.; Masion, A.; Bottero, J.Y.; Elmerich, P. Nucleation and Growth Mechanisms of Fe Oxyhydroxide in the Presence of PO4 Ions. 2. P K-Edge EXAFS Study. Langmuir 1997, 13, 1827–1834. [Google Scholar] [CrossRef]
- Tribe, L.; Kwon, K.D.; Trout, C.C.; Kubicki, J.D. Molecular Orbital Theory Study on Surface Complex Structures of Glyphosate on Goethite: Calculation of Vibrational Frequencies. Environ. Sci. Technol. 2006, 40, 3836–3841. [Google Scholar] [CrossRef]
- Anderson, G.; Arlidge, E.Z. The Adsorption of Inositol Phosphates and Glycerophosphate by Soil Clays, Clay Minerals, and Hydrated Sesquioxides in Acid Media. J. Soil Sci. 1962, 13, 216–224. [Google Scholar] [CrossRef]
- Celi, L.; Lamacchia, S.; Marsan, F.A.; Barberis, E. Interaction of Inositol Hexaphosphate on Clays: Adsorption and Charging Phenomena. Soil Sci. 1999, 164, 574–585. [Google Scholar] [CrossRef]
- Colthup, N.B.; Daly, L.H.; Wiberley, S.E. Compounds Containing Boron, Silicon, Phosphorus, Sulfur, or Halogen. In Introduction to Infrared and Raman Spectroscopy, 3rd ed.; Colthup, N.B., Daly, L.H., Wiberley, S.E., Eds.; Academic Press: San Diego, CA, USA, 1990. [Google Scholar] [CrossRef]
- Olsson, R.; Giesler, R.; Loring, J.S.; Persson, P. Adsorption, Desorption, and Surface-Promoted Hydrolysis of Glucose-1-Phosphate in Aqueous Goethite (Alpha-Feooh) Suspensions. Langmuir 2010, 26, 18760–18770. [Google Scholar] [CrossRef]
- Del Bene, J.E.; Jordan, M.J.T. Vibrational Spectroscopy of the Hydrogen Bond: An Ab Initio Quantum-Chemical Perspective. Null 1999, 18, 119–162. [Google Scholar] [CrossRef]
- Fornaro, T.; Burini, D.; Biczysko, M.; Barone, V. Hydrogen-Bonding Effects on Infrared Spectra from Anharmonic Computations: Uracil–Water Complexes and Uracil Dimers. J. Phys. Chem. A 2015, 119, 4224–4236. [Google Scholar] [CrossRef]
- Arai, Y.; Sparks, D. ATR–FTIR Spectroscopic Investigation on Phosphate Adsorption Mechanisms at the Ferrihydrite–Water Interface. J. Colloid Interface Sci. 2001, 241, 317–326. [Google Scholar] [CrossRef]
- Lincoln, S.; Stranks, D. Phosphato Complexes of Cobalt(III). I. General Structural and Hydrolytic Properties. Aust. J. Chem. 1968, 21, 37–56. [Google Scholar] [CrossRef]
- Nakagawa, H.; Oyama, T. Molecular Basis of Water Activity in Glycerol–Water Mixtures. Front. Chem. 2019, 7, 731. [Google Scholar] [CrossRef]
- Kataoka, Y.; Kitadai, N.; Hisatomi, O.; Nakashima, S. Nature of Hydrogen Bonding of Water Molecules in Aqueous Solutions of Glycerol by Attenuated Total Reflection (ATR) Infrared Spectroscopy. Appl. Spectrosc. 2011, 65, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wan, B.; Zhang, Y.; Zhang, L.; Liu, F.; Feng, X. In Situ ATR-FTIR Spectroscopic Study of the Co-Adsorption of Myo-Inositol Hexakisphosphate and Zn(II) on Goethite. Soil Res. 2018, 56, 526–534. [Google Scholar] [CrossRef]
- Peak Fit v4. Peak Separation and Analysis Software; SPSS Science, Inc.: Chicago, IL, USA, 2003. [Google Scholar]
- FAO. FAO’s Director-General on How to Feed the World in 2050. Popul. Dev. Rev. 2009, 35, 837–839. [Google Scholar] [CrossRef]
- De Groot, C.J.; Golterman, H.L. On the Presence of Organic Phosphate in Some Camargue Sediments: Evidence for the Importance of Phytate. Hydrobiologia 1993, 252, 117–126. [Google Scholar] [CrossRef]
| Study and Description | Wavenumber [cm] | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| goethite–OP | ||||||||||||
| Ref.Tejedor and Anderson [22] | ||||||||||||
| pH 6.0, 190 mol P/g of Gt | 1004 | 1030 | 1045 | 1099 | 1128 | |||||||
| 982 | 1006 | 1123 | ||||||||||
| 1044 | 1096 | |||||||||||
| FePO | 1001 | 1025 | ||||||||||
| Ref. Persson et al. [57] | ||||||||||||
| pH 4.2−5.7 [] | 1001 | 1049 | 1122 | 1178 | ||||||||
| pH 7.9 [] | 1001 | 1049 | 1122 | |||||||||
| pH 13 [] | 966 | 1057 | ||||||||||
| Ref. Kubicki et al. [44] | ||||||||||||
| pH 4.22 [] | 982 | 1009 | 1044 | 1091 | 1122 | 1157 | 1195 | |||||
| pH 7.51 [] | 1022 | 1043 | 1084 | 1124 | 1177 | |||||||
| Ref. Ahmed et al. [15] | ||||||||||||
| pH 6.3 | 1000 | 1110 | 1165 | |||||||||
| M@010 [] | 964 | 1021 | 1049 | 1207 | ||||||||
| B@010 [] | 957 | 992 | 1007 | 1035 | 1071 | 1227 | ||||||
| M@100 [] | 957 | 999 | 1044 | 1058 | ||||||||
| B@100 [] | 953 | 974 | 1048 | |||||||||
| goethite–[organic P-compounds] | ||||||||||||
| Ref. Persson et al. [43] (MMP) | ||||||||||||
| pH 2.6 | 1003 | 1051 | 1140 | 1185 | ||||||||
| pH 9.9 | 990 | 1051 | 1120 | 1185 | ||||||||
| Ref. Tribe et al. [83] (GLP) | ||||||||||||
| 1001 | 1020 | 1030 | 1063 | 1118 | ||||||||
| 973 | 979 | 1032 | 1068 | 1084 | 1095 | 1102 | ||||||
| Ref. Li et al. [29] (GP) | ||||||||||||
| pH 3 | 1008 | 1052 | 1098 | 1139 | ||||||||
| pH 9 | 998 | 1044 | 1092 | 1126 | ||||||||
| current work | ||||||||||||
| M [] | 975 | 998 | 1029 | 1042 | 1070 | 1120 | 1146 | 1176 | 1211 | 1244 | ||
| B [] | 951 | 994 | 1043 | 1088 | 1115 | 1148 | 1170 | 1191 | 1213 | 1236 | ||
| goethite–IHP | ||||||||||||
| Ref. Yan et al. [41] | ||||||||||||
| pH 5 | 998 | 1075 | 1135 | 1166 | ||||||||
| Ref. Yan et al. [94] | ||||||||||||
| pH 5 | 1010 | 1071 | 1134 | 1164 | ||||||||
| pH 6 | 998 | 1068 | 1131 | |||||||||
| Ref. Johnson et al. [30] | ||||||||||||
| fitted band centers | 974 | 991 | 1011 | 1043 | 1074 | 1099 | 1128 | 1160 | 1187 | 1220 | ||
| current work | ||||||||||||
| M | 970 | 991 | 1036 | 1063 | 1090 | 1148 | 1190 | 1214 | 1236 | |||
| 3M | 958 | 996 | 1023 | 1050 | 1065 | 1096 | 1108 | 1141 | 1164 | 1193 | 1219 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganta, P.B.; Kühn, O.; Ahmed, A.A. Ab Initio Molecular Dynamics Simulations of the Interaction between Organic Phosphates and Goethite. Molecules 2021, 26, 160. https://doi.org/10.3390/molecules26010160
Ganta PB, Kühn O, Ahmed AA. Ab Initio Molecular Dynamics Simulations of the Interaction between Organic Phosphates and Goethite. Molecules. 2021; 26(1):160. https://doi.org/10.3390/molecules26010160
Chicago/Turabian StyleGanta, Prasanth B., Oliver Kühn, and Ashour A. Ahmed. 2021. "Ab Initio Molecular Dynamics Simulations of the Interaction between Organic Phosphates and Goethite" Molecules 26, no. 1: 160. https://doi.org/10.3390/molecules26010160
APA StyleGanta, P. B., Kühn, O., & Ahmed, A. A. (2021). Ab Initio Molecular Dynamics Simulations of the Interaction between Organic Phosphates and Goethite. Molecules, 26(1), 160. https://doi.org/10.3390/molecules26010160







