Hydrodechlorination of 4-Chlorophenol on Pd-Fe Catalysts on Mesoporous ZrO2SiO2 Support
Abstract
1. Introduction
2. Results
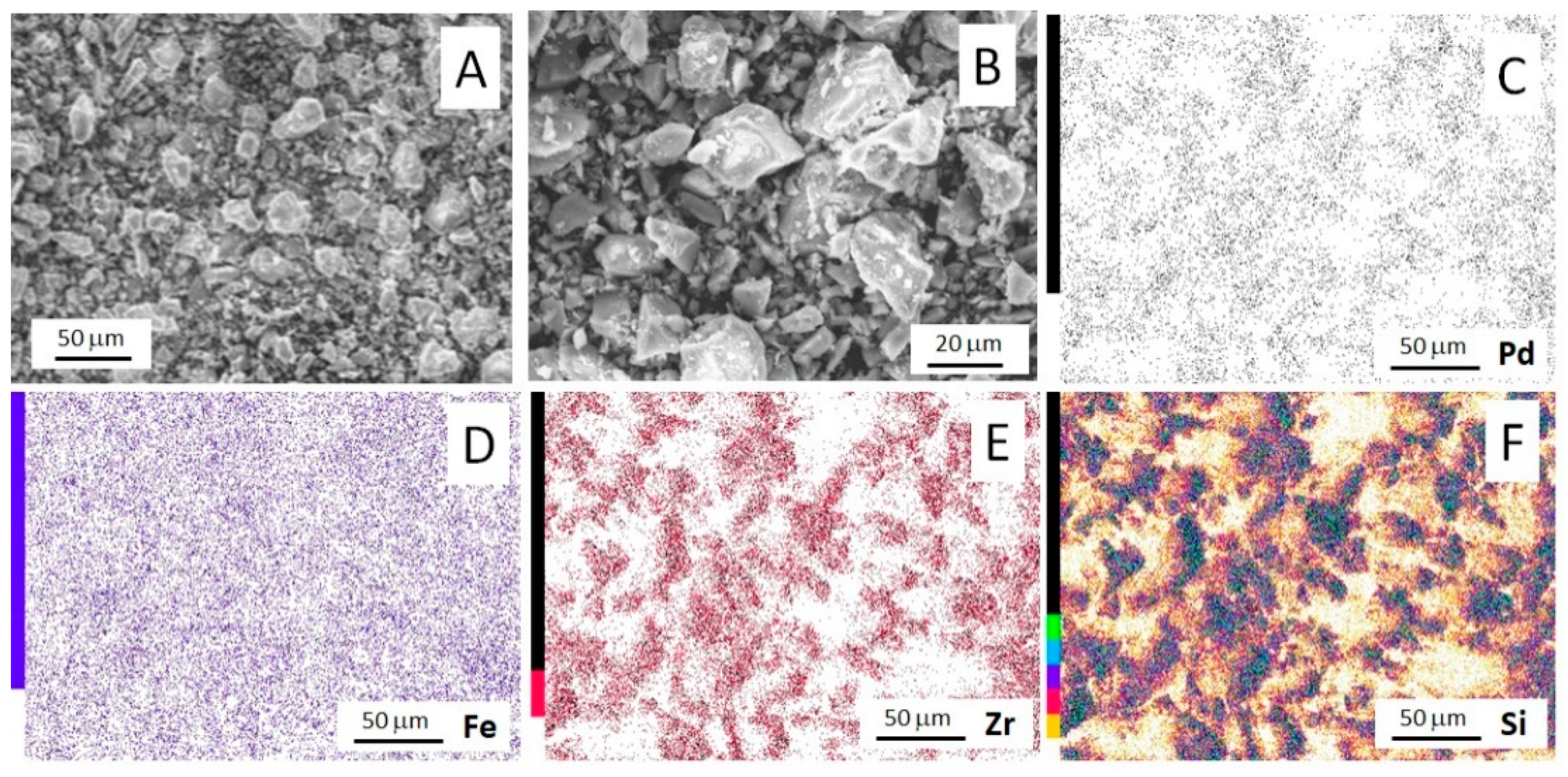
2.1. Texture and Morphology
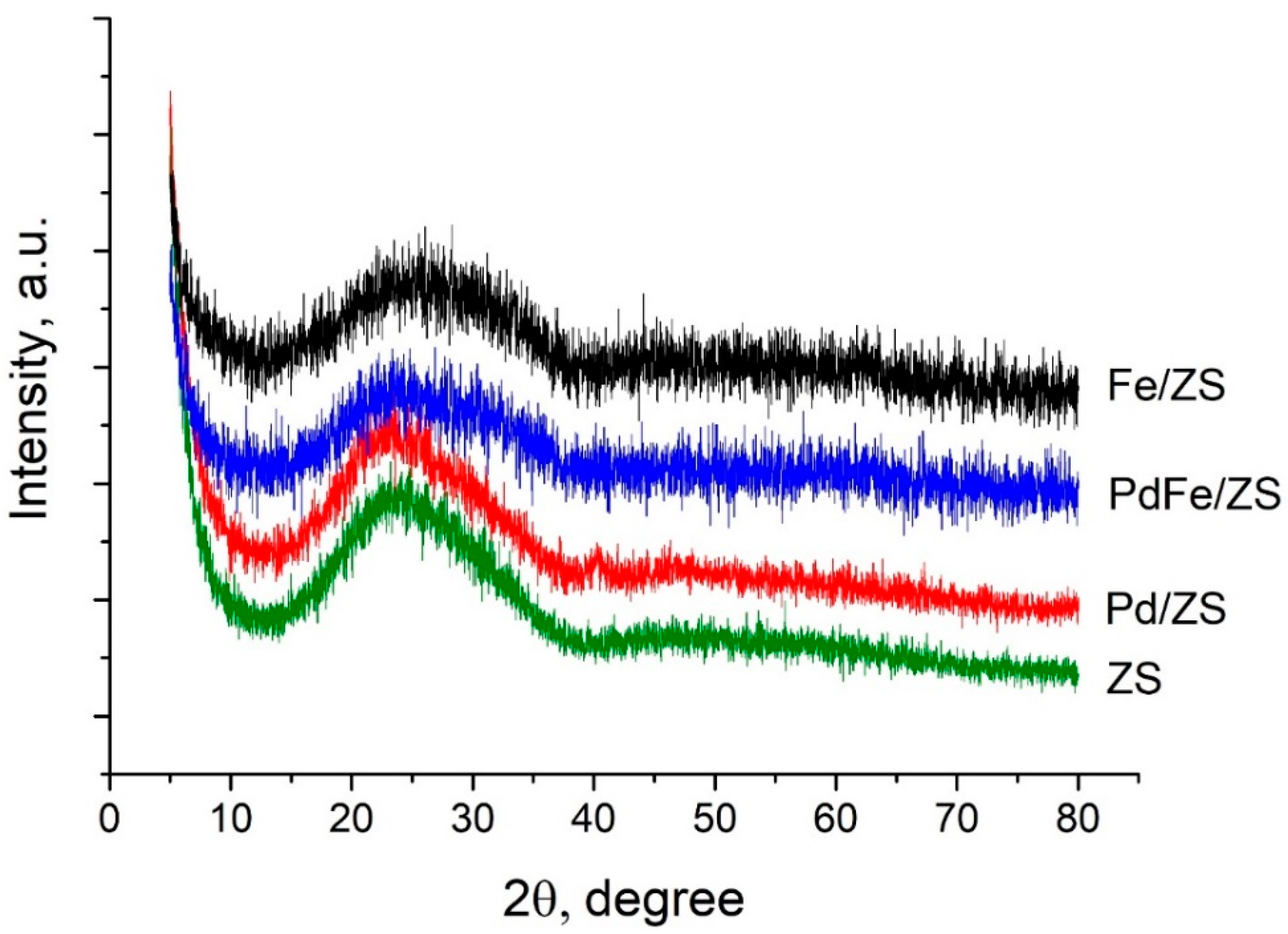
2.2. X-ray Diffraction (XRD) and Transmision Electron Microscopy (TEM) Analysis
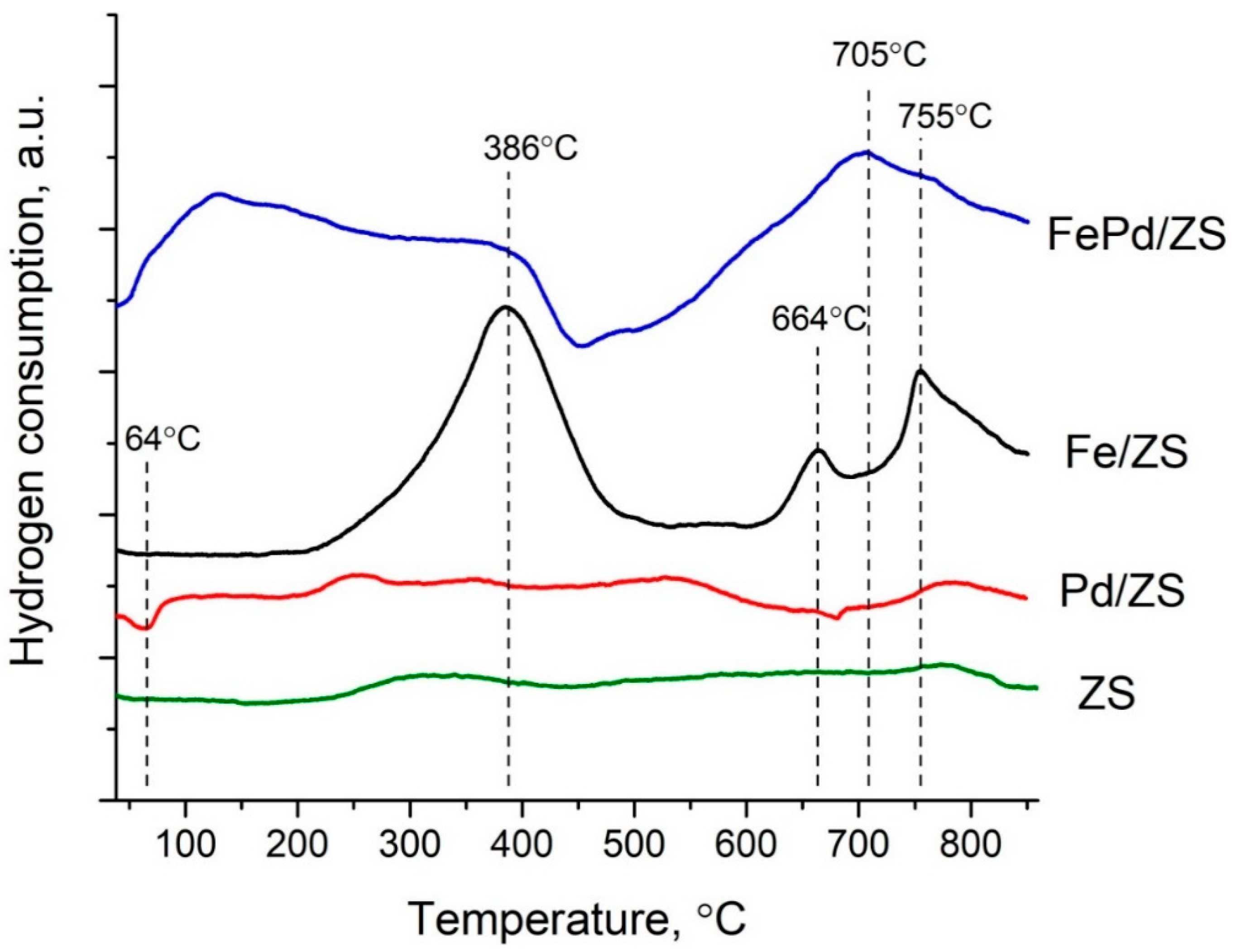
2.3. Reducibility of Metals in Catalysts
- Step 1:
- 3Fe2O3 + H2 → 2Fe3O4 + H2O
- Step 2:
- Fe3O4 + H2 → 3FeO + H2O
- Step 3:
- FeO + H2 → Fe + H2O
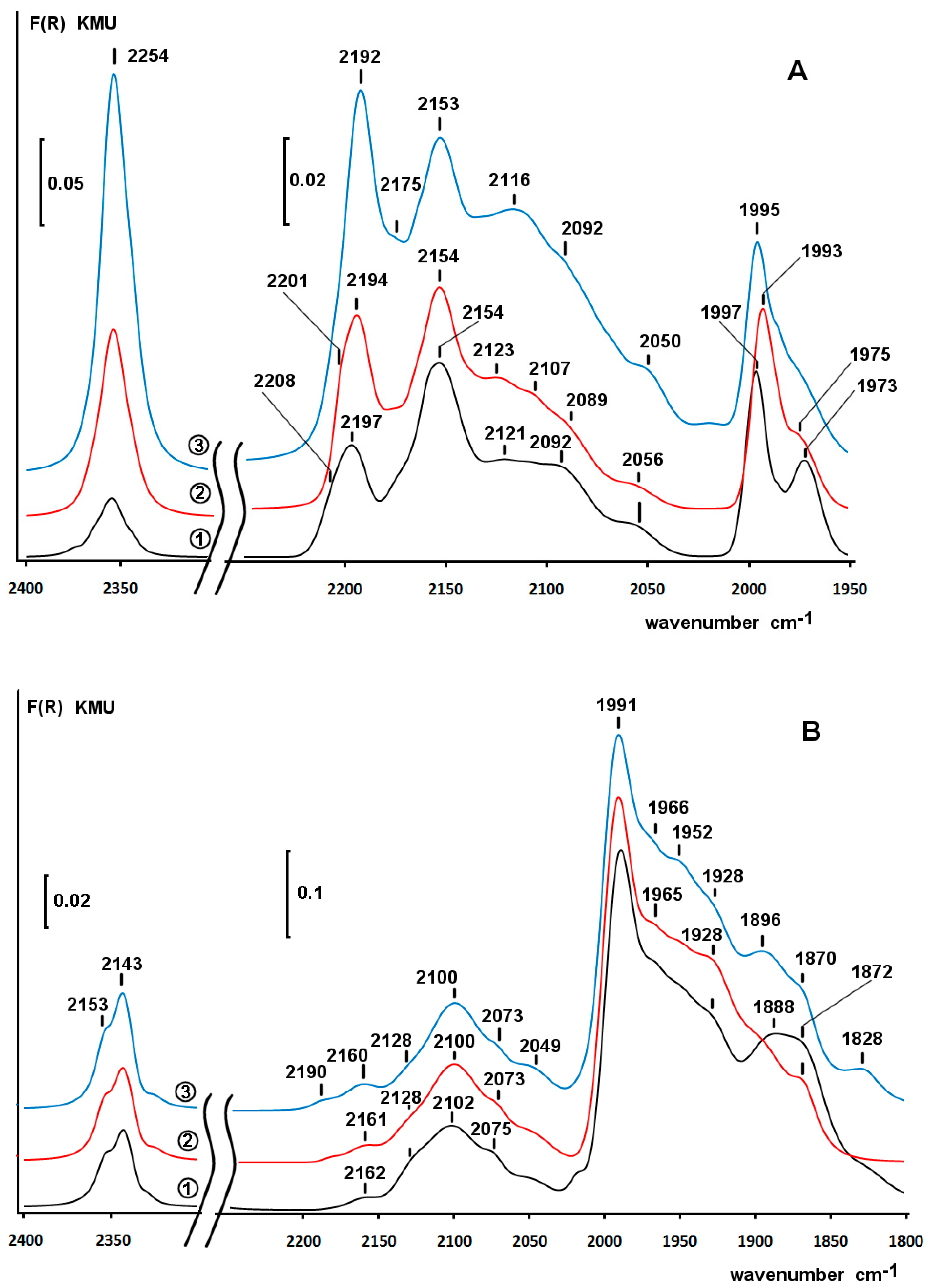
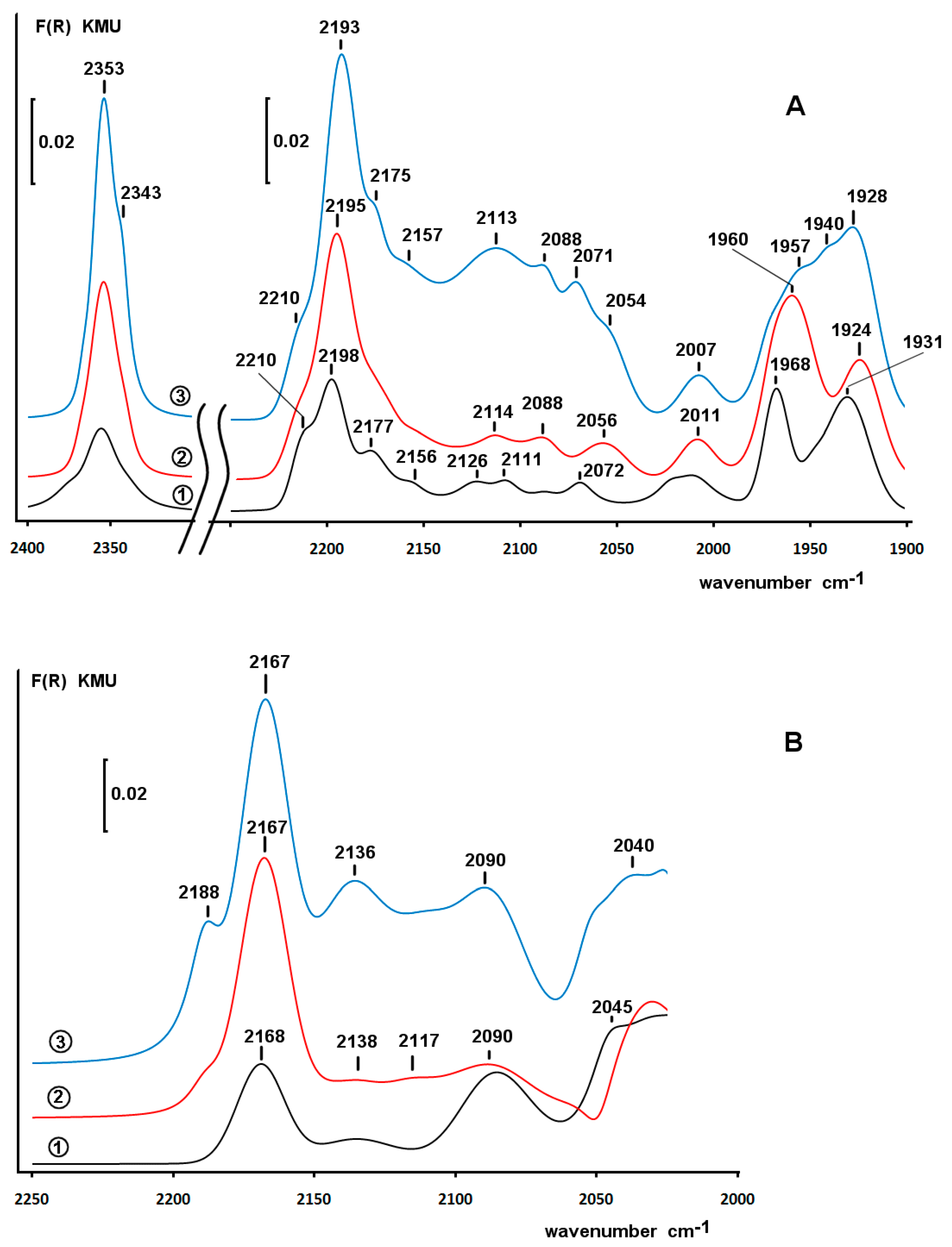
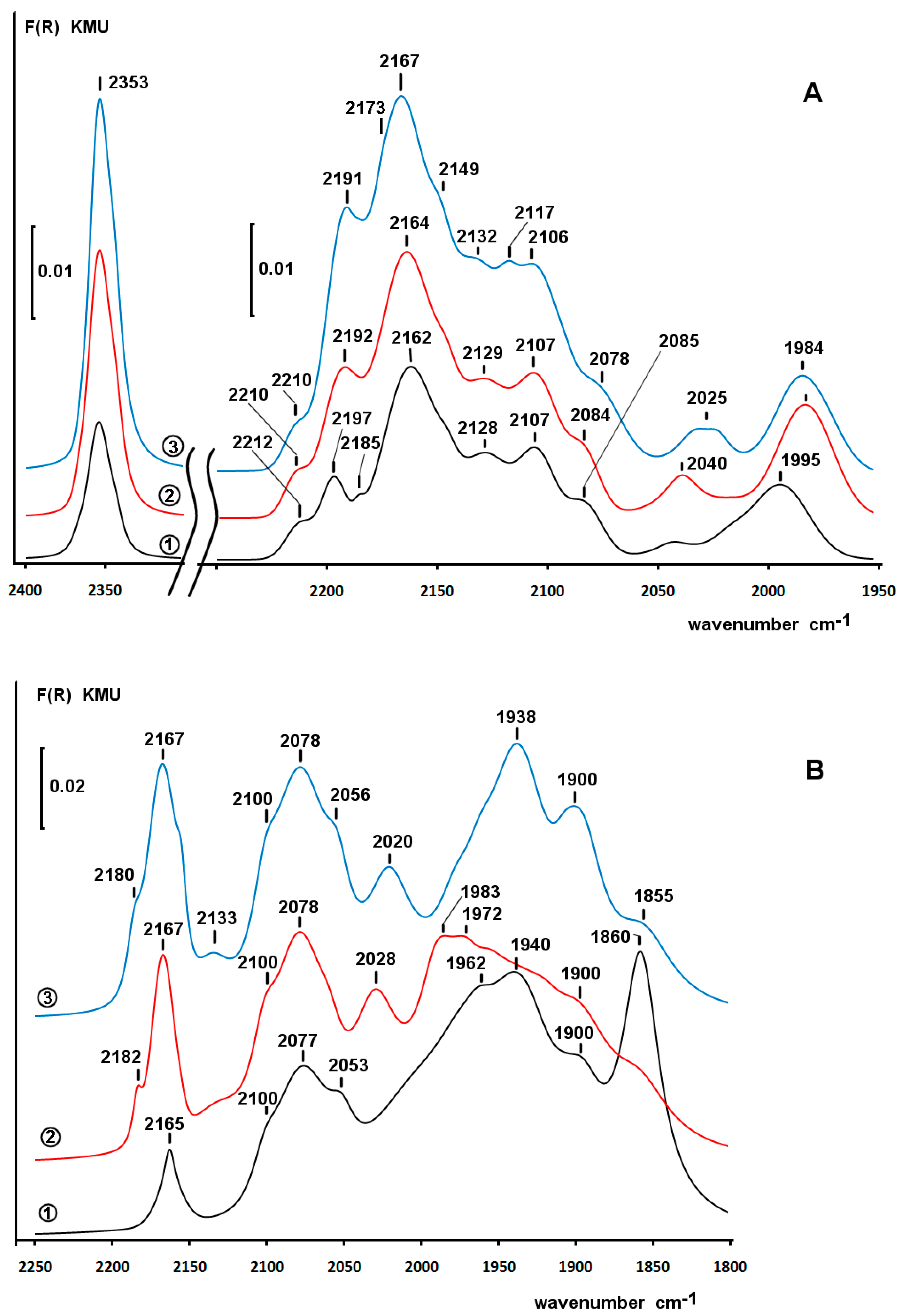
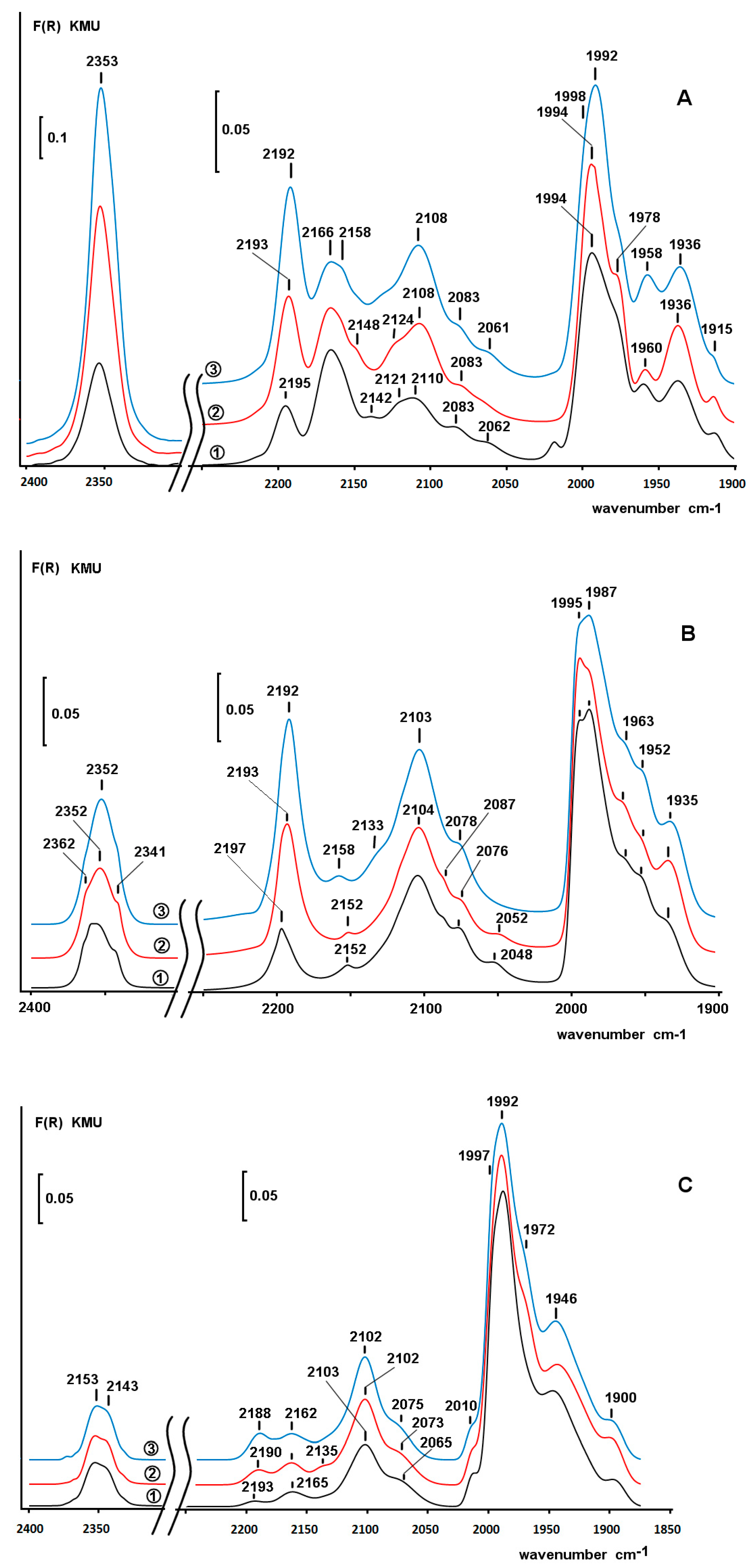
2.4. Diffuse Reflectance Infrared Fourier Transform Spectroscopy (DRIFT) Study of Adsorbed CO
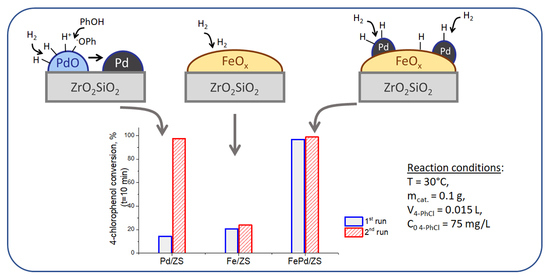
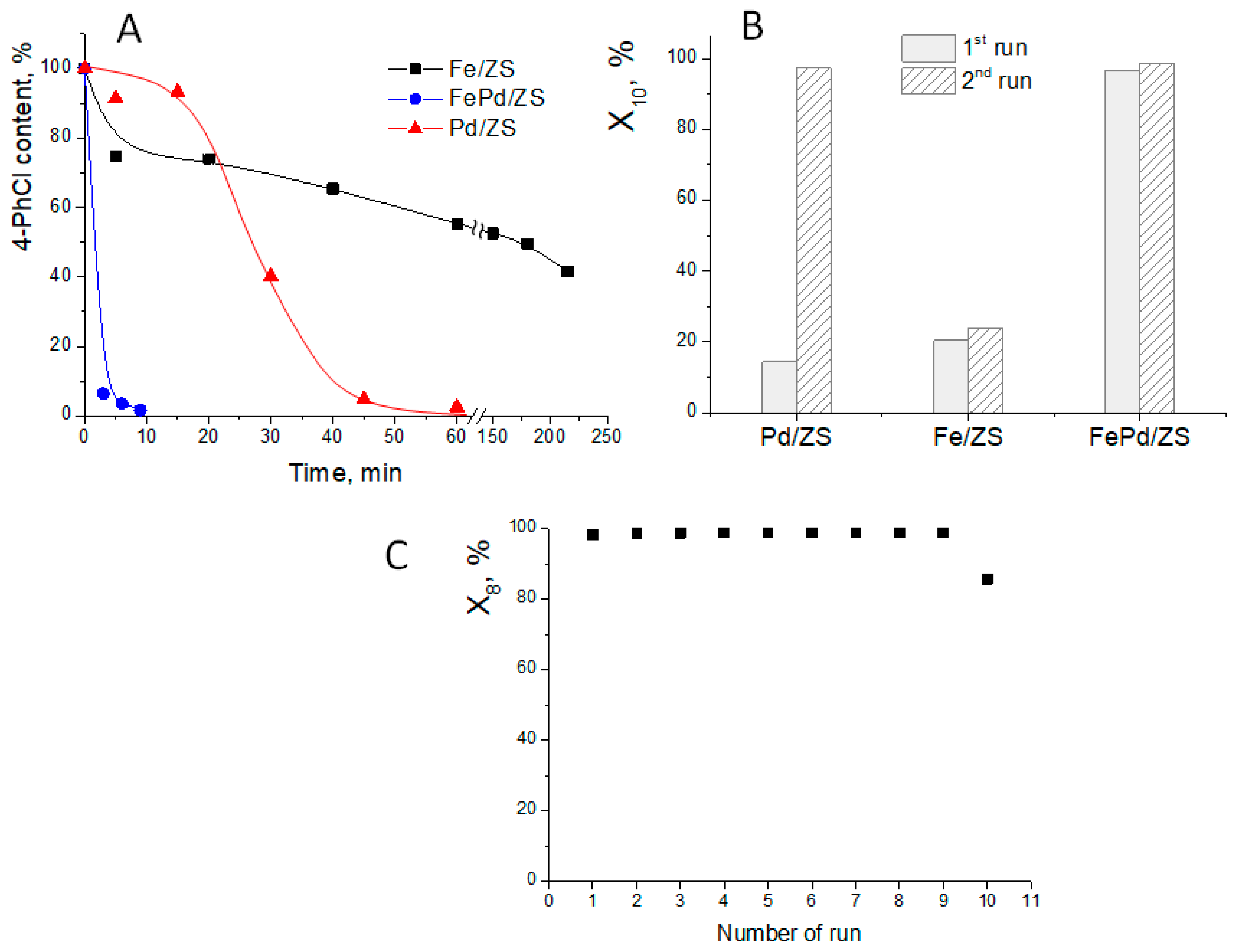
2.5. Catalytic Tests in 4-PhCl Reduction
2.6. DRIFT Spectroscopy of CO Adsorbed on Pd/ZS after Reduction with Phenol and H2 in Water
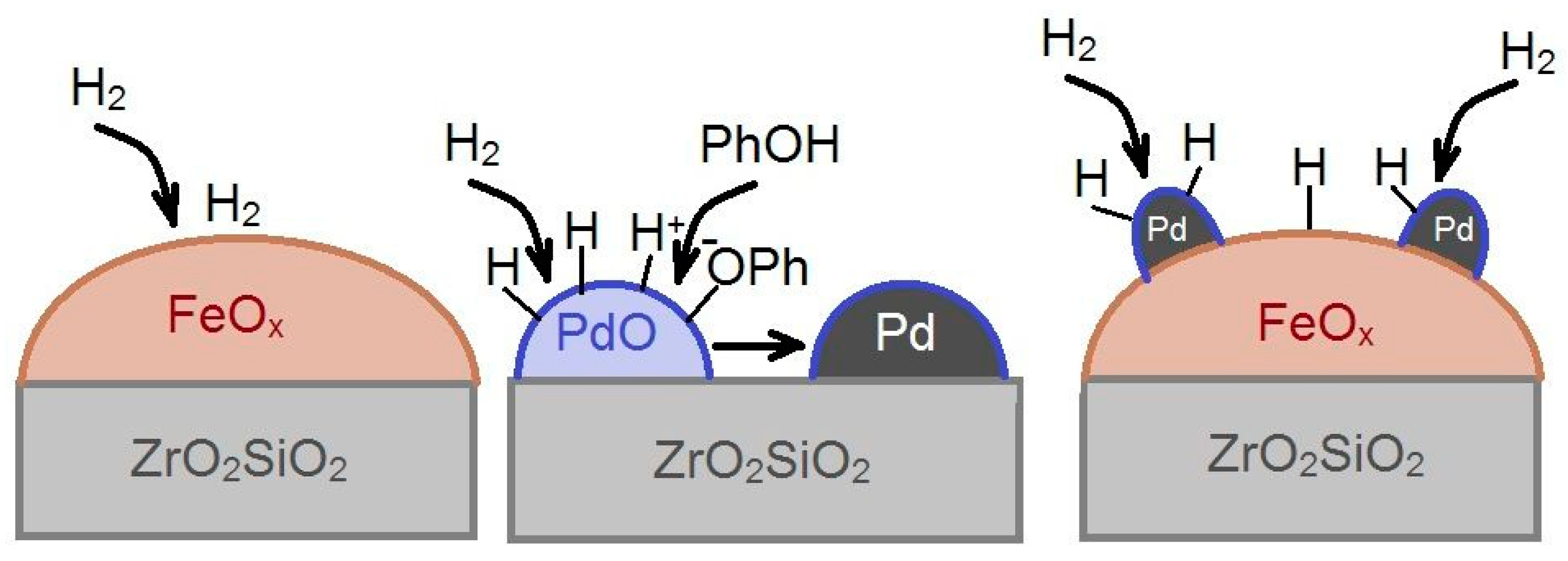
3. Discussion
4. Materials and Methods
4.1. Synthesis of the ZS Support
4.2. Synthesis of the Catalysts
4.3. Catalytic Tests
4.4. Characterization of the Catalysts
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lokteva, E.; Golubina, E.; Likholobov, V.; Lunin, V. Disposal of Chlorine-Containing Wastes. In Chemistry Beyond Chlorine; Tundo, P., He, L.-N., Lokteva, E., Mota, C., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 559–584. [Google Scholar] [CrossRef]
- Shi, W.; Yu, N.; Jiang, X.; Han, Z.; Wang, S.; Zhang, X.; Wei, S.; Giesy, J.P.; Yu, H. Influence of blooms of phytoplankton on concentrations of hydrophobic organic chemicals in sediments and snails in a hyper-eutrophic, freshwater lake. Water Res. 2017, 113, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Hung, H.; Tian, C.; Kallenborn, R. Revolatilization of persistent organic pollutants in the Arctic induced by climate change. Nat. Clim. Chang. 2011, 1, 255–260. [Google Scholar] [CrossRef]
- Juhler, R.K.; Sørensen, S.R.; Larsen, L. Analysing transformation products of herbicide residues in environmental samples. Water Res. 2001, 35, 1371–1378. [Google Scholar] [CrossRef]
- Yuan, G.; Keane, M.A. Liquid phase catalytic hydrodechlorination of chlorophenols at 273 K. Catal. Commun. 2003, 4, 195–201. [Google Scholar] [CrossRef]
- Tundo, P.; Perosa, A.; Selva, M.; Zinovyev, S.S. A mild catalytic detoxification method for PCDDs and PCDFs. Appl. Catal. B Environ. 2001, 32, L1–L7. [Google Scholar] [CrossRef]
- Simagina, V.I.; Netskina, O.V.; Tayban, E.S.; Komova, O.V.; Grayfer, E.D.; Ischenko, A.V.; Pazhetnov, E.M. The effect of support properties on the activity of Pd/C catalysts in the liquid-phase hydrodechlorination of chlorobenzene. Appl. Catal. A Gen. 2010, 379, 87–94. [Google Scholar] [CrossRef]
- Gómez-Quero, S.; Cárdenas-Lizana, F.; Keane, M.A. Liquid phase catalytic hydrodechlorination of 2,4-dichlorophenol over Pd/Al2O3: Batch vs. continuous operation. Chem. Eng. J. 2011, 166, 1044–1051. [Google Scholar] [CrossRef]
- Wu, Y.; Gan, L.; Zhang, S.; Song, H.; Lu, C.; Li, W.; Wang, Z.; Jiang, B.; Li, A. Carbon-nanotube-doped Pd-Ni bimetallic three-dimensional electrode for electrocatalytic hydrodechlorination of 4-chlorophenol: Enhanced activity and stability. J. Hazard. Mater. 2018, 356, 17–25. [Google Scholar] [CrossRef]
- Cheng, L.; Jin, Z.; Wang, X. Hydrodechlorination of chlorophenols at low temperature over highly defective Pd catalyst. Catal. Commun. 2013, 41, 60–64. [Google Scholar] [CrossRef]
- Raut, S.S.; Shetty, R.; Raju, N.M.; Kamble, S.P.; Kulkarni, P.S. Screening of zero valent mono/bimetallic catalysts and recommendation of Raney Ni (without reducing agent) for dechlorination of 4-chlorophenol. Chemosphere 2020, 250, 126298. [Google Scholar] [CrossRef]
- Ma, X.; Liu, S.; Liu, Y.; Li, X.; Li, Q.; Gu, G.; Xia, C. Promoted liquid-phase hydrodechlorination of chlorophenol over Raney Ni via controlling base: Performance, mechanism, and application. Chemosphere 2020, 242, 125202. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Huang, R.; Li, C.; Che, M.; Su, R.; Li, S.; Yu, J.; Qi, W.; He, Z. Continuous rapid dechlorination of p-chlorophenol by Fe-Pd nanoparticles promoted by procyanidin. Chem. Eng. Sci. 2019, 201, 121–131. [Google Scholar] [CrossRef]
- Han, B.; Liu, W.; Li, J.; Wang, J.; Zhao, D.; Xu, R.; Lin, Z. Catalytic hydrodechlorination of triclosan using a new class of anion-exchange-resin supported palladium catalysts. Water Res. 2017, 120, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Sandoval, J.; Munoz, M.; de Pedro, Z.M.; Casas, J.A. Fast degradation of diclofenac by catalytic hydrodechlorination. Chemosphere 2018, 213, 141–148. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Pro-file for Phenol (Update); Public Health Service, U.S., Department of Health and Human Services: Atlanta, GA, USA, 1998.
- Diaz, E.; Mohedano, A.F.; Casas, J.A.; Shalaby, C.; Eser, S.; Rodriguez, J.J. On the performance of Pd and Rh catalysts over different supports in the hydrodechlorination of the MCPA herbicide. Appl. Catal. B Environ. 2016, 186, 151–156. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Uemichi, Y.; Ayame, A. Low-temperature hydrodechlorination mechanism of chlorobenzenes over platinum-supported and palladium-supported alumina catalysts. Appl. Catal. A Gen. 2005, 287, 89–97. [Google Scholar] [CrossRef]
- Klokov, S.V.; Lokteva, E.S.; Golubina, E.V.; Maslakov, K.I.; Levanov, A.V.; Chernyak, S.A.; Likholobov, V.A. Effective Pd/C catalyst for chlorobenzene and hexachlorobenzene hydrodechlorination by direct pyrolysis of sawdust impregnated with palladium nitrate. Catal. Commun. 2016, 77, 37–41. [Google Scholar] [CrossRef]
- Golubina, E.V.; Lokteva, E.S.; Lunin, V.V.; Telegina, N.S.; Stakheev, A.Y.; Tundo, P. The role of Fe addition on the activity of Pd-containing catalysts in multiphase hydrodechlorination. Appl. Catal. A Gen. 2006, 302, 32–41. [Google Scholar] [CrossRef]
- Witońska, I.A.; Walock, M.J.; Binczarski, M.; Lesiak, M.; Stanishevsky, A.V.; Karski, S. Pd–Fe/SiO2 and Pd–Fe/Al2O3 catalysts for selective hydrodechlorination of 2,4-dichlorophenol into phenol. J. Mol. Catal. A Chem. 2014, 393, 248–256. [Google Scholar] [CrossRef]
- Jin, Z.; Yu, C.; Wang, X.; Wan, Y.; Li, D.; Lu, G. Liquid phase hydrodechlorination of chlorophenols at lower temperature on a novel Pd catalyst. J. Hazard. Mater. 2011, 186, 1726–1732. [Google Scholar] [CrossRef]
- de Pedro, Z.M.; Diaz, E.; Mohedano, A.F.; Casas, J.A.; Rodriguez, J.J. Compared activity and stability of Pd/Al2O3 and Pd/AC catalysts in 4-chlorophenol hydrodechlorination in different pH media. Appl. Catal. B Environ. 2011, 103, 128–135. [Google Scholar] [CrossRef]
- Shao, Y.; Xu, Z.; Wan, H.; Wan, Y.; Chen, H.; Zheng, S.; Zhu, D. Enhanced liquid phase catalytic hydrodechlorination of 2,4-dichlorophenol over mesoporous carbon supported Pd catalysts. Catal. Commun. 2011, 12, 1405–1409. [Google Scholar] [CrossRef]
- Yuan, G.; Keane, M.A. Liquid phase hydrodechlorination of chlorophenols over Pd/C and Pd/Al2O3: A consideration of HCl/catalyst interactions and solution pH effects. Appl. Catal. B Environ. 2004, 52, 301–314. [Google Scholar] [CrossRef]
- Yuan, G.; Keane, M.A. Catalyst deactivation during the liquid phase hydrodechlorination of 2,4-dichlorophenol over supported Pd: Influence of the support. Catal. Today 2003, 88, 27–36. [Google Scholar] [CrossRef]
- Yuan, G.; Keane, M.A. Role of base addition in the liquid-phase hydrodechlorination of 2,4-dichlorophenol over Pd/Al2O3 and Pd/C. J. Catal. 2004, 225, 510–522. [Google Scholar] [CrossRef]
- Xiong, J.; Ma, Y.; Yang, W.; Zhong, L. Rapid, highly efficient and stable catalytic hydrodechlorination of chlorophenols over novel Pd/CNTs-Ni foam composite catalyst in continuous-flow. J. Hazard. Mater. 2018, 355, 89–95. [Google Scholar] [CrossRef]
- Ding, X.; Yao, Z.; Xu, Y.; Liu, B.; Liu, Q.; She, Y. Aqueous-phase hydrodechlorination of 4-chlorophenol on palladium nanocrystals: Identifying the catalytic sites and unraveling the reaction mechanism. J. Catal. 2018, 368, 336–344. [Google Scholar] [CrossRef]
- Jadbabaei, N.; Ye, T.; Shuai, D.; Zhang, H. Development of palladium-resin composites for catalytic hydrodechlorination of 4-chlorophenol. Appl. Catal. B Environ. 2017, 205, 576–586. [Google Scholar] [CrossRef]
- Molina, C.B.; Pizarro, A.H.; Casas, J.A.; Rodriguez, J.J. Aqueous-phase hydrodechlorination of chlorophenols with pillared clays-supported Pt, Pd and Rh catalysts. Appl. Catal. B Environ. 2014, 148–149, 330–338. [Google Scholar] [CrossRef]
- Fan, M.; Long, Y.; Zhu, Y.; Hu, X.; Dong, Z. Two-dimensional covalent-organic-framework-derived nitroen-rich carbon nanosheets modified with small Pd nanoparticles for the hydrodechlorination of chlorophenols and hydrogenation of phenol. Appl. Catal. A Gen. 2018, 568, 130–138. [Google Scholar] [CrossRef]
- Deng, H.; Fan, G.; Wang, C.; Zhang, L. Aqueous phase catalytic hydrodechlorination of 4-chlorophenol over palladium deposited on reduced graphene oxide. Catal. Commun. 2014, 46, 219–223. [Google Scholar] [CrossRef]
- Choi, M.; Kleitz, F.; Liu, D.; Lee, H.Y.; Ahn, W.-S.; Ryoo, R. Controlled Polymerization in Mesoporous Silica toward the Design of Organic−Inorganic Composite Nanoporous Materials. J. Am. Chem. Soc. 2005, 127, 1924–1932. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.G.; Wang, H.; Li, J.P.; Zhao, N.; Wei, W.; Sun, Y.H. A facile route to synthesize mesoporous zirconia with ultra high thermal stability. Mater. Res. Bull. 2007, 42, 171–176. [Google Scholar] [CrossRef]
- Li, R.; Yu, F.; Li, F.; Zhou, M.; Xu, B.; Xie, K. One-pot synthesis of superacid catalytic material SO42−/ZrO2−SiO2 with thermostable well-ordered mesoporous structure. J. Solid State Chem. 2009, 182, 991–994. [Google Scholar] [CrossRef]
- Bacani, R.; Martins, T.S.; Fantini, M.C.A.; Lamas, D.G. Structural studies of mesoporous ZrO2−CeO2 and ZrO2−CeO2/SiO2 mixed oxides for catalytical applications. J. Alloy. Compd. 2016, 671, 396–402. [Google Scholar] [CrossRef]
- Chen, G.; Lei, T.; Wang, Z.; Liu, S.; He, X.; Guan, Q.; Xin, X.; Xu, H. Preparation of higher alcohols by biomass-based syngas from wheat straw over CoCuK/ZrO2−SiO2 catalyst. Ind. Crop. Prod. 2019, 131, 54–61. [Google Scholar] [CrossRef]
- Liu, W.; Li, L.; Zhang, X.; Wang, Z.; Wang, X.; Peng, H. Design of Ni-ZrO2@SiO2 catalyst with ultra-high sintering and coking resistance for dry reforming of methane to prepare syngas. J. CO2 Util. 2018, 27, 297–307. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Sharma, M.; Jain, P.; Mishra, A.; Mehta, A.; Choudhury, D.; Hazra, S.; Basu, S. Variation of surface area of silica monoliths by controlling ionic character/chain length of surfactants and polymers. Mater. Lett. 2017, 194, 213–216. [Google Scholar] [CrossRef]
- Christy, E.J.S.; Alagar, R.; Anitha Pius, D.M. Porous nonhierarchical CeO2/SiO2 monolith for effective degradation of organic pollutants. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100365. [Google Scholar] [CrossRef]
- Valenzuela, M.A.; Bosch, P.; Jiménez-Becerrill, J.; Quiroz, O.; Páez, A.I. Preparation, characterization and photocatalytic activity of ZnO, Fe2O3 and ZnFe2O4. J. Photochem. Photobiol. A Chem. 2002, 148, 177–182. [Google Scholar] [CrossRef]
- Armendariz, H.; Aguilar-Rios, G.; Salas, P.; Valenzuela, M.A.; Schifter, I.; Arriola, H.; Nava, N. Oxidative dehydrogenation of n-butane on iron-zinc oxide catalysts. Appl. Catal. A Gen. 1992, 92, 29–38. [Google Scholar] [CrossRef]
- Sepúlveda, J.H.; Fígoli, N.S. The influence of calcination temperature on Pd dispersion and hydrogen solubility in Pd/SiO2. Appl. Surf. Sci. 1993, 68, 257–264. [Google Scholar] [CrossRef]
- Boudart, M.; Hwang, H.S. Solubility of hydrogen in small particles of palladium. J. Catal. 1975, 39, 44–52. [Google Scholar] [CrossRef]
- Matam, S.K.; Otal, E.H.; Aguirre, M.H.; Winkler, A.; Ulrich, A.; Rentsch, D.; Weidenkaff, A.; Ferri, D. Thermal and chemical aging of model three-way catalyst Pd/Al2O3 and its impact on the conversion of CNG vehicle exhaust. Catal. Today 2012, 184, 237–244. [Google Scholar] [CrossRef]
- Niu, L.; Liu, X.; Liu, X.; Lv, Z.; Zhang, C.; Wen, X.; Yang, Y.; Li, Y.; Xu, J. In Situ XRD Study on Promotional Effect of Potassium on Carburization of Spray-dried Precipitated Fe2O3 Catalysts. ChemCatChem 2017, 9, 1691–1700. [Google Scholar] [CrossRef]
- Wendel, J.; Manchili, S.K.; Hryha, E.; Nyborg, L. Reduction of surface oxide layers on water-atomized iron and steel powder in hydrogen: Effect of alloying elements and initial powder state. Thermochim. Acta 2020, 692, 178731. [Google Scholar] [CrossRef]
- Wan, H.-J.; Wu, B.-S.; Zhang, C.-H.; Xiang, H.-W.; Li, Y.-W.; Xu, B.-F.; Yi, F. Study on Fe–Al2O3 interaction over precipitated iron catalyst for Fischer–Tropsch synthesis. Catal. Commun. 2007, 8, 1538–1545. [Google Scholar] [CrossRef]
- Nielsen, M.R.; Moss, A.B.; Bjørnlund, A.S.; Liu, X.; Knop-Gericke, A.; Klyushin, A.Y.; Grunwaldt, J.-D.; Sheppard, T.L.; Doronkin, D.E.; Zimina, A.; et al. Reduction and carburization of iron oxides for Fischer–Tropsch synthesis. J. Energy Chem. 2020, 51, 48–61. [Google Scholar] [CrossRef]
- Urasaki, K.; Tanimoto, N.; Hayashi, T.; Sekine, Y.; Kikuchi, E.; Matsukata, M. Hydrogen production via steam–iron reaction using iron oxide modified with very small amounts of palladium and zirconia. Appl. Catal. A Gen. 2005, 288, 143–148. [Google Scholar] [CrossRef]
- Narui, K.; Furuta, K.; Yata, H.; Nishida, A.; Kohtoku, Y.; Matsuzaki, T. Catalytic activity of PdO/ZrO2 catalyst for methane combustion. Catal. Today 1998, 45, 173–178. [Google Scholar] [CrossRef]
- Shi, C.-K.; Yang, L.-F.; Wang, Z.-C.; He, X.-E.; Cai, J.-X.; Li, G.; Wang, X.-S. Promotion effects of ZrO2 on the Pd/HZSM-5 catalyst for low-temperature catalytic combustion of methane. Appl. Catal. A Gen. 2003, 243, 379–388. [Google Scholar] [CrossRef]
- Hadjiivanov, K.I.; Vayssilov, G.N. Characterization of oxide surfaces and zeolites by carbon monoxide as an IR probe molecule. In Advances in Catalysis; Academic Press: Cambridge, MA, USA, 2002; Volume 47, pp. 307–511. [Google Scholar]
- Bertarione, S.; Scarano, D.; Zecchina, A.; Johánek, V.; Hoffmann, J.; Schauermann, S.; Frank, M.M.; Libuda, J.; Rupprechter, G.; Freund, H.-J. Surface Reactivity of Pd Nanoparticles Supported on Polycrystalline Substrates as Compared to Thin Film Model Catalysts: Infrared Study of CO Adsorption. J. Phys. Chem. B 2004, 108, 3603–3613. [Google Scholar] [CrossRef]
- Lear, T.; Marshall, R.; Antonio Lopez-Sanchez, J.; Jackson, S.D.; Klapötke, T.M.; Bäumer, M.; Rupprechter, G.; Freund, H.-J.; Lennon, D. The application of infrared spectroscopy to probe the surface morphology of alumina-supported palladium catalysts. J. Chem. Phys. 2005, 123, 174706. [Google Scholar] [CrossRef]
- Binet, C.; Jadi, A.; Lavalley, J.-C. Évaluation de l’importance relative des faces cristallographiques du palladium supporté sur alumine: Étude infrarouge à l’aide de la molécule sonde CO. J. Chim. Phys. 1989, 86, 451–470. [Google Scholar] [CrossRef]
- Davydov, A. Molecular Spectroscopy of Oxide Catalyst Surfaces; Sheppard, N.T., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2003; p. 684. [Google Scholar] [CrossRef]
- Angell, C.L.; Schaffer, P.C. Infrared Spectroscopic Investigations of Zeolites and Adsorbed Molecules. II. Adsorbed Carbon Monoxide1. J. Phys. Chem. 1966, 70, 1413–1418. [Google Scholar] [CrossRef]
- Ballivet-Tkatchenko, D.; Coudurier, G. Adduct formation and further reactivity of iron carbonyl complexes introduced into a zeolite matrix. Inorg. Chem. 1979, 18, 558–564. [Google Scholar] [CrossRef]
- Couble, J.; Bianchi, D. Heats of adsorption of linear CO species adsorbed on reduced Fe/Al2O3 catalysts using the AEIR method in diffuse reflectance mode. Appl. Catal. A Gen. 2011, 409–410, 28–38. [Google Scholar] [CrossRef]
- Fellah, M.F. CO and NO Adsorptions on Different Iron Sites of Fe-ZSM-5 Clusters: A Density Functional Theory Study. J. Phys. Chem. C 2011, 115, 1940–1951. [Google Scholar] [CrossRef]
- Mihaylov, M.; Ivanova, E.; Chakarova, K.; Novachka, P.; Hadjiivanov, K. Reduced iron sites in Fe–BEA and Fe–ZSM-5 zeolites: FTIR study of CO adsorption and 12C16O–13C18O co-adsorption. Appl. Catal. A Gen. 2011, 391, 3–10. [Google Scholar] [CrossRef]
- Wielers, A.F.H.; Kock, A.J.H.M.; Hop, C.E.C.A.; Geus, J.W.; van Der Kraan, A.M. The reduction behavior of silica-supported and alumina-supported iron catalysts: A Mössbauer and infrared spectroscopic study. J. Catal. 1989, 117, 1–18. [Google Scholar] [CrossRef]
- Lingaiah, N.; Sai Prasad, P.S.; Kanta Rao, P.; Berry, F.J.; Smart, L.E. Structure and activity of microwave irradiated silica supported Pd–Fe bimetallic catalysts in the hydrodechlorination of chlorobenzene. Catal. Commun. 2002, 3, 391–397. [Google Scholar] [CrossRef]
- Lieltz, G.; Nimz, M.; Völter, J.; Läzär, K.; Guczi, L. Double promotion of palladium/silica catalysts by iron and magnesium oxide in the synthesis of methanol from carbon monoxide and hydrogen. Appl. Catal. 1988, 45, 71–83. [Google Scholar] [CrossRef]
- Karski, S.; Witońska, I.; Rogowski, J.; Gołuchowska, J. Interaction between Pd and Ag on the surface of silica. J. Mol. Catal. A Chem. 2005, 240, 155–163. [Google Scholar] [CrossRef]
- Witonska, I.; Krolak, A. Catalytic hydrodechlorination of 2,4-dichlorophenol over Pd/SiO2 and Pd-Bi/SiO2 systems. Effect of the chemical nature of silica. Przem. Chem. 2011, 90, 636–640. [Google Scholar]
- Gómez-Quero, S.; Cárdenas-Lizana, F.; Keane, M.A. Effect of Metal Dispersion on the Liquid-Phase Hydrodechlorination of 2,4-Dichlorophenol over Pd/Al2O3. Ind. Eng. Chem. Res. 2008, 47, 6841–6853. [Google Scholar] [CrossRef]
- Xia, C.; Liu, Y.; Zhou, S.; Yang, C.; Liu, S.; Xu, J.; Yu, J.; Chen, J.; Liang, X. The Pd-catalyzed hydrodechlorination of chlorophenols in aqueous solutions under mild conditions: A promising approach to practical use in wastewater. J. Hazard. Mater. 2009, 169, 1029–1033. [Google Scholar] [CrossRef]
- Zinovyev, S.; Shelepchikov, A.; Tundo, P. Design of new systems for transfer hydrogenolysis of polychlorinated aromatics with 2-propanol using a Raney nickel catalyst. Appl. Catal. B Environ. 2007, 72, 289–298. [Google Scholar] [CrossRef]
- Ukisu, Y.; Kameoka, S.; Miyadera, T. Catalytic dechlorination of aromatic chlorides with noble-metal catalysts under mild conditions: Approach to practical use. Appl. Catal. B Environ. 2000, 27, 97–104. [Google Scholar] [CrossRef]
- Ukisu, Y.; Iimura, S.; Uchida, R. Catalytic dechlorination of polychlorinated biphenyls with carbon-supported noble metal catalysts under mild conditions. Chemosphere 1996, 33, 1523–1530. [Google Scholar] [CrossRef]
- Ukisu, Y.; Miyadera, T. Hydrogen-transfer hydrodehalogenation of aromatic halides with alcohols in the presence of noble metal catalysts. J. Mol. Catal. A Chem. 1997, 125, 135–142. [Google Scholar] [CrossRef]
- Li, C.; Hoffman, M.Z. One-Electron Redox Potentials of Phenols in Aqueous Solution. J. Phys. Chem. B 1999, 103, 6653–6656. [Google Scholar] [CrossRef]
- Pavitt, A.S.; Bylaska, E.J.; Tratnyek, P.G. Oxidation potentials of phenols and anilines: Correlation analysis of electrochemical and theoretical values. Environ. Sci. Process. Impacts 2017, 19, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Tanwongwan, W.; Eiad-ua, A.; Kraithong, W.; Viriya-empikul, N.; Suttisintong, K.; Klamchuen, A.; Kasamechonchung, P.; Khemthong, P.; Faungnawakij, K.; Kuboon, S. Simultaneous activation of copper mixed metal oxide catalysts in alcohols for gamma-valerolactone production from methyl levulinate. Appl. Catal. A Gen. 2019, 579, 91–98. [Google Scholar] [CrossRef]
- Kulkarni, D.; Wachs, I.E. Isopropanol oxidation by pure metal oxide catalysts: Number of active surface sites and turnover frequencies. Appl. Catal. A Gen. 2002, 237, 121–137. [Google Scholar] [CrossRef]
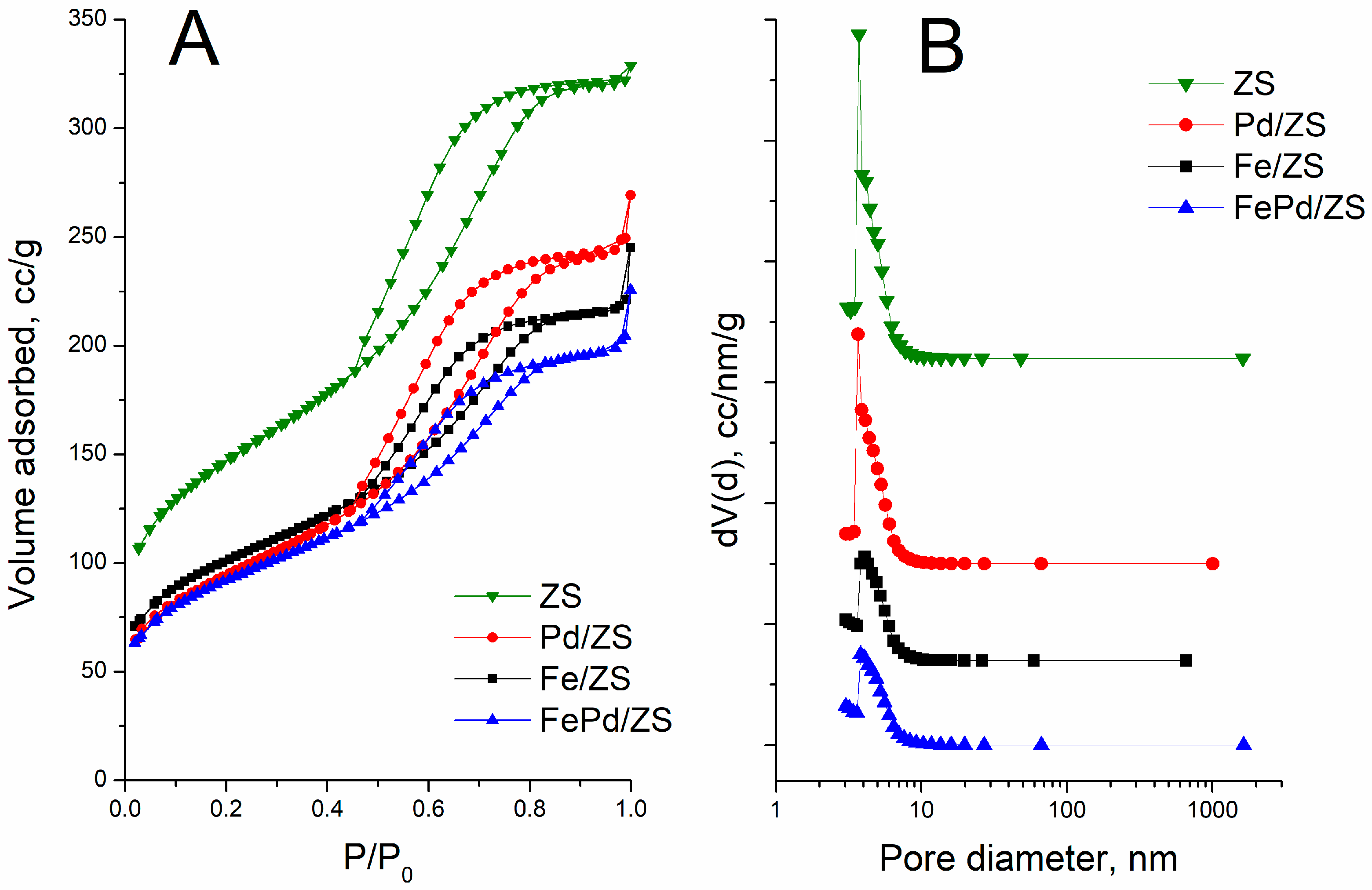
| Sample | SBET (m2/g) | Vpore (cm3/g) | Average Pore Width (nm) | |||
|---|---|---|---|---|---|---|
| Fresh | After Catalytic Test | Fresh | After Catalytic Test | Fresh | After Catalytic Test | |
| Pd/ZS | 339 ± 34 | N/D * | 0.374 | N/D | 3.7 | N/D |
| Fe/ZS | 331 ± 33 | 320 ± 32 | 0.266 | 0.297 | 4.1 | 4–5 |
| FePd/ZS | 364 ± 36 | 300 ± 30 | 0.292 | 0.303 | 4.1 | 4–5 |
| ZS | 529 ± 53 | - | 0.397 | - | 3.7 | - |
| Catalyst | Metal Content, wt% (AAS Data) | Composition, at % (SEM-EDS Data) | |||||
|---|---|---|---|---|---|---|---|
| Pd | Fe | O | Si | Zr | Pd | Fe | |
| Pd/ZS | 0.86 ± 0.02 | – | 79.3 | 18.9 | 1.7 | 0.1 | – |
| Fe/ZS | – | 9.6 ± 0.2 | 80.1 | 15.6 | 1.6 | – | 2.7 |
| FePd/ZS | 0.89 ± 0.01 | 8.2 ± 0.4 | 77.9 | 18.1 | 1.7 | 0.1 | 2.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lokteva, E.S.; Shishova, V.V.; Tolkachev, N.N.; Kharlanov, A.N.; Maslakov, K.I.; Kamaev, A.O.; Kaplin, I.Y.; Savina, I.N.; Golubina, E.V. Hydrodechlorination of 4-Chlorophenol on Pd-Fe Catalysts on Mesoporous ZrO2SiO2 Support. Molecules 2021, 26, 141. https://doi.org/10.3390/molecules26010141
Lokteva ES, Shishova VV, Tolkachev NN, Kharlanov AN, Maslakov KI, Kamaev AO, Kaplin IY, Savina IN, Golubina EV. Hydrodechlorination of 4-Chlorophenol on Pd-Fe Catalysts on Mesoporous ZrO2SiO2 Support. Molecules. 2021; 26(1):141. https://doi.org/10.3390/molecules26010141
Chicago/Turabian StyleLokteva, Ekaterina S., Vera V. Shishova, Nikolay N. Tolkachev, Andrey N. Kharlanov, Konstantin I. Maslakov, Alexey O. Kamaev, Igor Yu. Kaplin, Irina N. Savina, and Elena V. Golubina. 2021. "Hydrodechlorination of 4-Chlorophenol on Pd-Fe Catalysts on Mesoporous ZrO2SiO2 Support" Molecules 26, no. 1: 141. https://doi.org/10.3390/molecules26010141
APA StyleLokteva, E. S., Shishova, V. V., Tolkachev, N. N., Kharlanov, A. N., Maslakov, K. I., Kamaev, A. O., Kaplin, I. Y., Savina, I. N., & Golubina, E. V. (2021). Hydrodechlorination of 4-Chlorophenol on Pd-Fe Catalysts on Mesoporous ZrO2SiO2 Support. Molecules, 26(1), 141. https://doi.org/10.3390/molecules26010141