Comparative Study of Phosgene Gas Sensing Using Carbon and Boron Nitride Nanomaterials—A DFT Approach
Abstract
1. Introduction
2. Computational Methods
Adsorption Model
3. Results and Discussion
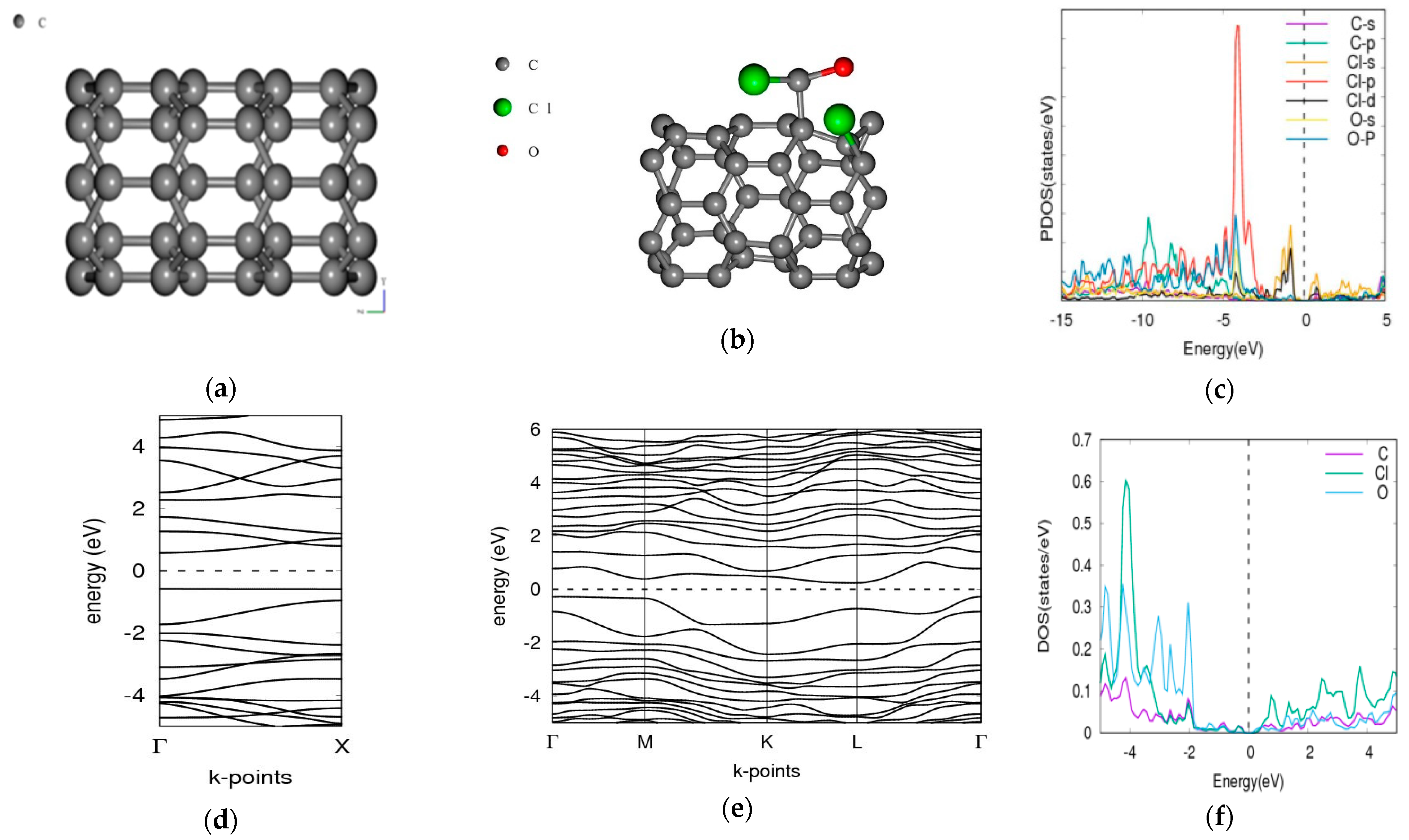
3.1. Phosgene on [5,0] CNT
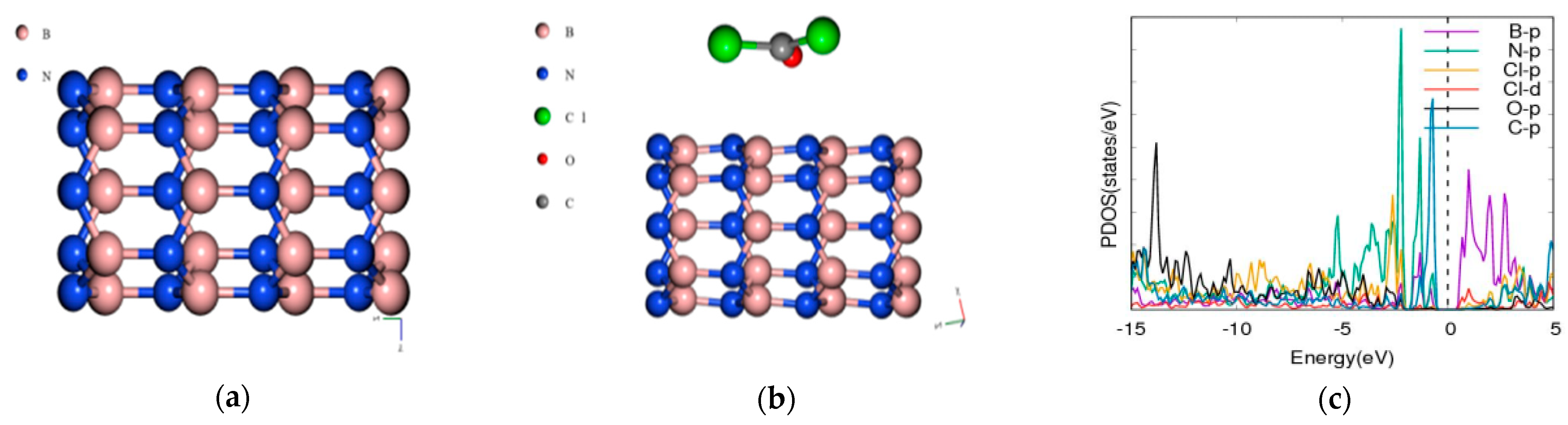
3.2. Phosgene on [5,0] BNNT
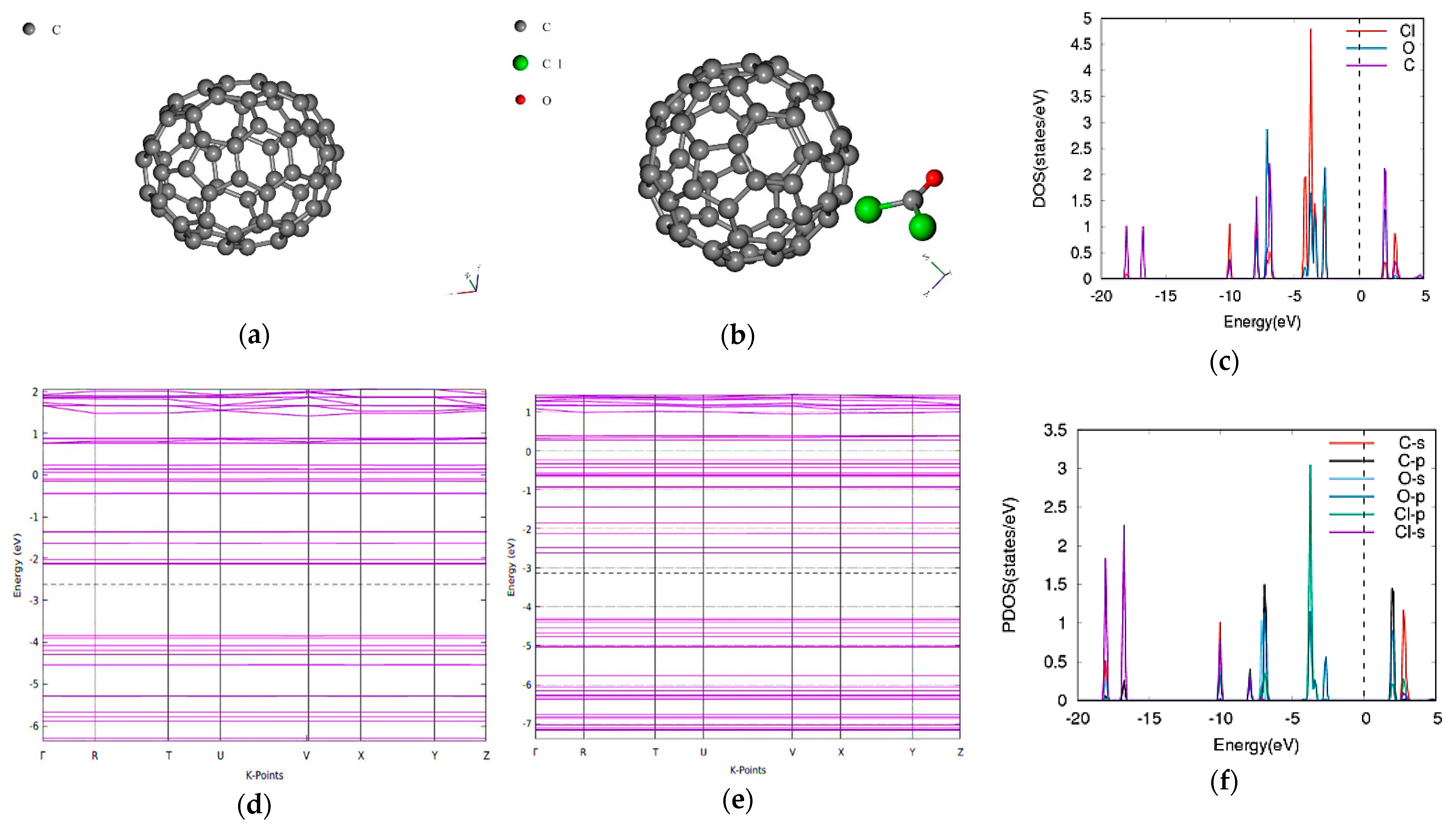
3.3. Phosgene on C70
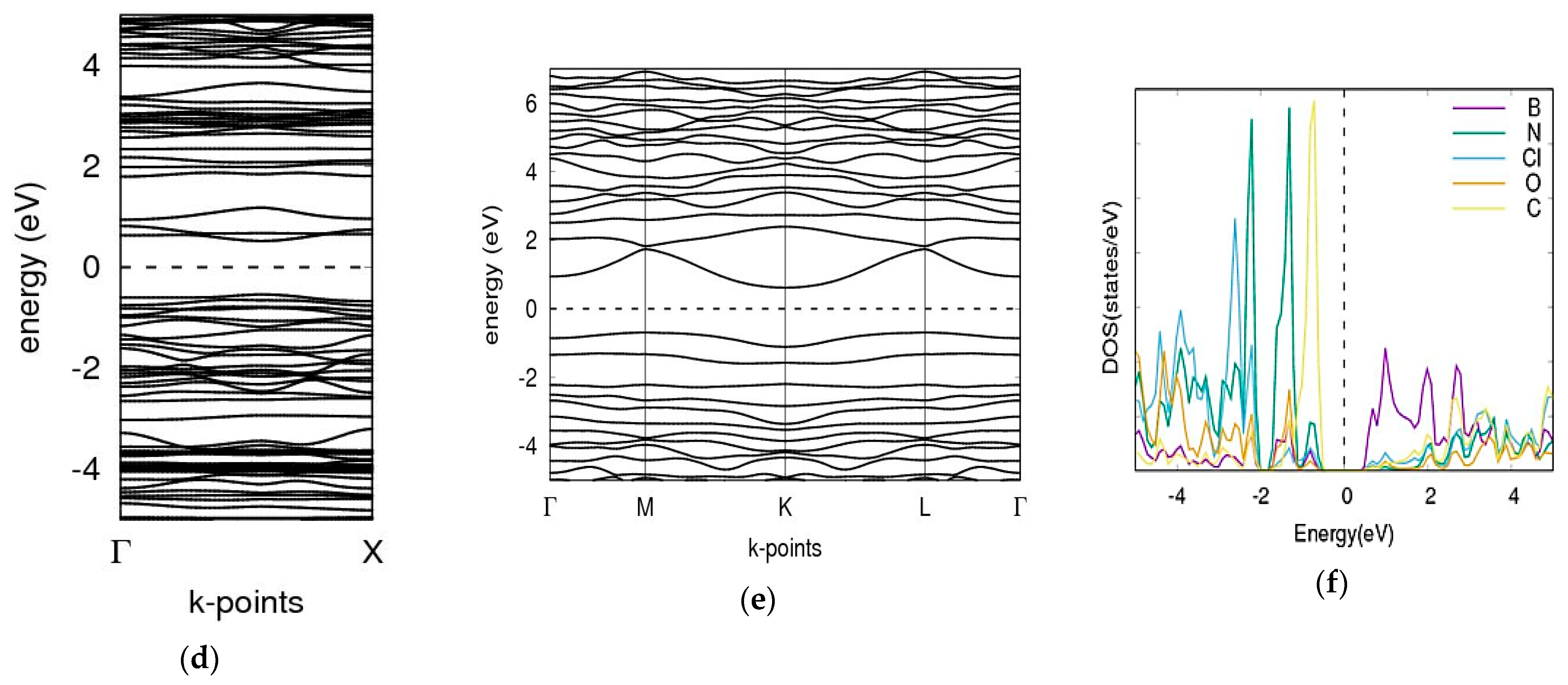
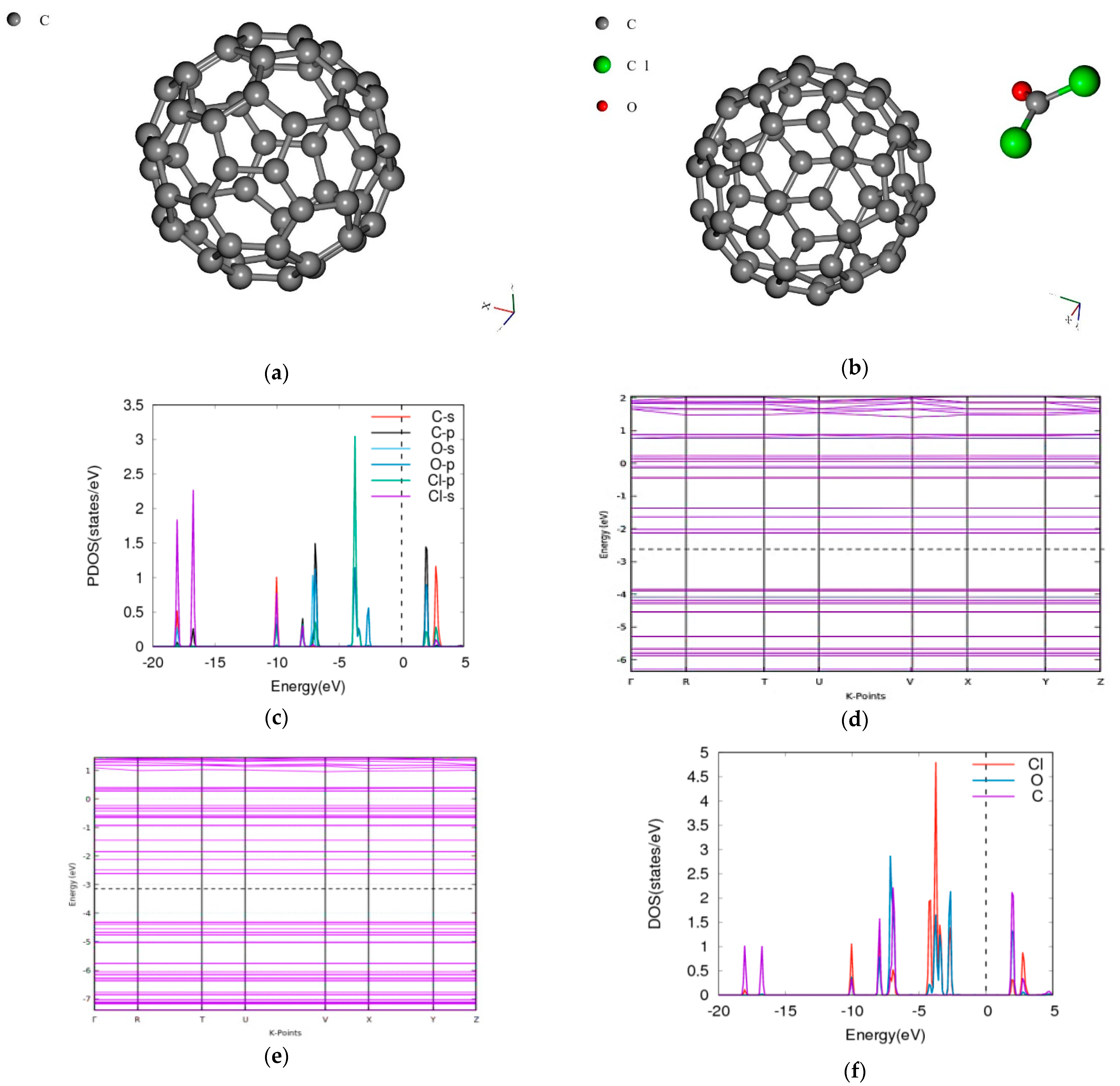
3.4. Phosgene on C60
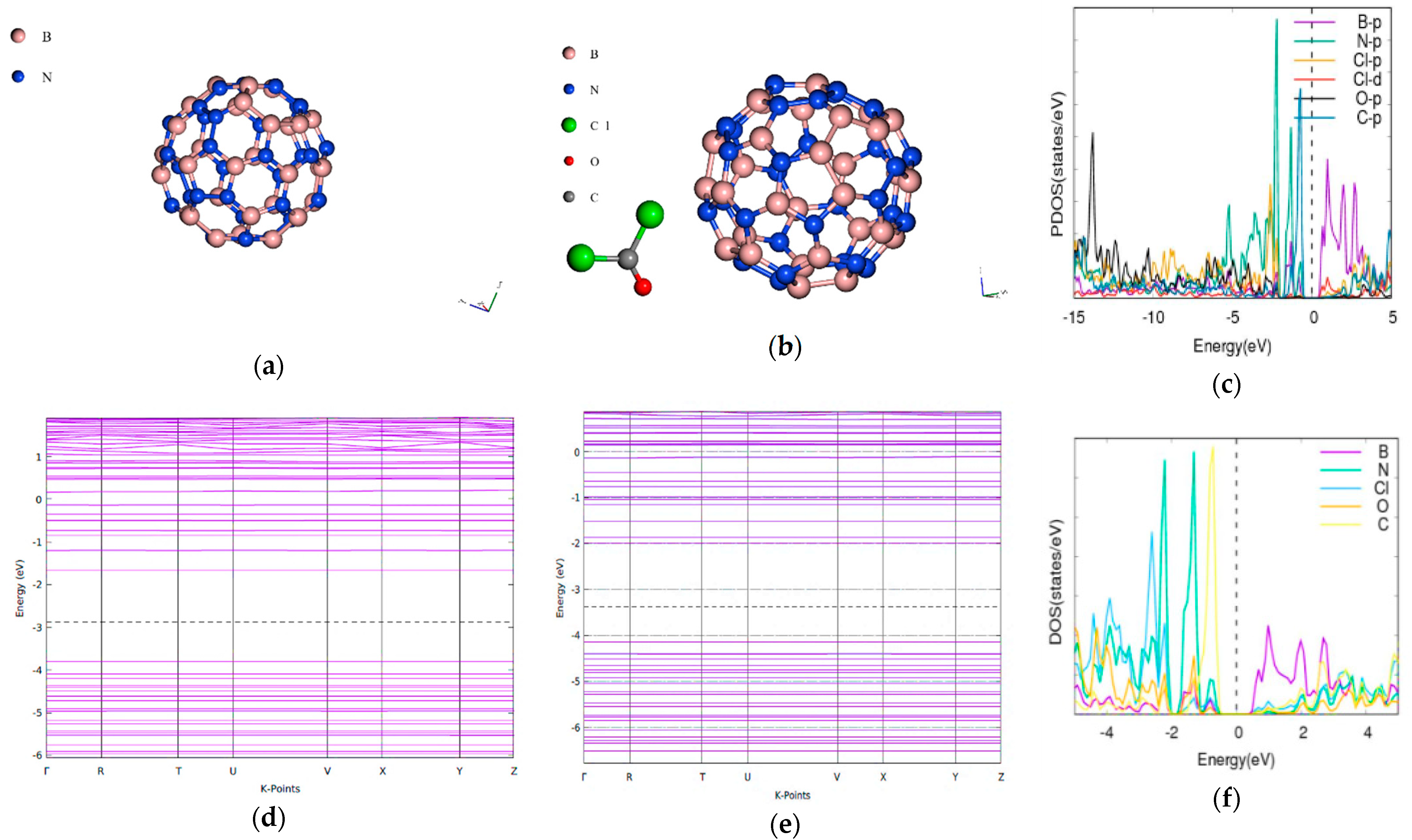
3.5. Phosgene on BN60
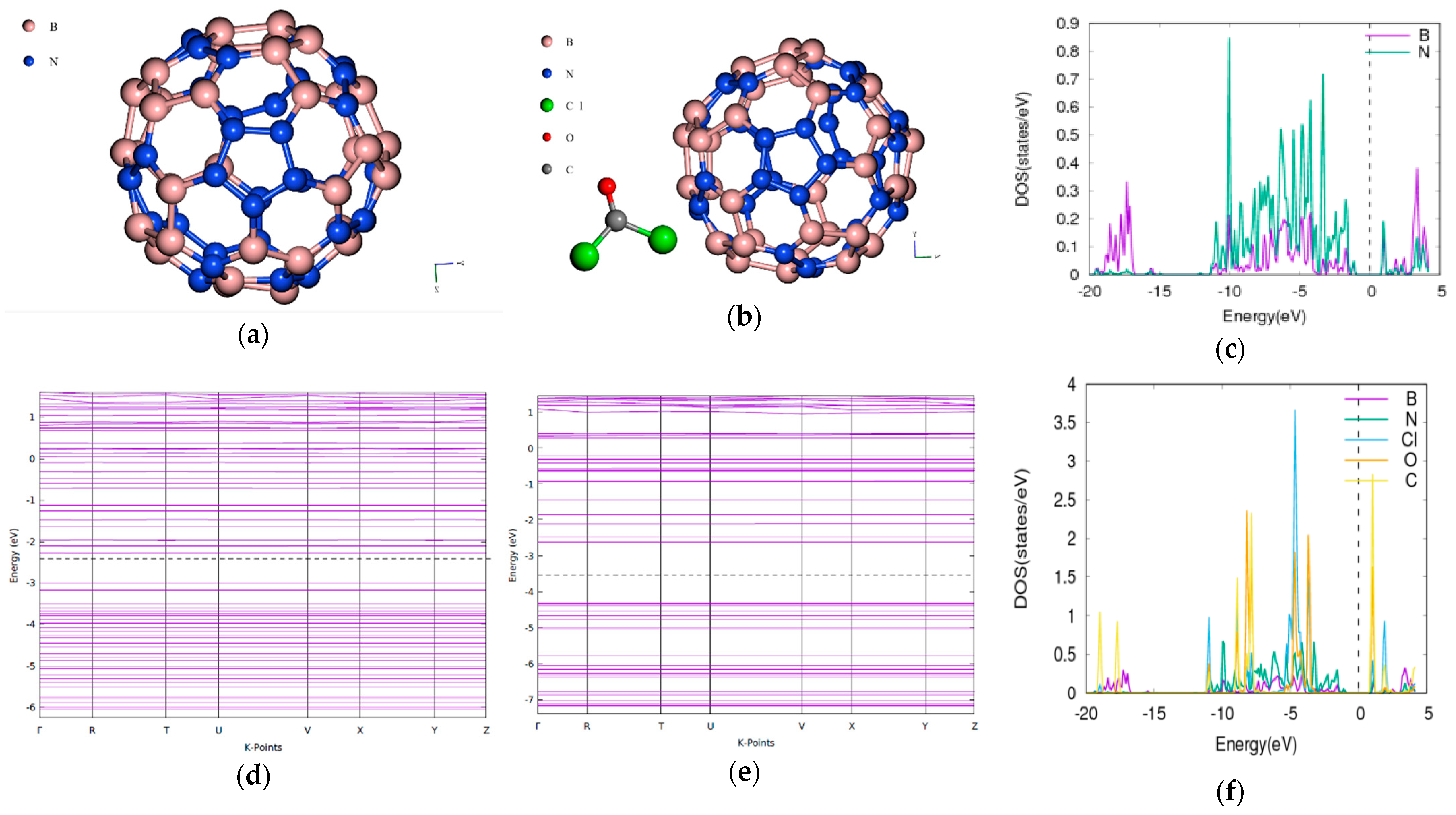
3.6. Phosgene on BN70
4. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gad, S. Phosgene. Encycl. Toxicol. 2014, 3, 904–906. [Google Scholar] [CrossRef]
- Virji, S.; Kojima, R.; Fowler, J.D.; Villanueva, J.G.; Kaner, R.B.; Weiller, B.H. Polyaniline nanofiber composites with amines: Novel materials for phosgene detection. Nano Res. 2009, 2, 135–142. [Google Scholar] [CrossRef]
- Greim, H.; Snyder, R. Toxicology and Risk Assessment: A Comprehensive Introduction; John Wiley & Sons, Ltd.: Chichester, West Sussex, UK, 2008. [Google Scholar] [CrossRef]
- Zhang, H.; Rudkevich, D.M. A FRET approach to phosgene detection. Chem. Commun. 2007, 1238–1239. [Google Scholar] [CrossRef] [PubMed]
- Frye-Mason, G.; Leuschen, M.L.; Wald, L.; Paul, K.; Hancock, L.F. Reactive chromophores for sensitive and selective detection of chemical warfare agents and toxic industrial chemicals. In Proceedings of the Sensors, and Command, Control, Communications, and Intelligence (C3I) Technologies for Homeland Security and Homeland Defense IV, Orlando, FL, USA, 28 March–1 April 2005; Volume 5778, pp. 337–347. [Google Scholar] [CrossRef]
- Nakano, N.; Yamamoto, A.; Kobayashi, Y.; Nagashima, K. Development of a monitoring tape for phosgene in air. Talanta 1995, 42, 641–645. [Google Scholar] [CrossRef]
- Hill, H.H.; Martin, S.J. Conventional analytical methods for chemical warfare agents. Pure Appl. Chem. 2002, 74, 2281–2291. [Google Scholar] [CrossRef]
- Henderson, T.J.; Cullinan, D.B. Purity analysis of hydrogen cyanide, cyanogen chloride and phosgene by quantitative 13C NMR spectroscopy. Magn. Reson. Chem 2007, 45, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Muir, B.; Cooper, D.B.; Carrick, W.A.; Timperley, C.M.; Slater, B.J.; Quick, S. Analysis of chemical warfare agents III. Use of bis-nucleophiles in the trace level determination of phosgene and perfluoroisobutylene. J. Chromatogr. A 2005, 1098, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Keil, A.; Hernandez-Soto, H.; Noll, R.J.; Fico, M.; Gao, L.; Ouyang, A.Z.; Cooks, R.G. Monitoring of Toxic Compounds in Air Using a Handheld Rectilinear Ion Trap Mass Spectrometer. Anal. Chem. 2008, 80, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Beheshtian, J.; Peyghan, A.A.; Bagheri, Z. Detection of phosgene by Sc-doped BN nanotubes: A DFT study. Sens. Actuators B Chem. 2012, 171–172, 846–852. [Google Scholar] [CrossRef]
- Felegari, Z.; Hamedani, S. Adsorption properties of the phosgene molecule on pristine graphyne, BN- and Si-doped graphynes: DFT study. Results Phys. 2017, 7, 2626–2631. [Google Scholar] [CrossRef]
- Moladoust, R.; Esrafili, M.D.; Hosseinian, A.; Alkorta, I.; Vessally, E. Adsorption sensitivity of pristine and Al- or Si-doped boron nitride nanoflake to COCl2: A DFT study. Mol. Phys. 2018, 117, 626–634. [Google Scholar] [CrossRef]
- Mella, A.; Cortés-Arriagada, D. Computational quest of adsorbents based on doped graphene nanosheets for phosgene uptake, and analysis of the co-adsorption phenomena. Synth. Met. 2019, 252, 142–150. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, H.; Wang, F.; Zhang, W.; Tang, S.; Ma, J.; Gong, H.; Zhang, J. Applied Surface Science Adsorption of phosgene molecule on the transition metal-doped graphene: First principles calculations. Appl. Surf. Sci. 2017, 425, 340–350. [Google Scholar] [CrossRef]
- Hosseinian, A.; Salary, M.; Arshadi, S.; Vessally, E.; Edjlali, L. The interaction of phosgene gas with different BN nanocones: DFT studies. Solid State Commun. 2018, 269, 23–27. [Google Scholar] [CrossRef]
- Chauhan, P.; Sharma, S. Nanomaterials for sensing applications. J. Nanomed. Res. 2016, 3, 1–8. [Google Scholar] [CrossRef][Green Version]
- Rad, A.S.; Ayub, K. A comparative density functional theory study of guanine chemisorption on Al12N12, Al12P12, B12N12, and B12P12 nano-cages. J. Alloys Compd. 2016, 672, 161–169. [Google Scholar] [CrossRef]
- Mahdavifar, Z.; Abbasi, N.; Shakerzadeh, E. A comparative theoretical study of CO2 sensing using inorganic AlN, BN and SiC single walled nanotubes. Sens. Actuators B Chem. 2013, 185, 512–522. [Google Scholar] [CrossRef]
- Wirtz, L.; Rubio, A.; De La Concha, R.A.; Loiseau, A. Ab initiocalculations of the lattice dynamics of boron nitride nanotubes. Phys. Rev. B Condens. Matter Mater. Phys. 2003, 68, 1. [Google Scholar] [CrossRef]
- Blase, X.; Rubio, A.; Louie, S.G.; Cohen, M.L. Quasiparticle band structure of bulk hexagonal boron nitride and related systems. Phys. Rev. 1995, 51, 6868–6875. [Google Scholar] [CrossRef]
- Noei, M.; Ahmadaghaei, N.; Salari, A.A. Ethyl benzene detection by BN nanotube: DFT studies. J. Saudi Chem. Soc. 2017, 21, S12–S16. [Google Scholar] [CrossRef]
- Beheshtian, J.; Baei, M.T.; Bagheri, Z.; Peyghan, A.A. AlN nanotube as a potential electronic sensor for nitrogen dioxide. Microelectron. J. 2012, 43, 452–455. [Google Scholar] [CrossRef]
- Elloh, V.; Mishra, A.K.; Dodoo-Arhin, D.; Abavare, E.K.; Gebreyesus, G.; Nyankson, E.; Efavi, J.; Onwona-Agyeman, B.; Yaya, A. Structural and Electronic properties of PVK/C60 Nanoheterostructure interfaces-A DFT Approach. Surf. Interfaces 2020, 20, 100556. [Google Scholar] [CrossRef]
- Liu, C.; Cheng, H.-M. Carbon nanotubes: Controlled growth and application. Mater. Today 2013, 16, 19–28. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effect. Phys. Rev. 1965, 140, A1133. [Google Scholar] [CrossRef]
- Kohn, W. Nobel Lecture: Electronic structure of matter—Wave functions and density functionals. Rev. Mod. Phys. 1999, 71, 1253–1266. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef]
- Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 1990, 41, 7892–7895. Available online: https://www.materialscloud.org/work/tools/seekpath (accessed on 8 September 2020). [CrossRef]
- Hinuma, Y.; Pizzi, G.; Kumagai, Y.; Oba, F.; Tanaka, I. Band structure diagram paths based on crystallography. Comput. Mater. Sci. 2017, 128, 140–184. [Google Scholar] [CrossRef]
- Kürti, J.; Zólyomi, V. First principles calculations for the electronic band structures of zone folding non-metallic single wall carbon nanotubes. Fourth Huntsville Gamma Ray Burst Symp. 2004, 723, 377–380. [Google Scholar] [CrossRef]
- Rabenau, T.; Simon, A.; Kremer, R.K.; Sohmen, E. The energy gaps of fullerene C60 and C70 determined from the temperature dependent microwave conductivity. Z. Für Phys. B Condens. Matter 1993, 90, 69–72. [Google Scholar] [CrossRef]
- Hazrati, M.K.; Hadipour, N.L. Adsorption behavior of 5-fluorouracil on pristine, B-, Si-, and Al-doped C60 fullerenes: A first-principles study. Phys. Lett. Sect. A Gen. At. Solid State Phys. 2016, 380, 937–941. [Google Scholar] [CrossRef]
- Hansen, P.; Fallon, P.; Krätschmer, W. An EELS study of fullerite—C60/C70. Chem. Phys. Lett. 1991, 181, 367–372. [Google Scholar] [CrossRef]
- Yin, D.; Yang, Y.; Yang, Y.; Fang, H. A novel fullerene-like B30N30 structure: Stability and electronic property. Carbon 2016, 102, 273–278. [Google Scholar] [CrossRef]
- Kayang, K.; Nyankson, E.; Efavi, J.; Abavare, E.; Garu, G.; Onwona-Agyeman, B.; Yaya, A. Single-Walled boron nitride nanotubes interaction with nickel, titanium, palladium, and gold metal atoms- A first-principles study. Results Mater. 2019, 2, 100029. [Google Scholar] [CrossRef]
- Akdim, B.; Kim, S.N.; Naik, R.R.; Maruyama, B.; Pender, M.J.; Pachter, R. Understanding effects of molecular adsorption at a single-wall boron nitride nanotube interface from density functional theory calculations. Nanotechnology 2009, 20, 355705. [Google Scholar] [CrossRef] [PubMed]
- Rostami, Z.; Hosseinian, A.; Monfared, A. DFT results against experimental data for electronic properties of C60 and C70 fullerene derivatives. J. Mol. Graph. Model. 2018, 81, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Yang, W.; Burke, K.; Yang, Z.; Gross, E.K.U.; Scheffler, M.; Scuseria, G.E.; Henderson, T.M.; Zhang, I.Y.; Ruzsinszky, A.; et al. Understanding band gaps of solids in generalized Kohn–Sham theory. Proc. Natl. Acad. Sci. USA 2017, 114, 2801–2806. [Google Scholar] [CrossRef]
- Hybertsen, M.S.; Louie, S.G. Electron correlation in semiconductors and insulators: Band gaps and quasiparticle energies. Phys. Rev. B 1986, 34, 5390–5413. [Google Scholar] [CrossRef]
| Model | Calculated Band Gap eV | Experimentally Determined Band Gap eV | Ab Initio Methods | References |
|---|---|---|---|---|
| [5,0] CNT | 1.90 | - | 2.03 (DFT) 2.32 (TB) | [31] |
| [5,0] BNNT | 3.41 | 5.5 | 2.73–4.6 (DFT) | [20,21,36,37] |
| C60 | 1.63 | 1.86 ± 0.1 (Microwave Absorption Method) 1.8 (EELS) | 1.79–2.76 | [32,34,38] |
| C70 | 1.72 | 1.57 ± 0.1(Microwave Absorption Method) | 1.74–2.69 | [32,38] |
| BN60 | 2.15 | - | 1.48–2.55 | [35] |
| BN70 | 0.73 | - | - |
| Model | Ef (eV) | HOMO (eV) | LUMO (eV) | Eg (eV) | %Δ |
|---|---|---|---|---|---|
| CNT | −0.2695 | –1.2174 | 0.6785 | 1.8959 | - |
| PCNT | 0.0756 | –1.4913 | 1.6424 | 3.1337 | 65.29↑ |
| BNNT | 0.5794 | –1.1297 | 2.2884 | 3.4181 | - |
| PBNNT | 0.1400 | –2.0414 | 2.3213 | 4.3627 | 27.64↑ |
| C60 | –3.4496 | –4.0030 | –2.3672 | 1.6358 | - |
| PC60 | –3.9954 | –4.4322 | –2.8253 | 1.6069 | –1.77↓ |
| C70 | –2.6274 | –3.8487 | –2.1214 | 1.7273 | - |
| PC70 | –3.1385 | –4.3108 | –2.6074 | 1.7033 | –1.39↓ |
| BN60 | –2.8907 | –3.8001 | –1.6547 | 2.1454 | - |
| PBN60 | –3.3846 | –4.1290 | –1.9795 | 2.1495 | 0.19↑ |
| BN70 | –2.4168 | –3.0131 | –2.2857 | 0.7274 | - |
| PBN70 | –3.6105 | –4.3106 | –2.6075 | 1.7031 | 134.12↑ |
| Model | Ead (Ry) | kJ/mol |
|---|---|---|
| CNT | 142.09 | 186,660.95 |
| BNNT | 136.96 | 179,921.26 |
| C60 | –1.93 | –2539.65 |
| C70 | –1.93 | –2538.68 |
| BN60 | –1.94 | –2548.05 |
| BN70 | –1.93 | –2541.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kweitsu, E.O.; Armoo, S.K.; Kan-Dapaah, K.; Abavare, E.K.K.; Dodoo-Arhin, D.; Yaya, A. Comparative Study of Phosgene Gas Sensing Using Carbon and Boron Nitride Nanomaterials—A DFT Approach. Molecules 2021, 26, 120. https://doi.org/10.3390/molecules26010120
Kweitsu EO, Armoo SK, Kan-Dapaah K, Abavare EKK, Dodoo-Arhin D, Yaya A. Comparative Study of Phosgene Gas Sensing Using Carbon and Boron Nitride Nanomaterials—A DFT Approach. Molecules. 2021; 26(1):120. https://doi.org/10.3390/molecules26010120
Chicago/Turabian StyleKweitsu, Emmanuel Obroni, Stephen Kanga Armoo, Kwabena Kan-Dapaah, Eric Kwabena Kyeh Abavare, David Dodoo-Arhin, and Abu Yaya. 2021. "Comparative Study of Phosgene Gas Sensing Using Carbon and Boron Nitride Nanomaterials—A DFT Approach" Molecules 26, no. 1: 120. https://doi.org/10.3390/molecules26010120
APA StyleKweitsu, E. O., Armoo, S. K., Kan-Dapaah, K., Abavare, E. K. K., Dodoo-Arhin, D., & Yaya, A. (2021). Comparative Study of Phosgene Gas Sensing Using Carbon and Boron Nitride Nanomaterials—A DFT Approach. Molecules, 26(1), 120. https://doi.org/10.3390/molecules26010120





