Specific Targeting of PEGylated Liposomal Doxorubicin (Doxil®) to Tumour Cells Using a Novel TIMP3 Peptide
Abstract
1. Introduction
2. Materials and Methods
2.1. H5V, 4T1 and MCF-7 Cell Culture
2.2. Conjugation of p700 Peptide to PEGylated Liposomal Doxorubicin
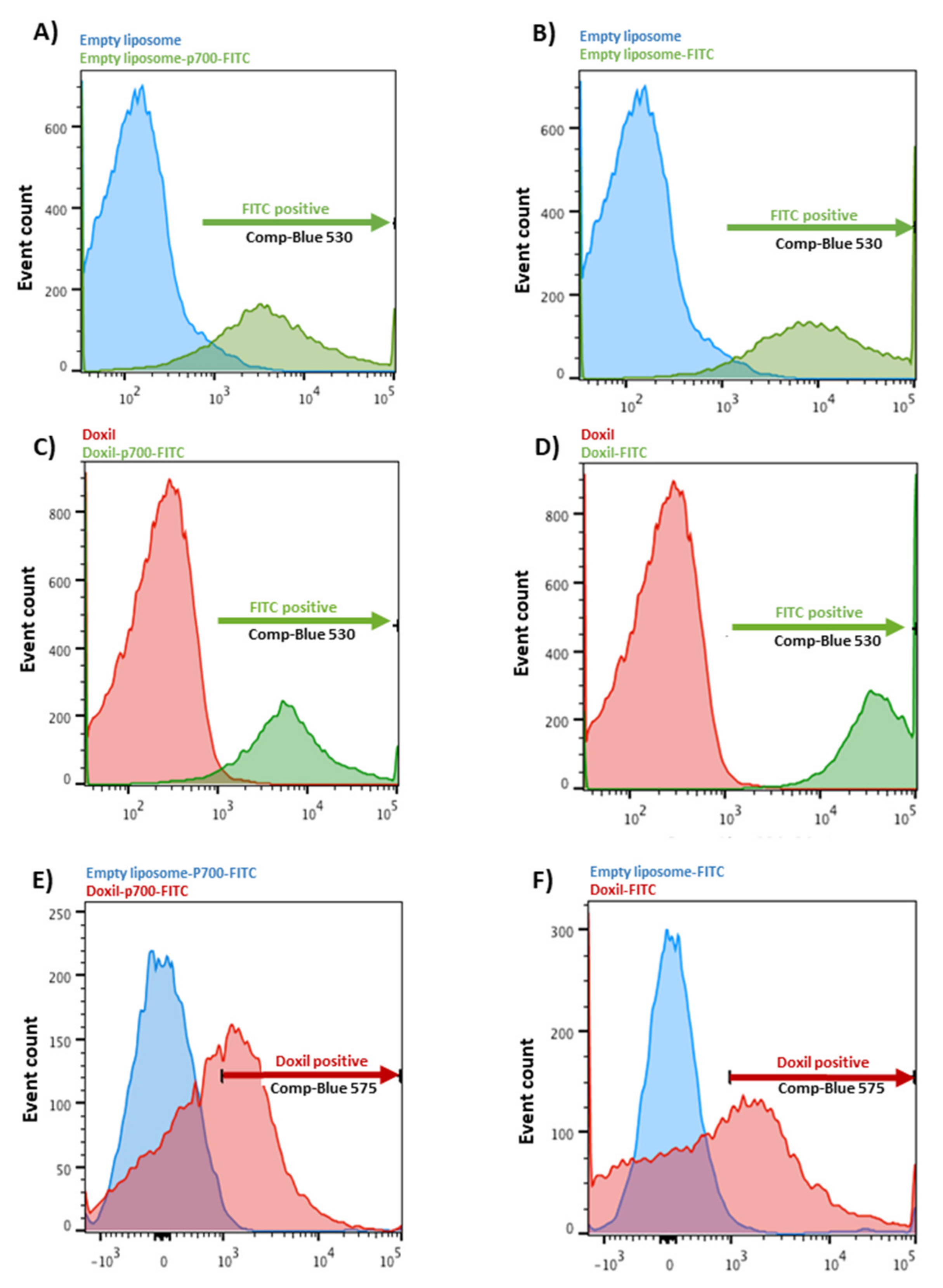
2.3. Confirmation of Coupling by Flow Cytometry
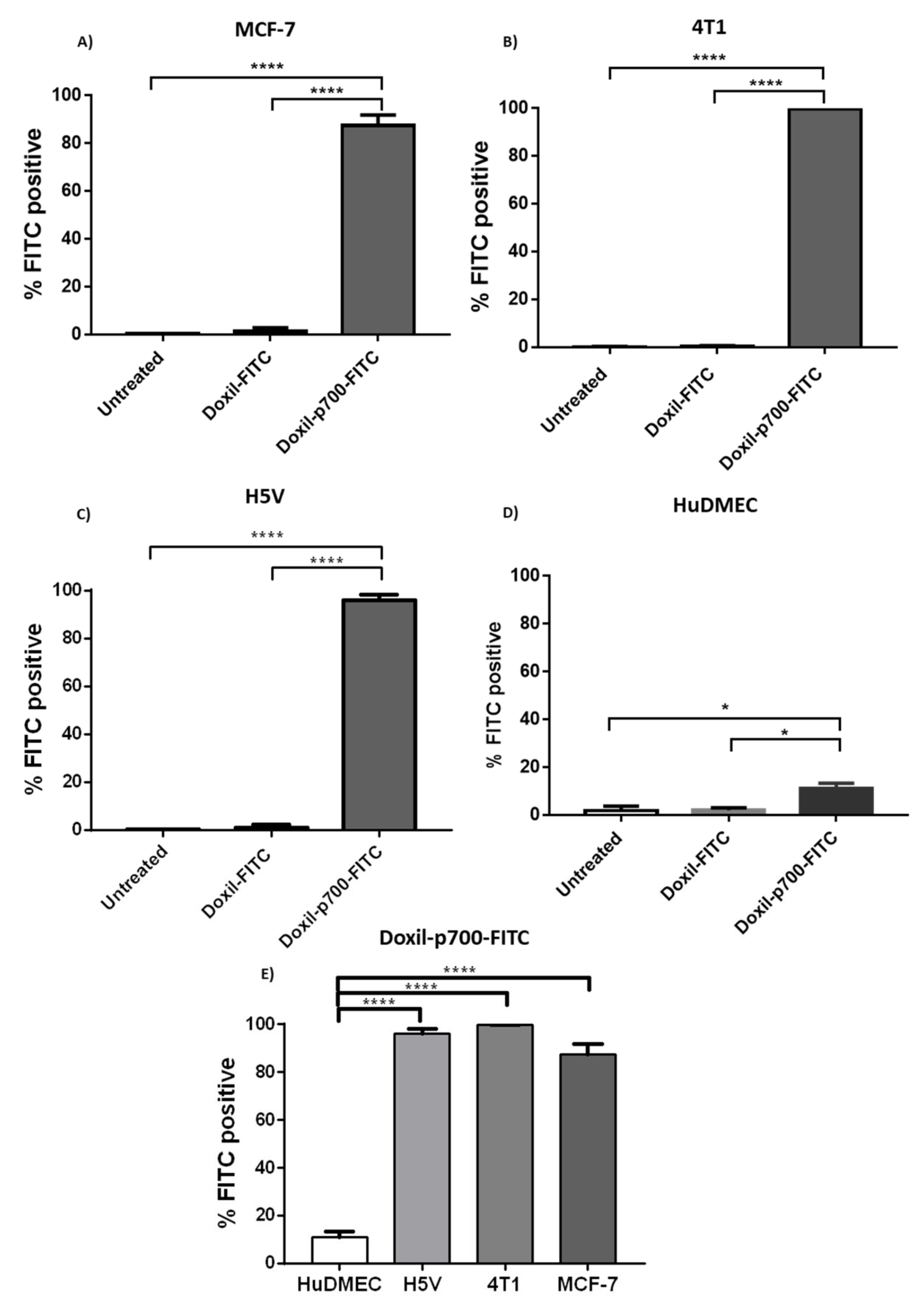
2.4. Confirmation of Cellular Binding of p700-Conjugated Liposomal Doxorubicin
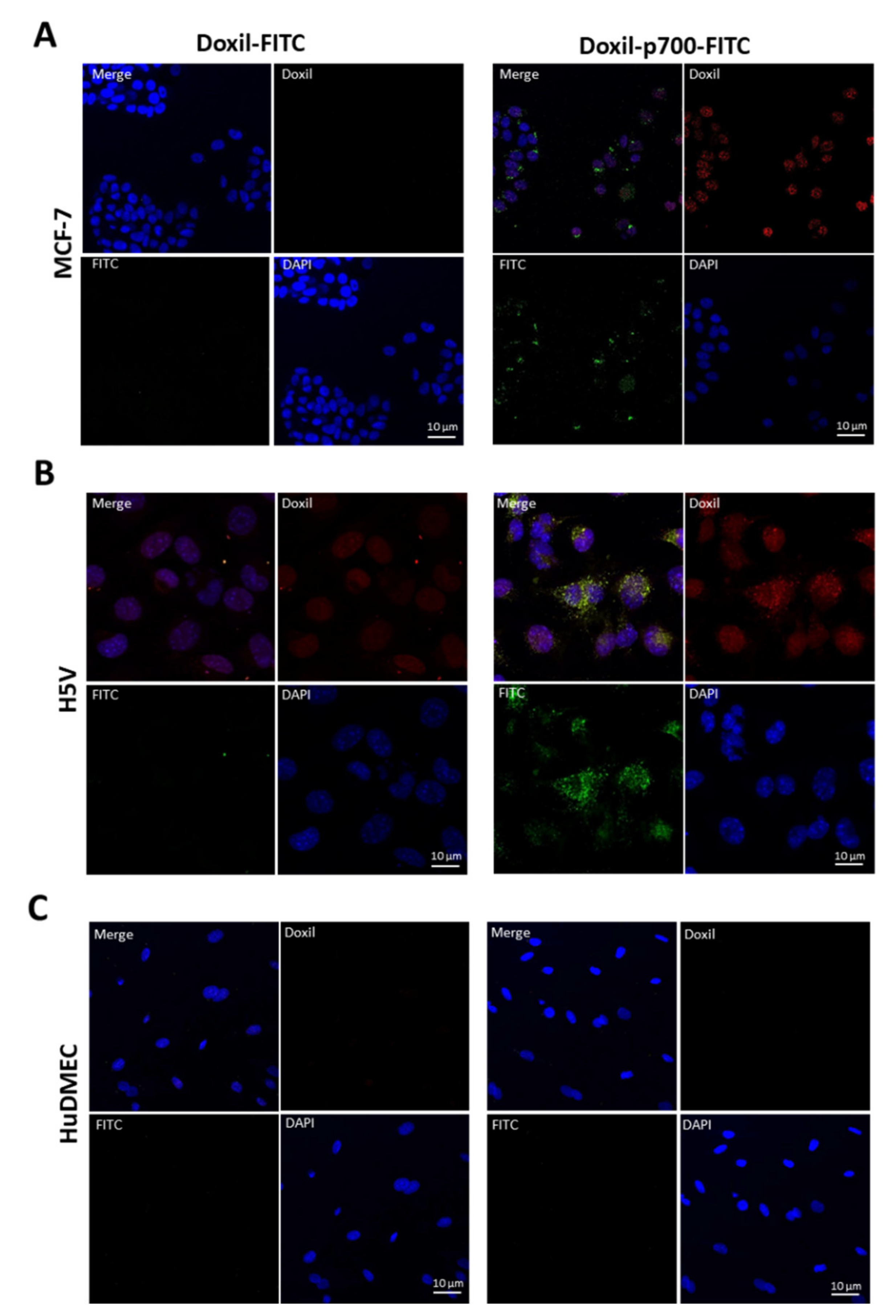
2.5. Confirmation to Cellular Uptake of p700-Conjugated Liposomal Doxorubicin
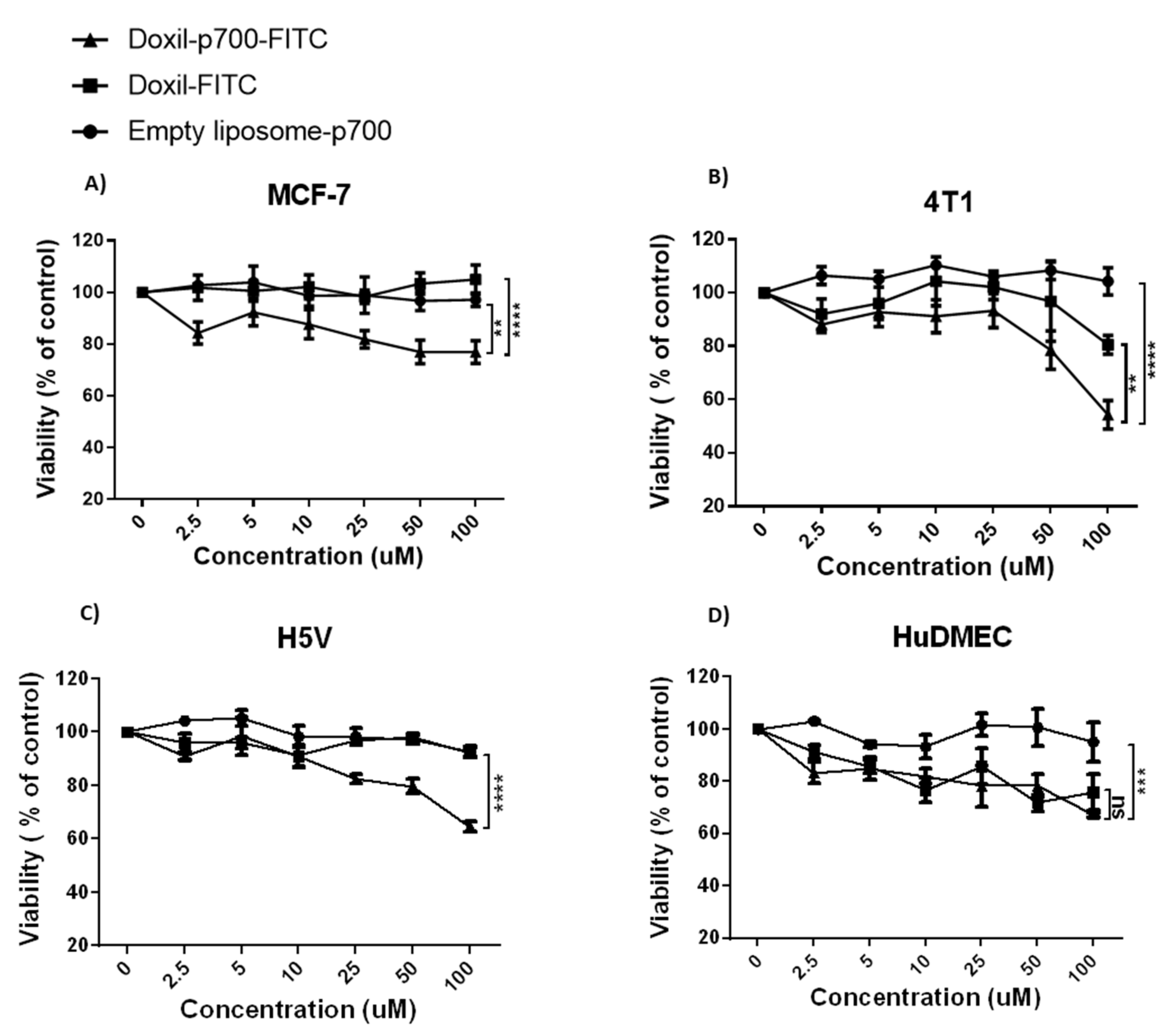
2.6. Cytotoxicity Assay
2.7. Statistical Analysis
3. Results
3.1. Coupling Assessment
3.2. Binding Evaluation
3.3. Cellular Uptake and Internalisation
3.4. Cytotoxic Effect of Conjugates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Westman, E.L.; Canova, M.J.; Radhi, I.J.; Koteva, K.; Kireeva, I.; Waglechner, N.; Wright, G.D. Bacterial inactivation of the anticancer drug doxorubicin. Chem. Biol. 2012, 19, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.; Khan, D.R. The treatment of breast cancer using liposome technology. J. Drug Deliv. 2012, 2012, 212965. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Guo, F.; Xu, H.; Liang, W.; Wang, C.; Yang, X.D. Combination therapy using co-encapsulated resveratrol and paclitaxel in liposomes for drug resistance reversal in breast cancer cells in vivo. Sci. Rep. 2016, 6, 22390. [Google Scholar] [CrossRef]
- Leriche, G.; Cifelli, J.L.; Sibucao, K.C.; Patterson, J.P.; Koyanagi, T.; Gianneschi, N.C.; Yang, J. Characterization of drug encapsulation and retention in archaea-inspired tetraether liposomes. Org. Biomol. Chem. 2017, 15, 2157–2162. [Google Scholar] [CrossRef]
- Green, A.E.; Rose, P.G. Pegylated liposomal doxorubicin in ovarian cancer. Int. J. Nanomed. 2006, 1, 229–239. [Google Scholar]
- Shoji, T.; Takatori, E.; Kaido, Y.; Omi, H.; Yokoyama, Y.; Mizunuma, H.; Kaiho, M.; Otsuki, T.; Takano, T.; Yaegashi, N.; et al. A phase I study of irinotecan and pegylated liposomal doxorubicin in recurrent ovarian cancer (Tohoku Gynecologic Cancer Unit 104 study). Cancer Chemother. Pharmacol. 2014, 73, 895–901. [Google Scholar] [CrossRef]
- Maeda, H. Vascular permeability in cancer and infection as related to macromolecular drug delivery, with emphasis on the EPR effect for tumor-selective drug targeting. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2012, 88, 53–71. [Google Scholar] [CrossRef]
- Tseng, Y.L.; Liu, J.J.; Hong, R.L. Translocation of liposomes into cancer cells by cell-penetrating peptides penetratin and tat: A kinetic and efficacy study. Mol. Pharmacol. 2002, 62, 864–872. [Google Scholar] [CrossRef]
- Patil, R.R.; Guhagarkar, S.A.; Devarajan, P.V. Engineered nanocarriers of doxorubicin: A current update. Crit. Rev. Ther. Drug Carrier Syst. 2008, 25, 1–61. [Google Scholar] [CrossRef]
- Northfelt, D.W.; Dezube, B.J.; Thommes, J.A.; Levine, R.; Von Roenn, J.H.; Dosik, G.M.; Rios, A.; Krown, S.E.; DuMond, C.; Mamelok, R.D. Efficacy of pegylated-liposomal doxorubicin in the treatment of AIDS-related Kaposi’s sarcoma after failure of standard chemotherapy. J. Clin. Oncol. 1997, 15, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Lukyanov, A.N.; Elbayoumi, T.A.; Chakilam, A.R.; Torchilin, V.P. Tumor-targeted liposomes: Doxorubicin-loaded long-circulating liposomes modified with anti-cancer antibody. J. Control. Release 2004, 100, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Wu, G.; Yang, W.; Barth, R.F.; Tjarks, W.; Lee, R.J. Synthesis of cetuximab-immunoliposomes via a cholesterol-based membrane anchor for targeting of EGFR. Bioconjug. Chem. 2007, 18, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, Q.; Lee, R.J. A folate receptor-targeted liposomal formulation for paclitaxel. Int. J. Pharm. 2006, 316, 148–153. [Google Scholar] [CrossRef]
- Futaki, S.; Suzuki, T.; Ohashi, W.; Yagami, T.; Tanaka, S.; Ueda, K.; Sugiura, Y. Arginine-rich peptides. An abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J. Biol. Chem. 2001, 276, 5836–5840. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Brown, N.J.; Jones, R.; Lewis, C.E.; Mujamammi, A.H.; Muthana, M.; Seed, M.P.; Barker, M.D. A peptide derived from TIMP-3 inhibits multiple angiogenic growth factor receptors and tumour growth and inflammatory arthritis in mice. Angiogenesis 2014, 17, 207–219. [Google Scholar] [CrossRef]
- Smith, N.R.; Baker, D.; James, N.H.; Ratcliffe, K.; Jenkins, M.; Ashton, S.E.; Sproat, G.; Swann, R.; Gray, N.; Ryan, A.; et al. Vascular endothelial growth factor receptors VEGFR-2 and VEGFR-3 are localized primarily to the vasculature in human primary solid cancers. Clin. Cancer Res. 2010, 16, 3548–3561. [Google Scholar] [CrossRef]
- Fan, F.; Wey, J.S.; McCarty, M.F.; Belcheva, A.; Liu, W.; Bauer, T.W.; Somcio, R.J.; Wu, Y.; Hooper, A.; Hicklin, D.J.; et al. Expression and function of vascular endothelial growth factor receptor-1 on human colorectal cancer cells. Oncogene 2005, 24, 2647–2653. [Google Scholar] [CrossRef]
- Hahn, D.; Simak, R.; Steiner, G.E.; Handisurya, A.; Susani, M.; Marberger, M. Expression of the VEGF-receptor Flt-1 in benign, premalignant and malignant prostate tissues. J. Urol. 2000, 164, 506–510. [Google Scholar] [CrossRef]
- Wu, Y.; Hooper, A.T.; Zhong, Z.; Witte, L.; Bohlen, P.; Rafii, S.; Hicklin, D.J. The vascular endothelial growth factor receptor (VEGFR-1) supports growth and survival of human breast carcinoma. Int. J. Cancer 2006, 119, 1519–1529. [Google Scholar] [CrossRef]
- Bairey, O.; Boycov, O.; Kaganovsky, E.; Zimra, Y.; Shaklai, M.; Rabizadeh, E. All three receptors for vascular endothelial growth factor (VEGF) are expressed on B-chronic lymphocytic leukemia (CLL) cells. Leuk. Res. 2004, 28, 243–248. [Google Scholar] [CrossRef]
- Graeven, U.; Fiedler, W.; Karpinski, S.; Ergun, S.; Kilic, N.; Rodeck, U.; Schmiegel, W.; Hossfeld, D.K. Melanoma-associated expression of vascular endothelial growth factor and its receptors FLT-1 and KDR. J. Cancer Res. Clin. Oncol. 1999, 125, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Matei, D.; Emerson, R.E.; Lai, Y.C.; Baldridge, L.A.; Rao, J.; Yiannoutsos, C.; Donner, D.D. Autocrine activation of PDGFRalpha promotes the progression of ovarian cancer. Oncogene 2006, 25, 2060–2069. [Google Scholar] [CrossRef] [PubMed]
- Sahadevan, K.; Darby, S.; Leung, H.Y.; Mathers, M.E.; Robson, C.N.; Gnanapragasam, V.J. Selective over-expression of fibroblast growth factor receptors 1 and 4 in clinical prostate cancer. J. Pathol. 2007, 213, 82–90. [Google Scholar] [CrossRef]
- Taeger, J.; Moser, C.; Hellerbrand, C.; Mycielska, M.E.; Glockzin, G.; Schlitt, H.J.; Geissler, E.K.; Stoeltzing, O.; Lang, S.A. Targeting FGFR/PDGFR/VEGFR impairs tumor growth, angiogenesis, and metastasis by effects on tumor cells, endothelial cells, and pericytes in pancreatic cancer. Mol. Cancer Ther. 2011, 10, 2157–2167. [Google Scholar] [CrossRef]
- Soudy, R.; Chen, C.; Kaur, K. Novel peptide-doxorubucin conjugates for targeting breast cancer cells including the multidrug resistant cells. J. Med. Chem. 2013, 56, 7564–7573. [Google Scholar] [CrossRef]
- Kanthou, C.; Dachs, G.U.; Lefley, D.V.; Steele, A.J.; Coralli-Foxon, C.; Harris, S.; Greco, O.; Dos Santos, S.A.; Reyes-Aldasoro, C.C.; English, W.R.; et al. Tumour cells expressing single VEGF isoforms display distinct growth, survival and migration characteristics. PLoS ONE 2014, 9, e104015. [Google Scholar] [CrossRef]
- Tao, K.; Fang, M.; Alroy, J.; Sahagian, G.G. Imagable 4T1 model for the study of late stage breast cancer. BMC Cancer 2008, 8, 228. [Google Scholar] [CrossRef]
- Fantozzi, A.; Christofori, G. Mouse models of breast cancer metastasis. Breast Cancer Res. BCR 2006, 8, 212. [Google Scholar] [CrossRef]
- Burks, S.R.; Legenzov, E.A.; Martin, E.W.; Li, C.; Lu, W.; Kao, J.P. Co-encapsulating the fusogenic peptide INF7 and molecular imaging probes in liposomes increases intracellular signal and probe retention. PLoS ONE 2015, 10, e0120982. [Google Scholar] [CrossRef]
- Rose, P.G. Pegylated liposomal doxorubicin: Optimizing the dosing schedule in ovarian cancer. Oncologist 2005, 10, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Coley, H.M. Mechanisms and strategies to overcome chemotherapy resistance in metastatic breast cancer. Cancer Treat. Rev. 2008, 34, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Garlanda, C.; Parravicini, C.; Sironi, M.; De Rossi, M.; Wainstok de Calmanovici, R.; Carozzi, F.; Bussolino, F.; Colotta, F.; Mantovani, A.; Vecchi, A. Progressive growth in immunodeficient mice and host cell recruitment by mouse endothelial cells transformed by polyoma middle-sized T antigen: Implications for the pathogenesis of opportunistic vascular tumors. Proc. Natl. Acad. Sci. USA 1994, 91, 7291–7295. [Google Scholar] [CrossRef] [PubMed]
- Kesisis, G.; Broxterman, H.; Giaccone, G. Angiogenesis inhibitors. Drug selectivity and target specificity. Curr. Pharm. Des. 2007, 13, 2795–2809. [Google Scholar] [CrossRef] [PubMed]
- Haas, N.B.; Manola, J.; Ky, B.; Flaherty, K.T.; Uzzo, R.G.; Kane, C.J.; Jewett, M.; Wood, L.; Wood, C.G.; Atkins, M.B.; et al. Effects of adjuvant sorafenib and sunitinib on cardiac function in renal cell carcinoma patients without overt metastases: Results from ASSURE, ECOG 2805. Clin. Cancer Res. 2015, 21, 4048–4054. [Google Scholar] [CrossRef]
- Randrup Hansen, C.; Grimm, D.; Bauer, J.; Wehland, M.; Magnusson, N.E. Effects and side effects of using sorafenib and sunitinib in the treatment of metastatic renal cell carcinoma. Int. J. Mol. Sci. 2017, 18, 461. [Google Scholar] [CrossRef]

| Abbreviation | Molecular Weight | Structure |
|---|---|---|
| Control-FITC | 1159.5 | Azido-PEG12-Lys(5-FITC)-amide |
| P700-FITC | 3032.4 |  Azido-PEG12-KIKSCYYLPCFVTSKN-Lys(5-FITC)-amide |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldughaim, M.S.; Muthana, M.; Alsaffar, F.; Barker, M.D. Specific Targeting of PEGylated Liposomal Doxorubicin (Doxil®) to Tumour Cells Using a Novel TIMP3 Peptide. Molecules 2021, 26, 100. https://doi.org/10.3390/molecules26010100
Aldughaim MS, Muthana M, Alsaffar F, Barker MD. Specific Targeting of PEGylated Liposomal Doxorubicin (Doxil®) to Tumour Cells Using a Novel TIMP3 Peptide. Molecules. 2021; 26(1):100. https://doi.org/10.3390/molecules26010100
Chicago/Turabian StyleAldughaim, Mohammed S., Munitta Muthana, Fatimah Alsaffar, and Michael D. Barker. 2021. "Specific Targeting of PEGylated Liposomal Doxorubicin (Doxil®) to Tumour Cells Using a Novel TIMP3 Peptide" Molecules 26, no. 1: 100. https://doi.org/10.3390/molecules26010100
APA StyleAldughaim, M. S., Muthana, M., Alsaffar, F., & Barker, M. D. (2021). Specific Targeting of PEGylated Liposomal Doxorubicin (Doxil®) to Tumour Cells Using a Novel TIMP3 Peptide. Molecules, 26(1), 100. https://doi.org/10.3390/molecules26010100





