Multivariate Analysis of Photoacoustic Spectra for the Detection of Short-Chained Hydrocarbon Isotopologues
Abstract
1. Introduction
2. Materials and Methods
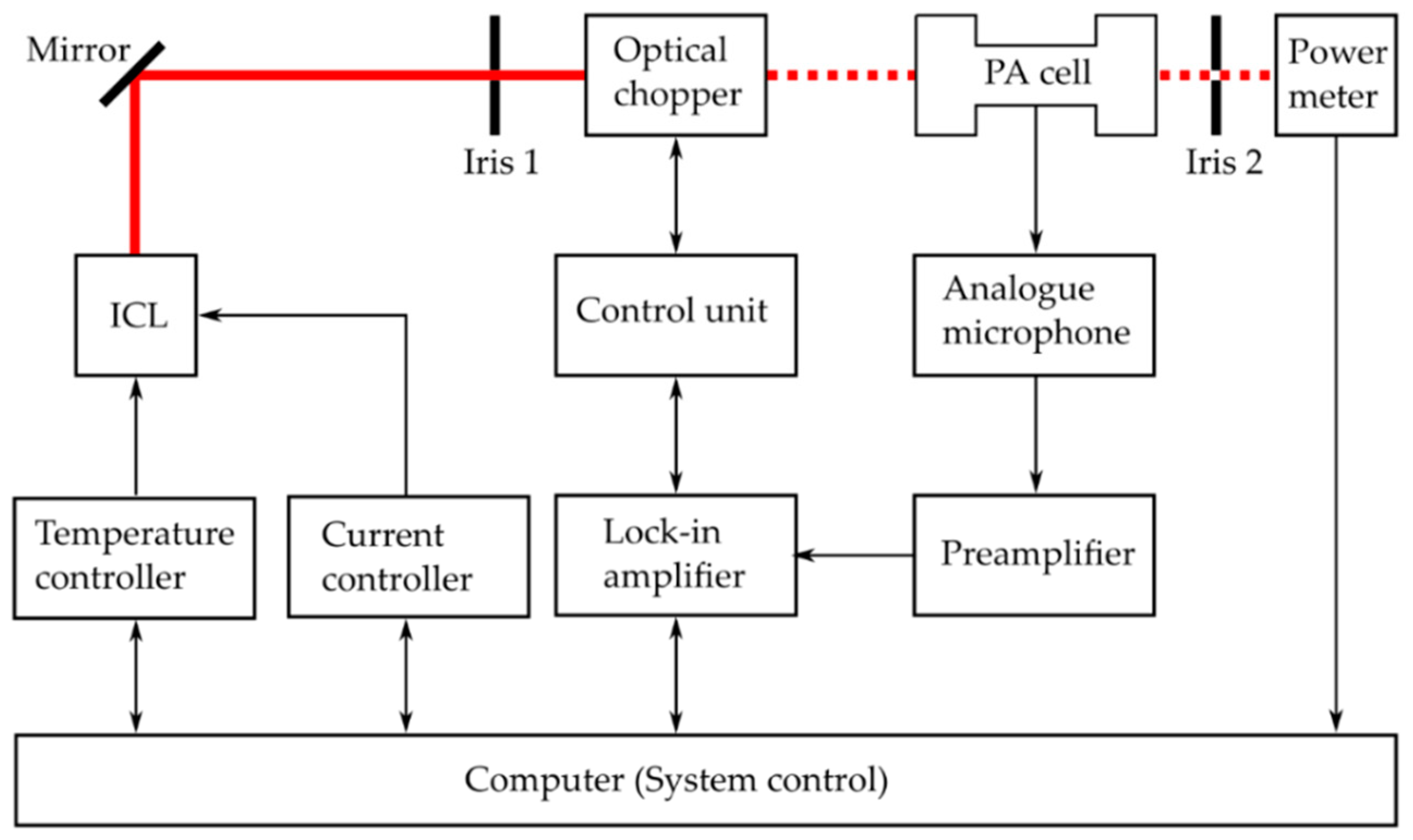
2.1. Photoacoustic Spectroscopy
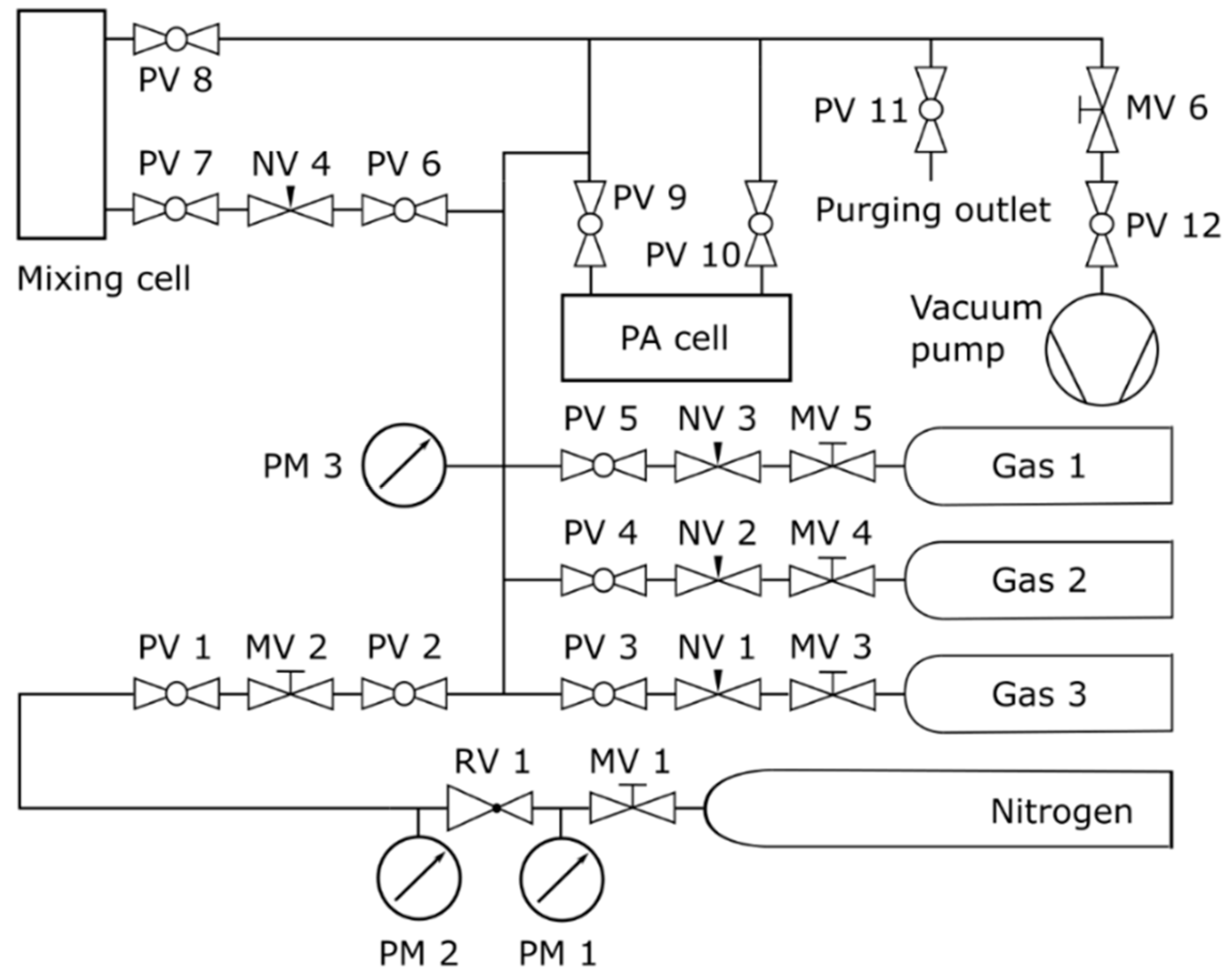
2.2. Experimental Setup
2.3. Measurement and Data Processing
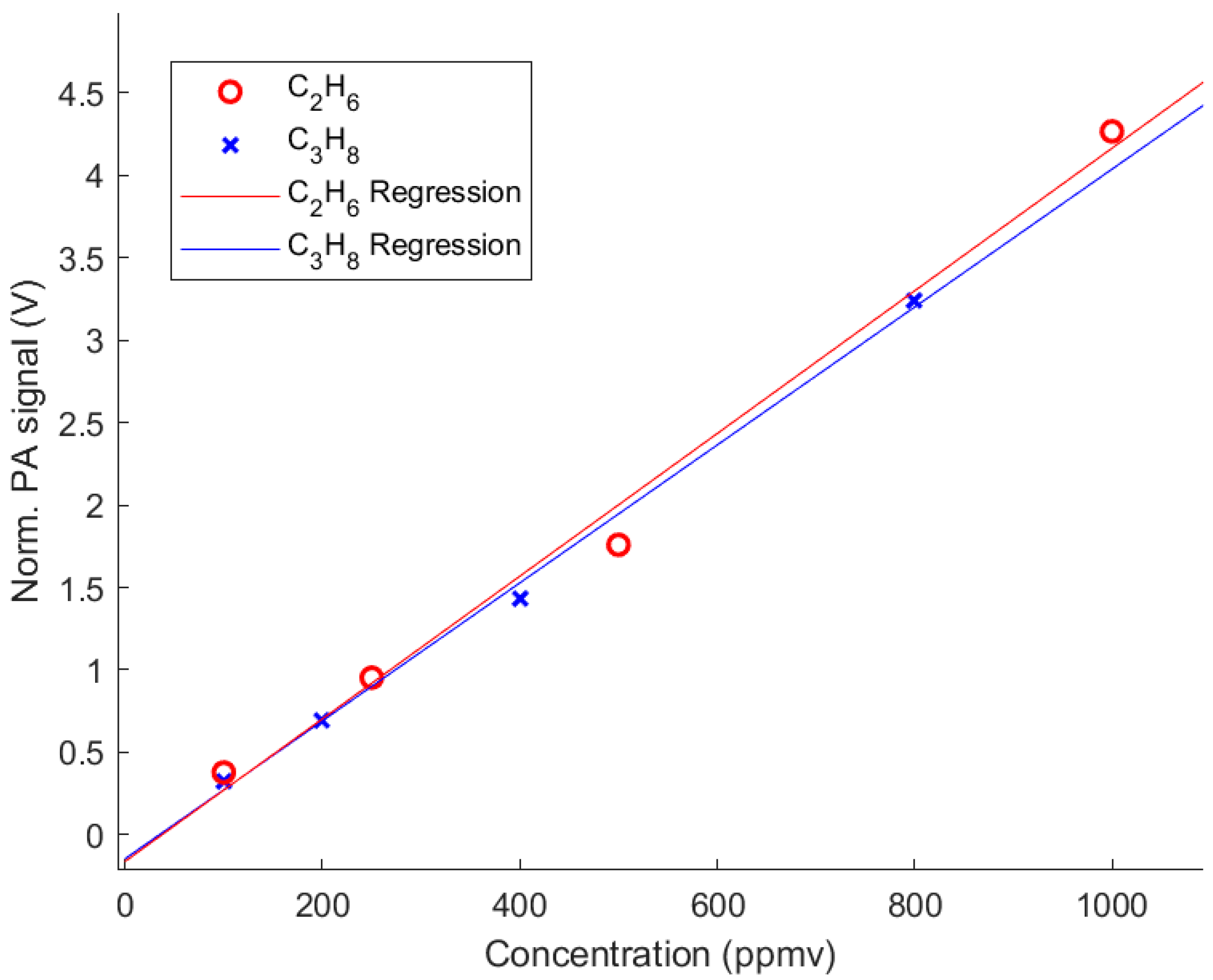
2.4. Detection Limits
2.5. Partial Least Squares Regression
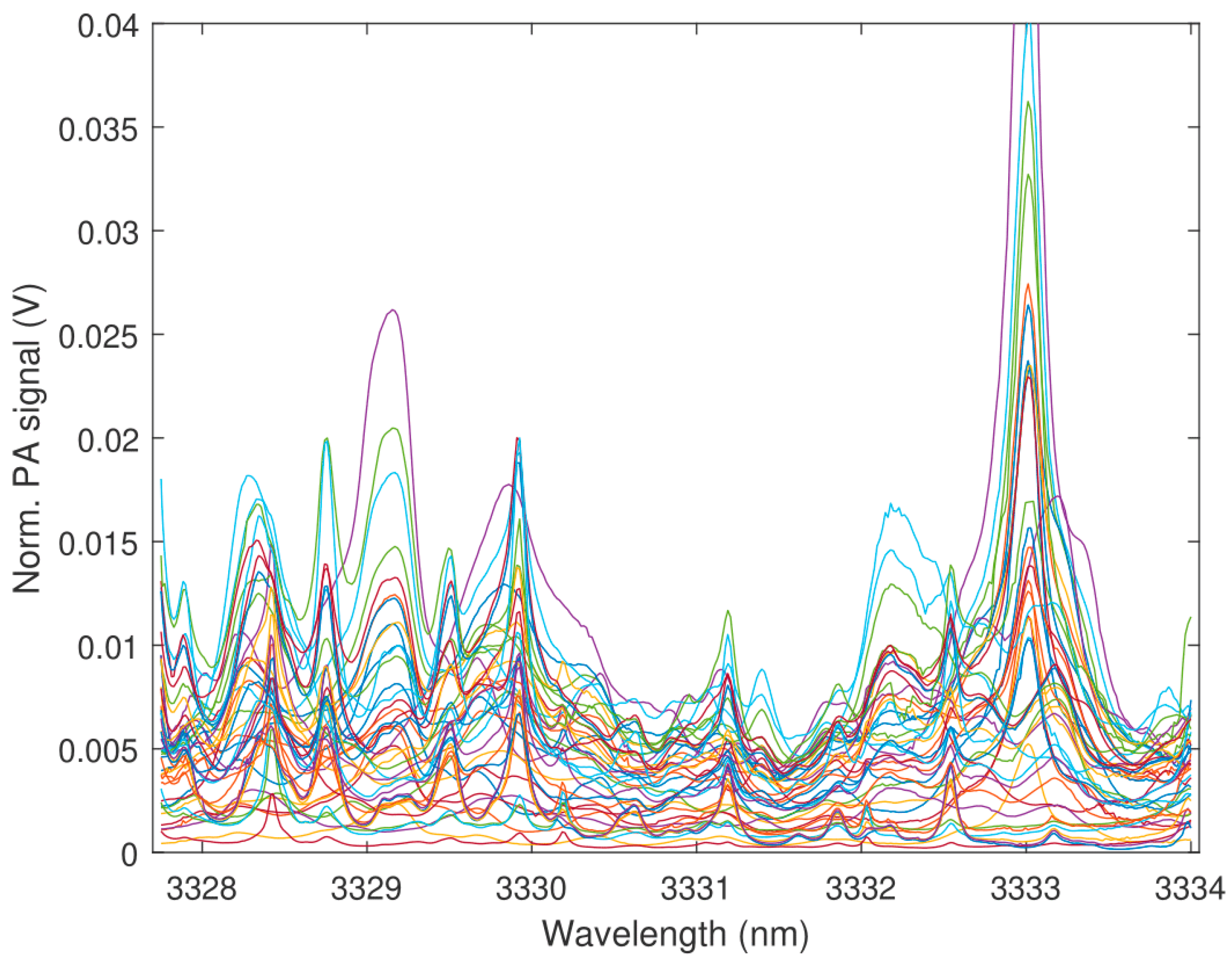
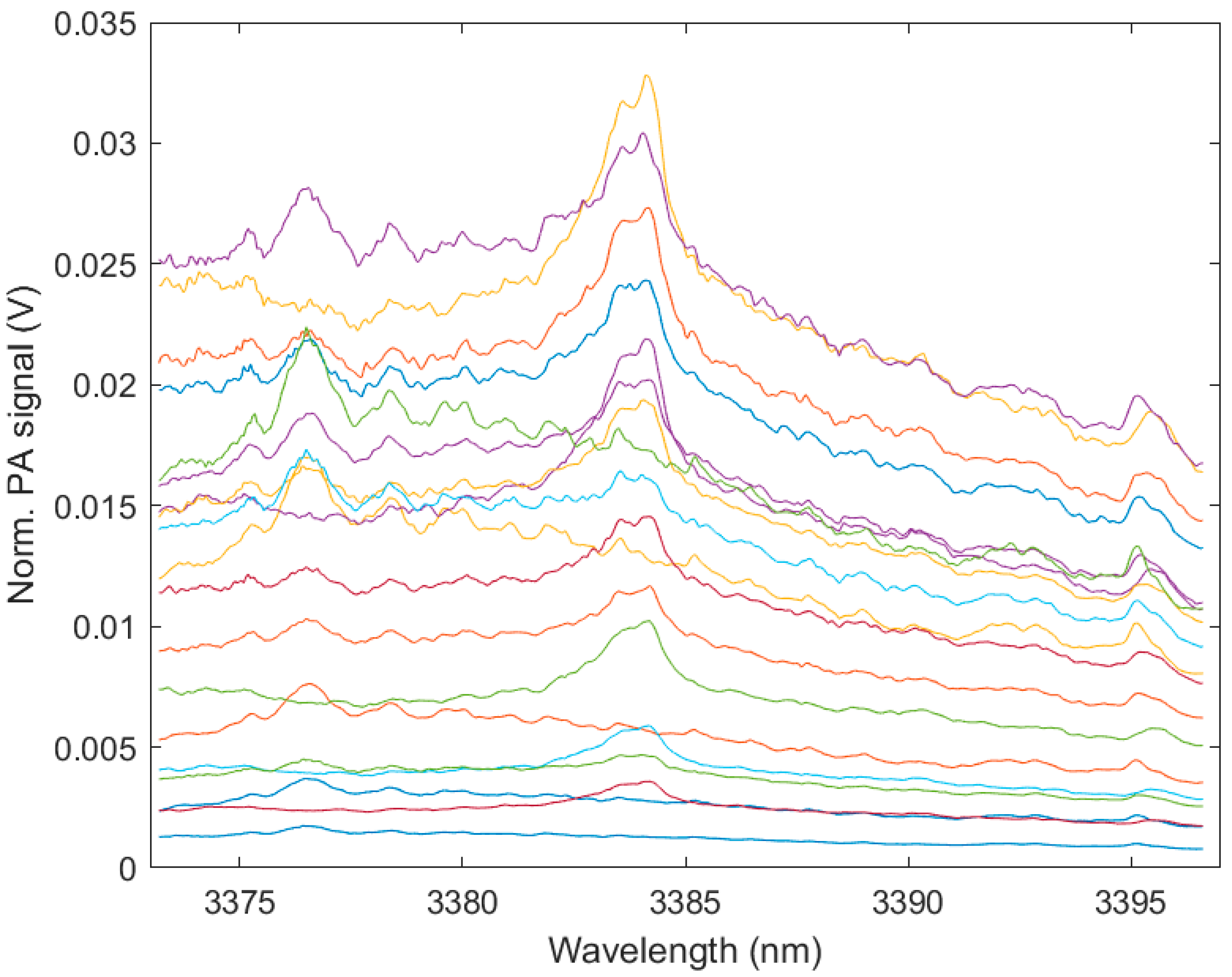
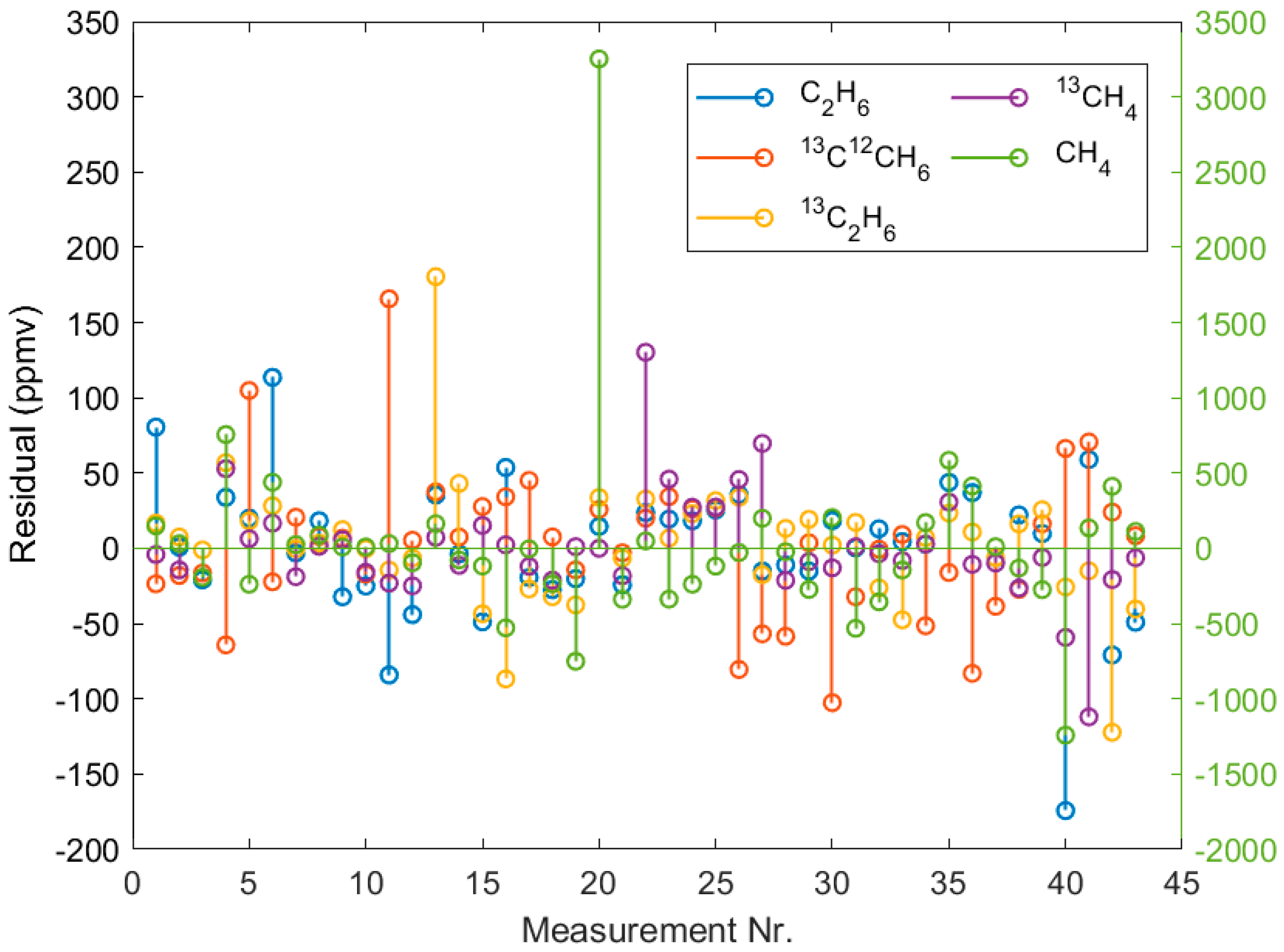
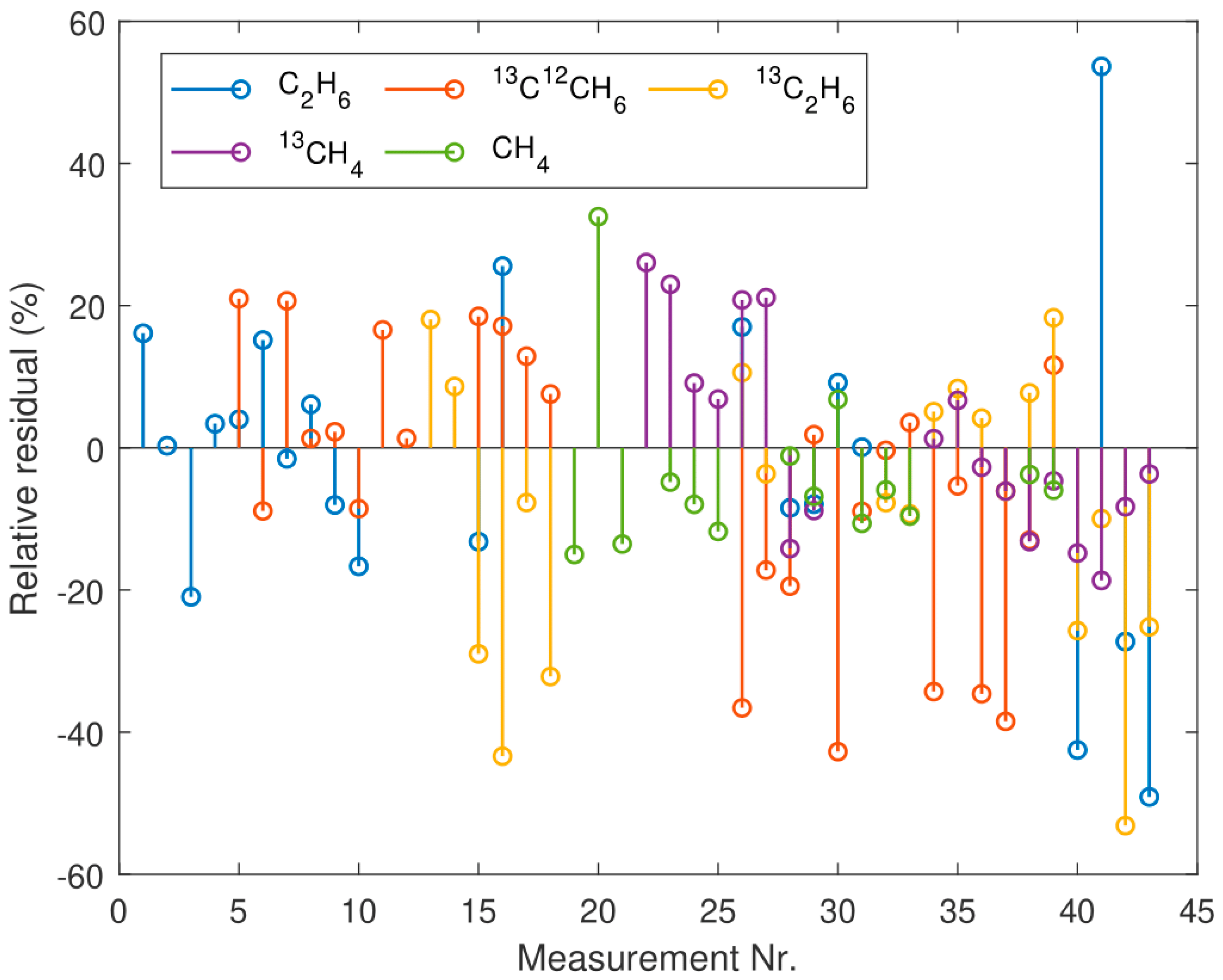
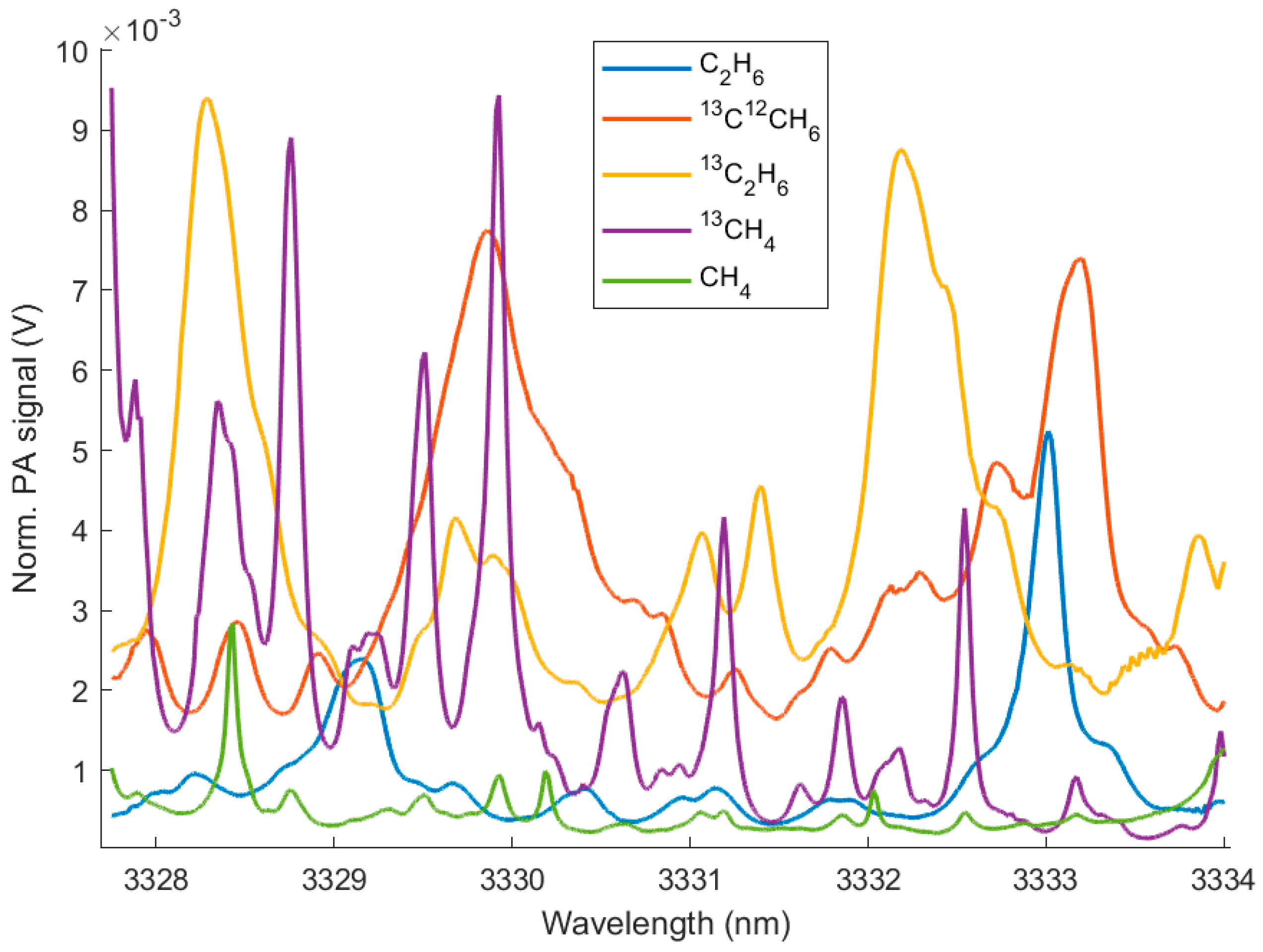
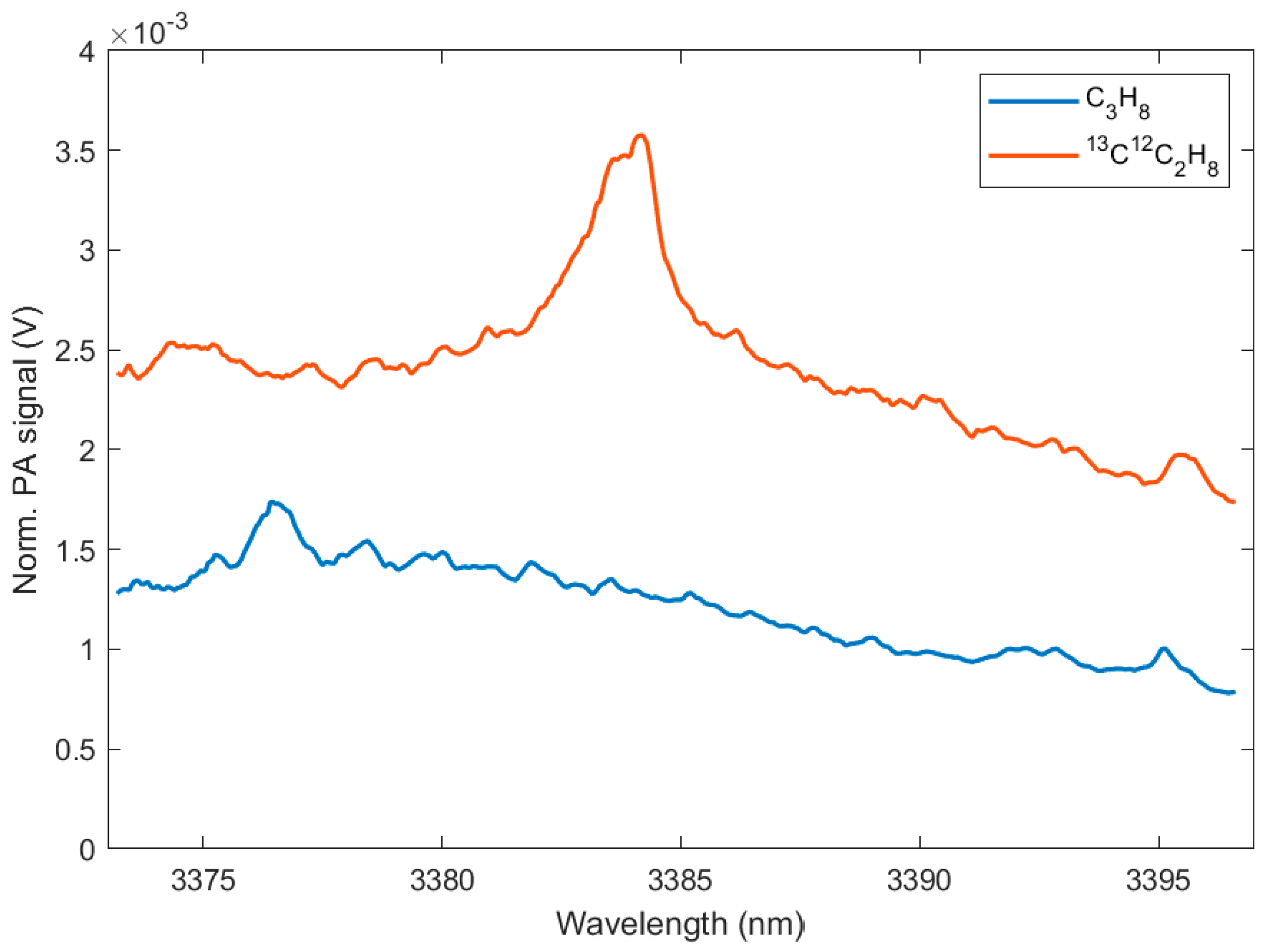
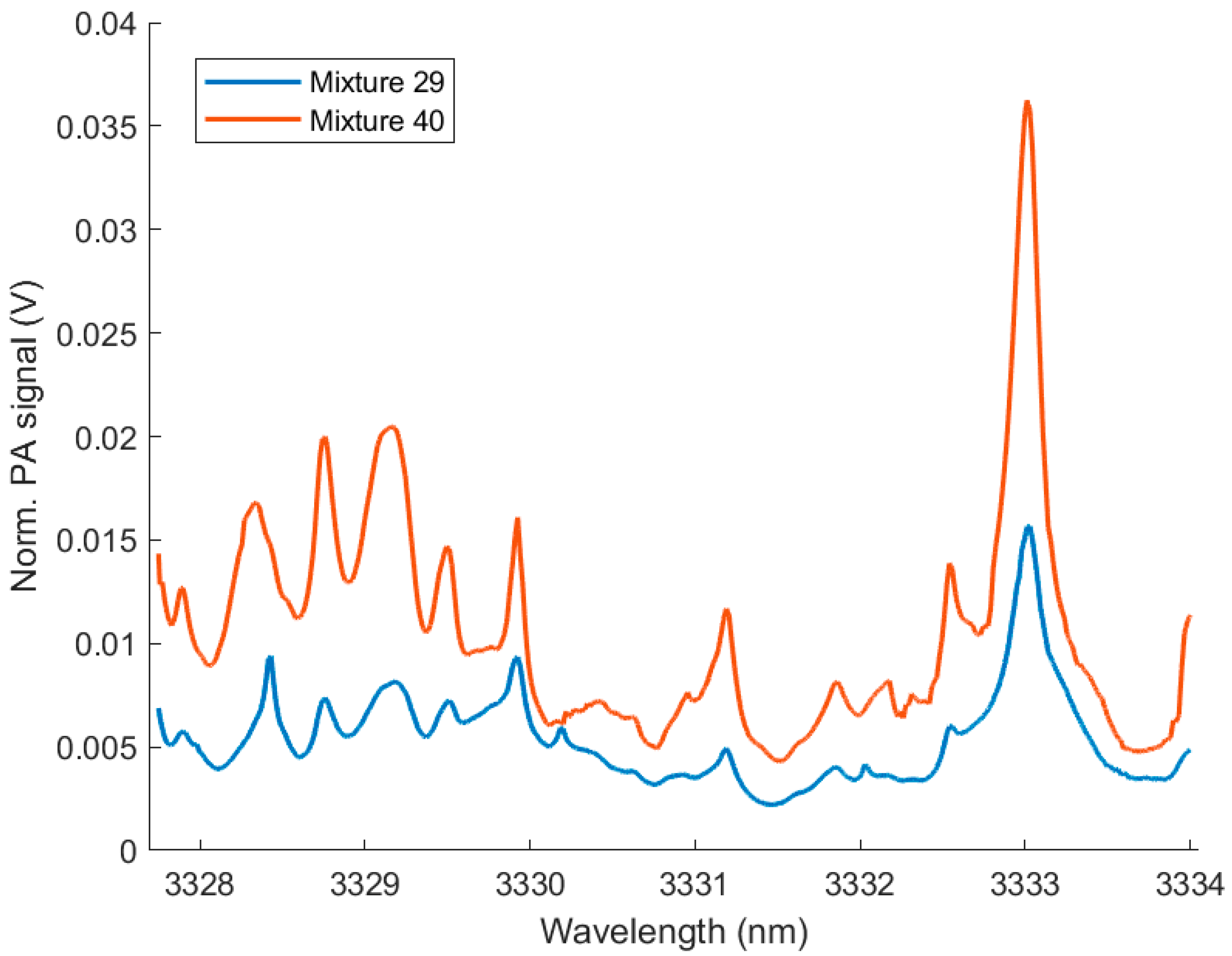
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| ICL 1541 | ICL 1638 | |||||||
|---|---|---|---|---|---|---|---|---|
| No. | C2H6 | 13C12CH6 | 13C2H6 | CH4 | 13CH4 | No. | C3H8 | 13C12C2H8 |
| 1 | 500 ± 11 | 0 | 0 | 0 | 0 | 1 | 200 ± 10 | 0 |
| 2 | 250 ± 10 | 0 | 0 | 0 | 0 | 2 | 400 ± 11 | 0 |
| 3 | 100 | 0 | 0 | 0 | 0 | 3 | 800 ± 11 | 0 |
| 4 | 1000 ± 12 | 0 | 0 | 0 | 0 | 4 | 0 | 800 ± 100 |
| 5 | 500 ± 11 | 500 ± 66 | 0 | 0 | 0 | 5 | 0 | 400 ± 55 |
| 6 | 750 ± 11 | 250 ± 38 | 0 | 0 | 0 | 6 | 0 | 210 ± 34 |
| 7 | 200 ± 10 | 100 ± 21 | 0 | 0 | 0 | 7 | 0 | 130 ± 25 |
| 8 | 300 ± 10 | 600 ± 77 | 0 | 0 | 0 | 8 | 100 | 0 |
| 9 | 400 ± 11 | 300 ± 44 | 0 | 0 | 0 | 9 | 240 ± 10 | 300 ± 44 |
| 10 | 150 ± 10 | 200 ± 32 | 0 | 0 | 0 | 10 | 350 ± 11 | 500 ± 66 |
| 11 | 0 | 1000 ± 122 | 0 | 0 | 0 | 11 | 500 ± 11 | 440 ± 59 |
| 12 | 0 | 400 ± 55 | 0 | 0 | 0 | 12 | 150 ± 10 | 100 ± 21 |
| 13 | 0 | 0 | 1000 ± 122 | 0 | 0 | 13 | 610 ± 11 | 250 ± 38 |
| 14 | 0 | 0 | 500 ± 66 | 0 | 0 | 14 | 310 ± 10 | 350 ± 49 |
| 15 | 370 ± 11 | 150 ± 27 | 150 ± 27 | 0 | 0 | 15 | 540 ± 11 | 540 ± 70 |
| 16 | 210 ± 10 | 200 ± 32 | 200 ± 32 | 0 | 0 | 16 | 450 ± 11 | 700 ± 88 |
| 17 | 0 | 350 ± 49 | 350 ± 49 | 0 | 0 | 17 | 200 ± 10 | 100 ± 21 |
| 18 | 0 | 100 ± 21 | 100 ± 21 | 0 | 0 | 18 | 900 ± 11 | 600 ± 77 |
| 19 | 0 | 0 | 0 | 5000 ± 18 | 0 | 19 | 1000 ± 12 | 50 ± 16 |
| 20 | 0 | 0 | 0 | 10000 | 0 | |||
| 21 | 0 | 0 | 0 | 2500 ± 14 | 0 | |||
| 22 | 0 | 0 | 0 | 0 | 500 ± 66 | |||
| 23 | 0 | 0 | 0 | 7000 ± 21 | 200 ± 32 | |||
| 24 | 0 | 0 | 0 | 3000 ± 15 | 300 ± 44 | |||
| 25 | 0 | 0 | 0 | 1000 ± 12 | 400 ± 55 | |||
| 26 | 210 ± 10 | 220 ± 35 | 320 ± 46 | 0 | 220 ± 35 | |||
| 27 | 0 | 330 ± 47 | 480 ± 64 | 0 | 330 ± 47 | |||
| 28 | 130 ± 10 | 300 ± 44 | 0 | 2000 ± 13 | 150 ± 27 | |||
| 29 | 190 ± 10 | 200 ± 32 | 0 | 4000 ± 16 | 100 ± 21 | |||
| 30 | 200 ± 10 | 240 ± 37 | 0 | 3000 ± 15 | 0 | |||
| 31 | 300 ± 10 | 360 ± 50 | 0 | 5000 ± 18 | 0 | |||
| 32 | 0 | 170 ± 29 | 340 ± 48 | 6000 ± 19 | 0 | |||
| 33 | 0 | 260 ± 39 | 510 ± 67 | 1500 ± 12 | 0 | |||
| 34 | 0 | 150 ± 27 | 140 ± 26 | 0 | 230 ± 36 | |||
| 35 | 0 | 300 ± 44 | 280 ± 41 | 0 | 460 ± 62 | |||
| 36 | 0 | 240 ± 37 | 260 ± 39 | 0 | 390 ± 54 | |||
| 37 | 0 | 100 ± 21 | 100 ± 21 | 0 | 160 ± 28 | |||
| 38 | 0 | 210 ± 34 | 210 ± 34 | 3500 ± 15 | 200 ± 32 | |||
| 39 | 0 | 140 ± 26 | 140 ± 26 | 4600 ± 17 | 130 ± 25 | |||
| 40 | 410 ± 11 | 0 | 100 ± 21 | 0 | 400 ± 55 | |||
| 41 | 110 ± 10 | 0 | 150 ± 27 | 0 | 600 ± 77 | |||
| 42 | 260 ± 10 | 0 | 230 ± 36 | 0 | 250 ± 38 | |||
| 43 | 100 | 0 | 160 ± 28 | 0 | 170 ± 29 | |||
| ICL 1541 | ICL 1638 | |||||||
|---|---|---|---|---|---|---|---|---|
| No. | C2H6 | 13C12CH6 | 13C2H6 | CH4 | 13CH4 | No. | C3H8 | 13C12C2H8 |
| 1 | 419.4 | 23.6 | −16.8 | −148.6 | 4.0 | 1 | 169.6 | 21.9 |
| 2 | 249.3 | 18.1 | −7.5 | −30.9 | 14.4 | 2 | 365.1 | 11.0 |
| 3 | 121.0 | 16.4 | 1.1 | 193.0 | 19.5 | 3 | 835.2 | 4.7 |
| 4 | 966.1 | 64.2 | −57.0 | −756.2 | −52.9 | 4 | 42.5 | 743.2 |
| 5 | 480.0 | 395.1 | −18.2 | 238.4 | −6.4 | 5 | 19.0 | 359.2 |
| 6 | 636.4 | 272.2 | −28.3 | −439.3 | −16.7 | 6 | 9.3 | 218.6 |
| 7 | 203.1 | 79.3 | 0.4 | −25.0 | 18.8 | 7 | −0.5 | 147.0 |
| 8 | 281.7 | 592.1 | −3.4 | −88.5 | −1.3 | 8 | 74.4 | 25.6 |
| 9 | 432.1 | 293.2 | −12.4 | −8.8 | −5.7 | 9 | 260.3 | 272.2 |
| 10 | 175.0 | 217.1 | 0.3 | −9.2 | 15.9 | 10 | 389.4 | 466.7 |
| 11 | 84.2 | 834.2 | 14.4 | −31.8 | 23.1 | 11 | 540.4 | 415.3 |
| 12 | 44.0 | 394.7 | 6.4 | 95.5 | 24.9 | 12 | 130.1 | 108.6 |
| 13 | −35.3 | −37.6 | 819.5 | −163.7 | −7.3 | 13 | 633.0 | 222.7 |
| 14 | 3.6 | −7.5 | 456.8 | 77.4 | 11.3 | 14 | 275.7 | 370.9 |
| 15 | 418.8 | 122.3 | 193.5 | 117.6 | −15.2 | 15 | 565.1 | 550.5 |
| 16 | 156.3 | 165.7 | 286.8 | 528.0 | −2.3 | 16 | 415.5 | 731.5 |
| 17 | 19.4 | 304.9 | 377.0 | 3.5 | 12.0 | 17 | 210.6 | 1054.1 |
| 18 | 27.5 | 92.4 | 132.2 | 236.4 | 21.1 | 18 | 777.5 | 619.7 |
| 19 | 20.2 | 14.3 | 37.6 | 5750.5 | −1.1 | 19 | 1038.9 | 43.9 |
| 20 | −14.5 | −25.8 | −33.8 | 6748.3 | 0.0 | |||
| 21 | 24.2 | 3.0 | 6.5 | 2838.3 | 18.2 | |||
| 22 | −23.7 | −20.0 | −32.8 | −49.5 | 369.7 | |||
| 23 | −19.4 | −34.3 | −6.8 | 7337.5 | 154.0 | |||
| 24 | −18.3 | −26.2 | −23.0 | 3238.5 | 272.7 | |||
| 25 | −25.4 | −26.8 | −31.7 | 1117.7 | 372.6 | |||
| 26 | 174.3 | 300.5 | 286.1 | 27.9 | 174.3 | |||
| 27 | 15.1 | 386.8 | 497.5 | −199.5 | 260.3 | |||
| 28 | 141.0 | 358.4 | −13.2 | 2022.8 | 171.3 | |||
| 29 | 205.1 | 196.3 | −19.3 | 4273.1 | 108.8 | |||
| 30 | 181.7 | 342.6 | −2.1 | 2795.9 | 13.0 | |||
| 31 | 299.7 | 392.2 | −17.2 | 5531.2 | −1.1 | |||
| 32 | −13.0 | 170.6 | 366.3 | 6355.6 | 3.6 | |||
| 33 | −4.5 | 250.8 | 557.5 | 1644.1 | 8.1 | |||
| 34 | −2.7 | 201.4 | 132.9 | −171.1 | 227.1 | |||
| 35 | −43.8 | 316.0 | 256.6 | −584.8 | 429.3 | |||
| 36 | −37.0 | 323.1 | 249.2 | −413.9 | 400.6 | |||
| 37 | 6.2 | 138.5 | 106.1 | −10.9 | 169.8 | |||
| 38 | −22.2 | 237.3 | 193.8 | 3630.9 | 226.4 | |||
| 39 | −9.8 | 123.7 | 114.4 | 4874.5 | 136.0 | |||
| 40 | 584.3 | −66.4 | 125.7 | 1241.4 | 459.2 | |||
| 41 | 51.0 | −70.7 | 164.9 | −135.6 | 712.0 | |||
| 42 | 330.9 | −24.1 | 352.2 | −412.2 | 270.7 | |||
| 43 | 149.1 | −8.4 | 200.3 | −112.3 | 176.2 | |||
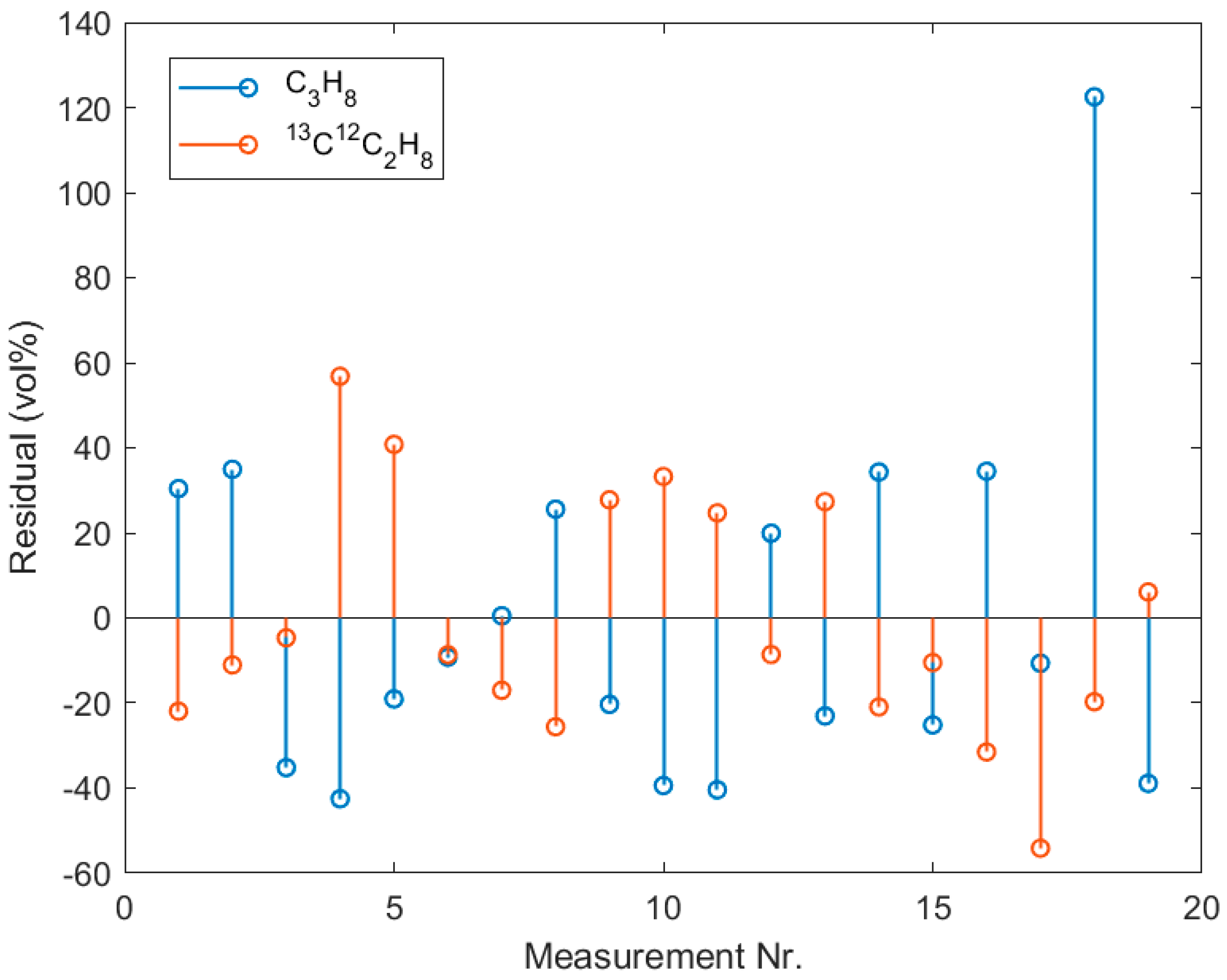
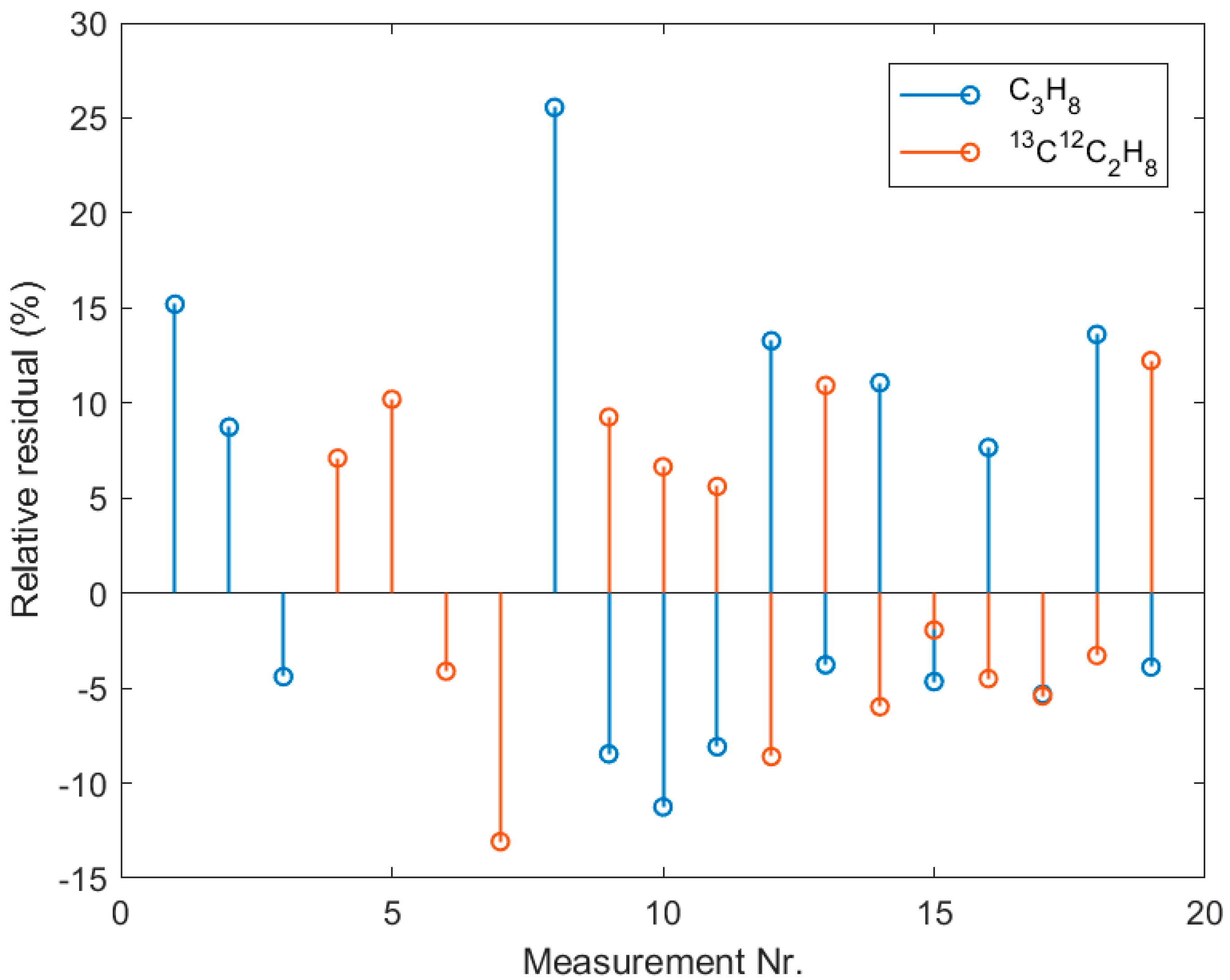
References
- Myhre, G.; Shindell, D.; Bréon, F.-M.; Collins, W.; Fuglestvedt, J.; Huang, J.; Koch, D.; Lamarque, J.-F.; Lee, D.; Mendoza, B.; et al. Anthropogenic and Natural Radiative Forcing. In Climate Change 2013: The Physical Science Basis; Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, Ed.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Warneck, P. Chemistry of the Natural Atmosphere, 2nd ed.; International geophysics series; Academic: San Diego, CA, USA; London, UK, 2000; ISBN 0-12-735632-0. [Google Scholar]
- Chen, T.-M.; Kuschner, W.G.; Gokhale, J.; Shofer, S. Outdoor air pollution: Ozone health effects. Am. J. Med. Sci. 2007, 334, 244–248. [Google Scholar] [CrossRef]
- van Dingenen, R.; Dentener, F.; Raes, F.; Krol, M.; Emberson, L.; Cofala, J. The global impact of ozone on agricultural crop yields under current and future air quality legislation. Atmos. Environ. 2009, 43, 604–618. [Google Scholar] [CrossRef]
- Shindell, D.; Kuylenstierna, J.C.I.; Vignati, E.; Van Dingenen, R.; Amann, M.; Klimont, Z.; Anenberg, S.C.; Muller, N.; Janssens-Maenhout, G.; Raes, F.; et al. Simultaneously mitigating near-term climate change and improving human health and food security. Science 2012, 335, 183–189. [Google Scholar] [CrossRef]
- Gensch, I.; Kiendler-Scharr, A.; Rudolph, J. Isotope ratio studies of atmospheric organic compounds: Principles, methods, applications and potential. Int. J. Mass Spectrom. 2014, 365, 206–221. [Google Scholar] [CrossRef]
- U.S. Energy Information Administration. International Energy Outlook 2019: With Projections to 2050; U.S. Energy Information Administration: Washington, DC, USA, 2019. Available online: https://www.eia.gov/outlooks/ieo/pdf/ieo2019.pdf (accessed on 10 May 2020).
- Faramawy, S.; Zaki, T.; Sakr, A.-E. Natural gas origin, composition, and processing: A review. J. Nat. Gas Sci. Eng. 2016, 34, 34–54. [Google Scholar] [CrossRef]
- Zumberge, J.E.; Ferworn, K.; Brown, S. Isotopic reversal (‘rollover’) in shale gases produced from the Mississippian Barnett and Fayetteville formations. Mar. Pet. Geol. 2012, 31, 43–52. [Google Scholar] [CrossRef]
- Xia, X.; Chen, J.; Braun, R.; Tang, Y. Isotopic reversals with respect to maturity trends due to mixing of primary and secondary products in source rocks. Chem. Geol. 2013, 339, 205–212. [Google Scholar] [CrossRef]
- Tilley, B.; Muehlenbachs, K. Isotope reversals and universal stages and trends of gas maturation in sealed, self-contained petroleum systems. Chem. Geol. 2013, 339, 194–204. [Google Scholar] [CrossRef]
- Ellis, L.; Brown, A.; Schoell, M.; Uchytil, S. Mud gas isotope logging (MGIL) assists in oil and gas drilling operations. Oil Gas J. 2003, 101, 32–41. [Google Scholar]
- Sessions, A.L. Isotope-ratio detection for gas chromatography. J. Sep. Sci. 2006, 29, 1946–1961. [Google Scholar] [CrossRef]
- Vurgaftman, I.; Weih, R.; Kamp, M.; Meyer, J.R.; Canedy, C.L.; Kim, C.S.; Kim, M.; Bewley, W.W.; Merritt, C.D.; Abell, J.; et al. Interband cascade lasers. J. Phys. D Appl. Phys. 2015, 48, 123001. [Google Scholar] [CrossRef]
- Loh, A.; Wolff, M. High resolution spectra of 13C ethane and propane isotopologues photoacoustically measured using interband cascade lasers near 3.33 and 3.38 µm, respectively. J. Quant. Spectrosc. Radiat. Transf. 2019, 227, 111–116. [Google Scholar] [CrossRef]
- Loh, A.; Wolff, M. Absorption cross sections of 13C ethane and propane isotopologues in the 3 µm region. J. Quant. Spectrosc. Radiat. Transf. 2017, 203, 517–521. [Google Scholar] [CrossRef]
- Bell, A.G. On the production and reproduction of sound by light. Am. J. Sci. 1880, 3, 305–324. [Google Scholar] [CrossRef]
- Harren, F.J.M.; Christescu, S.M. Photoacoustic Spectroscopy in Trace Gas Monitoring: (2019 version). In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; Wiley: Hoboken, NJ, USA, 2006; ISBN 9780470027318. [Google Scholar]
- Elia, A.; Lugarà, P.M.; Di Franco, C.; Spagnolo, V. Photoacoustic techniques for trace gas sensing based on semiconductor laser sources. Sensors 2009, 9, 9616–9628. [Google Scholar] [CrossRef]
- Patimisco, P.; Scamarcio, G.; Tittel, F.K.; Spagnolo, V. Quartz-enhanced photoacoustic spectroscopy: A review. Sensors 2014, 14, 6165–6206. [Google Scholar] [CrossRef]
- Kessler, W. Multivariate Datenanalyse in der Bio- und Prozessanalytik: Mit Beispielen aus der Praxis; Wiley-VCH: Weinheim, Germany, 2006; ISBN 9783527312627. [Google Scholar]
- Hair, J.F.; Black, W.C.; Babin, B.J.; Anderson, R.E. Multivariate Data Analysis, 7th ed.; Pearson Custom Library Prentice Hall: Upper Saddle River, NJ, USA, 2010; ISBN 978-1-292-02190-4. [Google Scholar]
- Saalberg, Y.; Wolff, M. Multivariate Analysis as a Tool to Identify Concentrations from Strongly Overlapping Gas Spectra. Sensors 2018, 18, 1562. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Sigrist, M.W. Laser photoacoustic spectrometry for trace gas monitoring. Analyst 1994, 119, 525–531. [Google Scholar] [CrossRef]
- Nodo, E. Optimization of resonant cell design for optoacoustic gas spectroscopy (H-type). Appl. Opt. 1978, 17, 1110–1119. [Google Scholar] [CrossRef]
- Stolper, D.; Sessions, A.L.; Ferreira, A.; Neto, E.S.; Schimmelmann, A.; Shusta, S.; Valentine, D.L.; Eiler, J. Combined 13C–D and D–D clumping in methane: Methods and preliminary results. Geochim. Cosmochim. Acta 2014, 126, 169–191. [Google Scholar] [CrossRef]
- de Jong, S. SIMPLS: An alternative approach to partial least squares regression. Chemom. Intell. Lab. Syst. 1993, 18, 251–263. [Google Scholar] [CrossRef]
- Patimisco, P.; Sampaolo, A.; Dong, L.; Tittel, F.K.; Spagnolo, V. Recent advances in quartz enhanced photoacoustic sensing. Appl. Phys. Rev. 2018, 5, 011106. [Google Scholar] [CrossRef]
- Schilt, S.; Thévenaz, L. Wavelength modulation photoacoustic spectroscopy: Theoretical description and experimental results. Infrared Phys. Technol. 2006, 48, 154–162. [Google Scholar] [CrossRef]
- Li, J.; Du, Z.; An, Y. Frequency modulation characteristics for interband cascade lasers emitting at 3 μm. Appl. Phys. B 2015, 121, 7–17. [Google Scholar] [CrossRef]
- Sampaolo, A.; Csutak, S.; Patimisco, P.; Giglio, M.; Menduni, G.; Passaro, V.; Tittel, F.K.; Deffenbaugh, M.; Spagnolo, V. Methane, ethane and propane detection using a compact quartz enhanced photoacoustic sensor and a single interband cascade laser. Sens. Actuators B Chem. 2019, 282, 952–960. [Google Scholar] [CrossRef]
| Sample Gas | Concentration | Purity | Supplier |
|---|---|---|---|
| CH4 (at NA) | 1 vol% | 3.5 | Air Liquide S.A., Paris, France |
| 13CH4 | pure | 2.0 | Sigma-Aldrich, Inc., St. Louis, MO, United States |
| C2H6 (at NA) | 100 ppmv | 6.0 | Linde AG., Dublin, Ireland |
| 1 vol% | 3.5 | Air Liquide S.A. | |
| 13C12CH6 | pure | 2.0 | Sigma-Aldrich, Inc. |
| 13C2H6 | pure | 2.0 | Sigma-Aldrich, Inc. |
| C3H8 (at NA) | 100 ppmv | 6.0 | Linde AG. |
| 1 vol% | 3.5 | Air Liquide S.A. | |
| 13C12C2H8 | pure | 2.0 | Cambridge Isotope Laboratories, Inc. Tewksbury, MA, United States |
| N2 | pure | 5.0 | Linde AG. |
| Sample Gas | Concentration (ppmv) | Wavelength (nm) | SNR | LOD (ppmv) |
|---|---|---|---|---|
| CH4 (at NA) | 2500 | 3328.4 | 728.9 | 3.4 |
| 13CH4 | 500 | 3329.9 | 4292.5 | 0.12 |
| C2H6 (at NA) | 100 | 3333.0 | 1449.7 | 0.069 |
| 13C12CH6 | 400 | 3329.9 | 3515.0 | 0.11 |
| 13C2H6 | 500 | 3332.2 | 3968.5 | 0.13 |
| C3H8 (at NA) | 100 | 3381.9 | 1564.3 | 0.064 |
| 13C12C2H8 | 130 | 3382.2 | 3039.8 | 0.043 |
| Sample Gas | Concentration of Mixture 29 (ppmv) | PLRS Concentration (ppmv) | Concentration of Mixture 40 (ppmv) | PLRS Concentration (ppmv) |
|---|---|---|---|---|
| CH4 (at NA) | 4000 ± 16 | 4273 | 0 | 1241 |
| 13CH4 | 100 ± 21 | 108 | 400 ± 55 | 459 |
| C2H6 (at NA) | 190 ± 10 | 205 | 410 ± 11 | 584 |
| 13C12CH6 | 200 ± 32 | 196 | 0 | −66 |
| 13C2H6 | 0 | −19 | 100 ± 21 | 126 |
| Sample Gas | RMSECV (ppmv) | RMSECV0 (ppmv) | MRR (%) |
|---|---|---|---|
| CH4 (at NA) | 604 | 572 | 9.7 |
| 13CH4 | 35 | 34 | 12.4 |
| C2H6 (at NA) | 46 | 44 | 17.3 |
| 13C12CH6 | 49 | 45 | 15.6 |
| 13C2H6 | 43 | 41 | 17.6 |
| C3H8 (at NA) | 40 | 40 | 9.7 |
| 13C12C2H8 | 28 | 28 | 7.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loh, A.; Wolff, M. Multivariate Analysis of Photoacoustic Spectra for the Detection of Short-Chained Hydrocarbon Isotopologues. Molecules 2020, 25, 2266. https://doi.org/10.3390/molecules25092266
Loh A, Wolff M. Multivariate Analysis of Photoacoustic Spectra for the Detection of Short-Chained Hydrocarbon Isotopologues. Molecules. 2020; 25(9):2266. https://doi.org/10.3390/molecules25092266
Chicago/Turabian StyleLoh, Alain, and Marcus Wolff. 2020. "Multivariate Analysis of Photoacoustic Spectra for the Detection of Short-Chained Hydrocarbon Isotopologues" Molecules 25, no. 9: 2266. https://doi.org/10.3390/molecules25092266
APA StyleLoh, A., & Wolff, M. (2020). Multivariate Analysis of Photoacoustic Spectra for the Detection of Short-Chained Hydrocarbon Isotopologues. Molecules, 25(9), 2266. https://doi.org/10.3390/molecules25092266






