Phenolic and Non-Polar Fractions of the Extracts from Fruits, Leaves, and Twigs of Elaeagnus rhamnoides (L.) A. Nelson—The Implications for Human Barrier Cells
Abstract
1. Introduction
2. Results and Discussion
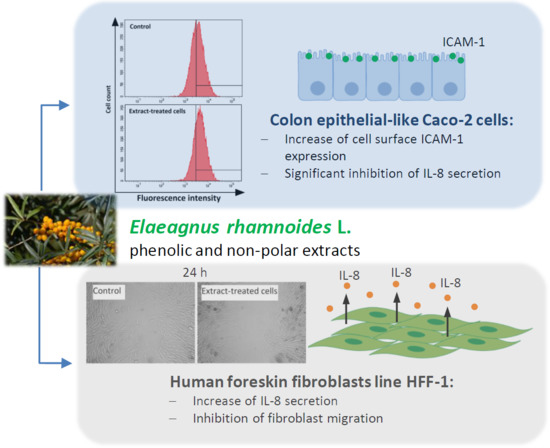
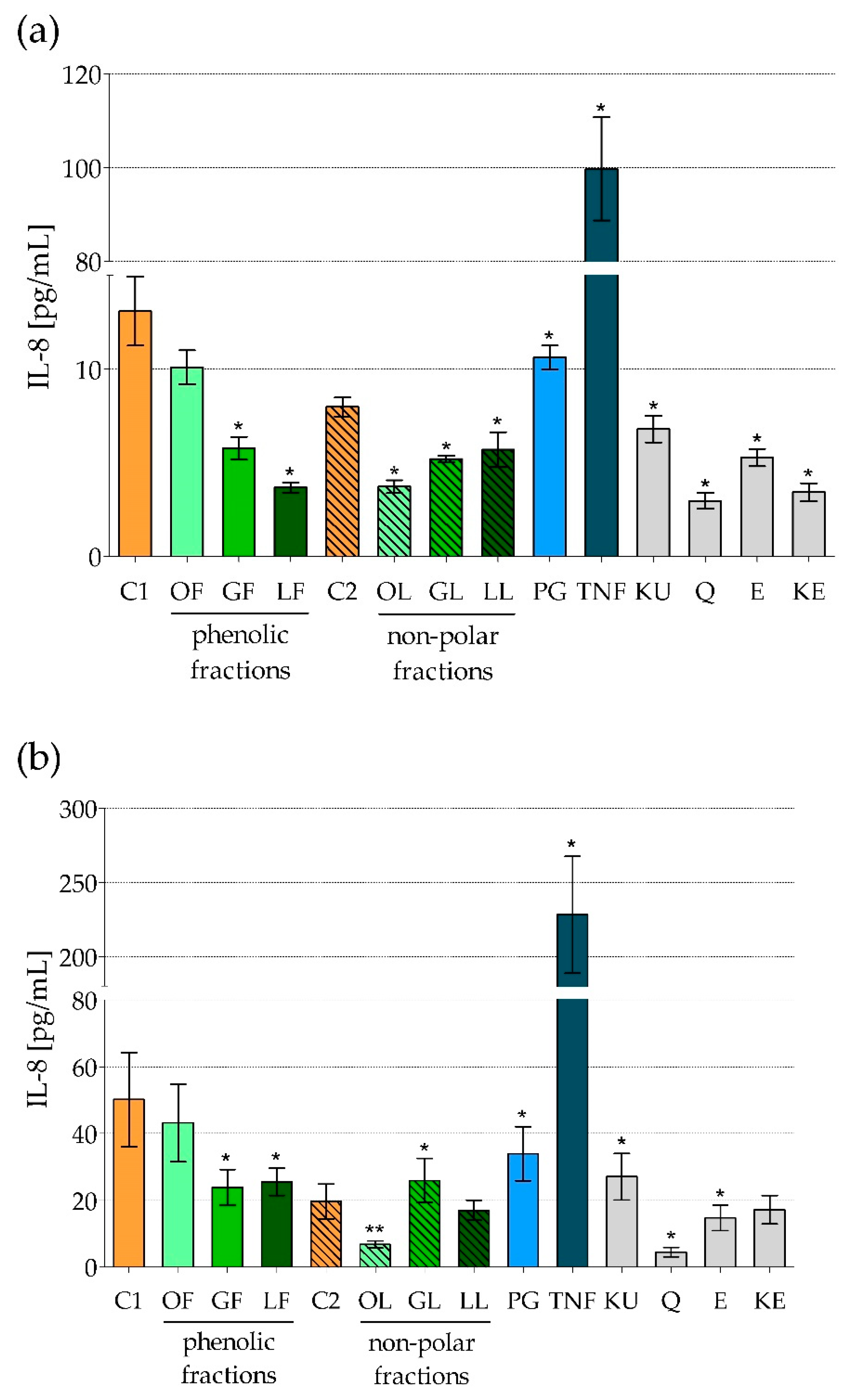
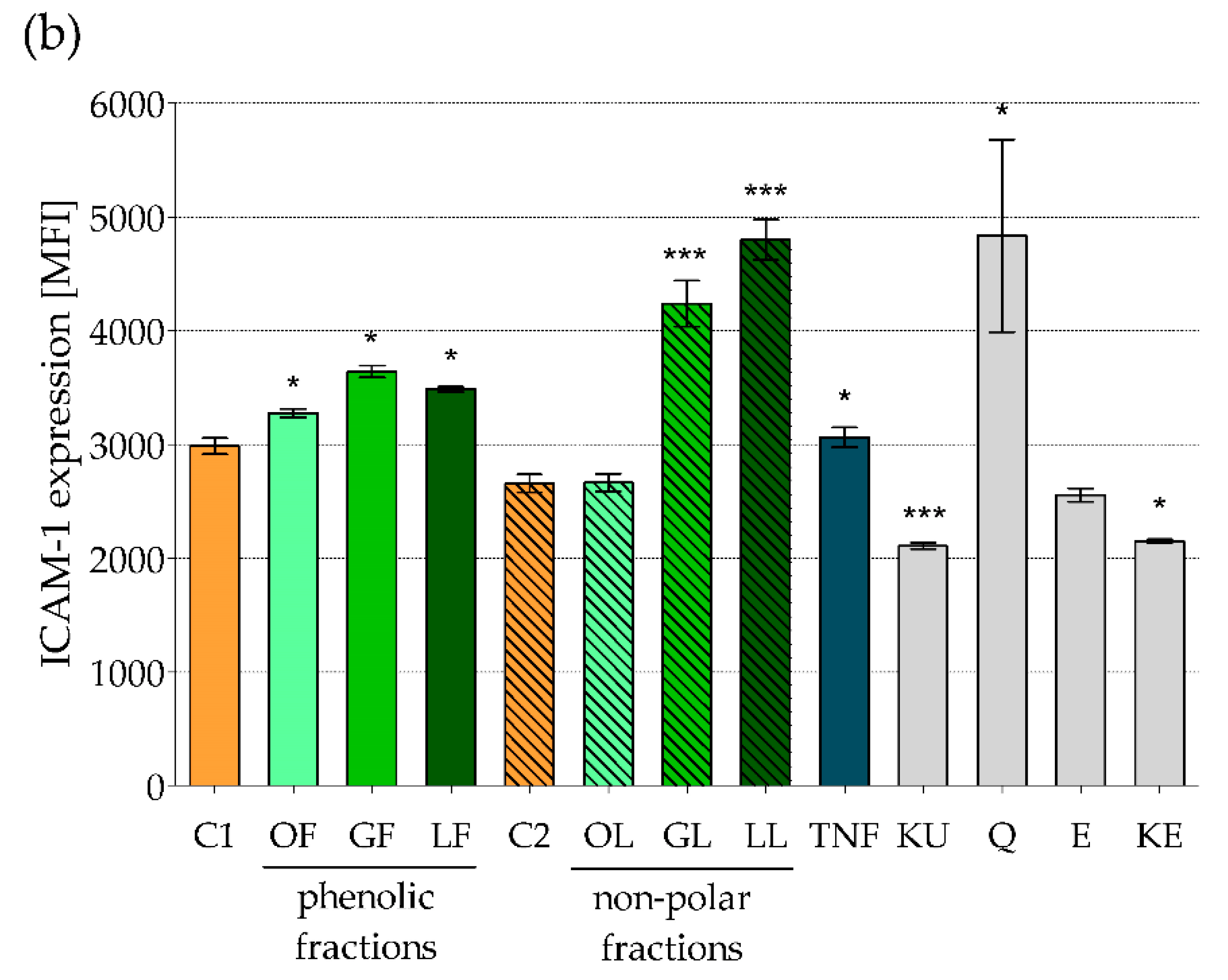
2.1. The Effect of Fractionated E. rhamnoides Extracts and Reference Compounds on Caco-2 Cells
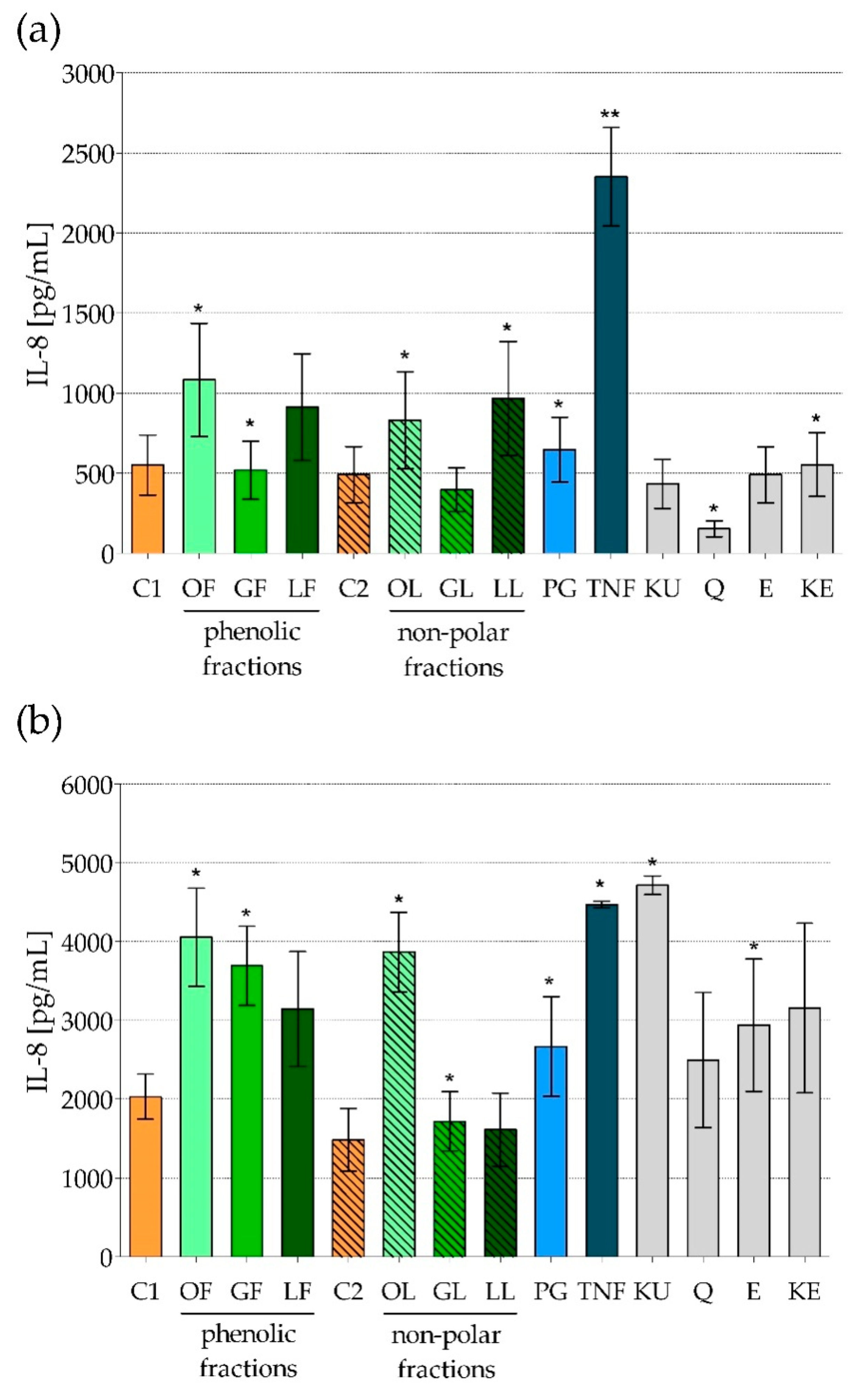
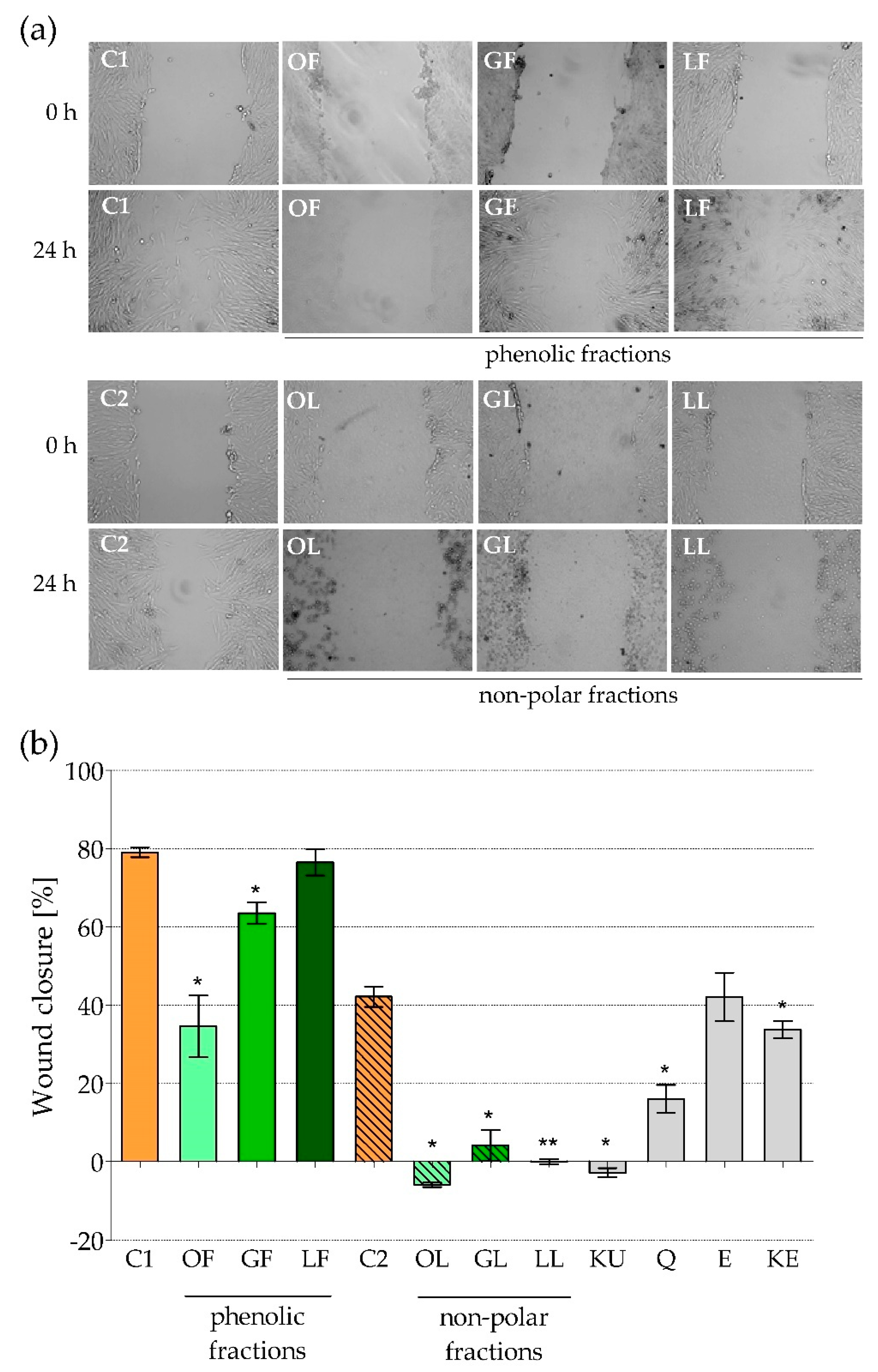
2.2. The Impact of Fractionated E. rhamnoides Extracts and Reference Compounds on HFF-1 Cells
3. Materials and Methods
3.1. Chemicals and Media
3.2. Plant Material
3.3. Preparation of Fractionated Sea Buckthorn Extracts and Phytochemical LC-MS Analyses
3.4. Preparation of the Solutions of Fractionated Sea Buckthorn Extracts and Reference Compounds
3.5. Cell Cultures
3.6. ELISA Assay for Assessment of IL-8, MIP-1α, and TNF-α Production
3.7. Scratch Assay to Determine of the Fibroblasts Migration
3.8. Assessment of ICAM-1 Expression on Caco-2 Cells Using Flow Cytometry
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brglez, M.E.; Knez, H.M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Asp. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef] [PubMed]
- González-Gallego, J.; García-Mediavilla, M.V.; Sánchez-Campos, S.; Tuñón, M.J. Fruit polyphenols, immunity and inflammation. Br. J. Nutr. 2010, 104, S15–S27. [Google Scholar] [CrossRef] [PubMed]
- Michel, T.; Destandau, E.; Le Floch, G.; Lucchesi, M.E.; Elfakir, C. Antimicrobial, antioxidant and phytochemical investigations of sea buckthorn (Hippophaë rhamnoides L.) leaf, stem, root and seed. Food. Chem. 2012, 131, 754–760. [Google Scholar] [CrossRef]
- Olas, B. Sea buckthorn as a source of important bioactive compounds in cardiovascular diseases. Food. Chem. Toxicol. 2016, 97, 199–204. [Google Scholar] [CrossRef]
- Olas, B. Berry phenolic antioxidants—Implications for human health? Front. Pharmacol. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Essafi-Benkhadir, K.; Refai, A.; Riahi, I.; Fattouch, S.; Karoui, H.; Essafi, M. Quince (Cydonia oblonga Miller) peel polyphenols modulate LPS-induced inflammation in human THP-1-derived macrophages through NF-κB, p38MAPK and Akt inhibition. Biochem. Biophys. Res. Commun. 2012, 418, 180–185. [Google Scholar] [CrossRef]
- Mendes, L.F.; Gaspar, V.M.; Conde, T.A.; Mano, J.F.; Duarte, I. Flavonoid-mediated immunomodulation of human macrophages involves key metabolites and metabolic pathways. Sci Rep. 2019, 9, 14906. [Google Scholar] [CrossRef]
- Che, D.N.; Cho, B.O.; Kim, J.S.; Shin, J.Y.; Kang, H.J.; Jang, S.I. Effect of luteolin and apigenin on the production of IL-31 and IL-33 in lipopolysaccharides-activated microglia cells and their mechanism of action. Nutrients 2020, 12, 811. [Google Scholar] [CrossRef]
- Edwards, C.A.; Havlik, J.; Cong, W.; Mullen, W.; Preston, T.; Morrison, D.J.; Combet, E. Polyphenols and health: Interactions between fibre, plant polyphenols and the gut microbiota. Nutr. Bull. 2017, 42, 356–360. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.Y. Metabolism of green tea catechins: An overview. Curr. Drug. Metab. 2006, 7, 755–809. [Google Scholar] [CrossRef] [PubMed]
- Botting, R.A.; Haniffa, M. The immunological network in the developing human skin. Immunology 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; Emanuel, C.; Cutting, K.F.; Williams, D.W. Microbiology of the skin and the role of biofilms in infection. Int. Wound J. 2012, 9, 14–32. [Google Scholar] [CrossRef]
- Radloff, J.; Cornelius, V.; Markov, A.G.; Amasheh, S. Caprate modulates intestinal barrier function in porcine peyer’s patch follicle-associated epithelium. Int. J. Mol. Sci. 2019, 20, 1418. [Google Scholar] [CrossRef]
- Schwerdtfeger, L.A.; Tobet, S.A. Vasoactive intestinal peptide regulates ileal goblet cell production in mice. Physiol. Rep. 2020, 8, e14363. [Google Scholar] [CrossRef]
- Toncic, R.J.; Jakasa, I.; Hadzavdic, S.L.; Goorden, S.M.; Vlugt, K.J.; Stet, F.S.; Balic, A.; Petkovic, M.; Pavicic, B.; Zuzul, K.; et al. Altered levels of sphingosine, sphinganine and their ceramides in atopic dermatitis are related to skin barrier function, disease severity and local cytokine milieu. Int. J. Mol. Sci. 2020, 21, 1958. [Google Scholar] [CrossRef]
- Larmo, P.; Alin, J.; Salminen, E.; Kallio, H.; Tahvonen, R. Effects of sea buckthorn berries on infections and inflammation: A double-blind, randomized, placebo-controlled trial. Eur. J. Clin. Nutr. 2008, 62, 1123–1130. [Google Scholar] [CrossRef]
- Suryakumar, G.; Gupta, A. Medicinal and therapeutic potential of sea buckthorn (Hippophae rhamnoides L.). J. Ethnopharmacol. 2011, 138, 268–278. [Google Scholar] [CrossRef]
- Różalska, B.; Sadowska, B.; Żuchowski, J.; Więckowska-Szakiel, M.; Budzyńska, A.; Wójcik, U.; Stochmal, A. Phenolic and nonpolar fractions of Elaeagnus rhamnoides (L.) A. Nelson extracts as virulence modulators —in vitro study on bacteria, fungi, and epithelial cells. Molecules 2018, 23, 1498. [Google Scholar] [CrossRef]
- Olas, B.; Żuchowski, J.; Lis, B.; Skalski, B.; Kontek, B.; Grabarczyk, Ł.; Stochmal, A. Comparative chemical composition, antioxidant and anticoagulant properties of phenolic fraction (a rich in non-acylated and acylated flavonoids and non-polar compounds) and non-polar fraction from Elaeagnus rhamnoides (L.) A. Nelson fruits. Food. Chem. 2018, 247, 39–45. [Google Scholar] [CrossRef]
- Sandner, G.; Mueller, A.S.; Zhou, X.; Stadlbauer, V.; Schwarzinger, B.; Schwarzinger, C.; Wenzel, U.; Maenner, K.; van der Klis, J.D.; Hirtenlehner, S.; et al. Ginseng extract ameliorates the negative physiological effects of heat stress by supporting heat shock response and improving intestinal barrier integrity: Evidence from studies with heat-stressed Caco-2 cells, C. elegans and growing broilers. Molecules 2020, 25, 835. [Google Scholar] [CrossRef]
- Gonzalez-Aparicio, M.; Alfaro, C. Influence of interleukin-8 and neutrophil extracellular trap (net) formation in the tumor microenvironment: Is there a pathogenic role? J. Immunol. Res. 2019, 2019, 6252138. [Google Scholar] [CrossRef] [PubMed]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [PubMed]
- Anand, D.A.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef]
- Lakhanpal, P.; Rai, D.K. Quercetin: A versatile flavonoid. Internet J. Med. Updat. 2007, 2, 20–35. [Google Scholar] [CrossRef]
- Marunaka, Y.; Marunaka, R.; Sun, H.; Yamamoto, T.; Kanamura, N.; Inui, T.; Taruno, A. Actions of quercetin, a polyphenol, on blood pressure. Molecules 2017, 22, 209. [Google Scholar] [CrossRef]
- Ncube, B.; Van Staden, J. Tilting plant metabolism for improved metabolite biosynthesis and enhanced human benefit. Molecules 2015, 20, 12698–12731. [Google Scholar] [CrossRef]
- Michalski, J.; Deinzer, A.; Stich, L.; Zinser, E.; Steinkasserer, A.; Knippertz, I. Quercetin induces an immunoregulatory phenotype in maturing human dendritic cells. Immunobiology 2020, 151929, in press. [Google Scholar] [CrossRef]
- Kim, I.B.; Kim, D.Y.; Lee, S.J.; Sun, M.J.; Lee, M.S.; Li, H.; Cho, J.J.; Park, C.S. Inhibition of IL-8 production by green tea polyphenols in human nasal fibroblasts and A549 epithelial cells. Biol. Pharm. Bull. 2006, 29, 1120–1125. [Google Scholar] [CrossRef]
- Guan, Q. A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J. Immunol. Res. 2019, 2019, 7247238. [Google Scholar] [CrossRef] [PubMed]
- Hazel, K.; O’Connor, A. Emerging treatments for inflammatory bowel disease. Ther. Adv. Chronic. Dis. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Chow, M.P.; Huang, W.C.; Lin, Y.C.; Chang, Y.J. Flavonoids inhibit tumor necrosis factor-alpha-induced up-regulation of intercellular adhesion molecule-1 (ICAM-1) in respiratory epithelial cells through activator protein-1 and nuclear factor-kappaB: Structure-activity relationships. Mol. Pharmacol. 2004, 66, 683–693. [Google Scholar] [PubMed]
- Freese, R.; Vaarala, O.; Turpeinen, A.M.; Mutanen, M. No difference in platelet activation and inflammation markers after diets rich or poor in vegetables, berries and apple in healthy subjects. Eur. J. Nutr. 2004, 43, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Ohkita, M.; Hayashi, H.; Ito, K.; Shigematsu, N.; Tanaka, R.; Tsutsui, H.; Matsumura, Y. Preventive effects of grape extract on ischemia/reperfusion-induced acute kidney injury in mice. Biol. Pharm. Bull. 2019, 42, 1883–1890. [Google Scholar] [CrossRef]
- Skalski, B.; Stochmal, A.; Żuchowski, J.; Grabarczyk, Ł.; Olas, B. Response of blood platelets to phenolic fraction and non-polar fraction from the leaves and twigs of Elaeagnus rhamnoides (L.) A. Nelson in vitro. Biomed. Pharmacother. 2020, 124, 109897. [Google Scholar] [CrossRef]
- Hao, W.; He, Z.; Zhu, H.; Liu, J.; Kwek, E.; Zhao, Y.; Ma, K.Y.; He, W.S.; Chen, Z.Y. Sea buckthorn seed oil reduces blood cholesterol and modulates gut microbiota. Food Funct. 2019, 10, 5669–5681. [Google Scholar] [CrossRef]
- Li, T.S.C.; Schroeder, W.R. Sea buckthorn (Hippophae rhamnoides L.): A multipurpose plant. HortTechnology 1996, 6, 370–380. [Google Scholar] [CrossRef]
- Bourke, C.D.; Prendergast, C.; Sanin, D.E.; Oulton, T.E.; Hall, R.J.; Mountford, A.P. Epidermal keratinocytes initiate wound healing and pro-inflammatory immune responses following percutaneous schistosome infection. Int. J. Parasitol. 2015, 45, 215–224. [Google Scholar] [CrossRef]
- Ganesh, G.V.; Ramkumar, K.M. Macrophage mediation in normal and diabetic wound healing responses. Inflamm. Res. 2020, 69, 347–363. [Google Scholar] [CrossRef]
- Habbu, P.V.; Joshi, H.; Patil, B.S. Potential wound healers from plant origin. Pharmacogn. Rev. 2007, 1, 271–282. [Google Scholar]
- Shaw, T.J.; Martin, P. Wound repair at a glance. J. Cell. Sci. 2009, 122, 3209–3213. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Jain, U.K. Many of medicinal plants act as wound healers, which has been confirmed in both in vitro and in vivo models. J. Nat. Pharmac. 2010, 1, 2–13. [Google Scholar]
- Sánchez, M.; González-Burgos, E.; Iglesias, I.; Gómez-Serranillos, M.P. Pharmacological update properties of Aloe vera and its major active constituents. Molecules 2020, 25, 1324. [Google Scholar] [CrossRef] [PubMed]
- Hormozi, M.; Assaei, R.; Boroujeni, M.B. The effect of Aloe vera on the expression of wound healing factors (TGF-β1 and bFGF) in mouse embryonic fibroblast cell: In vitro study. Biomed. Pharmacother. 2017, 88, 610–616. [Google Scholar] [CrossRef]
- Negahdari, S.; Galehdari, H.; Kesmati, M.; Rezaie, A.; Shariati, G. Wound healing activity of extracts and formulations of Aloe vera, henna, Adiantum capillus-veneris, and myrrh on mouse dermal fibroblast cells. Int. J. Prev. Med. 2017, 8, 18. [Google Scholar] [CrossRef]
- Moriyama, M.; Moriyama, H.; Uda, J.; Kubo, H.; Nakajima, Y.; Goto, A.; Akaki, J.; Yoshida, I.; Matsuoka, N.; Hayakawa, T. Beneficial effects of the genus aloe on wound healing, cell proliferation, and differentiation of epidermal keratinocytes. PLoS ONE 2016, 11, e0164799. [Google Scholar] [CrossRef]
- Atiba, A.; Ueno, H.; Uzuka, Y. The effect of Aloe vera oral administration on cutaneous wound healing in type 2 diabetic rats. J. Vet. Med. Sci. 2011, 73, 583–589. [Google Scholar] [CrossRef]
- Moghadam, S.E.; Moridi Farimani, M.; Soroury, S.; Ebrahimi, S.N.; Jabbarzadeh, E. Hypermongone C accelerates wound healing through the modulation of inflammatory factors and promotion of fibroblast migration. Molecules 2019, 24, 2022. [Google Scholar] [CrossRef]
- Nagori, B.P.; Solanki, R. Role of medicinal plants in wound healing. Res. J. Med. Plants. 2011, 5, 392–405. [Google Scholar] [CrossRef]
- Pereira, R.F.; Bártolo, P.J. Traditional therapies for skin wound healing. Adv. Wound Care 2016, 5, 208–229. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, N.K.; Kumar, R.; Siddiqui, M.S.; Gupta, A. Mechanism of wound-healing activity of Hippophae rhamnoides L. leaf extract in experimental burns. Evid. Based Complement. Altern. Med. 2011, 2011, 659705. [Google Scholar] [CrossRef] [PubMed]
- Żuchowski, J.; Pecio, Ł.; Marciniak, B.; Kontek, R.; Stochmal, A. Unusual isovalerylated flavonoids from the fruit of sea buckthorn (Elaeagnus rhamnoides) grown in Sokółka, Poland. Phytochemistry 2019, 163, 178–186. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

| Group of Compounds | Relative Peak Area (%) | Dominant Compounds |
|---|---|---|
| phenolic fraction of the fruits (OF) | ||
| Flavonol glycosides | 67.5 | I-3-O-Rut *, I-3-O-Glc *, I-3-O-Glc-7-O-Rha *, Q-3-O-Glc *, rutin *, I-3-O-Glc-7-O-(3′-O-IvA)-Rha |
| Other polar | 21.0 | unidentified, hexose, quinic acid/isomer |
| Triterpenoids and acylated triterpenoids | 9.1 | C30H48O5, C30H48O6 |
| Other non-polar | 2.4 | unidentified |
| non-polar fraction of the fruits (OL) | ||
| Flavonol glycosides | 0.9 | as in the phenolic fraction |
| Other polar | 0.1 | unidentified |
| Triterpenoids and acylated triterpenoids | 83.6 | oleanolic acid *, ursolic acid *, C30H48O5, C30H48O4, C30H48O5-pCouA, C30H48O4-pCouA, C30H48O6-pCouA |
| Other non-polar | 15.4 | unidentified |
| Group of Compounds | Relative Peak Area (%) | Dominant Compounds |
|---|---|---|
| phenolic fraction of the twigs (GF) | ||
| Proanthocyanidins and catechin | 54.3 | catechin, (epi)C-(epi)C, (epi)C-(epi)C-(epi)C, (epi)C-(epi)C-(epi)C-(epi)C, (epi)GC, (epi)C-(epi)GC |
| Flavonol glycosides | 2.3 | I-3-O-Glu-7-O-Rha *, I-3-O-Rut *, K-Hex-CouA |
| Hydrolysable tannins and ellagic acid | 1.9 | ellagic acid, casuarinin/isomer |
| Other polar | 19.8 | dihexose, quinic acid/isomer, unidentified |
| Spermidine derivatives | 10.7 | tricoumaroyl spermidine, feruloyl dicoumaroyl spermidine, diferuloyl coumaroyl spermidine, triferuloyl spermidine |
| Triterpenoids and acylated triterpenoids | 6.7 | C30H48O5, C30H48O4 |
| Other non-polar | 4.3 | unidentified |
| non-polar fraction of the twigs (GL) | ||
| Proanthocyanidins and catechin | 1.3 | catechin, (epi)C-(epi)C, (epi)C-(epi)C-(epi)C |
| Other polar | 1.8 | unidentified |
| Spermidine derivatives | 2.0 | as in the phenolic fraction |
| Triterpenoids and acylated triterpenoids | 89.0 | oleanolic acid *, ursolic acid *, C30H48O5, C30H48O4, C30H48O5-pCouA, C30H48O4-CafA |
| Other non-polar | 5.9 | unidentified |
| Group of Compounds | Relative Peak Area (%) | Dominant Compounds |
|---|---|---|
| phenolic fraction of the leaves (LF) | ||
| Hydrolysable tannins and ellagic acid | 31.3 | casuarinin, casuarictin, strictinin, hippophaenin B or their isomers, ellagic acid |
| Flavonol glycosides | 24.5 | I-3-O-Glc-7-O-Rha *, I-Hex-dHex, I-3-O-Rut *, rutin *, Q-Hex, K-Hex-CouA, I-dHex-Hex-LinA |
| Other polar | 17.4 | dihexose, quinic acid/isomer, (epi)gallocatechin, unidentified |
| Triterpenoid saponins | 15.0 | C71H112O31, C69H110O29, C65H102O27, C65H102O26 |
| Triterpenoids and acylated triterpenoids | 7.6 | C30H48O5, C30H48O4 |
| Other non-polar | 4.2 | unidentified |
| non-polar fraction of the leaves (LL) | ||
| Hydrolysable tannins | 2.7 | casuarinin, hippophaenin B or their isomers |
| Flavonol glycosides | 2.6 | As in the phenolic fraction |
| Other polar | 1.2 | dihexose, quinic acid/isomer, unidentified |
| Triterpenoid saponins | 30.5 | C69H110O29, C63H100O25, C63H100O24, C57H90O20 |
| Triterpenoids and acylated triterpenoids | 50.7 | oleanolic acid *, ursolic acid *, C30H48O5, C30H48O4, C30H48O5-pCouA, C30H48O4-pCouA, C30H48O5-FerA |
| Other non-polar | 12.3 | unidentified |
| Preparation * | Action 6 h | Action 24 h | Control/Significance 6 h; 24 h |
|---|---|---|---|
| OF | ↓ 1.3× | ↓ 1.2× | C1/NS; NS |
| GF | ↓ 2.3× | ↓ 2.1× | C1/p = 0.0138; p = 0.0345 |
| LF | ↓ 3.5× | ↓ 2.0× | C1/p = 0.0138; p = 0.0345 |
| OL | ↓ 2.2× | ↓ 2.9× | C2/p = 0.0138; p = 0.0048 |
| GL | ↓ 1.5× | ↑ 1.3× | C2/p = 0.0138; p = 0.0345 |
| LL | ↓ 1.4× | ↓ 1.2× | C2/p = 0.0138; NS |
| PG | ↑ 1.3× | ↑ 1.7× | C2/p = 0.0138; p = 0.0345 |
| TNF | ↑ 12.5× | ↑ 11.6× | C2/p = 0.0138; p = 0.0345 |
| KU | ↓ 1.2× | ↑ 1.4× | C2/p = 0.0345; p = 0.0345 |
| Q | ↓ 2.7× | ↓ 4.4× | C2/p = 0.0138; p = 0.0345 |
| E | ↓ 1.5× | ↓ 1.3× | C2/p = 0.0138; p = 0.0345 |
| KE | ↓ 2.3× | ↓ 1.1× | C2/p = 0.0138; NS |
| Preparation * | Action 6 h | Action 24 h | Control/Significance 6 h; 24 h |
|---|---|---|---|
| OF | ↑ 2.0× | ↑ 2.0× | C1/p = 0.0138; p = 0.0138 |
| GF | ↓ 1.1× | ↑ 1.8× | C1/p = 0.0138; p = 0.0138 |
| LF | ↑ 1.7× | ↑ 1.5× | C1/NS; NS |
| OL | ↑ 1.7× | ↑ 2.6× | C2/p = 0.0138; p = 0.0133 |
| GL | ↓ 1.2× | ↑ 1.2× | C2/NS; p = 0.0138 |
| LL | ↑ 2.0× | ↑ 1.1× | C2/p = 0.0138; NS |
| PG | ↑ 1.3× | ↑ 1.8× | C2/p = 0.0138; p = 0.0138 |
| TNF | ↑ 4.8× | ↑ 3.0× | C2/p = 0.009; p = 0.0138 |
| KU | ↓ 1.1× | ↑ 3.2× | C2/NS; p = 0.0138 |
| Q | ↓ 3.2× | ↑ 1.7× | C2/p = 0.0138; NS |
| E | no change | ↑ 2.0× | C2/NS; p = 0.0138 |
| KE | ↑ 1.1× | ↑ 2.1× | C2/p = 0.0138; NS |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadowska, B.; Rywaniak, J.; Cichocka, A.; Cichocka, K.; Żuchowski, J.; Wójcik-Bojek, U.; Więckowska-Szakiel, M.; Różalska, B. Phenolic and Non-Polar Fractions of the Extracts from Fruits, Leaves, and Twigs of Elaeagnus rhamnoides (L.) A. Nelson—The Implications for Human Barrier Cells. Molecules 2020, 25, 2238. https://doi.org/10.3390/molecules25092238
Sadowska B, Rywaniak J, Cichocka A, Cichocka K, Żuchowski J, Wójcik-Bojek U, Więckowska-Szakiel M, Różalska B. Phenolic and Non-Polar Fractions of the Extracts from Fruits, Leaves, and Twigs of Elaeagnus rhamnoides (L.) A. Nelson—The Implications for Human Barrier Cells. Molecules. 2020; 25(9):2238. https://doi.org/10.3390/molecules25092238
Chicago/Turabian StyleSadowska, Beata, Joanna Rywaniak, Anna Cichocka, Kinga Cichocka, Jerzy Żuchowski, Urszula Wójcik-Bojek, Marzena Więckowska-Szakiel, and Barbara Różalska. 2020. "Phenolic and Non-Polar Fractions of the Extracts from Fruits, Leaves, and Twigs of Elaeagnus rhamnoides (L.) A. Nelson—The Implications for Human Barrier Cells" Molecules 25, no. 9: 2238. https://doi.org/10.3390/molecules25092238
APA StyleSadowska, B., Rywaniak, J., Cichocka, A., Cichocka, K., Żuchowski, J., Wójcik-Bojek, U., Więckowska-Szakiel, M., & Różalska, B. (2020). Phenolic and Non-Polar Fractions of the Extracts from Fruits, Leaves, and Twigs of Elaeagnus rhamnoides (L.) A. Nelson—The Implications for Human Barrier Cells. Molecules, 25(9), 2238. https://doi.org/10.3390/molecules25092238