A Heterometallic Three-Dimensional Metal−Organic Framework Bearing an Unprecedented One-Dimensional Branched-Chain Secondary Building Unit
Abstract
1. Introduction
2. Results and Discussion
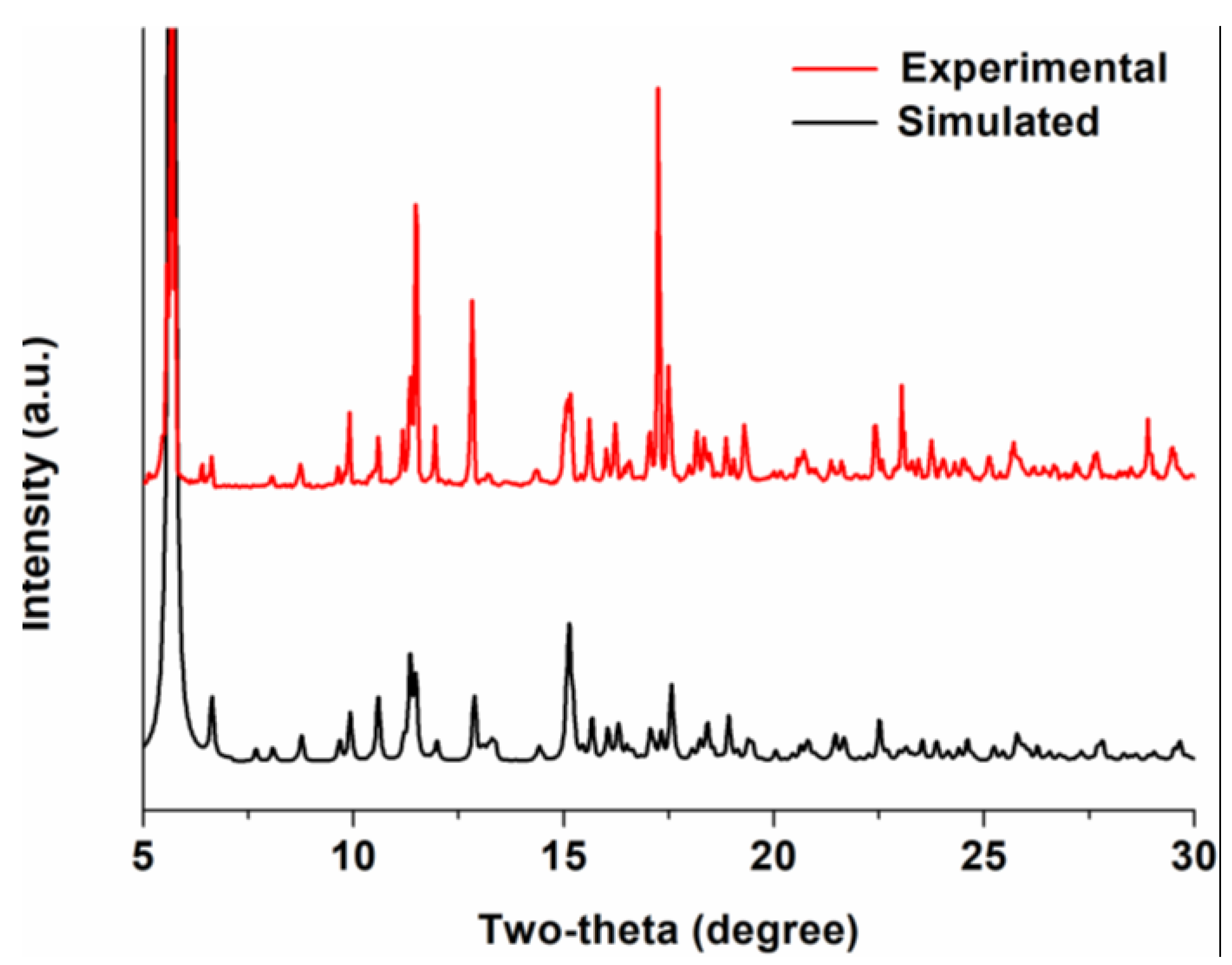
2.1. Synthesis and Material Characterization of MOF 1
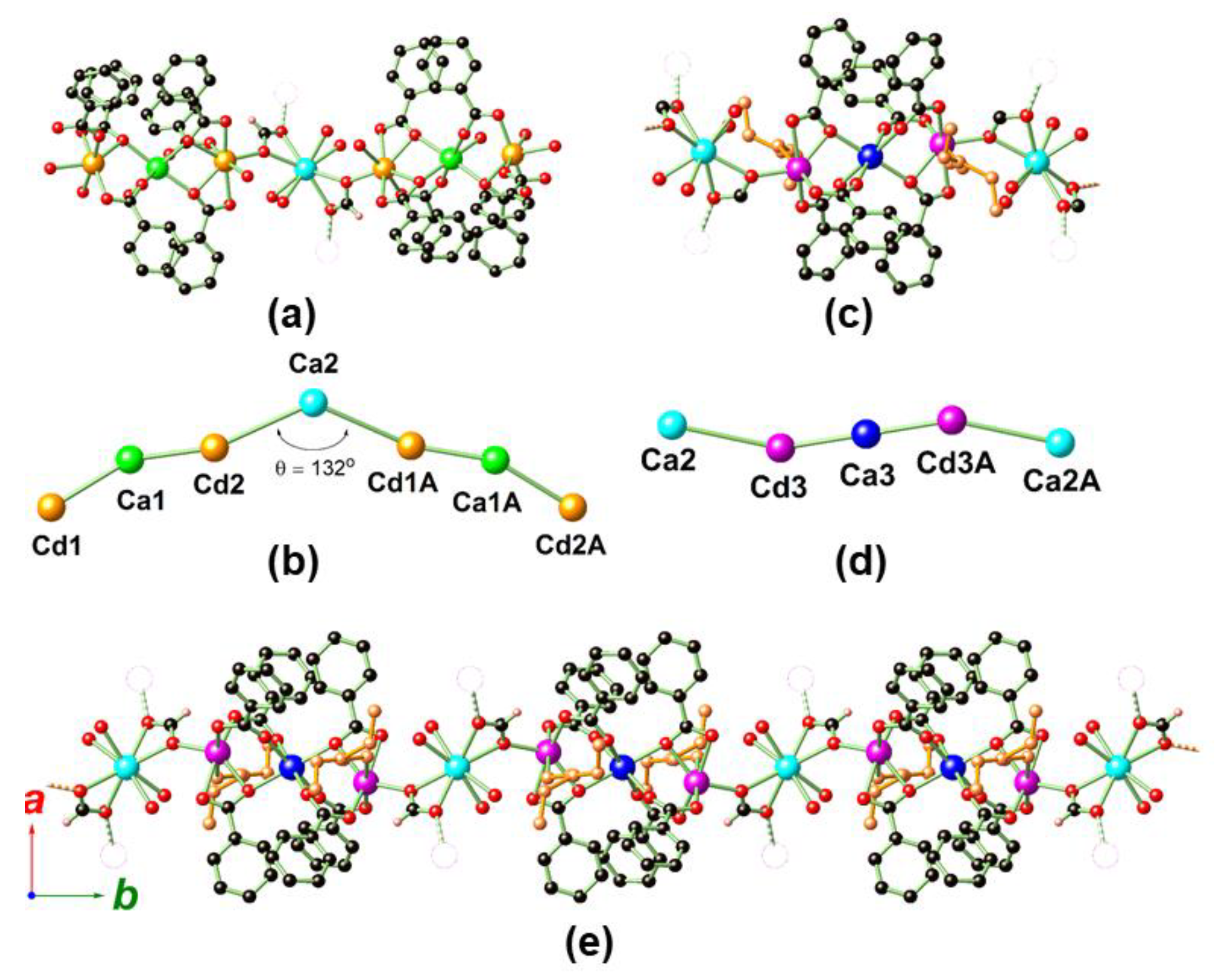
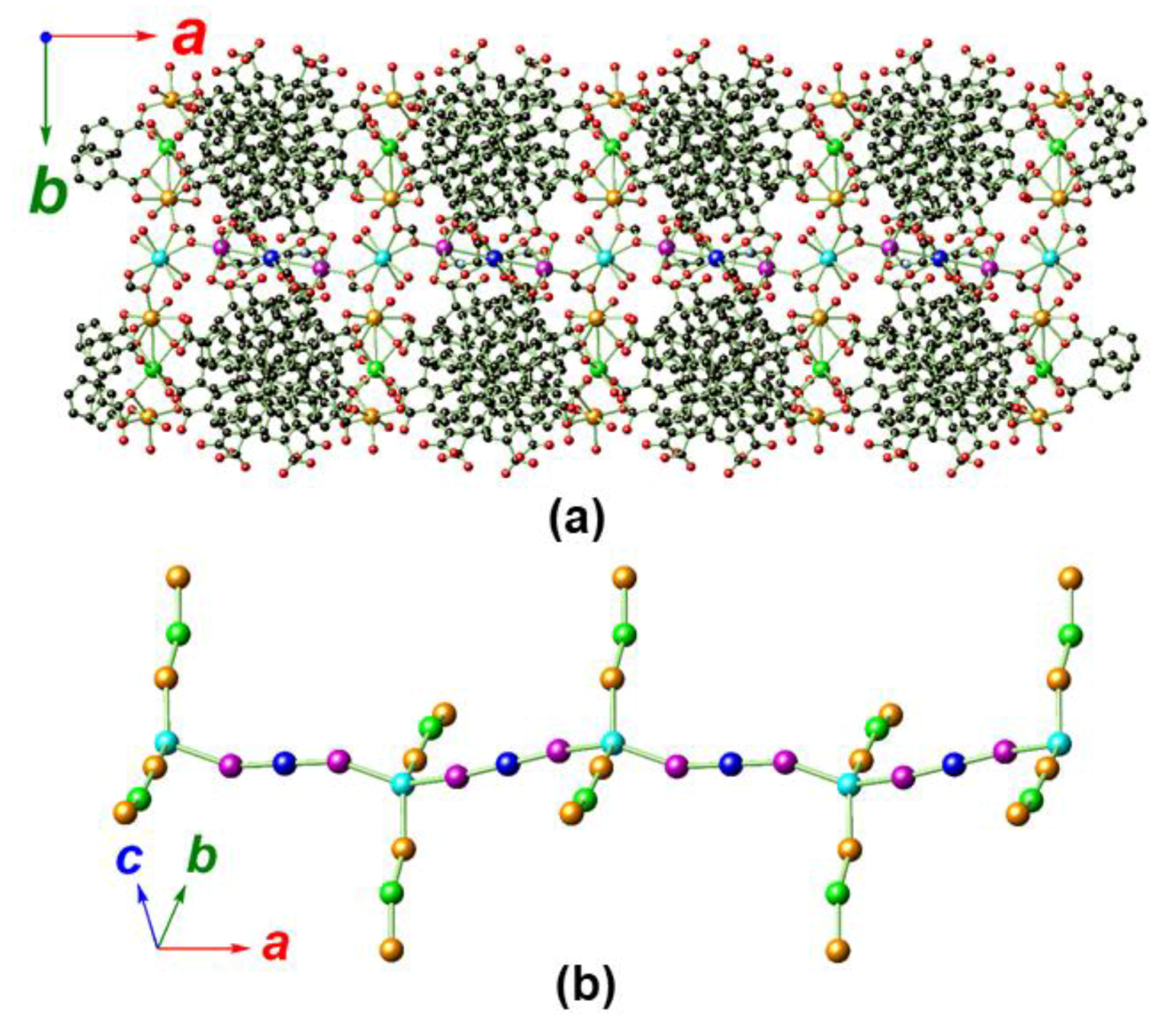
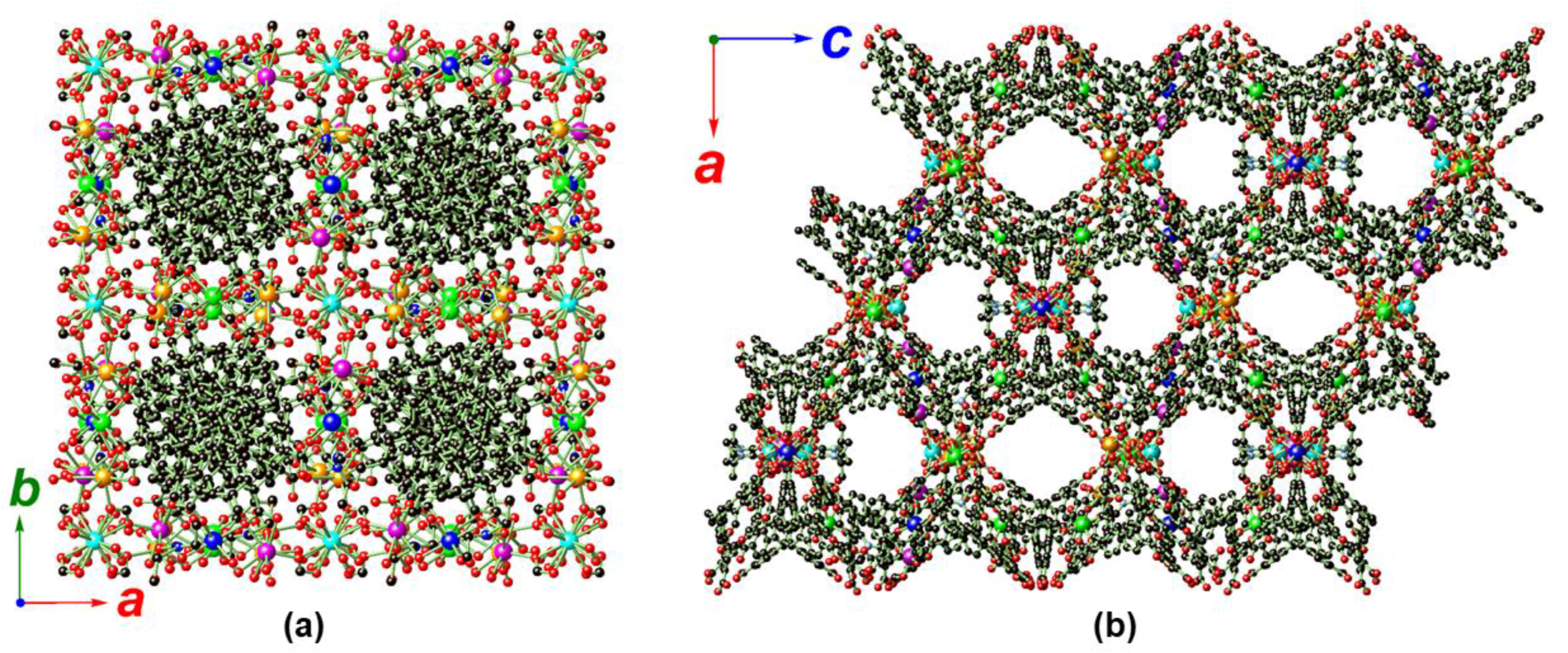
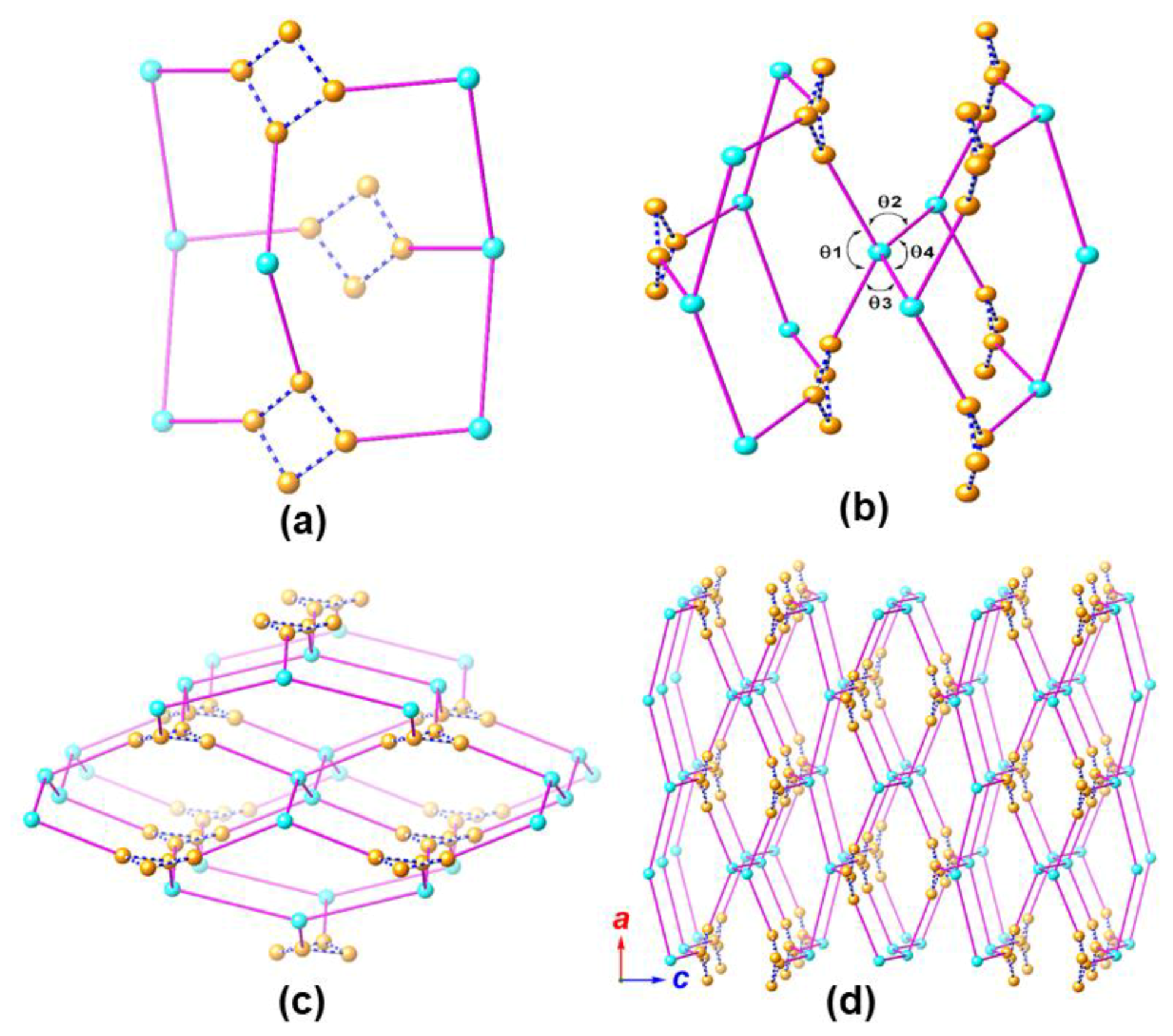
2.2. Crystal Structure Analysis of MOF 1
3. Synthesis and Material Characterization
3.1. General
3.2. Synthesis of [Cd6Ca4(BTB)6(HCOO)2(DEF)2(H2O)12]∙DEF∙xSol. (MOF 1)
3.3. X-Ray Crystallography for MOF 1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, L.; Liu, X.-Q.; Jiang, H.-L.; Sun, L.-B. Metal–organic frameworks for heterogeneous basic catalysis. Chem. Rev. 2017, 117, 8129–8176. [Google Scholar] [CrossRef] [PubMed]
- Buchwalter, P.; Rosé, J.; Braunstein, P. Multimetallic catalysis based on heterometallic complexes and clusters. Chem. Rev. 2015, 115, 28–126. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Zhang, J.; Xu, Q.; Wu, X.-T.; Zhu, Q.-L. Pore surface engineering of metal–organic frameworks for heterogeneous catalysis. Coord. Chem. Rev. 2018, 376, 248–276. [Google Scholar] [CrossRef]
- Sun, W.; He, C.; Liu, T.; Duan, C. Synergistic catalysis for light-driven proton reduction using a polyoxometalate-based Cu–Ni heterometallic–organic framework. Chem. Commmun. 2019, 55, 3805–3808. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, G.-P.; Jin, J.; Wu, D.; Ma, L.-F.; Wang, Y.-Y. A first new porous d–p HMOF material with multiple active sites for excellent CO2 capture and catalysis. Chem. Commmun. 2020, 56, 2395–2398. [Google Scholar] [CrossRef] [PubMed]
- Broere, D.L.J.; Modder, D.K.; Blokker, E.; Siegler, M.A.; van der Vlugt, J.I. Metal–metal interactions in heterobimetallic complexes with dinucleating redox-active ligands. Angew. Chem. Int. Ed. 2016, 55, 2406–2410. [Google Scholar] [CrossRef] [PubMed]
- Hinde, C.S.; Webb, W.R.; Chew, B.K.J.; Tan, H.R.; Zhang, W.-H.; Hor, T.S.A.; Raja, R. Utilisation of gold nanoparticles on amine-functionalised UiO-66 (NH2-UiO-66) nanocrystals for selective tandem catalytic reactions. Chem. Commun. 2016, 52, 6557–6560. [Google Scholar] [CrossRef]
- Nguyen, A.I.; Suess, D.L.M.; Darago, L.E.; Oyala, P.H.; Levine, D.S.; Ziegler, M.S.; Britt, R.D.; Tilley, T.D. Manganese–cobalt oxido cubanes relevant to manganese-doped water oxidation catalysts. J. Am. Chem. Soc. 2017, 139, 5579–5587. [Google Scholar] [CrossRef]
- Botas, J.A.; Calleja, G.; Sánchez-Sánchez, M.; Orcajo, M.G. Cobalt doping of the MOF-5 framework and its effect on gas-adsorption properties. Langmuir 2010, 26, 5300–5303. [Google Scholar] [CrossRef]
- Song, X.; Jeong, S.; Kim, D.; Lah, M.S. Transmetalations in two metal-organic frameworks with different framework flexibilities: Kinetics and core-shell heterostructure. CrystEngComm 2012, 14, 5753–5756. [Google Scholar] [CrossRef]
- Song, X.; Kim, T.K.; Kim, H.; Kim, D.; Jeong, S.; Moon, H.R.; Lah, M.S. Post-synthetic modifications of framework metal ions in isostructural metal–organic frameworks: Core–shell heterostructures via selective transmetalations. Chem. Mater. 2012, 24, 3065–3073. [Google Scholar] [CrossRef]
- Zhai, Q.-G.; Bu, X.; Mao, C.; Zhao, X.; Feng, P. Systematic and dramatic tuning on gas sorption performance in heterometallic metal−organic frameworks. J. Am. Chem. Soc. 2016, 138, 2524–2527. [Google Scholar] [CrossRef] [PubMed]
- Dincă, M.; Long, J.R. High-enthalpy hydrogen adsorption in cation-exchanged variants of the microporous metal−organic framework Mn3[(Mn4Cl)3(BTT)8(CH3OH)10]2. J. Am. Chem. Soc. 2007, 129, 11172–11176. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Dhas, N.; Deshmukh, P.; Caro, C.; Patil, P.; Luisa García-Martín, M.; Padya, B.; Nikam, A.; Mehta, T.; Mutalik, S. Heterogeneous surface architectured metal-organic frameworks for cancer therapy, imaging, and biosensing: A state-of-the-art review. Coord. Chem. Rev. 2020, 409, 213212. [Google Scholar] [CrossRef]
- Hao, C.; Wu, X.; Sun, M.; Zhang, H.; Yuan, A.; Xu, L.; Xu, C.; Kuang, H. Chiral core–shell upconversion nanoparticle@MOF nanoassemblies for quantification and bioimaging of reactive oxygen species in vivo. J. Am. Chem. Soc. 2019, 141, 19373–19378. [Google Scholar] [CrossRef]
- Wang, H.-S. Metal–organic frameworks for biosensing and bioimaging applications. Coord. Chem. Rev. 2017, 349, 139–155. [Google Scholar] [CrossRef]
- Zhang, W.-H.; Ren, Z.-G.; Lang, J.-P. Rational construction of functional molybdenum (tungsten)-copper-sulfur coordination oligomers and polymers from preformed cluster precursors. Chem. Soc. Rev. 2016, 45, 4995–5019. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, W.-H.; Lang, J.-P. Versatile thiomolybdate(thiotungstate)–copper–sulfide clusters and multidimensional polymers linked by cyanides. Coord. Chem. Rev. 2017, 350, 248–274. [Google Scholar] [CrossRef]
- Qiao, X.; Ma, Z.; Si, L.; Ding, W.; Xu, G. Doping metal-organic framework with a series of europium-antenna cations: Obviously improved spectral response for O2 gas via long-range energy roll-back procedure. Sens. Actuators B Chem. 2019, 299, 126978. [Google Scholar] [CrossRef]
- Rybak, J.-C.; Hailmann, M.; Matthes, P.R.; Zurawski, A.; Nitsch, J.; Steffen, A.; Heck, J.G.; Feldmann, C.; Goetzendoerfer, S.; Meinhardt, J.; et al. Metal−organic framework luminescence in the yellow gap by codoping of the homoleptic imidazolate ∞3[Ba(Im)2] with divalent europium. J. Am. Chem. Soc. 2013, 135, 6896–6902. [Google Scholar] [CrossRef]
- Zheng, F.; Yang, Y.; Chen, Q. High lithium anodic performance of highly nitrogen-doped porous carbon prepared from a metal−organic framework. Nat. Commun. 2014, 5, 5261. [Google Scholar] [CrossRef] [PubMed]
- Sapianik, A.A.; Zorina-Tikhonova, E.N.; Kiskin, M.A.; Samsonenko, D.G.; Kovalenko, K.A.; Sidorov, A.A.; Eremenko, I.L.; Dybtsev, D.N.; Blake, A.J.; Argent, S.P.; et al. Rational synthesis and investigation of porous metal−organic framework materials from a preorganized heterometallic carboxylate building block. Inorg. Chem. 2017, 56, 1599–1608. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C.A.; Jones, S.C.; Kinnibrugh, T.L.; Tongwa, P.; Farrell, R.A.; Vakil, A.; Timofeeva, T.V.; Khrustalev, V.N.; Allendorf, M.D. Homo- and heterometallic luminescent 2D stilbene metal−organic frameworks. Dalton Trans. 2014, 43, 2925–2935. [Google Scholar] [CrossRef] [PubMed]
- Abednatanzi, S.; Gohari Derakhshandeh, P.; Depauw, H.; Coudert, F.-X.; Vrielinck, H.; Van Der Voort, P.; Leus, K. Mixed-metal metal–organic frameworks. Chem. Soc. Rev. 2019, 48, 2535–2565. [Google Scholar] [CrossRef]
- Castells-Gil, J.; Padial Natalia, M.; Almora-Barrios, N.; Albero, J.; Ruiz-Salvador, A.R.; González-Platas, J.; García, H.; Martí-Gastaldo, C. Chemical engineering of photoactivity in heterometallic titanium–organic frameworks by metal doping. Angew. Chem. Int. Ed. 2018, 57, 8453–8457. [Google Scholar] [CrossRef]
- Chao, M.-Y.; Chen, J.; Young, D.J.; Zhang, W.-H.; Lang, J.-P. Smoothing the single-crystal to single-crystal conversions of a two-dimensional metal–organic framework via the hetero-metal doping of the linear trimetallic secondary building unit. Dalton. Trans. 2018, 47, 13722–13729. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, W.-H.; Lang, J.-P. Site-selective homo- and hetero-metallic doping of a 1D Zn-based coordination polymer to enhance the dimensionality and photocurrent responses. CrystEngComm 2016, 18, 3048–3054. [Google Scholar] [CrossRef]
- Chen, C.; Wang, N.; Long, Y.; Gao, J.; Xie, W.; Ran, X.; Yue, S. Series of novel 3D microporous heterometallic 3d-4f coordination frameworks with (5,6)-connected topology: Synthesis, crystal structure and magnetic properties. CrystEngComm 2013, 15, 4611–4616. [Google Scholar] [CrossRef]
- Bo, Q.-B.; Wang, H.-Y.; Wang, D.-Q.; Zhang, Z.-W.; Miao, J.-L.; Sun, G.-X. Structure and photoluminescence tuning features of Mn2+- and Ln3+-activated Zn-based heterometal–organic frameworks (MOFs) with a single 5-methylisophthalic acid ligand. Inorg. Chem. 2011, 50, 10163–10177. [Google Scholar] [CrossRef]
- Schoedel, A.; Zaworotko, M.J. [M3(μ3-O)(O2CR)6] and related trigonal prisms: Versatile molecular building blocks for crystal engineering of metal−organic material platforms. Chem. Sci. 2014, 5, 1269–1282. [Google Scholar] [CrossRef]
- Perry, J.J.I.V.; Perman, J.A.; Zaworotko, M.J. Design and synthesis of metal−organic frameworks using metal−organic polyhedra as supermolecular building blocks. Chem. Soc. Rev. 2009, 38, 1400–1417. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Wang, K.-Y.; Day, G.S.; Zhou, H.-C. The chemistry of multi-component and hierarchical framework compounds. Chem. Soc. Rev. 2019, 48, 4823–4853. [Google Scholar] [CrossRef] [PubMed]
- Tu, B.; Diestel, L.; Shi, Z.-L.; Bandara, W.R.L.N.; Chen, Y.; Lin, W.; Zhang, Y.-B.; Telfer, S.G.; Li, Q. Harnessing bottom-up self-assembly to position five distinct components in an ordered porous framework. Angew. Chem. Int. Ed. 2019, 58, 5348–5353. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.; Tu, B.; Li, Q. Metal–organic frameworks with multicomponents in order. Coord. Chem. Rev. 2019, 388, 107–125. [Google Scholar] [CrossRef]
- Lin, Q.; Bu, X.; Kong, A.; Mao, C.; Zhao, X.; Bu, F.; Feng, P. New heterometallic zirconium metalloporphyrin frameworks and their heteroatom-activated high-surface-area carbon derivatives. J. Am. Chem. Soc. 2015, 137, 2235–2238. [Google Scholar] [CrossRef]
- Zhang, W.-H.; Liu, Q.; Lang, J.-P. Heterometallic transition metal clusters and cluster-supported coordination polymers derived from Tp- and Tp*-based Mo(W) sulfido precursors. Coord. Chem. Rev. 2015, 293–294, 187–210. [Google Scholar] [CrossRef]
- Kim, I.S.; Ahn, S.; Vermeulen, N.A.; Webber, T.E.; Gallington, L.C.; Chapman, K.W.; Penn, R.L.; Hupp, J.T.; Farha, O.K.; Notestein, J.M.; et al. The synthesis science of targeted vapor-phase metal–organic framework postmodification. J. Am. Chem. Soc. 2020, 142, 242–250. [Google Scholar] [CrossRef]
- Zheng, J.; Ye, J.; Ortuño, M.A.; Fulton, J.L.; Gutiérrez, O.Y.; Camaioni, D.M.; Motkuri, R.K.; Li, Z.; Webber, T.E.; Mehdi, B.L.; et al. Selective methane oxidation to methanol on Cu-oxo dimers stabilized by zirconia nodes of an NU-1000 metal–organic framework. J. Am. Chem. Soc. 2019, 141, 9292–9304. [Google Scholar] [CrossRef]
- Dunning Samuel, G.; Nandra, G.; Conn Adam, D.; Chai, W.; Sikma, R.E.; Lee Ji, S.; Kunal, P.; Reynolds Joseph, E.; Chang, J.-S.; Steiner, A.; et al. A metal–organic framework with cooperative phosphines that permit post-synthetic installation of open metal sites. Angew. Chem. Int. Ed. 2018, 57, 9295–9299. [Google Scholar] [CrossRef]
- Huxley, M.; Coghlan, C.J.; Burgun, A.; Tarzia, A.; Sumida, K.; Sumby, C.J.; Doonan, C.J. Site-specific metal and ligand substitutions in a microporous Mn2+-based metal−organic framework. Dalton Trans. 2016, 45, 4431–4438. [Google Scholar] [CrossRef]
- Bloch, W.M.; Burgun, A.; Doonan, C.J.; Sumby, C.J. Probing post-synthetic metallation in metal−organic frameworks: Insights from X-ray crystallography. Chem. Commun. 2015, 51, 5486–5489. [Google Scholar] [CrossRef]
- Evans, J.D.; Sumby, C.J.; Doonan, C.J. Post-synthetic metalation of metal−organic frameworks. Chem. Soc. Rev. 2014, 43, 5933–5951. [Google Scholar] [CrossRef]
- Blake, A.J.; Champness, N.R.; Easun, T.L.; Allan, D.R.; Nowell, H.; George, M.W.; Jia, J.; Sun, X.-Z. Photoreactivity examined through incorporation in metal−organic frameworks. Nat. Chem. 2010, 2, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-X.; Pan, W.-L.; Niu, R.-J.; Liu, Y.; Chen, J.-X.; Zhang, W.-H.; Lang, J.-P.; Young, D.J. Effective loading of cisplatin into a nanoscale UiO-66 metal–organic framework with preformed defects. Dalton Trans. 2019, 48, 5308–5314. [Google Scholar] [CrossRef] [PubMed]
- Yee, K.-K.; Reimer, N.; Liu, J.; Cheng, S.-Y.; Yiu, S.-M.; Weber, J.; Stock, N.; Xu, Z. Effective mercury sorption by thiol-laced metal−organic frameworks: In strong acid and the vapor phase. J. Am. Chem. Soc. 2013, 135, 7795–7798. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.-L.; Yee, K.-K.; Wong, Y.-L.; Zha, M.; He, J.; Zeller, M.; Hunter, A.D.; Yang, K.; Xu, Z. Metalation triggers single crystalline order in a porous solid. J. Am. Chem. Soc. 2016, 138, 14852–14855. [Google Scholar] [CrossRef]
- Zanon, A.; Verpoort, F. Metals@ZIFs: Catalytic applications and size selective catalysis. Coord. Chem. Rev. 2017, 353, 201–222. [Google Scholar] [CrossRef]
- Smith, S.J.D.; Ladewig, B.P.; Hill, A.J.; Lau, C.H.; Hill, M.R. Post-synthetic Ti exchanged UiO-66 metal-organic frameworks that deliver exceptional gas permeability in mixed matrix membranes. Sci. Rep. 2015, 5, 7823. [Google Scholar] [CrossRef]
- Lalonde, M.; Bury, W.; Karagiaridi, O.; Brown, Z.; Hupp, J.T.; Farha, O.K. Transmetalation: Routes to metal exchange within metal-organic frameworks. J. Mater. Chem. A 2013, 1, 5453–5468. [Google Scholar] [CrossRef]
- Chen, J.-X.; Chen, M.; Ding, N.-N.; Chen, W.-H.; Zhang, W.-H.; Hor, T.S.A.; Young, D.J. Transmetalation of a dodecahedral Na9 aggregate-based polymer: A facile route to water stable Cu(II) coordination networks. Inorg. Chem. 2014, 53, 7446–7454. [Google Scholar] [CrossRef]
- Jin, L.; Li, X.-X.; Qi, Y.-J.; Niu, P.-P.; Zheng, S.-T. Giant hollow heterometallic polyoxoniobates with sodalite-type lanthanide–tungsten–oxide cages: Discrete nanoclusters and extended frameworks. Angew. Chem. Int. Ed. 2016, 55, 13793–13797. [Google Scholar] [CrossRef] [PubMed]
- Brozek, C.K.; Dincă, M. Cation exchange at the secondary building units of metal−organic frameworks. Chem. Soc. Rev. 2014, 43, 5456–5467. [Google Scholar] [CrossRef]
- Albalad, J.; Arinez-Soriano, J.; Vidal-Gancedo, J.; Lloveras, V.; Juanhuix, J.; Imaz, I.; Aliaga-Alcalde, N.; Maspoch, D. Hetero-bimetallic paddlewheel clusters in coordination polymers formed by a water-induced single-crystal-to-single-crystal transformation. Chem. Commun. 2016, 52, 13397–13400. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Kim, H.; Kim, K. Metathesis in single crystal: Complete and reversible exchange of metal ions constituting the frameworks of metal−organic frameworks. J. Am. Chem. Soc. 2009, 131, 3814–3815. [Google Scholar] [CrossRef] [PubMed]
- Brozek, C.K.; Dincă, M. Ti3+-, V2+/3+-, Cr2+/3+-, Mn2+-, and Fe2+-substituted MOF-5 and redox reactivity in Cr- and Fe-MOF-5. J. Am. Chem. Soc. 2013, 135, 12886–12891. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Feng, D.; Liu, T.-F.; Chen, Y.-P.; Yuan, S.; Wang, K.; Wang, X.; Fordham, S.; Zhou, H.-C. A versatile synthetic route for the preparation of titanium metal−organic frameworks. Chem. Sci. 2016, 7, 1063–1069. [Google Scholar] [CrossRef]
- Liu, T.-F.; Zou, L.; Feng, D.; Chen, Y.-P.; Fordham, S.; Wang, X.; Liu, Y.; Zhou, H.-C. Stepwise synthesis of robust metal−organic frameworks via postsynthetic metathesis and oxidation of metal nodes in a single-crystal to single-crystal transformation. J. Am. Chem. Soc. 2014, 136, 7813–7816. [Google Scholar] [CrossRef]
- Zhu, X.-W.; Zhou, X.-P.; Li, D. Exceptionally water stable heterometallic gyroidal MOFs: Tuning the porosity and hydrophobicity by doping metal ions. Chem. Commun. 2016, 52, 6513–6516. [Google Scholar] [CrossRef]
- Jiang, L.; Wu, N.; Li, Q.; Li, J.; Wu, D.; Li, Y. Heterometallic strategy for enhancing the dynamic separation of C2H2/CO2: A linear pentanuclear cluster-based metal–organic framework. Inorg. Chem. 2019, 58, 4080–4084. [Google Scholar] [CrossRef]
- Marcus, Y. Ionic radii in aqueous solutions. Chem. Rev. 1988, 88, 1475–1498. [Google Scholar] [CrossRef]
- Banerjee, D.; Parise, J.B. Recent advances in s-block metal carboxylate networks. Cryst. Growth Des. 2011, 11, 4704–4720. [Google Scholar] [CrossRef]
- Zhang, Z.-X.; Ding, N.-N.; Zhang, W.-H.; Chen, J.-X.; Young, D.J.; Hor, T.S.A. Stitching 2D polymeric layers into flexible interpenetrated metal−organic frameworks within single crystals. Angew. Chem. Int. Ed. 2014, 53, 4628–4632. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.-Y.; Chen, J.; Hao, Z.-M.; Tang, X.-Y.; Ding, L.; Zhang, W.-H.; Young, D.J.; Lang, J.-P. A single-crystal to single-crystal conversion scheme for a two-dimensional metal–organic framework bearing linear Cd3 secondary building units. Cryst. Growth Des. 2019, 19, 724–729. [Google Scholar] [CrossRef]
- Xiang, H.; Gao, W.-Y.; Zhong, D.-C.; Jiang, L.; Lu, T.-B. The diverse structures of Cd(II) coordination polymers with 1,3,5-benzenetribenzoate tuned by organic bases. CrystEngComm 2011, 13, 5825–5832. [Google Scholar] [CrossRef]
- Chao, M.-Y.; Chen, J.; Wu, X.-Y.; Wang, R.-Y.; Wang, P.-P.; Ding, L.; Young, D.J.; Zhang, W.-H. Unconventional pyridyl ligand inclusion within a flexible metal-organic framework bearing an N,N′-diethylformamide (DEF)-solvated Cd5 cluster secondary building unit. ChemPlusChem 2020, 85, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bredenkoetter, B.; Rieger, B.; Volkmer, D. Two-dimensional metal−organic frameworks (MOFs) constructed from heterotrinuclear coordination units and 4,4′-biphenyldicarboxylate ligands. Dalton Trans. 2007, 689–696. [Google Scholar] [CrossRef]
- Paul, A.; Connolly, D.; Schulz, M.; Pryce, M.T.; Vos, J.G. Effect of water during the quantitation of formate in photocatalytic studies on CO2 reduction in dimethylformamide. Inorg. Chem. 2012, 51, 1977–1979. [Google Scholar] [CrossRef]
- Burrows, A.D.; Cassar, K.; Friend, R.M.W.; Mahon, M.F.; Rigby, S.P.; Warren, J.E. Solvent hydrolysis and templating effects in the synthesis of metal−organic frameworks. CrystEngComm 2005, 7, 548–550. [Google Scholar] [CrossRef]
- Armaghan, M.; Shang, X.J.; Yuan, Y.Q.; Young, D.J.; Zhang, W.H.; Hor, T.S.A.; Lang, J.P. Metal−organic frameworks via emissive metal-carboxylate zwitterion intermediates. ChemPlusChem 2015, 80, 1231–1234. [Google Scholar] [CrossRef]
- Shi, Y.-X.; Hu, F.-L.; Zhang, W.-H.; Lang, J.-P. A unique Zn(II)-based MOF fluorescent probe for the dual detection of nitroaromatics and ketones in water. CrystEngComm 2015, 17, 9404–9412. [Google Scholar] [CrossRef]
- Mu, B.; Huang, Y.; Walton, K.S. A metal−organic framework with coordinatively unsaturated metal centers and microporous structure. CrystEngComm 2010, 12, 2347–2349. [Google Scholar] [CrossRef]
- Chao, M.-Y.; Zhang, W.-H.; Lang, J.-P. Co2 and Co3 mixed cluster secondary building unit approach toward a three-dimensional metal-organic framework with permanent porosity. Molecules 2018, 23, 755. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.-L.; Yuan, Y.-Q.; Chao, M.-Y.; Young, D.J.; Zhang, W.-H.; Lang, J.-P. Deciphering the structural relationships of five Cd-based metal–organic frameworks. Inorg. Chem. 2017, 56, 6522–6531. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Cryst. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Bruker. APEX2, SAINT and SADABS; Bruker ACS Inc.: Madison, WI, USA, 2014. [Google Scholar]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. Sect. C 2015, 71, 9–18. [Google Scholar] [CrossRef]
Sample Availability: Sample of MOF 1 as single crystals is available from the authors. |

| Parameter | Value |
|---|---|
| Molecular formula | C179H141Ca4Cd6N3O55 |
| Formula weight | 4048.66 |
| Crystal system | Tetragonal |
| Space group | I41/a |
| a (Å) | 30.7009 (10) |
| b (Å) | 30.7009 (10) |
| c (Å) | 54.251 (4) |
| α (°) | 90 |
| β (°) | 90 |
| γ (°) | 90 |
| V (Å3) | 51,134 (5) |
| Z | 8 |
| ρcalc (g cm−3) | 1.052 |
| F(000) | 16,352 |
| µ (mm−1) | 0.629 |
| Total reflections | 438,339 |
| Unique reflections | 31,735 |
| Observed reflections | 28,071 |
| Rint | 0.1169 |
| Variables | 1154 |
| R1a | 0.0633 |
| wR2b | 0.1615 |
| GOF c | 1.087 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Chao, M.-Y.; Liu, Y.; Xu, B.-W.; Zhang, W.-H.; Young, D.J. A Heterometallic Three-Dimensional Metal−Organic Framework Bearing an Unprecedented One-Dimensional Branched-Chain Secondary Building Unit. Molecules 2020, 25, 2190. https://doi.org/10.3390/molecules25092190
Chen J, Chao M-Y, Liu Y, Xu B-W, Zhang W-H, Young DJ. A Heterometallic Three-Dimensional Metal−Organic Framework Bearing an Unprecedented One-Dimensional Branched-Chain Secondary Building Unit. Molecules. 2020; 25(9):2190. https://doi.org/10.3390/molecules25092190
Chicago/Turabian StyleChen, Jing, Meng-Yao Chao, Yan Liu, Bo-Wei Xu, Wen-Hua Zhang, and David J. Young. 2020. "A Heterometallic Three-Dimensional Metal−Organic Framework Bearing an Unprecedented One-Dimensional Branched-Chain Secondary Building Unit" Molecules 25, no. 9: 2190. https://doi.org/10.3390/molecules25092190
APA StyleChen, J., Chao, M.-Y., Liu, Y., Xu, B.-W., Zhang, W.-H., & Young, D. J. (2020). A Heterometallic Three-Dimensional Metal−Organic Framework Bearing an Unprecedented One-Dimensional Branched-Chain Secondary Building Unit. Molecules, 25(9), 2190. https://doi.org/10.3390/molecules25092190








