Screening Suitability of Northern Hemisphere Algal Strains for Heterotrophic Cultivation and Fatty Acid Methyl Ester Production
Abstract
1. Introduction
2. Results and Discussion
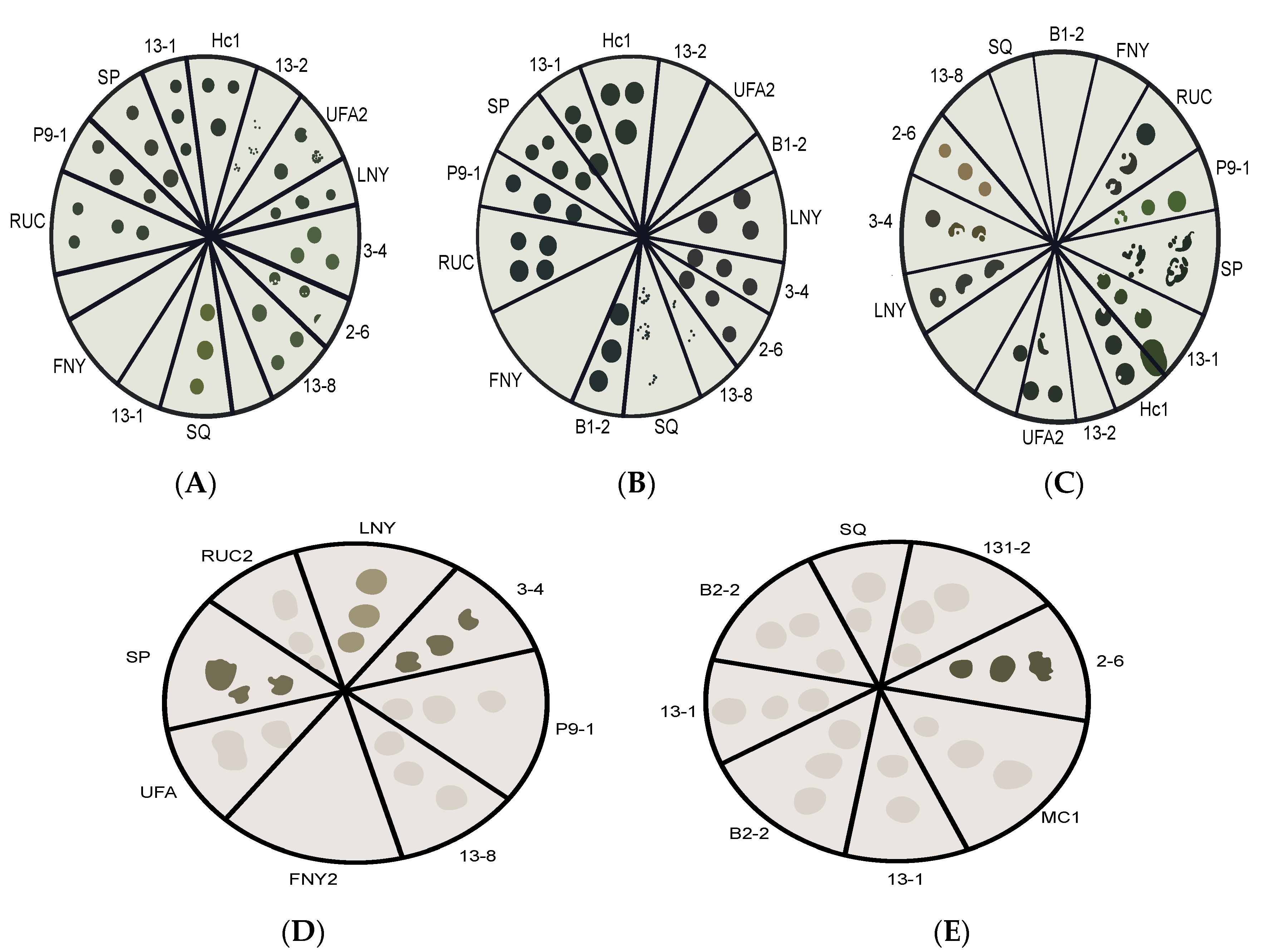
2.1. Pre-Screening
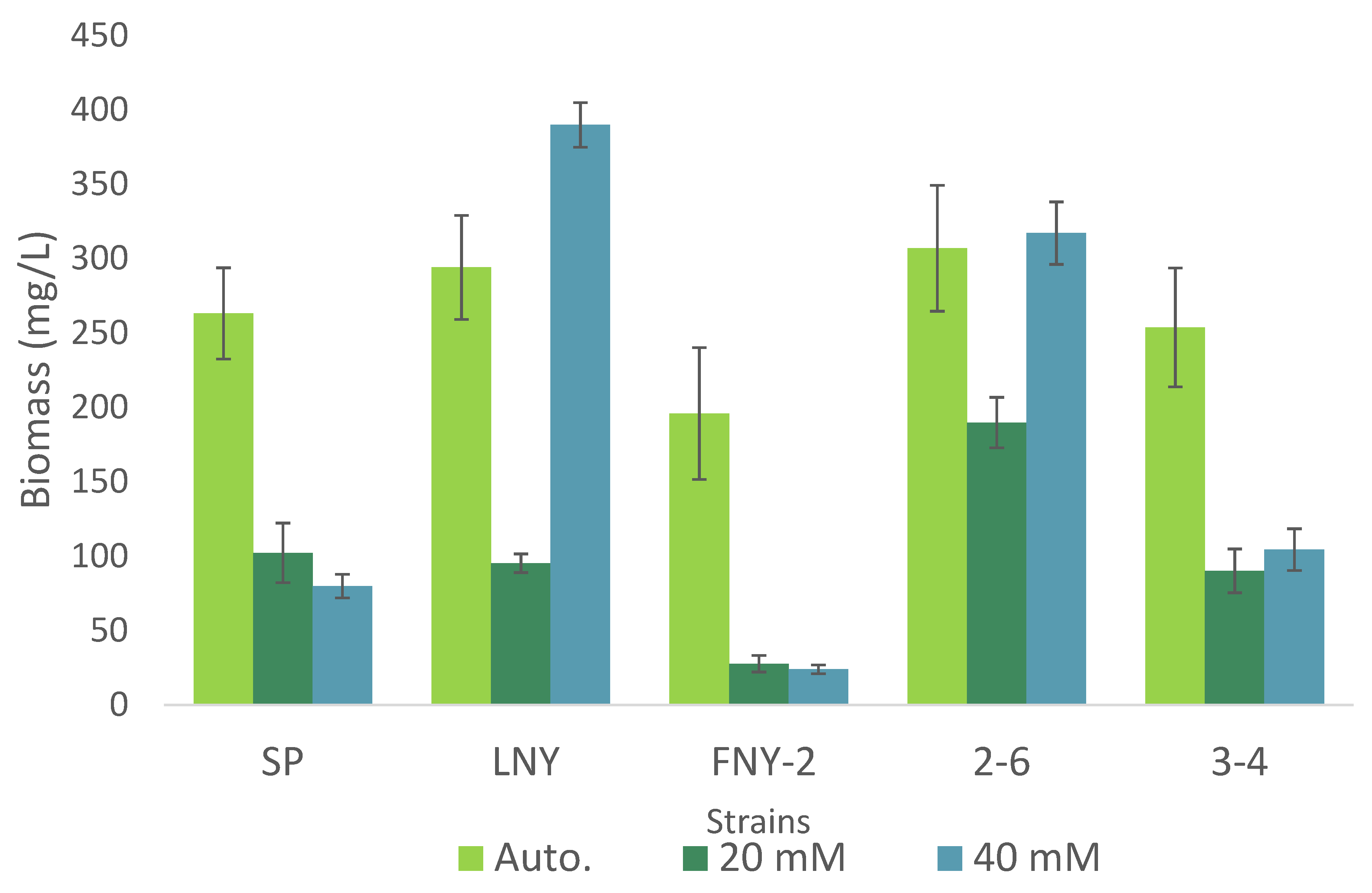
2.2. Comparison of Heterotrophic and Autotrophic Algal Growth
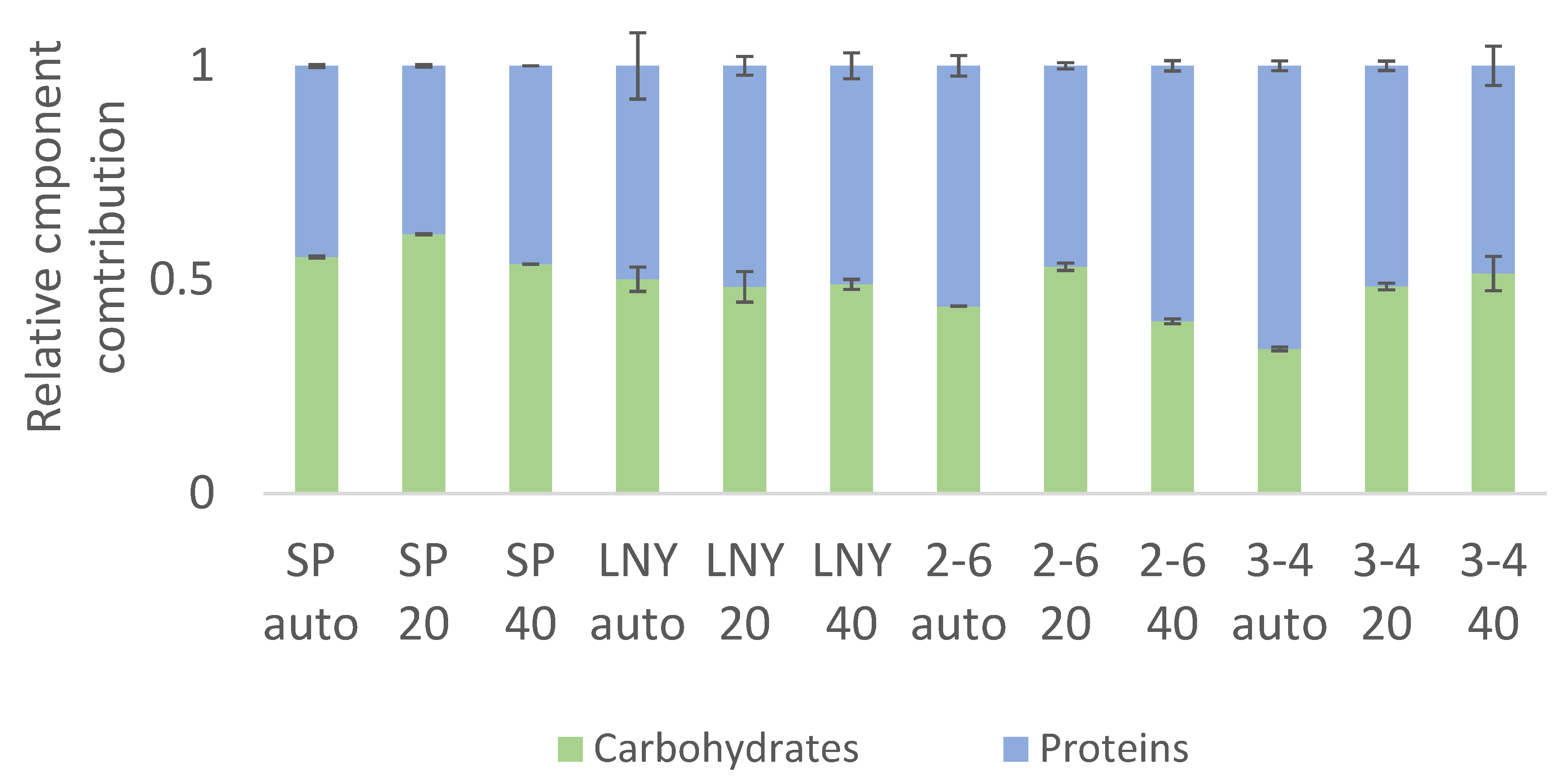
2.3. Biochemical Component Analysis
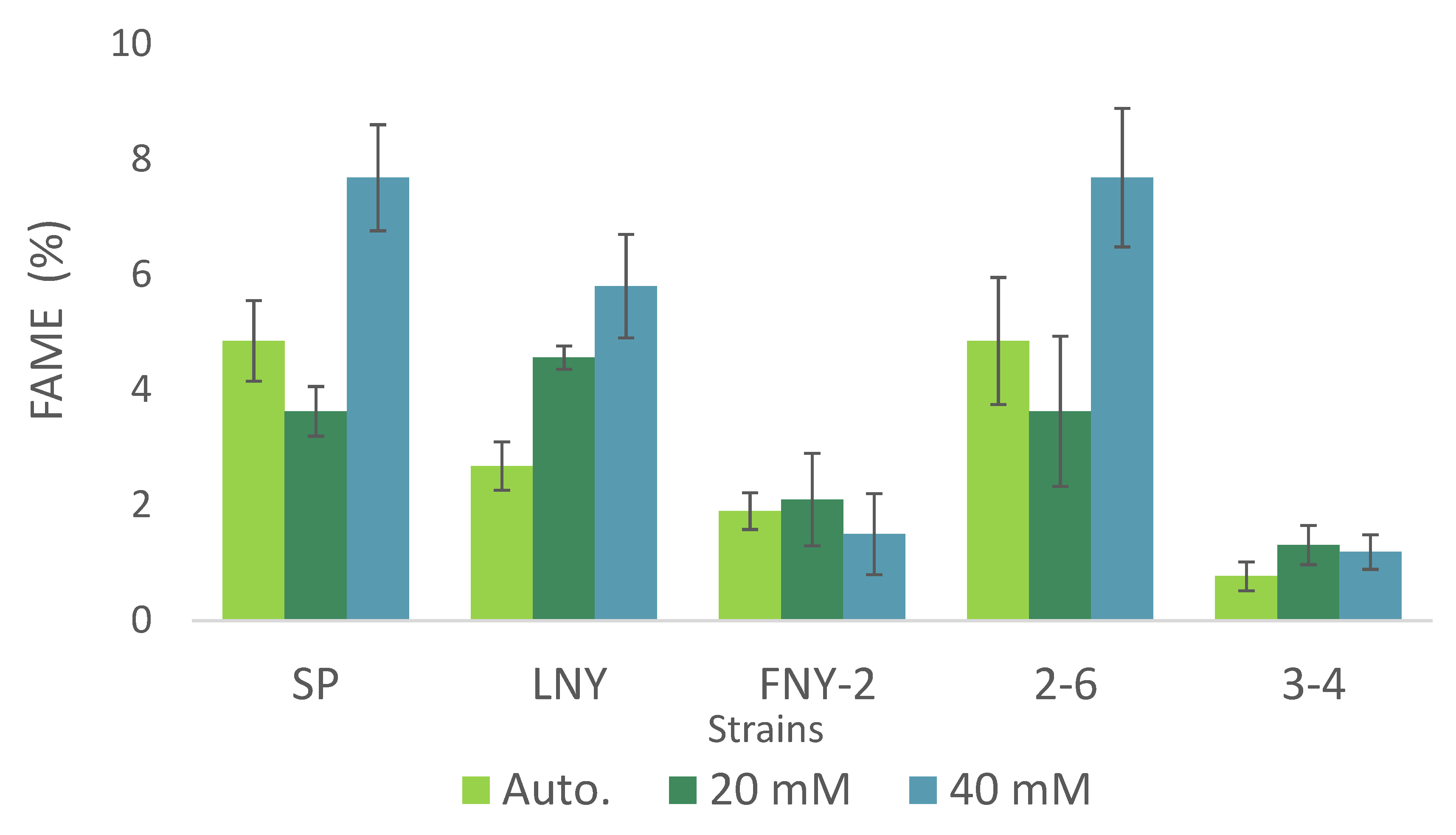
2.4. Fatty Acid Ester Content and Composition
2.5. Conclusions
3. Materials and Methods
3.1. Algae Strains and Growth Conditions
3.2. Assessments of Biomass
3.3. Lipid Analysis
3.4. Trans-Methylation
3.5. Analysis of Proportions of Lipids, Carbohydrates and Protein Proportions
3.6. Fatty Acid Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abdelaziz, A.E.; Leite, G.B.; Hallenbeck, P.C. Addressing the challenges for sustainable production of algal biofuels: I. Algal strains and nutrient supply. Environ. Technol. 2013, 34, 1783–1805. [Google Scholar] [CrossRef] [PubMed]
- McFadden, G.I.; van Dooren, G.G. Evolution: Red algal genome affirms a common origin of all plastids. Curr. Biol. 2004, 14, R514–R516. [Google Scholar] [CrossRef] [PubMed]
- Ukeles, R.; Rose, W.E. Observations on organic carbon utilization by photosynthetic marine microalgae. Mar. Biol. 1976, 37, 11–28. [Google Scholar] [CrossRef]
- Nzayisenga, J.C.; Eriksson, K.; Sellstedt, A. Mixotrophic and heterotrophic production of lipids and carbohydrates by a locally isolated microalga using wastewater as a growth medium. Bioresour. Technol. 2018, 257, 260–265. [Google Scholar] [CrossRef]
- Randrianarison, G.; Ashraf, M.A. Microalgae: A potential plant for energy production. Geol. Ecol. Landsc. 2017, 1, 104–120. [Google Scholar] [CrossRef]
- Knothe, G. Improving biodiesel fuel properties by modifying fatty ester composition. Energy Environ. Sci. 2009, 2, 759–766. [Google Scholar] [CrossRef]
- Ferro, L.; Gentili, F.G.; Funk, C. Isolation and characterization of microalgal strains for biomass production and wastewater reclamation in Northern Sweden. Algal Res. 2018, 32, 44–53. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Factor. 2018, 17, 36. [Google Scholar] [CrossRef]
- Benedetti, M.; Vecchi, V.; Barera, S.; Dall’Osto, L. Biomass from microalgae: The potential of domestication towards sustainable biofactories. Microb. Cell Factor. 2018, 17, 173. [Google Scholar] [CrossRef]
- Chen, Y.H.; Walker, T.H. Biomass and lipid production of heterotrophic microalgae Chlorella protothecoides by using biodiesel-derived crude glycerol. Biotechnol. Lett. 2011, 33, 1973–1983. [Google Scholar] [CrossRef]
- Leite, G.B.; Paranjape, K.; Abdelaziz, A.E.M.; Hallenbeck, P.C. Utilization of biodiesel-derived glycerol or xylose for increased growth and lipid production by indigenous microalgae. Bioresour. Technol. 2015, 184, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Stehfest, K.; Toepel, J.; Wilhelm, C. The application of micro-FTIR spectroscopy to analyze nutrient stress-related changes in biomass composition of phytoplankton algae. Plant Physiol. Biochem. 2005, 43, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Ferro, L.; Gojkovic, Z.; Gorzsas, A.; Funk, C. Statistical Methods for Rapid Quantification of Proteins, Lipids, and Carbohydrates in Nordic Microalgal Species Using ATR-FTIR Spectroscopy. Molecules 2019, 24, 3237. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Liu, Z.; Langner, U.; Stehfest, K.; Wilhelm, C. The use of FTIR spectroscopy to assess quantitative changes in the biochemical composition of microalgae. J. Biophotonics 2010, 3, 557–566. [Google Scholar] [CrossRef]
- Dean, A.P.; Sigee, D.C.; Estrada, B.; Pittman, J.K. Using FTIR spectroscopy for rapid determination of lipid accumulation in response to nitrogen limitation in freshwater microalgae. Bioresour. Technol. 2010, 101, 4499–4507. [Google Scholar] [CrossRef]
- Hess, S.K.; Lepetit, B.; Kroth, P.G.; Mecking, S. Production of chemicals from microalgae lipids—Status and perspectives. Eur. J. Lipid Sci. Technol. 2018, 120, 1700152. [Google Scholar] [CrossRef]
- Monirul, I.M.; Masjuki, H.H.; Kalam, M.A.; Zulkifli, N.W.M.; Rashedul, H.K.; Rashed, M.M.; Imdadul, H.K.; Mosarof, M.H. A comprehensive review on biodiesel cold flow properties and oxidation stability along with their improvement processes. RSC Adv. 2015, 5, 86631–86655. [Google Scholar] [CrossRef]
- Standardization Institute. Automotive Fuels-Fatty Acid Methyl Esters (FAME) for Diesel Engines, Requirements and Test Methods; EN14214, E.S.; Standardization Institute: Belgrade, Serbia, 2004. [Google Scholar]
- Liu, J.; Huang, J.; Sun, Z.; Zhong, Y.; Jiang, Y.; Chen, F. Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: Assessment of algal oils for biodiesel production. Bioresour. Technol. 2011, 102, 106–110. [Google Scholar] [CrossRef]
- Lopez, G.; Yate, C.; Ramos, F.A.; Cala, M.P.; Restrepo, S.; Baena, S. Production of Polyunsaturated Fatty Acids and Lipids from Autotrophic, Mixotrophic and Heterotrophic cultivation of Galdieria sp. strain USBA-GBX-832. Sci. Rep. 2019, 9, 10791. [Google Scholar] [CrossRef]
- Milledge, J.J.; Heaven, S. Methods of energy extraction from microalgal biomass: A review. Rev. Environ. Sci. Bio/Technol. 2014, 13, 301–320. [Google Scholar] [CrossRef]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Selstam, E.; Öquist, G. Effects of frost hardening on the composition of galactolipids and phospholipids occurring during isolation of chloroplast thylakoids from needles of scots pine. Plant Sci. 1985, 42, 41–48. [Google Scholar] [CrossRef]
Sample Availability: Samples of the metabolic compounds are not available from the authors. |
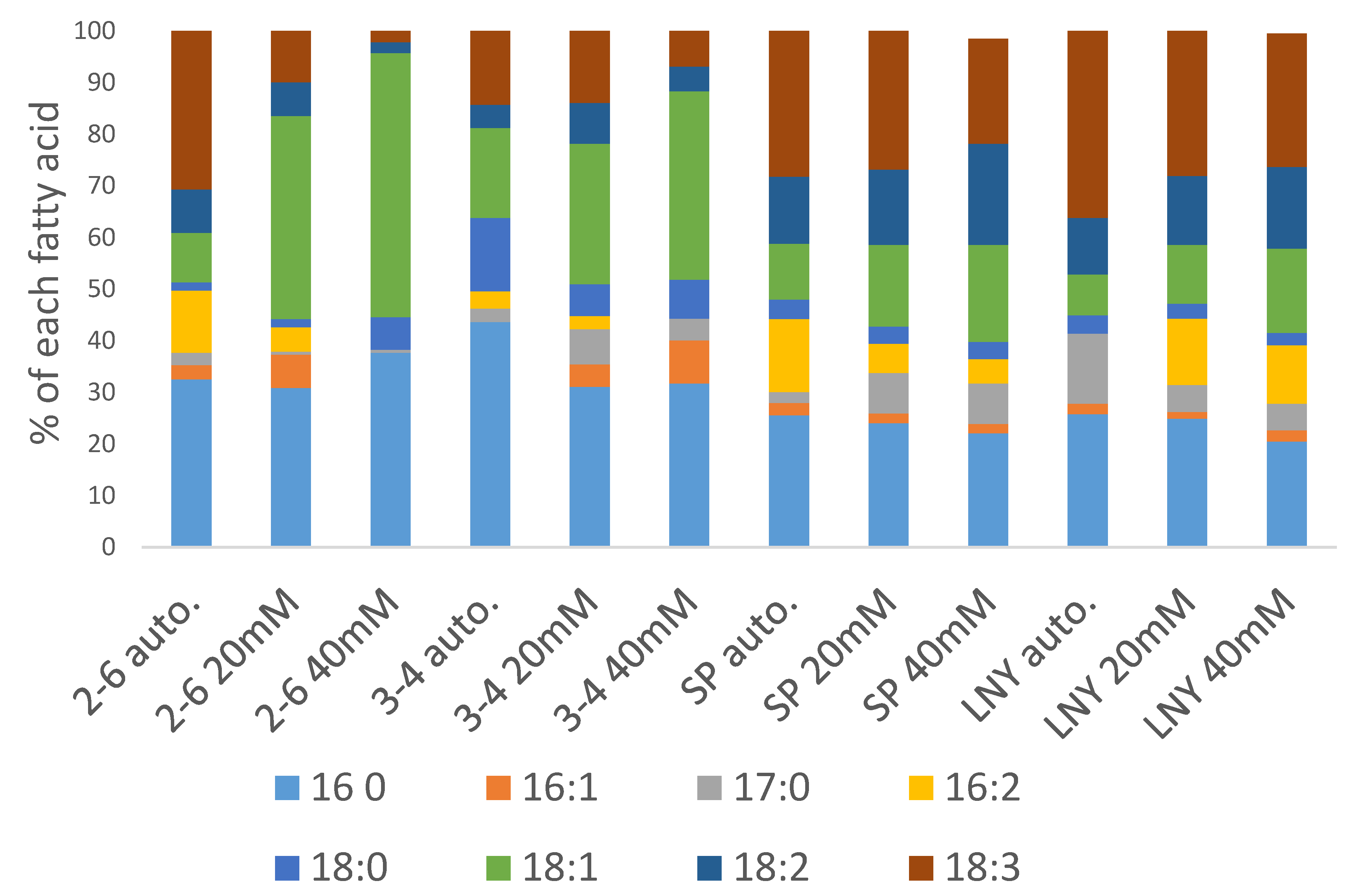
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nzayisenga, J.C.; Niemi, C.; Ferro, L.; Gorzsas, A.; Gentili, F.G.; Funk, C.; Sellstedt, A. Screening Suitability of Northern Hemisphere Algal Strains for Heterotrophic Cultivation and Fatty Acid Methyl Ester Production. Molecules 2020, 25, 2107. https://doi.org/10.3390/molecules25092107
Nzayisenga JC, Niemi C, Ferro L, Gorzsas A, Gentili FG, Funk C, Sellstedt A. Screening Suitability of Northern Hemisphere Algal Strains for Heterotrophic Cultivation and Fatty Acid Methyl Ester Production. Molecules. 2020; 25(9):2107. https://doi.org/10.3390/molecules25092107
Chicago/Turabian StyleNzayisenga, Jean Claude, Calle Niemi, Lorenza Ferro, Andras Gorzsas, Francesco G. Gentili, Christiane Funk, and Anita Sellstedt. 2020. "Screening Suitability of Northern Hemisphere Algal Strains for Heterotrophic Cultivation and Fatty Acid Methyl Ester Production" Molecules 25, no. 9: 2107. https://doi.org/10.3390/molecules25092107
APA StyleNzayisenga, J. C., Niemi, C., Ferro, L., Gorzsas, A., Gentili, F. G., Funk, C., & Sellstedt, A. (2020). Screening Suitability of Northern Hemisphere Algal Strains for Heterotrophic Cultivation and Fatty Acid Methyl Ester Production. Molecules, 25(9), 2107. https://doi.org/10.3390/molecules25092107






