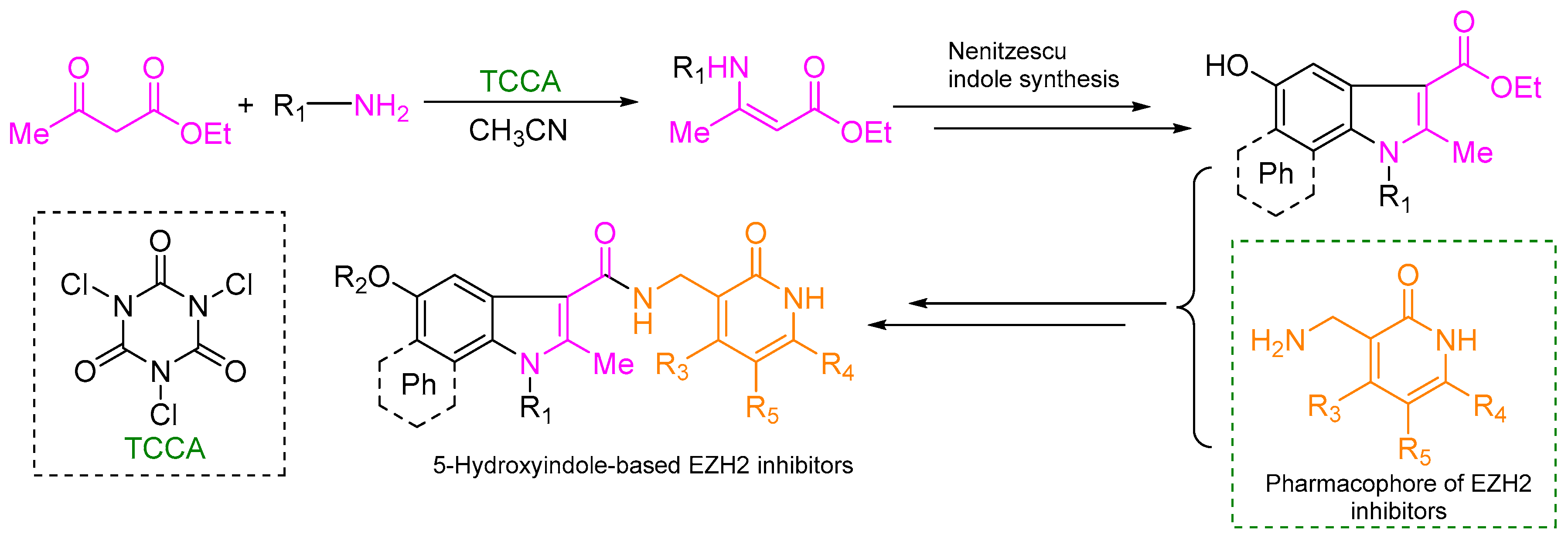
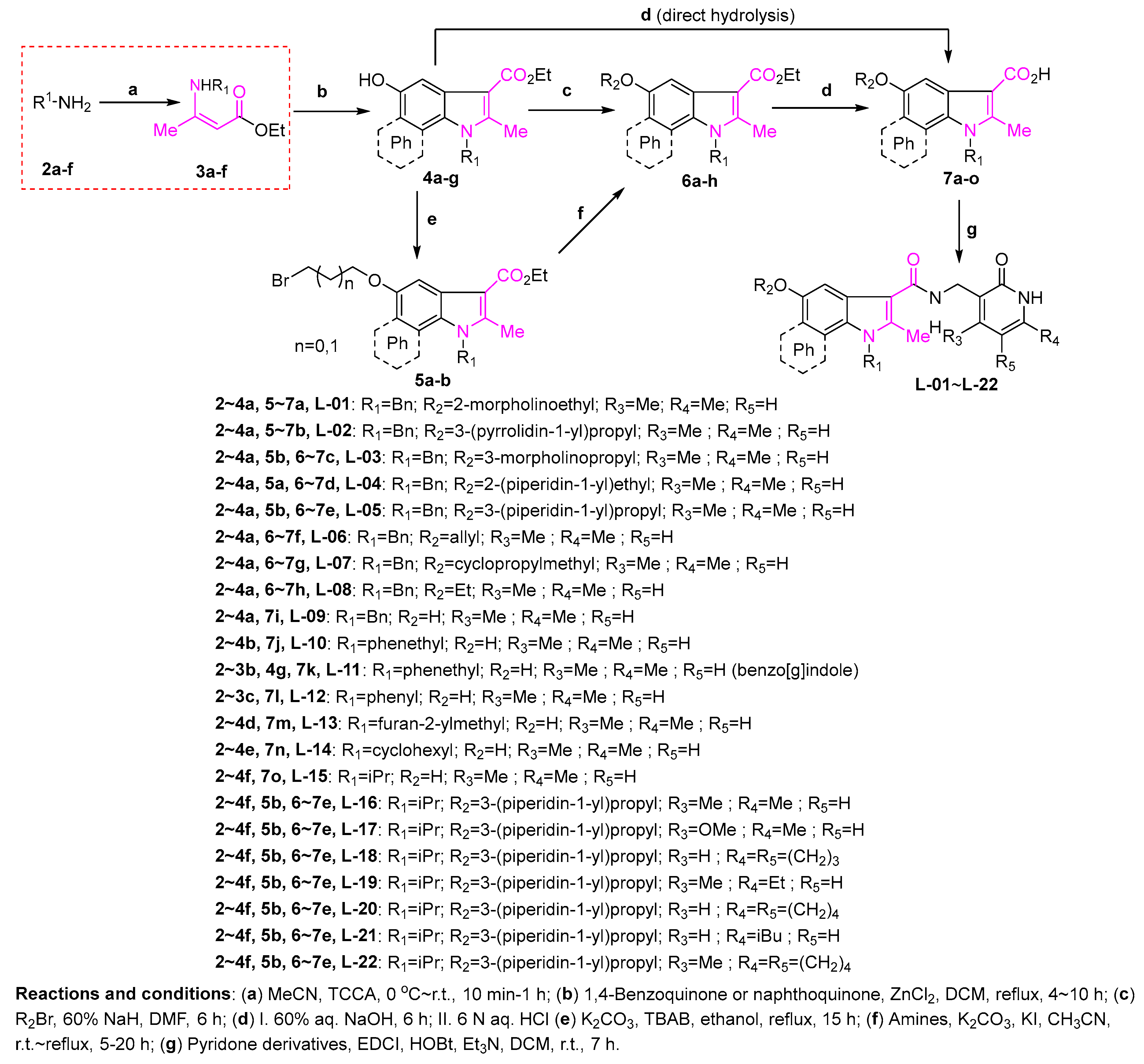
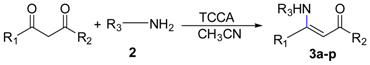
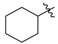
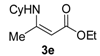
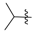
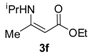
3.2.1. General Procedure for the Syntheses of Ethyl Enoates 3a–p
TCCA (714/34.8 mg, 31/0.15 mmol) was added to ethyl acetoacetate (20/1 g, 154/7.68 mmol) or tert-butyl 3-oxobutanoate (1.2 g, 7.59 mmol) or acetylacetone (0.77 g, 7.69 mmol) or ethyl benzoylacetate (1.48 g, 7.71 mmol) in acetonitrile (20 mL) under ice bath cooling, and the appropriate amine (0.23/0.012 mol) was added dropwise. The mixture was stirred under ice bath conditions and allowed to naturally warm up to room temperature for the indicated time. After the reaction was complete, the mixture was poured into water, extracted with dichloromethane (3 × 50 mL), washed with water (3 × 50 mL) and brine (50 mL). The organic solution was dried over magnesium sulfate, filtered, evaporated under reduced pressure and used in the following reaction without any further purification.
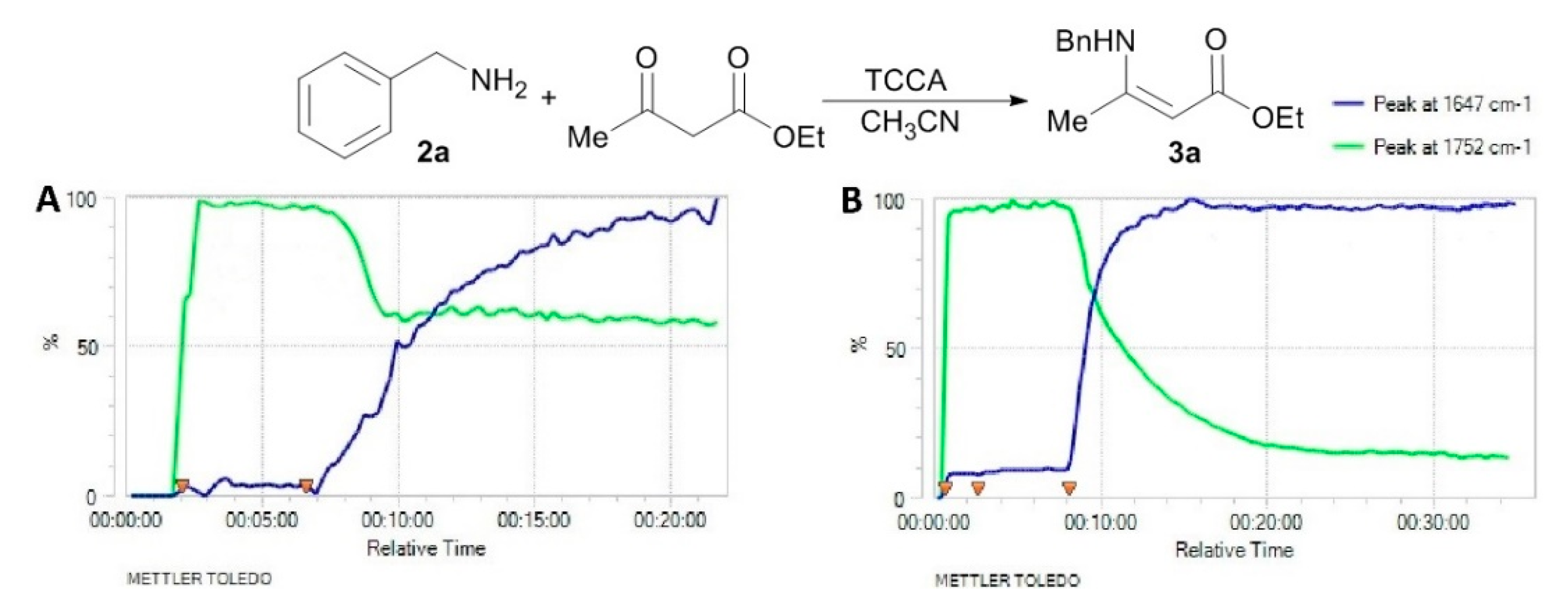
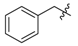
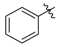
Ethyl 3-(benzylamino)but-2-enoate (3a). Light yellow solid (32.0 g, 95%). M.p. 49–50 °C.1H-NMR (CDCl3) δ 8.95 (brs, 1H, NH), 7.34 (t, J = 7.6 Hz, 2H, ArH), 7.27–7.26 (m, 3H, ArH), 4.53 (s, 1H, C=C-H), 4.43 (d, J = 6.3 Hz, 2H, OCH2), 4.10 (q, J = 6.4 Hz, 2H, NCH2), 1.92 (s, 3H, CH3), 1.26 (t, J = 7.1 Hz, 3H, CH3). HRMS (ESI) calcd for C13H17NO2Na [M+Na]+: 242.1151, found: 242.1151.
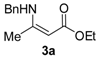
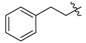
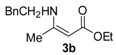
Ethyl 3-(phenethylamino)but-2-enoate (3b). Colorless liquid (35.1 g, 98%). 1H-NMR (CDCl3) δ 8.65 (brs, 1H, NH), 7.30 (t, J = 7.3 Hz, 2H, ArH), 7.24–7.19 (m, 3H, ArH), 4.42 (s, 1H, C=C-H), 4.08 (q, J = 7.1 Hz, 2H, OCH2), 3.45–3.42 (m, 2H, NCH2), 2.85 (t, J = 7.6 Hz, 2H, PhCH2), 1.82 (s, 3H, CH3), 1.25 (t, J = 7.1 Hz, 3H, CH3). HRMS (ESI) calcd for C14H19NO2Na [M+Na]+: 256.1308, found: 256.1307.
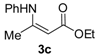
Ethyl 3-(phenylamino)but-2-enoate (3c). Colorless liquid (29.0 g, 92%). 1H-NMR (DMSO-d6) δ 10.38 (s, 1H, NH), 7.39–7.34 (m, 2H, ArH), 7.19–7.17 (m, 3H, ArH), 4.69 (d, J = 0.4 Hz, 1H, C=C-H), 4.06 (q, J = 7.1 Hz, 2H, CH2), 2.01 (s, 3H, CH3), 1.20 (t, J = 7.1 Hz, 3H, CH3). HRMS (ESI) calcd for C12H16NO2 [M+H]+: 206.1176, found: 206.1170.
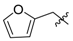
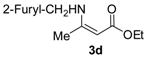
Ethyl 3-((furan-2-ylmethyl)amino)but-2-enoate (3d). Colorless liquid (30.2 g, 94%). 1H-NMR (CDCl3) δ 8.80 (brs, 1H, NH), 7.35 (d, J = 1.4 Hz, 1H, ArH), 6.31–6.30 (m, 1H, ArH), 6.19 (d, J = 3.2 Hz, 1H, ArH), 4.52 (s, 1H, C=C-H), 4.37 (d, J = 6.3 Hz, 2H, NCH2), 4.08 (q, J = 7.1 Hz, 2H, OCH2), 1.99 (s, 3H, CH3), 1.24 (t, J = 7.1 Hz, 3H, CH3). HRMS (ESI) calcd for C11H15NO3Na [M+Na]+: 232.0949, found: 232.0949.
Ethyl 3-(cyclohexylamino)but-2-enoate (3e). Colorless liquid (30.8 g, 95%). 1H-NMR (CDCl3) δ 8.63 (brs, 1H, NH), 4.39–4.37 (m, 1H, C=C-H), 4.10–4.05 (m, 2H, OCH2), 3.32–3.27 (m, 1H, NCH), 1.94–1.92 (m, 3H, CH3), 1.88–1.85 (m, 2H, CH2), 1.77–1.73 (m, 2H, CH2), 1.60–1.20 (m, 9H, CH2, CH3). HRMS (ESI) calcd for C12H21NO2Na [M+Na]+: 234.1465, found: 234.1461.
Ethyl 3-(isopropylamino)but-2-enoate (3f). Colorless liquid (25.3 g, 96%). 1H-NMR (CDCl3) δ 8.50 (brs, 1H, NH), 4.39 (s, 1H, C=C-H), 4.10–4.06 (m, 2H, OCH2), 3.70–3.66 (m, 1H, CH), 1.94 (s, 3H, CH3), 1.26–1.23 (m, 3H, CH3), 1.21-1.20 (m, 6H, 2´CH3). HRMS (ESI) calcd for C9H18NO2 [M+H]+: 172.1332, found: 172.1335.
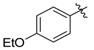
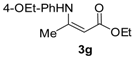
Ethyl 3-((4-ethoxyphenyl)amino)but-2-enoate (3g). White solid (1.82 g, 95%). M.p. 53–54 °C. 1H-NMR (CDCl3) δ 10.14 (brs, 1H, NH), 7.01 (d, J = 8.8 Hz, 2H, ArH), 6.84 (d, J = 8.9 Hz, 2H, ArH), 4.64 (s, 1H, C=C-H), 4.16–4.12 (14.2 Hz, 7.1 Hz, 2 H, OCH2), 4.01 (q, J = 7.0 Hz, 2H, OCH2), 1.88 (s, 3H, CH3), 1.41 (t, J = 7.0 Hz, 3H, CH3), 1.28 (t, J = 7.1 Hz, 3H, CH3). HRMS (ESI) calcd for C14H19NO3Na [M+Na]+: 272.1257, found: 272.1252.
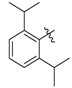
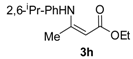
Ethyl 3-((2,6-diisopropylphenyl)amino)but-2-enoate (3h). White solid (2.0 g, 90%). M.p. 79–81 °C. 1H-NMR (CDCl3) δ 9.84 (brs, 1H, NH), 7.30–7.26 (m, 1H, ArH), 7.17 (d, J = 7.7 Hz, 2H, ArH), 4.68 (brs, 1 H, C=C-H), 4.17 (q, J = 7.1 Hz, 2 H, OCH2), 3.13–3.07 (m, 2H, 2´CH), 1.62 (s, 3H, CH3), 1.31 (t, J = 7.1 Hz, 3H, CH3), 1.22 (d, J = 6.9 Hz, 6H, 2´CH3), 1.12 (d, J = 6.8 Hz, 6H, 2´CH3). HRMS (ESI) calcd for C18H27NO2Na [M+Na]+: 312.1934, found: 312.1933.
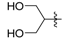
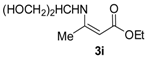
Ethyl 3-((1,3-dihydroxypropan-2-yl)amino)but-2-enoate (3i). White solid (1.45 g, 94%). M.p. 74–75 oC. 1H-NMR (CDCl3) δ 8.76 (d, J = 9.4 Hz, 1H, NH), 4.53 (s, 1H, C=C-H), 4.09 (q, J = 7.1 Hz, 2H, OCH2), 3.79–3.64 (m, 4H, 2´CH2), 3.69–3.65 (m, 1H, CH), 1.98 (s, 3H, CH3), 1.25 (t, J = 7.1 Hz, 3H, CH3). HRMS (ESI) calcd for C9H17NO4Na [M+Na]+: 226.1050, found: 226.1044.
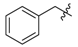
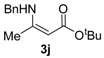
tert-Butyl 3-(benzylamino)but-2-enoate (3j). White solid (1.96 g, 99%). M.p. 59~60 °C. 1H-NMR (CDCl3) δ 8.89 (brs, 1H, NH), 7.37–7.32 (m, 2H, ArH), 7.30–7.24 (m, 3H, ArH), 4.46 (s, 1H, C=C-H), 4.42 (d, J = 6.4 Hz, 2H, NCH2), 1.87 (s, 3H, CH3), 1.47 (s, 9H, 3´CH3). HRMS (ESI) calcd for C15H21NO2Na [M+Na]+: 270.1465, found: 270.1461.
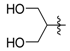
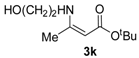
tert-Butyl 3-((2-hydroxyethyl)amino)but-2-enoate (3k). White crystals (1.65 g, 94%). M.p. 51–53 °C. 1H-NMR (CDCl3) δ 8.59 (brs, 1H, NH), 4.43 (s, 1H, C=C-H), 3.74 (t, J = 5.3 Hz, 2H, CH2), 3.37 (q, J = 5.6 Hz, 2H, CH2), 1.92 (s, 3H, CH3), 1.46(s, 9H, 3´CH3). HRMS (ESI) calcd for C10H19NO3 Na [M+Na]+: 224.1257, found: 224.1252.
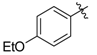
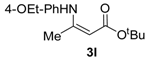
tert-Butyl 3-((4-ethoxyphenyl)amino)but-2-enoate (3l). White crystals (1.96 g, 93%). 1H-NMR (CDCl3) δ 10.10 (brs, 1H, NH), 7.01 (d, J = 8.8 Hz, 2H, ArH), 6.83 (d, J = 8.8 Hz, 2H, ArH), 4.58 (s, 1H, C=C-H), 4.01 (q, J = 7.0 Hz, 2H, OCH2), 1.86 (s, 3H, CH3), 1.50 (s, 9H, 3´CH3), 1.41 (t, J = 7.0 Hz, 3H, CH3). HRMS (ESI): calcd for C16H23NO3Na [M+Na]+: 300.1576; found: 300.1567.
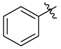
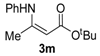
tert-Butyl 3-(phenylamino)but-2-enoate (3m). White crystals (1.63 g, 92%). 1H-NMR (CDCl3) δ 10.34 (brs, 1H, NH), 7.30 (t, J = 7.8 Hz, 2H, ArH), 7.13 (t, J = 7.4 Hz, 1H, ArH), 7.08 (d, J = 7.6 Hz, 2H, ArH), 4.62 (s, 1H, C=C-H), 1.50 (s, 9H, 3´CH3). HRMS (ESI): calcd for C14H19NO2Na [M+Na]+: 256.1313, found: 256.1307.
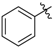
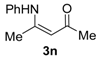
4-(Phenylamino)pent-3-en-2-one (3n). White crystals (1.21 g, 90%). M.p. 49–51 °C. 1H-NMR (CDCl3) δ 12.47 (brs, 1H, NH), 7.35–7.33 (m, 2H, ArH), 7.20 (t, J = 7.4 Hz, 1H, ArH), 7.11 (d, J = 7.5 Hz, 2H, ArH), 5.19 (brs, 1H, C=C-H), 2.10 (s, 3H, CH3), 2.00 (s, 3H, CH3). HRMS (ESI) calcd for C11H13NONa [M+Na]+: 198.0889, found: 198.0885.
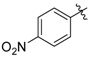
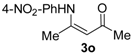
4-((4-Nitrophenyl)amino)pent-3-en-2-one (3o). Yellow solid (1.46 g, 86%). M.p. 143–144 °C. 1H-NMR (CDCl3) δ 12.78 (brs, 1H, NH), 8.21–8.20 (dd, J = 7.2 Hz, 2.0 Hz, 2H, ArH), 7.19–7.18 (dd, J = 7.2 Hz, 2.0 Hz, 2H, ArH), 5.33 (brs, 1H, C=C-H), 2.19 (s, 3H, CH3), 2.15 (s, 3H, CH3). HRMS (ESI) calcd for C11H13N2O3 [M+H]+: 221.0921, found: 221.0922.
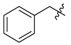
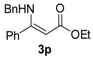
Ethyl 3-(benzylamino)-3-phenylacrylate (3p). White solid (1.99 g, 92%). M.p. 71–72 °C. 1H-NMR (CDCl3) δ 8.90 (brs, 1H, NH), 7.39–7.33 (m, 5H, ArH), 7.29 (t, J = 7.2 Hz, 2H, ArH), 7.23 (t, J = 7.3 Hz, 1H, ArH), 7.18 (d, J = 7.2 Hz, 2H, ArH), 4.67 (s, 1H, C=C-H), 4.26 (d, J = 6.5 Hz, 2H, NCH2), 4.15 (q, J = 7.1 Hz, 2H, OCH2), 1.28 (t, J = 7.1 Hz, 3H, CH3). HRMS (ESI) calcd for C18H19NO2Na [M+Na]+: 304.1308, found: 304.1307.
3.2.2. General Procedure for the Syntheses of Ethyl 5-Hydroxyindolecarboxylates 4a–g
Anhydrous ZnCl2 (1.26 g, 9.25 mmol) was added to a stirred suspension of p-benzoquinone (10 g, 92.5 mmol) or naphthoquinone (14.6 g, 92.5 mmol) in dry DCM (50 mL). After heating at reflux, a solution of crotonamine (92.5 mmol) in dry DCM (50 mL) was added into the mixture over 40 min and stirred at reflux for a further 45 min. The mixture was cooled to room temperature and held at 4 °C for 30 min to allow product precipitation. The solid was filtered and washed with DCM, water and acetonitrile to give a corresponding products 4a–g.
Ethyl 1-benzyl-5-hydroxy-2-methyl-1H-indole-3-carboxylate (4a). The title product was obtained from ethyl enoate 3a and p-benzoquinone according to the general procedure described above as an off white solid (14.3 g, 50%). 1H-NMR (DMSO-d6) δ 8.96 (s, 1H, OH), 7.40 (d, J = 2.3 Hz, 1H, ArH), 7.33–7.23 (m, 4H, ArH), 7.01–7.00 (d, J = 7.3 Hz, 2H, ArH), 6.63 (dd, J = 8.7 Hz, 2.4 Hz, 1H, ArH), 5.44 (s, 2H, NCH2), 4.27 (q, J = 7.1 Hz, 2H, OCH2), 2.64 (s, 1H, CH3), 1.36 (t, J = 7.1 Hz, 3H, CH3). 13C-NMR (DMSO-d6) δ 165.6 (C=Oester), 153.3 (HOAr-Cindole), 145.4 (ArC), 137.8 (ArC), 130.8 (ArC), 129.2 (ArC), 127.7 (ArC), 127.6 (ArC), 126.6 (ArC), 112.1 (ArC), 111.3 (C-C=O), 106.0 (ArC), 103.1 (ArC), 59.2 (OCH2), 46.3 (NCH2benzyl), 15.0 (CH2CH3), 12.3 (CH3indole). HRMS (ESI) calcd for C19H19NO3Na [M+Na]+: 332.1257, found: 332.1255.
Ethyl 5-hydroxy-2-methyl-1-phenethyl-1H-indole-3-carboxylate (4b). The title product was obtained from ethyl enoate 3b and p-benzoquinone according to the general procedure described above as an off white solid (21.5 g, 72%). M.p. 175–177 °C. 1H-NMR (DMSO-d6) δ 8.97 (s, 1H, OH), 7.38 (d, J = 2.2 Hz, 1H, ArH), 7.33 (d, J = 8.7 Hz, 1H, ArH), 7.27–7.20 (m, 3H, ArH), 7.12 (d, J = 7.1 Hz, 2H, ArH), 6.68–6.66 (dd, J = 8.6 Hz, 2.3 Hz, 1H, ArH), 4.32 (t, J = 7.3 Hz, 2H, OCH2), 4.24 (q, J = 7.1 Hz, 2H, NCH2), 2.95 (t, J = 7.3 Hz, 2H, CH2), 2.4 (s, 3H, CH3), 1.34 (t, J = 7.1 Hz, 3H, CH3). 13C-NMR (DMSO-d6) δ 165.6 (C=Oester), 153.1 (HOAr-Cindole), 145.5 (ArC), 138.7 (ArC), 130.1 (ArC), 129.4 (ArC), 128.8 (ArC), 127.7 (ArC), 127.0 (ArC), 111.8 (ArC), 111.0 (C-C=O), 106.0 (ArC), 102.4 (ArC), 59.1 (OCH2), 44.8 (NCH2), 35.6 (CH2Ph), 15.0 (CH2CH3), 11.7 (CH3indole). HRMS (ESI) calcd for C20H21NO3Na [M+Na]+: 346.1414, found: 346.1403.
Ethyl 5-hydroxy-2-methyl-1-phenyl-1H-indole-3-carboxylate (4c). The title product was obtained from ethyl enoate 3c and p-benzoquinone according to the general procedure described above as a brown solid (16.1 g, 59%). M.p. 203–205 °C. 1H-NMR (DMSO-d6) δ 9.06 (s, 1H, OH), 7.63 (t, J = 7.4 Hz, 2H, ArH), 7.57 (t, J = 7.3 Hz, 1H, ArH), 7.47 (d, J = 2.3 Hz, 1H, ArH), 7.45 (d, J = 7.3 Hz, 2H, ArH), 6.78 (d, J = 8.7 Hz, 1H, ArH), 6.65–6.63 (dd, J = 8.8 Hz, 2.4 Hz, 1H, ArH), 4.32 (q, J = 7.1 Hz, 2H, OCH2), 2.49 (s, 3H, CH3), 1.38 (t, J = 7.1 Hz, 3H, CH3). 13C-NMR (DMSO-d6) δ 165.6 (C=Oester), 153.6 (HOAr-Cindole), 145.3 (ArC), 136.5 (ArC), 131.9 (ArC), 130.3 (ArC), 129.3 (ArC), 128.4 (ArC), 127.5 (ArC), 112.5 (ArC), 111.2 (ArC), 105.9 (ArC), 104.0 (ArC), 59.4 (OCH2), 15.0 (CH2CH3), 13.3 (CH3indole). HRMS (ESI) calcd for C18H17NO3Na [M+Na]+: 318.1101, found: 318.1100.
Ethyl 1-(furan-2-ylmethyl)-5-hydroxy-2-methyl-1H-indole-3-carboxylate (4d). The title product was obtained from ethyl enoate 3d and p-benzoquinone according to the general procedure described above as an off white solid (15.8 g, 57%). M.p. 207–211 °C. 1H-NMR (DMSO-d6) δ 8.95 (s, 1H, OH), 7.55 (d, J = 0.8 Hz, 1H, ArH), 7.44 (d, J = 8.7 Hz, 1H, ArH), 7.36 (d, J = 2.2 Hz, 1H, ArH), 6.68–6.66 (dd, J = 8.8 Hz, 2.3 Hz, 1H, ArH), 6.46 (d, J = 3.1 Hz, 1H, ArH), 6.39–3.38 (m, 1H, ArH), 5.39 (s, 2H, NCH2), 4.26 (q, J = 7.1 Hz, 2H, OCH2), 2.77 (s, 3H, CH3), 1.34 (t, J = 7.1 Hz, 3H, CH3). 13C-NMR (DMSO-d6) δ 165.5 (C=Oester), 153.2 (HOAr-Cindole), 150.5 (ArC), 145.3 (ArC), 143.5 (ArC), 130.4 (ArC), 127.6 (ArC), 111.9 (ArC), 111.3 (ArC), 111.0 (ArC), 109.0 (ArC), 105.9 (ArC), 103.1 (ArC), 59.2 (OCH2), 15.0(CH2CH3), 12.2 (CH3indole). HRMS (ESI) calcd for C17H17NO4Na [M+Na]+: 322.1050, found: 322.1049.
Ethyl 1-cyclohexyl-5-hydroxy-2-methyl-1H-indole-3-carboxylate (4e). The title product was obtained from ethyl enoate 3e and p-benzoquinone according to the general procedure described above as a pink solid (16.3 g, 67%). M.p. 245–247 °C. 1H-NMR (DMSO-d6) δ 8.90 (s, 1H, OH), 7.50 (d, J = 8.8 Hz, 1H, ArH), 7.40 (d, J = 2.3 Hz, 1H, ArH), 6.64–6.62 (dd, J = 8.8 Hz, 2.4 Hz, 1H, ArH), 4.28–4.24 (m, 3H, NCH, OCH2), 2.73 (s, 3H, CH3), 2.22–2.20 (m, 2H, CH2), 1.86–1.84 (m, 2H, CH2), 1.76–1.75 (m, 2H, CH2), 1.70–1.68 (m, 1H, CH2), 1.50–1.44 (m, 2H), 1.38–1.33 (m, 4H, CH3, CH2). 13C-NMR (DMSO-d6) δ 165.7 (C=Oester), 152.5 (HOAr-Cindole), 145.0 (ArC), 129.0 (ArC), 128.6 (ArC), 113.5 (ArC), 111.4 (ArC), 106.1 (ArC), 102.5 (ArC), 59.1 (OCH2), 55.5 (NCH2), 30.8 (CH2), 26.1 (CH2), 25.2 (CH2), 15.0 (CH2CH3), 12.4 (CH3indole). HRMS (ESI) calcd for C18H23NO3Na [M+Na]+: 324.1570, found: 324.1567.
Ethyl 5-hydroxy-1-isopropyl-2-methyl-1H-indole-3-carboxylate (4f). The title product was obtained from ethyl enoate 3f and p-benzoquinone according to the general procedure described above as a white solid (16.4 g, 68%). M.p. 168–170 °C. 1H-NMR (DMSO-d6) δ 8.86 (s, 1H, OH), 7.42 (d, J = 8.8 Hz, 1H, ArH), 7.38 (d, J = 2.2 Hz, 1H, ArH), 6.63–6.61 (dd, J = 8.8 Hz, 2.3 Hz, 1H, ArH), 4.77–4.73 (m, 1H, NCH), 2.69 (s, 3H, CH3), 1.57 (s, 6H, 2´CH3), 1.52 (d, J = 6.9 Hz, 3H, CH3). 13C-NMR (DMSO-d6) δ 165.3 (C=Oester), 152.5, 144.4 (ArC), 128.7 (ArC), 112.7 (ArC), 111.4 (ArC), 106.2 (ArC), 103.8 (ArC), 59.1 (OCH2), 47.0 (NCH2), 26.1 (CH3), 15.0 (CH2CH3), 12.5 (CH3indole). HRMS (ESI) calcd for C17H23NO3Na [M+Na]+: 312.1570, found: 312.1571.
Ethyl 5-hydroxy-2-methyl-1-phenethyl-1H-benzo[g]indole-3-carboxylate (4g). The title product was obtained from ethyl enoate 3b and naphthoquinone according to the general procedure described above for the ethyl 5-hydroxyindolecarboxylate as a white solid (21.4 g, 62%). 1H-NMR (DMSO-d6) δ 9.77 (s, 1H, OH), 8.45 (d, J = 8.6 Hz, 1H, ArHnaphthalene), 8.32 (dd, J = 8.3 Hz, 0.8 Hz, 1H, ArHnaphthalene), 7.73 (s, 1H, ArHnaphthalene), 7.67–7.64 (m, 1H, ArHnaphthalene), 7.46 (t, J = 7.6 Hz, 1H, ArHnaphthalene), 7.31 (t, J = 7.0 Hz, 2H, PhH), 7.27–7.25 (m, 1H, PhH), 7.15 (d, J = 7.0 Hz, 2H, PhH), 4.77 (t, J = 7.3 Hz, 2H, NCH2), 4.30 (q, J = 7.1 Hz, 2H, OCH2), 3.14 (t, J = 7.3 Hz, 2H, PhCH2), 2.49 (s, 3H, CH3), 1.39 (t, J = 7.1 Hz, 3H, CH3). 13C-NMR (DMSO-d6) δ 165.6 (C=Oester), 148.8 (HOAr-C), 143.6 (ArC), 138.3 (ArC), 129.3 (ArC), 129.1 (ArC), 127.2 (ArC), 126.9 (ArC), 125.0 (ArC), 124.0 (ArC), 123.6 (ArC), 123.1 (ArC), 123.0 (ArC), 122.7 (ArC), 120.7 (ArC), 104.2 (ArC), 102.3 (ArC), 59.4 (OCH2), 47.17 (NCH2), 35.7 (PhCH2), 15.0 (CH3), 11.9 (CH3). HRMS (ESI) calcd for C24H23NO3Na [M+Na]+: 396.1570, found: 396.1564.
3.2.7. General Procedure for the Syntheses of the Target Compounds L–01~L–22
To a solution of indole derivatives (1 mmol), EDCI (0.29 g, 1.5 mmol), HOBt (0.20 g, 1.5 mmol) in dry dichloromethane (10 mL) Et3N (0.76 g, 5 mmol) was added and the mixture was stirred at room temperature for 30 min. Then, the corresponding pyridone derivatives (1 mmol) was added, and the resulting mixture was reacted at room temperature for indicated time while monitoring by TLC. When the reaction was complete the reaction mixture was poured into water, extracted with dichloromethane (15 × 3 mL), and washed with water (3 × 15 mL) and brine (15 mL). The organic solution was dried with anhydrous sodium sulfate, filtered and evaporated under reduced pressure. The solid residue was purified by column chromatography on silica gel.
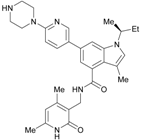
1-Benzyl-N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-2-methyl-5-(2-morpholinoethoxy)-1H-indole-3-carboxamide (L–01). The title product was obtained from 7a and 3-(amino- methyl)-4,6-dimethylpyridin-2(1H)-one hydrochloride according to the general procedure described above as a white solid (0.29 g, 55%). M.p.158–160 °C. 1H-NMR (DMSO-d6) δ 11.45 (s, 1H, NHpyridone), 7.65 (t, J = 5.1 Hz, 1H, NHCH2), 7.32–7.21 (m, 5H, ArH), 6.98 (d, J = 7.4 Hz, 2H, ArH), 6.74 (dd, J = 8.8 Hz, 2.3 Hz, 1H, ArH), 5.89 (s, 1H, ArHpyridone), 5.41 (s, 2H, NCH2benzyl), 4.33 (d, J = 5.4 Hz, 2H, NHCH2), 4.10 (t, J = 5.6 Hz, 2H, OCH2), 3.58 (t, J = 4.5 Hz, 4H, 2 × OCH2), 2.70 (t, J = 5.6 Hz, 2H, NCH2), 2.50 (s, 3H, CH3), 2.49 (t, J = 10.0 Hz, 4H, 2 × NCH2), 2.26 (s, 3H, CH3), 2.12 (s, 3H, CH3). 13C-NMR (DMSO-d6) δ 165.1 (C=Oindole), 163.9 (C=Opyridone), 154.1 (ArCHpyridone), 148.9 (ArCHindole), 142.1 (ArCHpyridone), 140.9 (N-C=Cindole), 138.1 (ArCHbenzyl), 131.4 (ArCHbenzyl), 129.1 (ArCHindole), 127.7 (ArCHbenzyl), 126.6 (ArCHindole), 126.5 (ArCHpyridone), 123.0 (ArCHbenzyl), 111.8 (ArCHindole), 111.3 (N-C=Cindole), 108.7 (ArCHpyridone), 108.1 (ArCHindole), 103.1 (ArCHindole), 66.7 (CH2OCH2), 66.4 (NCH2CH2O), 57.7 (NCH2CH2O), 54.2 (CH2NCH2), 46.2 (NCH2Ph), 35.6 (NHCH2), 19.4 (CH3pyridone), 18.7 (CH3pyridone), 11.9 (CH3indole). ESI-HRMS: m/z [M+Na]+ calcd for C31H36O4N4Na: 551.2629; found: 551.2621.
1-Benzyl-N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-2-methyl-5-(3-(pyrrolidin-1-yl)propoxy)-1H-indole-3-carboxamide (L–02). The title product was obtained from 7b and 3-(aminomethyl)-4,6-dimethylpyridin-2(1H)-one hydrochloride according to the general procedure described above as a white solid (0.23 g, 34%). M.p.195-196 °C. 1H-NMR (DMSO-d6) δ 11.57 (brs, 1H, NHpyridone), 7.65 (t, J = 5.2 Hz, 1H, NHCH2), 7.32–7.18 (m, 5H, ArH), 6.98 (d, J = 7.4 Hz, 2H, ArH), 6.73–6.71 (dd, J = 8.8 Hz, 2.2 Hz, 1H, ArH), 5.90 (s, 1H, ArHpyridone), 5.41 (s, 2H, NCH2benzyl), 4.33 (d, J = 5.3 Hz, 2H, NHCH2), 4.03 (t, J = 6.2 Hz, 2H, OCH2), 2.71 (brs, 2H, CH2), 2.63 (brs, 4H, 2 × CH2), 2.53 (s, 3H, CH3), 2.27 (s, 3H, CH3), 2.12 (s, 3H, CH3), 1.96–1.94 (m, 2H, CH2), 1.73 (brs, 4H, 2 × CH2). 13C-NMR (DMSO-d6) δ 165.1 (C=Oindole), 163.9 (C=Opyridone), 154.2 (ArCHpyridone), 148.8 (ArCHindole), 143.1 (ArCHpyridone), 140.8 (N-C=Cindole), 138.1 (ArCHbenzyl), 131.4 (ArCHbenzyl), 129.1 (ArCHindole), 127.7 (ArCHbenzyl), 126.6 (ArCHindole), 126.3 (ArCHpyridone), 123.0 (ArCHbenzyl), 111.9 (ArCHindole), 111.3 (N-C=Cindole), 108.7 (ArCHpyridone), 108.1 (ArCHindole), 103.0 (ArCHindole), 66.4 (OCH2), 53.9 (NCH2CH2), 52.6 (CH2NCH2tetrahydropyrrole), 46.2 (NCH2Ph), 35.6 (NHCH2), 28.1 (CH2CH2CH2), 23.5 (CH2CH2tetrahydropyrrole), 19.4 (CH3pyridone), 18.7 (CH3pyridone), 11.9 (CH3indole). ESI-HRMS: m/z [M+H]+ calcd for C32H39O3N4: 527.3017; found: 527.3046.
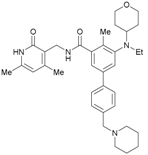
1-Benzyl-N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-2-methyl-5-(3-morpholinopropoxy)-1H-indole-3-carboxamide (L–03). The title product was obtained from 7c and 3-(aminomethyl)-4,6-dimethylpyridin-2(1H)-one hydrochloride according to the general procedure described above as a yellow solid (0.30 g, 86%). M.p. 158-160 °C. 1H-NMR (DMSO-d6) δ 11.53 (s, 1H, NHpyridone), 7.65 (t, J = 5.1 Hz, 1H, NHCH2), 7.31–7.21 (m, 5H, ArH), 6.98 (d, J = 7.5 Hz, 2H, ArH), 6.72 (dd, J = 8.8 Hz, 2.3 Hz, 1H, ArH), 5.89 (s, 1H, ArHpyridone), 5.41 (s, 2H, NCH2benzyl), 4.33 (d, J = 5.3 Hz, 2H, NHCH2), 4.10 (t, J = 6.3 Hz, 2H, OCH2), 3.57 (t, J = 4.4 Hz, 4H, 2 × OCH2), 2.50 (s, 3H, CH3), 2.40 (t, J = 7.2 Hz, 2H, CH2), 2.37 (s, 4H, 2 × NCH2), 2.26 (s, 3H, CH3), 2.12 (s, 3H, CH3), 1.91–1.86 (m, 2H, CH2). 13C-NMR (DMSO-d6) δ 165.1 (C=Oindole), 163.9 (C=Opyridone), 154.3 (ArCHpyridone), 148.9 (ArCHindole), 143.0 (ArCHpyridone), 140.8 (N-C=Cindole), 138.2 (ArCHbenzyl), 131.3 (ArCHbenzyl), 129.1 (ArCHbenzyl), 127.6 (ArCHindole), 126.6 (ArCHindole), 126.3 (ArCHpyridone), 123.0 (ArCHbenzyl), 111.8 (ArCHindole), 111.3 (N-C=Cindole), 108.7 (ArCHpyridone), 108.1 (ArCHindole), 103.0 (ArCHindole), 66.8 (CH2CH2O), 66.6 (CH2OCH2), 55.5 (CH2NCH2), 53.9 (NCH2CH2CH2O), 46.2 (NCH2Ph), 35.6 (NHCH2), 26.6 (NCH2CH2CH2O), 19.4 (CH3pyridone), 18.7 (CH3pyridone), 12.0 (CH3indole). ESI-HRMS: m/z [M+Na]+ calcd for C32H38O4N4Na: 565.2785; found: 565.2797.
1-Benzyl-N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-2-methyl-5-(2-(piperidin-1-yl)ethoxy-1H-indole-3-carboxamide (L–04). The title product was obtained from 7d and 3-(aminomethyl)-4,6-dimethylpyridin-2(1H)-one hydrochloride according to the general procedure described above as a white solid (0.26 g, 50%). M.p.215–217 °C. 1H-NMR (DMSO-d6) δ 11.54 (s, 1H, NHpyridone), 7.65 (t, J = 4.9 Hz, 1H, NHCH2), 7.32–7.21 (m, 5H, ArH), 6.98 (d, J = 7.5 Hz, 2H, ArH), 6.73 (m, 1H, ArH), 5.89 (s, 1H, ArHpyridone), 5.41 (s, 2H, NCH2benzyl), 4.33 (d, J = 5.1Hz, 2H, NHCH2), 4.06 (t, J = 5.6 Hz, 2H, OCH2), 2.66 (t, J = 5.6 Hz, 2H, NCH2), 2.53 (s, 3H, CH3), 2.43 (s, 4H, 2 × CH2), 2.26 (s, 3H, CH3), 2.12 (s, 3H, CH3), 1.49 (t, J = 5.3 Hz, 4H, 2 × CH2), 1.37 (m, 2H, CH2). 13C-NMR (DMSO-d6) δ 165.1 (C=Oindole), 163.9 (C=Opyridone), 154.2 (ArCHpyridone), 148.7 (ArCHindole), 143.1 (ArCHpyridone), 140.9 (N-C=Cindole), 138.1 (ArCHbenzyl), 131.3 (ArCHbenzyl), 129.1 (ArCHbenzyl), 127.6 (ArCHindole), 126.6 (ArCHindole), 126.3 (ArCHpyridone), 123.0 (ArCHbenzyl), 111.7 (ArCHindole), 111.3 (N-C=Cindole), 108.7 (ArCHpyridone), 108.1 (ArCHindole), 103.1 (ArCHindole), 66.6 (NCH2CH2O), 58.1 (CH2NCH2), 54.9 (NCH2Ph), 46.2 (NCH2CH2O), 35.6 (NHCH2), 26.1 (CH2CH2CH2piperidine), 24.5 (CH2CH2CH2piperidine), 19.4 (CH3pyridone), 18.7 (CH3pyridone), 11.9 (CH3indole). ESI-HRMS: m/z [M+H]+ calcd for C32H38O4N4Na: 527.3017; found: 527.3018.
1-Benzyl-N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-2-methyl-5-(3-(piperidin-1-yl)propoxy)-1H-indole-3-carboxamide (L–05). The title product was obtained from 7e and 3-(aminomethyl)-4,6-dimethylpyridin-2(1H)-one hydrochloride according to the general procedure described above as a white solid (0.20 g, 41%). M.p. 210–212 °C. 1H-NMR (DMSO-d6) δ 11.53 (s, 1H, NHpyridone), 7.68 (s, 1H, NHCH2), 7.31–7.21 (m, 5H, ArH), 6.98 (d, J = 7.5 Hz, 2H, ArH), 6.72 (dd, J = 8.8 Hz, 1.9 Hz, 1H, ArH), 5.89 (s, 1H, ArHpyridone), 5.41 (s, 2H, NCH2benzyl), 4.33 (d, J = 5.2 Hz, 2H, NHCH2), 3.99 (t, J = 6.2 Hz, 2H, OCH2), 2.53 (s, 3H, CH3), 2.38 (t, J = 7.1 Hz, 2H, NCH2), 2.33 (s, 4H, 2 × NCH2), 2.26 (s, 3H, CH3), 2.12 (s, 3H, CH3), 1.89–1.84 (m, 2H, CH2), 1.49 (t, J = 5.3 Hz, 4H, 2 × CH2), 1.37 (s, 2H, CH2). 13C-NMR (DMSO-d6) δ 165.1 (C=Oindole), 163.9 (C=Opyridone), 154.3 (ArCHpyridone), 148.8 (ArCHindole), 143.1 (ArCHpyridone), 140.8 (N-C=Cindole), 138.1 (ArCHbenzyl), 131.3 (ArCHbenzyl), 129.1 (ArCHbenzyl), 127.6 (ArCHindole), 126.6 (ArCHindole), 126.3 (ArCHpyridone), 123.0 (ArCHbenzyl), 111.8 (ArCHindole), 111.3 (N-C=Cindole), 108.7 (ArCHpyridone), 108.1 (ArCHindole), 103.0 (ArCHindole), 66.8 (CH2O), 55.8 (NCH2CH2CH2O), 54.6 (CH2NCH2), 46.2 (NCH2Ph), 35.6 (NHCH2), 27.0 (NCH2CH2CH2O), 26.1 (CH2CH2CH2piperidine), 24.6 (CH2CH2CH2piperidine), 19.4 (CH3pyridone), 18.7 (CH3pyridone), 11.9 (CH3indole). ESI-HRMS: m/z [M+H]+ calcd for C33H41O3N4: 541.3173; found: 541.3179.
5-(Allyloxy)-1-benzyl-N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-2-methyl-1H-indole-3-carboxamide (L–06). The title product was obtained from 7f and 3-(amino- methyl)-4,6-dimethylpyridin-2(1H)-one hydrochloride according to the general procedure described above as a white solid (0.35 g, 78%). M.p. 245–47 °C. 1H-NMR (DMSO-d6) δ 11.58 (brs, 1H, NHpyridone), 7.69 (m, 1H, NHCH2), 7.33–7.21 (m, 5H, ArH), 6.98 (d, J = 7.4 Hz, 2H, ArH), 6.77–6.75 (dd, J = 8.9 Hz, 2.3 Hz, 1H, ArH), 6.10–6.04 (m, 1H, ArH), 5.89 (s, 1H, ArHpyridone), 5.41 (s, 2H, NCH2benzyl), 5.39 (d, J = 1.6 Hz, 1H, ArH), 5.24–5.23 (m, ArH), 4.58 (d, J = 5.1 Hz, 2H, OCH2), 4.33 (d, J = 5.3 Hz, 2H, NHCH2), 2.54 (s, 3H, CH3), 2.26 (s, 3H, CH3), 2.12 (s, 3H, CH3). 13C-NMR (DMSO-d6) δ 165.0 (C=Oindole), 163.9 (C=Opyridone), 153.9 (ArCHpyridone), 148.8 (ArCHindole), 143.1 (ArCHpyridone), 141.1 (N-C=Cindole), 138.1 (ArCHbenzyl), 134.8 (CH2=CH), 131.4 (ArCHbenzyl), 129.1 (ArCHbenzyl), 127.7 (ArCHindole), 126.6 (ArCHindole), 126.2 (ArCHpyridone), 122.9 (ArCHbenzyl), 117.5 (CH2=CH), 111.8 (ArCHindole), 111.3 (N-C=Cindole), 108.6 (ArCHpyridone), 108.1 (ArCHindole), 103.4 (ArCHindole), 69.1 (CH2O), 46.2 (NCH2Ph), 35.6 (NHCH2), 19.4 (CH3pyridone), 18.7 (CH3pyridone), 11.9 (CH3indole). ESI-HRMS: m/z [M+Na]+ calcd for C28H29O3N3Na: 478.2101; found: 478.2097.
1-Benzyl-5-(cyclopropylmethoxy)-N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-2-methyl-1H-indole-3-carboxamide (L–07). The titled product was obtained from 7g and 3-(aminomethyl)-4,6-dimethylpyridin-2(1H)-one hydrochloride according to the general procedure described above as a white solid (0.30 g, 64%). M.p.136–138 °C. 1H-NMR (DMSO-d6) δ 11.53 (s, 1H, NHpyridone), 7.63 (t, J = 5.2 Hz, 1H, NHCH2), 7.32–7.21 (m, 5H, ArH), 6.98 (d, J = 7.4 Hz, 2H, ArH), 6.74–6.73 (dd, J = 8.8 Hz, 2.3 Hz, 1H, ArH), 5.89 (s, 1H, ArHpyridone), 5.41 (s, 2H, NCH2benzyl), 4.33 (d, J = 8.6 Hz, 2H, NHCH2), 3.81 (d, J = 6.9 Hz, 2H, OCH2), 2.53 (s, 3H, CH3), 2.26 (s, 3H, CH3), 2.12 (s, 3H, CH3), 1.24–1.19 (m, 1H, CH), 0.58–0.55 (m, 2H, CH2), 0.33 (q, J = 5.2 Hz, 2H, CH2). 13C-NMR (DMSO-d6) δ 165.1 (C=Oindole), 163.9 (C=Opyridone), 154.3 (ArCHpyridone), 148.9 (ArCHindole), 143.1 (ArCHpyridone), 140.9 (N-C=Cindole), 138.1 (ArCHbenzyl), 131.3 (ArCHbenzyl), 129.1 (ArCHbenzyl), 127.6 (ArCHindole), 126.6 (ArCHindole), 126.3 (ArCHpyridone), 122.9 (ArCHbenzyl), 111.8 (ArCHindole), 111.3 (N-C=Cindole), 108.6 (ArCHpyridone), 108.1 (ArCHindole), 103.8 (ArCHindole), 72.9 (CH2O), 46.2 (NCH2Ph), 35.6 (NHCH2), 19.4 (CH3pyridone), 18.7 (CH3pyridone), 11.9 (CH3indole), 10.9 (CH), 3.6 (CH2CH2). ESI-HRMS: m/z [M+Na]+ calcd for C29H31O3N3Na: 492.2258; found: 492.2266.
1-Benzyl-N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-5-ethoxy-2-methyl-1H-indole-3-carboxamide (L–08). The title product was obtained from 7h and 3-(amino- methyl)-4,6-dimethylpyridin-2(1H)-one hydrochloride according to the general procedure described above as a pink solid (0.30 g, 43%). M.p. 263–264 °C. 1H-NMR (DMSO-d6) δ 11.54 (s, 1H, NHpyridone), 7.65 (s, 1H, NHCH2), 7.32–7.21 (m, 5H, ArH), 6.98 (d, J = 7.6 Hz, 2H, ArH), 6.73 (d, J = 8.8 Hz, 1H, ArH), 5.89 (s, 1H, ArHpyridone), 5.41 (s, 2H, NCH2benzyl), 4.33 (d, J = 5.3 Hz, 2H, NHCH2), 4.03 (q, J = 6.8 Hz, 2H, CH2), 2.54 (s, 3H, CH3), 2.26 (s, 3H, CH3), 2.12 (s, 3H, CH3), 1.34 (t, J = 6.9 Hz, 3H, CH3). 13C-NMR (DMSO-d6) δ 165.1 (C=Oindole), 164.0 (C=Opyridone), 154.2 (ArCHpyridone), 148.7 (ArCHindole), 143.2 (ArCHpyridone), 141.0 (N-C=Cindole), 138.1 (ArCHbenzyl), 131.3 (ArCHbenzyl), 129.1 (ArCHbenzyl), 127.6 (ArCHindole), 126.6 (ArCHindole), 126.2 (ArCHpyridone), 122.9 (ArCHbenzyl), 111.8 (ArCHindole), 111.3 (N-C=Cindole), 108.6 (ArCHpyridone), 108.1 (ArCHindole), 102.8 (ArCHindole), 63.8 (CH2O), 46.2 (NCH2Ph), 35.7 (NHCH2), 19.4 (CH3pyridone), 18.7 (CH3pyridone), 15.4 (CH2CH3), 11.9 (CH3indole). ESI-HRMS: m/z [M+Na]+ calcd for C27H29O3N3Na: 466.2101; found: 466.2107.
1-Benzyl-N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-5-hydroxy-2-methyl-1H-indole-3-carboxamide (L–09). The title product was obtained from 7i and 3-(aminomethyl)-4,6-dimethylpyridin-2(1H)-one hydrochloride according to the general procedure described above as a white solid (0.40, 61%). M.p. 288–290 °C. 1H-NMR (DMSO-d6) δ 11.55 (s, 1H, NHpyridone), 8.85 (s, 1H, OH), 7.44 (t, J = 5.3 Hz, 1H, NHCH2), 7.29–7.20 (m, 4H, ArH), 7.13 (d, J = 2.0 Hz, 1H, ArH), 6.99 (d, J = 7.6 Hz, 2H, ArH), 6.61–6.59 (dd, J = 8.6 Hz, 2.0 Hz, 1H, ArH), 5.89 (s, 1H, ArHpyridone), 5.37 (s, 2H, NCH2benzyl), 4.33 (d, J = 5.4 Hz, 2H, NHCH2), 2.52 (s, 3H, CH3), 2.26 (s, 3H, CH3), 2.12 (s, 3H, CH3). 13C-NMR (DMSO-d6) δ 165.4 (C=Oindole), 163.9 (C=Opyridone), 152.6 (ArCHpyridone), 148.9 (ArCHindole), 143.1 (ArCHpyridone), 140.7 (N-C=Cindole), 138.2 (ArCHbenzyl), 130.7 (ArCHbenzyl), 129.1 (ArCHbenzyl), 127.6 (ArCHindole), 126.6 (ArCHindole), 122.9 (ArCHbenzyl), 111.6 (ArCHindole), 111.0 (N-C=Cindole), 108.1 (ArCHpyridone), 108.0 (ArCHindole), 104.4 (ArCHindole), 46.1 (NCH2Ph), 35.6 (NHCH2), 19.4 (CH3pyridone), 18.7 (CH3pyridone), 12.0 (CH3indole). ESI-HRMS: m/z [M+Na]+ calcd for C25H25O3N3Na: 438.1788; found: 438.1798.
N-((4,6-Dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-5-hydroxy-2-methyl-1-phenethyl-1H-indole-3-carboxamide (L–10). The title product was obtained from 7j and 3-(amino- methyl)-4,6-dimethylpyridin-2(1H)-one hydrochloride according to the general procedure described above as a white solid (0.25 g, 58%). M.p.242–245 °C. 1H-NMR (DMSO-d6) δ 11.54 (brs, 1H, NHpyridone), 8.84 (brs, 1H, OH), 7.32 (t, J = 5.0 Hz, 1H, NHCH2), 7.28–7.19 (m, 4H, ArH), 7.16 (d, J = 7.1 Hz, 2H, ArH), 7.10 (d, J = 1.7 Hz, 1H, ArH), 6.65–6.63 (dd, J = 8.6 Hz, 1.9 Hz, 1H, ArH), 5.89 (s, 1H, ArHpyridone), 4.30 (d, J = 5.3 Hz, 2H, NHCH2), 4.26 (t, J = 7.3 Hz, 2H, NCH2), 2.91 (t, J = 7.3 Hz, 2H, PhCH2), 2.37 (s, 3H, CH3), 2.25 (s, 3H, CH3), 2.12 (s, 3H, CH3). 13C-NMR (DMSO-d6) δ 165.4 (C=Oindole), 163.9 (C=Opyridone), 152.4 (ArCHpyridone), 148.8 (ArCHindole), 143.1 (ArCHpyridone), 140.8 (N-C=Cindole), 138.9 (PhCH), 130.1 (PhCH), 129.4 (PhCH), 128.8 (ArCHindole), 126.9 (ArCHindole), 126.5 (ArCHpyridone), 123.0 (PhCH), 111.4 (ArCHindole), 110.8 (N-C=Cindole), 108.1 (ArCHpyridone), 107.4 (ArCHindole), 104.3 (ArCHindole), 44.6 (NCH2CH2Ph), 35.8 (NCH2CH2Ph), 35.6 (NHCH2), 19.4 (CH3pyridone), 18.6 (CH3pyridone), 11.6 (CH3indole). ESI-HRMS: m/z [M+Na]+ calcd for C26H27O3N3Na: 452.1945; found: 452.1980.
N-((4,6-Dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-5-hydroxy-2-methyl-1-phenethyl-1H-benzo- [g]indole-3-carboxamide (L–11). The title product was obtained from 7k and 3-(amino- methyl)-4,6-dimethylpyridin-2(1H)-one hydrochloride according to the general procedure described above as a white solid (0.35 g, 64%). M.p. 240 °C (decomposes). 1H-NMR (DMSO-d6) δ 11.36 (brs, 1H, NHpyridone), 8.42 (d, J=8.6 Hz, 1H, ArH), 8.29 (d, J=8.3 Hz, 1H, ArH), 7.62 (t, J=7.5 Hz, 1H, NHCH2), 7.44-7.41 (m, 2H, ArH), 7.37 (s, 1H, ArH), 7.34-7.29 (m, 2H, ArH), 7.27-7.25 (m, 1H, ArH), 7.21 (d, J=7.4 Hz, 2H, ArH), 5.89 (s, 1H, ArHpyridone), 4.70 (t, J=7.4 Hz, 2H, NCH2), 4.34 (d, J=5.0 Hz, 2H, NHCH2), 3.08 (t, J=7.3 Hz, 2H, PhCH2), 2.37 (s, 3H, CH3), 2.27 (s, 3H, CH3), 2.12 (s, 3H, CH3). 13C-NMR (DMSO-d6) δ 165.6 (C=Oindole), 163.9 (C=Opyridone), 149.2 (ArCHpyridone), 148.0 (ArCHindole), 143.1 (ArCHpyridone), 138.5 (N-C=Cindole), 137.6 (PhCH), 129.3 (PhCH), 129.1 (ArCHindole), 127.2 (ArCHindole), 126.7 (ArCHpyridone), 124.0 (ArCH), 124.0 (ArCH), 123.5 (ArCH), 122.9 (ArCH), 122.8 (ArCH), 122.7 (ArCH), 122.6 (ArCH), 120.6 (ArCH), 110.3 (N-C=Cindole), 108.1 (ArCHpyridone), 101.2 (ArCHindole), 47.1 (NCH2CH2Ph), 36.0 (NCH2CH2Ph), 35.6 (NHCH2), 19.4 (CH3pyridone), 18.7 (CH3pyridone), 11.7 (CH3indole). ESI-HRMS: m/z [M+Na]+ calcd for C30H29O3N3Na: 502.2101; found: 502.2124.
N-((4,6-Dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-5-hydroxy-2-methyl-1-phenyl-1H-indole-3- carboxamide (L–12). The title product was obtained from 7l and 3-(amino- methyl)-4,6-dimethylpyridin-2(1H)-one hydrochloride according to the general procedure described above as a white solid (0.15 g, 38%). M.p. 285–287 °C. 1H-NMR (DMSO-d6) δ 11.56 (brs, 1H, NHpyridone), 8.94 (s, 1H, OH), 7.62–7.59 (m, 2H, ArH), 7.54–7.50 (m, 2H, ArH), 7.40 (d, J = 7.3 Hz, 2H, ArH), 7.18 (d, J = 2.1 Hz, 1H), 6.78 (d, J = 8.7 Hz, 1H, ArH), 6.61–6.59 (dd, J = 8.7 Hz, 2.2 Hz, 1H, ArH), 5.90 (s, 1H, ArHpyridone), 4.35 (d, J = 5.4 Hz, 2H, NHCH2), 2.38 (s, 3H, CH3), 2.27 (s, 3H, CH3), 2.12 (s, 3H, CH3). 13C-NMR (DMSO-d6) δ 165.3 (C=Oindole), 163.9 (C=Opyridone), 152.9 (ArCHpyridone), 149.0 (ArCHindole), 143.1 (ArCHpyridone), 140.3 (N-C=Cindole), 137.0 (PhCH), 131.7 (ArCHindole), 130.2 (PhCH), 128.8 (ArCHindole), 128.4 (PhCH), 126.7 (ArCHpyridone), 122.9 (PhCH), 122.1 (ArCHindole), 111.0 (N-C=Cindole), 109.1 (ArCHindole), 108.1 (ArCHpyridone), 104.4 (ArCHindole), 35.6 (NHCH2), 19.4 (CH3pyridone), 18.7 (CH3pyridone), 13.0 (CH3indole). ESI-HRMS: m/z [M+Na]+ calcd for C24H23O3N3Na: 424.1632; found: 424.1662.
N-((4,6-Dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-1-(furan-2-ylmethyl)-5-hydroxy-2-methyl-1H-indole-3-carboxamide (L–13). The title product was obtained from 7m and 3-(amino- methyl)-4,6-dimethylpyridin-2(1H)-one hydrochloride according to the general procedure described above as a white solid (0.30 g, 75%). M.p. 290–292 °C. 1H-NMR (DMSO-d6) δ 11.54 (s, 1H, NHpyridone), 8.85 (s, 1H, OH), 7.53 (s, 1H, NHCH2), 7.39 (dd, J = 13.6 Hz, 5.2 Hz, 2H, ArH), 7.08 (d, J = 2.0 Hz, 1H, ArH), 6.64-6.63 (dd, J = 8.6 Hz, 2.0 Hz, 1H), 6.40–6.36 (m, 2H, ArH), 5.88 (s, 1H, ArHpyridone), 5.32 (s, 2H, NCH2), 4.31 (d, J = 5.3 Hz, 2H, NHCH2), 2.64 (s, 3H, CH3), 2.25 (s, 3H, CH3), 2.11 (s, 3H, CH3). 13C-NMR (DMSO-d6) δ 165.3 (C=Oindole), 163.9 (C=Opyridone), 152.5 (ArCHpyridone), 151.0 (ArCHfuran), 148.9 (ArCHindole), 143.3 (ArCHpyridone), 143.1 (ArCHfuran), 140.5 (N-C=Cindole), 130.4 (ArCHfuran), 126.5 (ArCHindole), 122.9 (ArCHfuran), 111.5 (ArCHindole), 111.0 (N-C=Cindole), 108.6 (ArCHindole), 108.1 (ArCHpyridone), 108.0 (ArCHindole), 104.3 (ArCHindole), 46.2 (NCH2), 35.5 (NHCH2), 19.4 (CH3pyridone), 18.6 (CH3pyridone), 11.9 (CH3indole). ESI-HRMS: m/z [M+Na]+ calcd for C23H23O4N3Na: 428.1581; found: 428.1587.
1-Cyclohexyl-N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-5-hydroxy-2-methyl-1H-indole-3-carboxamide (L–14). The title product was obtained from 7n and 3-(amino- methyl)-4,6-dimethylpyridin-2(1H)-one hydrochloride according to the general procedure described above as a white solid (0.36 g, 90%). M.p. 268–270 °C. 1H-NMR (DMSO-d6) δ 11.53 (s, 1H, NHpyridone), 8.80 (s, 1H, OH), 7.44 (t, J = 5.3 Hz, 1H, NHCH2), 7.32 (t, J = 5.3 Hz, 1H, ArH), 7.05 (d, J = 1.9 Hz, 1H, ArH), 6.60–6.58 (dd, J = 2.0 Hz, 1H, ArH), 5.88 (s, 1H, ArHpyridone), 4.31 (d, J = 5.3 Hz, 2H, NHCH2), 4.20 (brs, 1H, NCH), 2.57 (s, 3H, CH3), 2.25 (s, 3H, CH3), 2.11 (s, 3H, CH3), 2.19–2.17 (m, 2H, CH2), 1.86 (d, J = 13.0 Hz, 2H, CH2), 1.74–1.67 (m, 3H, CH2+1/2CH2), 1.48–1.30 (m, 3H, CH2+1/2CH2). 13C-NMR (DMSO-d6) δ 165.7 (C=Oindole), 163.9 (C=Opyridone), 151.8 (ArCHpyridone), 148.9 (ArCHindole), 143.0 (ArCHpyridone), 140.1 (N-C=Cindole), 129.0 (ArCHindole), 127.5 (ArCHpyridone), 122.9 (ArCHindole), 113.1 (ArCHindole), 111.0 (N-C=Cindole), 108.1 (ArCHpyridone), 107.9 (ArCHindole), 104.2 (ArCHindole), 55.2 (NCH), 35.5 (NHCH2), 31.0 (CH2cyclohexyl), 26.2 (CH2cyclohexyl), 25.3 (CH2cyclohexyl), 19.4 (CH3pyridone), 18.6 (CH3pyridone), 12.5 (CH3indole). ESI-HRMS: m/z [M+Na]+ calcd for C24H29O3N3Na: 430.2101; found: 430.2116.
N-((4,6-Dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-5-hydroxy-1-isopropyl-2-methyl-1H-indole-3-carboxamide (L–15). The titled product was obtained from 7o and 3-(amino- methyl)-4,6-dimethylpyridin-2(1H)-one hydrochloride according to the general procedure described above as a white solid (0.24 g, 65%). M.p. 234–236 °C. 1H-NMR (DMSO-d6) δ 11.53 (s, 1H, NHpyridone), 8.80 (s, 1H, OH), 7.39 (d, J = 8.8 Hz, 1H, ArH), 7.32 (t, J = 5.2 Hz, 1H, NHCH2), 7.06 (d, J = 2.1 Hz, 1H, ArH), 6.61–6.59 (dd, J = 8.8 Hz, 2.2 Hz, 1H, ArH), 5.88 (s, 1H, ArHpyridone), 4.71–4.66 (m, 1H, NCH), 4.31 (d, J = 5.3 Hz, 2H, NHCH2), 2.56 (s, 3H, CH3), 2.25 (s, 3H, CH3), 2.11 (s, 3H, CH3), 1.50 (d, J = 7.0 Hz, 6H, 2 × CH3). 13C-NMR (DMSO-d6) δ 165.6 (C=Oindole), 163.8 (C=Opyridone), 151.9 (ArCHpyridone), 148.9 (ArCHindole), 143.0 (ArCHpyridone), 139.9 (N-C=Cindole), 128.7 (ArCHindole), 127.5 (ArCHindole), 122.9 (ArCHindole), 112.5 (ArCHindole), 111.1 (N-C=Cindole), 108.1 (ArCHpyridone), 107.7 (ArCHpyridone), 104.3 (ArCHindole), 46.8 (NCH), 35.5 (NHCH2), 21.5 (CH3), 19.4 (CH3pyridone), 18.6 (CH3pyridone), 12.4 (CH3indole). ESI-HRMS: m/z [M+Na]+ calcd for C21H25O3N3Na: 390.1788; found: 390.1821.
N-((4,6-Dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-1-isopropyl-2-methyl-5-(3-(piperidin-1-yl)- propoxy)-1H-indole-3-carboxamide (L–16). The title product was obtained from 7e and 3 according to the general procedure described above as a white solid (80 mg, 40%). M.p. 200–202 °C. 1H-NMR (CDCl3) δ 12.06 (brs, 1H, NHpyridone), 7.46 (t, J = 5.7 Hz, 1H, NHCH2), 7.34–7.32 (m, 2H, ArH), 6.73 (dd, J = 7.1 Hz, 2.2 Hz, 1H, ArH), 5.87 (s, 1H, ArHpyridone), 4.70–4.65 (m, 1H, NCH), 4.58 (d, J = 6.0 Hz, 2H, NHCH2), 4.12 (t, J = 6.6 Hz, 2H, OCH2), 2.72 (brs, 2H, CH2), 2.71 (s, 3H, CH3), 2.60 (brs, 4H, CH2NCH2), 2.41 (s, 3H), 2.23 (s, 3H, CH3), 2.08–2.06 (m, 2H, CH2), 1.71–1.70 (m, 4H, 2 × CH2), 1.58 (d, J = 7.0 Hz, 6H, 2 × CH3), 1.48 (brs, 2H, CH2). 13C-NMR (CDCl3) δ 165.9 (C=Oindole), 165.0 (C=Opyridone), 153.8 (ArCHpyridone), 149.3 (ArCHindole), 142.3 (ArCHpyridone), 141.0 (N-C=Cindole), 129.3 (ArCHindole), 126.7 (ArCHindole), 123.8 (ArCHindole), 112.3 (ArCHindole), 111.6 (N-C=Cindole), 109.5 (ArCHpyridone), 108.0 (ArCHpyridone), 102.2 (ArCHindole), 66.9 (CH2O), 55.8 (NCH2CH2CH2O), 54.2 (CH2NCH2), 46.9 (NCH), 35.6 (NHCH2), 26.1 (NCH2CH2CH2O), 24.8 (CH2piperidine), 23.7 (CH2piperidine), 21.4 (CH3), 19.6 (CH3pyridone), 19.0 (CH3pyridone), 12.1 (CH3indole). ESI-HRMS: m/z [M+H]+ calcd for C29H41O3N4: 493.3173; found: 493.3174.
1-Isopropyl-N-((4-methoxy-6-methyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-2-methyl-5-(3-(piperidin-1-yl)propoxy)-1H-indole-3-carboxamide (L–17). The title product was obtained from 7e and pyridone 25 according to the general procedure described above as a white solid (100 mg, 48%). M.p. 181–188 °C. 1H-NMR (CDCl3) δ 7.66 (t, J = 5.0 Hz, 1H, NHCH2), 7.42 (d, J = 1.9 Hz, 1H, ArH), 7.35 (d, J = 8.9 Hz, 1H, ArH), 6.74–6.72 (dd, J = 8.9 Hz, 2.2 Hz, 1H, ArH), 5.90 (s, 1H, ArHpyridone), 4.71–4.67 (m, 1H, NCH), 4.63 (d, J = 5.5 Hz, 2H, OCH2), 4.15 (t, J = 6.1 Hz, 2H, NCH2), 3.88 (s, 3H, OCH3), 2.90 (brs, 2H, CH2), 2.74–2.62 (m, 6H, CH2), 2.32 (s, 3H, CH3), 2.14 (t, J = 6.8 Hz, 2H, CH2), 1.77 (brs, 4H, 2 × CH2), 1.59 (d, J = 7.0 Hz, 6H, 2 × CH3), 1.50 (brs, 2H, CH2). 13C-NMR (CDCl3) δ 165.8 (C=Oindole), 165.7 (ArCOCH3pyridone), 165.4 (C=Opyridone), 153.5 (ArCHpyridone), 146.0 (ArCHindole), 141.5 (N-C=Cindole), 129.5 (ArCHindole), 126.7 (ArCHindole), 112.3 (ArCHindole), 111.4 (N-C=Cindole), 108.2 (ArCHpyridone), 107.5 (ArCHindole), 103.0 (ArCHindole), 94.5 (ArCHpyridone), 66.8 (CH2O), 56.1 (OCH3), 55.6 (NCH2CH2CH2O), 53.9 (CH2NCH2), 46.9 (NCH), 32.7 (NHCH2), 25.4 (NCH2CH2CH2O), 24.1 (CH2piperidine), 23.2 (CH2piperidine), 21.4 (CH3), 19.5 (CH3pyridone), 12.2 (CH3indole). ESI-HRMS: m/z [M+H]+ calcd for C29H41O4N4: 509.3122; found: 509.3127.
1-Isopropyl-2-methyl-N-((2-oxo-2,5,6,7-tetrahydro-1H-cyclopenta[b]pyridin-3-yl)methyl)-5-(3-(piperidin-1-yl)propoxy)-1H-indole-3-carboxamide (L–18). The title product was obtained from 7e and pyridone 17 according to the general procedure described above as a white solid (65 mg, 31%). M.p. 141–144 °C. 1H-NMR (CDCl3) δ 7.45 (s, 1H, OH), 7.43 (t, J = 5.5 Hz, 1H, NHCH2), 7.35–7.33 (m, 2H, ArH), 6.73–6.71 (dd, J = 8.9 Hz, 1.9 Hz, 1H, ArH), 4.70–4.66 (m, 1H, NCH), 4.50 (d, J = 5.9 Hz, 2H, OCH2), 4.20 (t, J = 6.7 Hz, 2H, NCH2), 2.95 (brs, 2H, CH2), 2.85 (t, J = 7.2 Hz, 2H, CH2), 2.82 (brs, 2H, CH2), 2.71 (s, 3H, CH3), 2.68 (t, J = 7.3 Hz, 2H, CH2), 2.22 (t, J = 6.4 Hz, 2H, CH2), 2.10–2.05 (m, 2H, CH2), 1.82 (brs, 4H, 2 × CH2), 1.58 (d, J = 7.0 Hz, 6H, 2 × CH3), 1.55 (brs, 2H, CH2). 13C-NMR (CDCl3) δ 166.0 (C=Oindole), 164.5 (C=Opyridone), 153.7 (ArCHpyridone), 147.9 (ArCHindole), 141.3 (ArCHpyridone), 137.2 (N-C=Cindole), 129.3 (ArCHindole), 126.6 (ArCHindole), 126.3 (ArCHindole), 119.0 (ArCHindole), 112.4 (N-C=Cindole), 111.8 (ArCHpyridone), 108.1 (ArCHpyridone), 102.2 (ArCHindole), 66.9 (CH2O), 55.6 (NCH2CH2CH2O), 54.0 (CH2NCH2), 46.9 (NCH), 40.5 (NHCH2), 31.5 (CH2pyridone), 29.6 (CH2pyridone), 25.6 (NCH2CH2CH2O), 24.2 (CH2piperidine), 23.3 (CH2piperidine), 23.0 (CH2pyridone), 21.4 (CH3), 12.0 (CH3indole). ESI-HRMS: m/z [M+H]+ calcd for C30H41O3N4: 505.3173; found: 505.3188.
N-((6-Ethyl-4-methyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-1-isopropyl-2-methyl-5-(3-(piperidin-1-yl)propoxy)-1H-indole-3-carboxamide (L–19). The title product was obtained from 7e and pyridone 22 according to the general procedure described above as a yellow solid (75 mg, 36%). M.p. 180–182 oC. 1H-NMR (CDCl3) δ 7.42 (t, J = 5.5 Hz, 1H, NHCH2), 7.34–7.33 (m, 2H, ArH), 6.74–6.72 (dd, J = 8.9 Hz, 1.9 Hz, 1H, ArH), 5.90 (s, 1H, ArHpyridone), 4.71–4.64 (m, 1H, NCH), 4.59 (d, J = 6.1 Hz, 2H, OCH2), 4.10 (t, J = 6.2 Hz, 2H, NCH2), 2.71 (s, 3H, CH3), 2.69 (brs, 2H, CH2), 2.55 (brs, 4H, 2 × CH2), 2.52 (q, J = 7.6 Hz, 2H, CH2), 2.43 (s, 3H), 2.03 (brs, 2H, CH2), 1.67 (brs, 4H, 2 × CH2), 1.58 (s, 6H, 2 × CH3), 1.45 (brs, 2H, CH2), 1.18 (t, J = 7.5 Hz, 3H, CH3). 13C-NMR (CDCl3) δ 165.9 (C=Oindole), 165.1 (C=Opyridone), 153.9 (ArCHpyridone), 149.5 (ArCHindole), 148.1 (ArCHpyridone), 141.0 (N-C=Cindole), 129.3 (ArCHindole), 126.7 (ArCHindole), 123.7 (ArCHindole), 112.3 (ArCHindole), 111.5 (N-C=Cindole), 108.0 (ArCHpyridone), 107.8 (ArCHpyridone), 102.4 (ArCHindole), 66.9 (CH2O), 55.9 (NCH2CH2CH2O), 54.2 (CH2NCH2), 46.9 (NCH), 35.7 (NHCH2), 26.1 (NCH2CH2CH2O), 26.0 (CH2CH3pyridone), 24.9 (CH2piperidine), 23.7 (CH2piperidine), 21.4 (CH3), 19.8 (CH3pyridone), 12.7 (CH2CH3pyridone), 12.2 (CH3indole). ESI-HRMS: m/z [M+H]+ calcd for C30H43O3N4: 507.3330; found: 507.3330.
1-Isopropyl-2-methyl-N-((2-oxo-1,2,5,6,7,8-hexahydroquinolin-3-yl)methyl)-5-(3-(piperidin-1-yl)propoxy)-1H-indole-3-carboxamide (L–20). The title product was obtained from 7e and pyridone 16 according to the general procedure described above as a white solid (52 mg, 25%). M.p. 127–130 °C. 1H-NMR (CDCl3) δ 11.61 (brs, 1H, NHpyridone), 7.41 (brs, 2H, ArH), 7.36–7.34 (m, 2H, ArH), 6.74–6.73 (dd, J = 8.9 Hz, 1.9 Hz, 1H, ArH), 4.73–4.67 (m, 1H, NCH), 4.49 (d, J = 5.8 Hz, 2H, OCH2), 4.21 (t, J = 5.8 Hz, 2H, NCH2), 3.06 (brs, 2H, CH2), 2.86 (brs, 3H, CH2), 2.73 (s, 3H, CH3), 2.65 (brs, 2H, CH2), 2.46–2.45 (m, 3H, CH2), 2.25 (brs, 2H, CH2), 1.85 (brs, 4H, CH2), 1.71–1.70 (m, 5H, CH2), 1.61 (brs, 3H, CH2), 1.59 (d, J = 7.0 Hz, 6H, 2 × CH3). 13C-NMR (CDCl3) δ 165.9 (C=Oindole), 163.7 (C=Opyridone), 153.6 (ArCHpyridone), 141.9 (ArCHindole), 141.6 (ArCHpyridone), 141.5 (N-C=Cindole), 129.5 (ArCHindole), 126.6 (ArCHindole), 125.9 (ArCHpyridone), 113.9 (ArCHindole), 112.4 (ArCHindole), 111.8 (N-C=Cindole), 107.9 (ArCHpyridone), 102.9 (ArCHindole), 67.0 (CH2O), 55.6 (NCH2CH2CH2O), 53.8 (CH2NCH2), 46.9 (NCH), 40.2 (NHCH2), 29.7 (CH2pyridone), 26.7 (CH2pyridone), 26.1 (NCH2CH2CH2O), 23.8 (CH2piperidine), 22.4 (CH2pyridone), 21.7 (CH2pyridone), 21.4 (CH3), 12.1 (CH3indole). ESI-HRMS: m/z [M+H]+ calcd for C31H43O3N4: 519.3330; found: 519.3329.
N-((6-Isobutyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-1-isopropyl-2-methyl-5-(3-(piperidin-1-yl)propoxy)-1H-indole-3-carboxamide (L–21). The title product was obtained from 7e and pyridone 20 according to the general procedure described above as a white solid (26 mg, 12%). M.p. 99–101 °C. 1H-NMR (CDCl3) δ 12.23 (brs, 1H, NHpyridone), 7.47 (d, J = 6.9 Hz, 1H, ArH), 7.37–7.35 (m, 2H, ArH), 7.31 (t, J = 5.5 Hz, 1H, ArH), 6.75–6.74 (dd, J = 8.9 Hz, 2.0 Hz, 1H, ArH), 6.00 (d, J = 6.1 Hz, 1H, ArHpyridone), 4.71–4.65 (m, 1H, NCH), 4.53 (d, J = 5.8 Hz, 2H, OCH2), 4.09 (t, J = 6.1 Hz, 2H, NCH2), 2.74 (brs, 2H, CH2), 2.72 (s, 3H, CH3), 2.61 (brs, 4H, 2 × CH2), 2.40 (d, J = 7.2 Hz, 2H, CH2), 2.08–2.07 (m, 2H, CH2), 2.00–1.93 (m, 1H, CH), 1.71 (brs, 4H, 2 × CH2), 1.59 (d, J = 7.0 Hz, 6H, 2 × CH3), 1.47 (brs, 2H, CH2), 0.85 (d, J = 6.6 Hz, 6H, 2 × CH3). 13C-NMR (CDCl3) δ 166.1 (C=Oindole), 165.0 (C=Opyridone), 153.8 (ArCHpyridone), 148.1 (ArCHpyridone), 141.1 (N-C=Cindole), 139.5, 129.4 (ArCHindole), 126.8 (ArCHindole), 125.7 (ArCHindole), 111.5 (N-C=Cindole), 108.0 (ArCHpyridone), 105.9 (ArCHpyridone), 102.7 (ArCHindole), 66.9 (CH2O), 55.8 (NCH2CH2CH2O), 54.1 (CH2NCH2), 46.9 (NCH), 42.1 (NHCH2), 39.9 (CH2CH), 29.7 (CH(CH3)2), 28.5 (CH2piperidine), 25.9 (NCH2CH2CH2O), 24.7 (CH2piperidine), 23.6 (CH2piperidine), 22.1 (CH(CH3)2), 21.4 (CH3), 12.2 (CH3indole). ESI-HRMS: m/z [M+H]+ calcd for C31H45O3N4: 521.3486; found: 521.3488.
1-Isopropyl-2-methyl-N-((4-methyl-2-oxo-1,2,5,6,7,8-hexahydroquinolin-3-yl)methyl)-5-(3-(piperidin-1-yl)propoxy)-1H-indole-3-carboxamide (L–22). The title product was obtained from 7e and pyridone 15 according to the general procedure described above as a white solid (38 mg, 17%). M.p. 194–196 °C. 1H-NMR (CDCl3) δ 13.10 (brs, 1H, NHpyridone), 7.55 (t, J = 5.8 Hz, 1H, NHCH2), 7.34–7.31 (m, 2H, ArH), 6.74–6.72 (dd, J = 8.9 Hz, 2.2 Hz, 1H, ArH), 4.70–4.65 (m, 1H, NCH), 4.63 (d, J = 5.9 Hz, 2H, OCH2), 4.00 (t, J = 6.4 Hz, 2H, NCH2), 3.02 (t, J = 5.9 Hz, 2H, CH2), 2.71 (s, 3H, CH3), 2.42–2.34 (m, 8H, 4 × CH2), 2.14 (s, 3H, CH3), 1.86–1.84 (m, 2H, CH2), 1.75–1.71 (m, 4H, 2 × CH2), 1.58 (d, J = 7.0 Hz, 6H, 2 × CH3), 1.56–1.54 (m, 4H, 2 × CH2), 1.39 (brs, 2H, CH2). 13C-NMR (CDCl3) δ 165.9 (C=Oindole), 163.7 (C=Opyridone), 154.0 (ArCHpyridone), 150.1 (ArCHpyridone), 140.7 (N-C=Cindole), 140.4 (ArCHindole), 130.9 (ArCHindole), 128.8 (ArCHindole), 126.8 (ArCHindole), 122.8 (ArCHpyridone), 114.4 (ArCHindole), 111.3 (N-C=Cindole), 108.1 (ArCHpyridone), 102.3 (ArCHindole), 66.9 (CH2O), 56.0 (NCH2CH2CH2O), 54.4 (CH2NCH2), 46.9 (NCH), 35.0 (NHCH2), 27.4 (NCH2CH2CH2O), 26.7 (CH2pyridone), 25.5 (CH2piperidine), 25.1 (CH2piperidine), 24.2 (CH2piperidine), 22.4 (CH2pyridone), 22.3 (CH2pyridone), 21.4 (CH3), 19.2 (CH3pyridone), 16.7 (CH2pyridone), 12.2 (CH3indole). ESI-HRMS: m/z [M+H]+ calcd for C32H45O3N4: 533.3486; found: 533.3495.