Identification of Transient Receptor Potential Vanilloid 3 Antagonists from Achillea alpina L. and Separation by Liquid-Liquid-Refining Extraction and High-Speed Counter-Current Chromatography
Abstract
1. Introduction
2. Results and Discussion
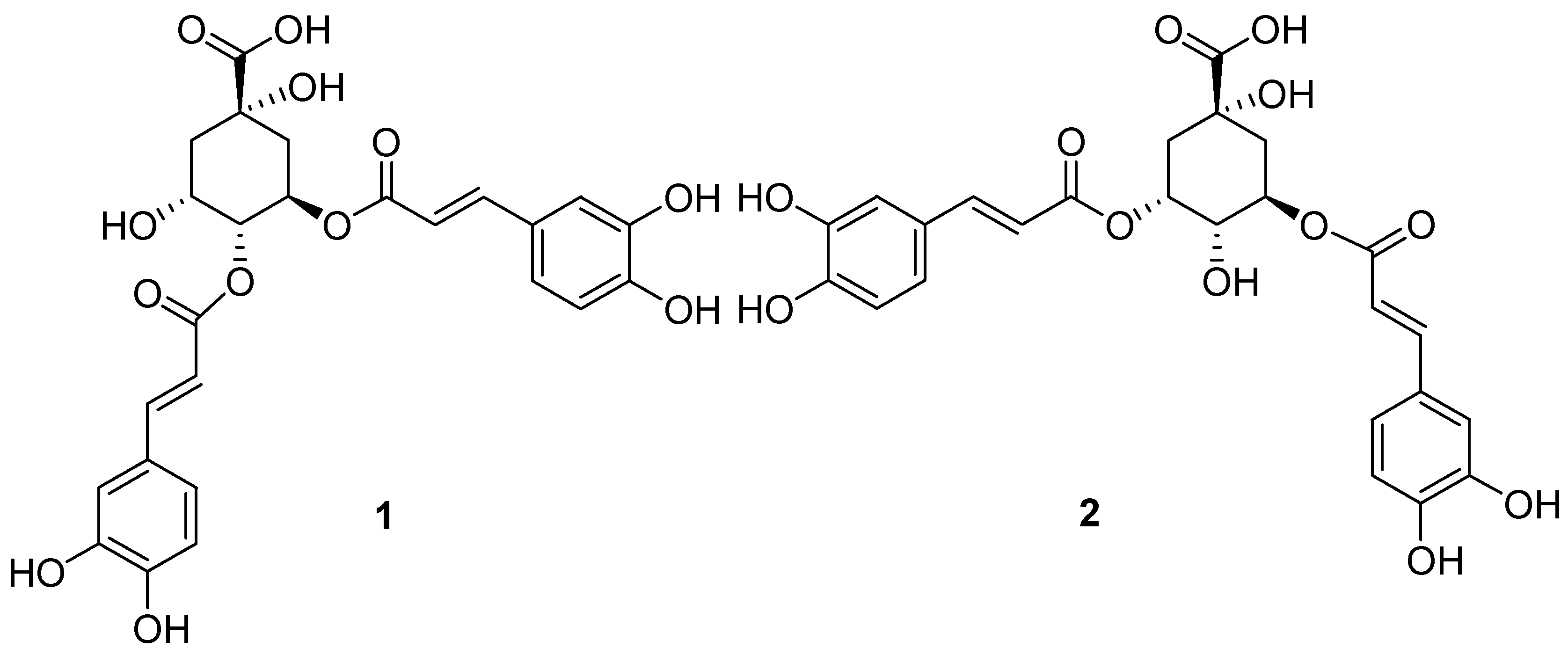
2.1. Bioassay-Guided Fractionation and Structure Identification
2.2. Identification of Isochlorogenic Acids A and B as TRPV3 Channel Antagonists
2.3. Molecular Docking Analysis
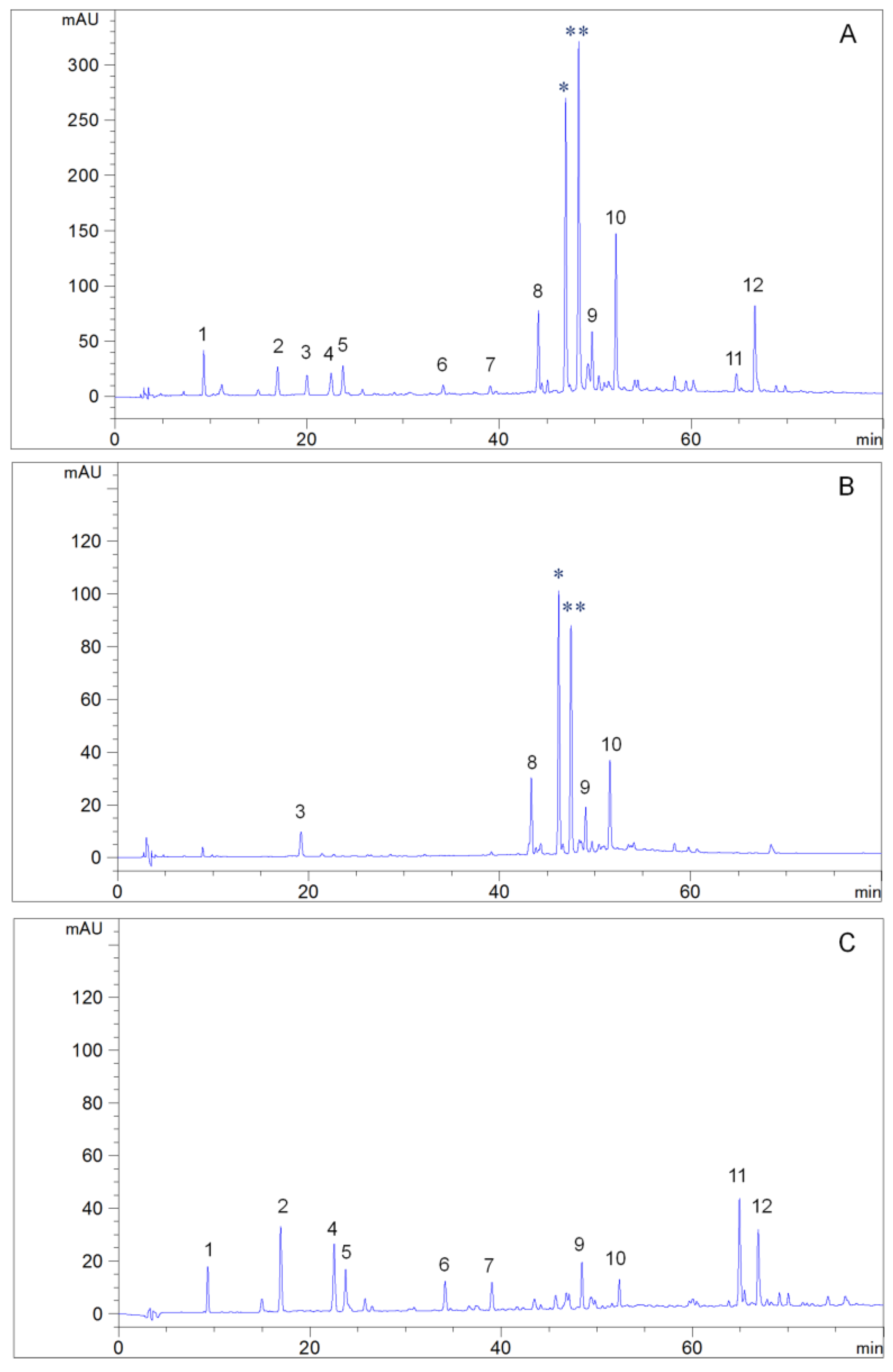
2.4. HPLC Analysis
2.5. Selection of Extraction Solvent Systems for Sample Pretreatment
2.6. Selection of Two-Phase Solvent System for HSCCC
2.7. HSCCC Separation
3. Materials and Methods
3.1. Apparatus and Reagents
3.2. Plant Materials
3.3. Isolation and Identification
3.4. Cell Culture and Electrophysiology
3.5. Molecular Docking
3.6. Selection of Extraction Solvent Systems for Sample Pretreatment
3.7. Refinement of Crude Extract
3.8. Selection and Preparation of Two-Phase Solvent System for HSCCC
3.9. HSCCC Separation Procedure
3.10. HPLC Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Singh, A.K.; McGoldrick, L.L.; Sobolevsky, A.I. Structure and gating mechanism of the transient receptor potential channel TRPV3. Nat. Struct. Mol. Biol. 2018, 25, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Jin, J.; Hu, L.; Shen, D.; Dong, X.P.; Samie, M.A.; Knoff, J.; Eisinger, B.; Liu, M.L.; Huang, S.M.; et al. TRP channel regulates EGFR signaling in hair morphogenesis and skin barrier formation. Cell 2010, 141, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Duchatelet, S.; Pruvost, S.; de Veer, S.; Fraitag, S.; Nitschké, P.; Bole-Feysot, C.; Bodemer, C.; Hovnanian, A. A new TRPV3 missense mutation in a patient with Olmsted syndrome and erythromelalgia. JAMA Dermatol. 2014, 250, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Bíró, T. TRPV3: A ‘more than skinny’ channel. Exp. Dermatol. 2013, 22, 447–452. [Google Scholar] [CrossRef]
- Broad, L.M.; Mogg, A.J.; Eberle, E.; Tolley, M.; Li, D.L.; Knopp, K.L. TRPV3 in drug development. Pharmaceuticals 2016, 9, 55. [Google Scholar] [CrossRef]
- Bang, S.; Yoo, S.; Yang, T.J.; Cho, H.; Hwang, S.W. 17(R)-resolvin D1 specifically inhibits transient receptor potential ion channel vanilloid 3 leading to peripheral antinociception. Br. J. Pharmacol. 2012, 165, 683–692. [Google Scholar] [CrossRef]
- Sun, X.Y.; Sun, L.L.; Qi, H.; Gao, Q.; Wang, G.X.; Wei, N.N.; Wang, K.W. Antipruritic effect of natural coumarin osthole through selective inhibition of thermosensitive TRPV3 channel in the skin. Mol. Pharmacol. 2018, 94, 1164–1173. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, X.Y.; Qi, H.; Ma, Q.X.; Zhou, Q.Q.; Wang, W.; Wang, K.W. Pharmacological inhibition of the temperature-sensitive and Ca2+-permeable transient receptor potential vanilloid TRPV3 channel by natural forsythoside B attenuates pruritus and cytotoxicity of keratinocytes. J. Pharmacol. Exp. Ther. 2019, 368, 21–31. [Google Scholar] [CrossRef]
- Chen, X.Q.; Wang, M.; Zhang, X.; Guo, W.W.; Wu, X. Study on chemical constituents of Achillea alpine. Chin. J. Chin. Mater. Med. 2015, 40, 1330–1333. [Google Scholar] [CrossRef]
- Lee, H.J.; Sim, M.O.; Woo, K.W.; Jeong, D.; Jung, H.K.; An, B.; Cho, H.W. Antioxidant and antimelanogenic activities of compounds isolated from the aerial parts of Achillea alpina L. Chem. Biodivers. 2019, 16, e1900033. [Google Scholar] [CrossRef]
- Zhang, R.Z.; Xu, Y.J.; Xiong, J.; Hu, J.F.; Yang, G.X. Chemical constituents from Achillea alpine. Chin. Tradit. Herb. Drugs 2013, 44, 2812–2815. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, Z.; Ren, T.K.; Ge, Y.; Zheng, Y.; Yao, D.G.; He, X.J.; Gu, Y.C.; Shi, Q.W.; Huo, C.H. Chemical composition of Achillea alpine. Chem. Nat. Compd. 2014, 50, 534–536. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, Q.Q.; Huo, C.H.; Wang, Y.F.; Wu, Y.B.; Zhang, M.L.; Li, L.G.; Jin, S.M.; Shi, Q.W.; Gu, Y.C. Phenolic components of the aerial parts of Achillea alpine. Chem. Nat. Compd. 2019, 55, 337–339. [Google Scholar] [CrossRef]
- Zhou, F.; Li, S.; Yang, J.; Ding, J.W.; He, C.; Teng, L. In-vitro cardiovascular protective activity of a new achillinoside from Achillea alpine. Rev. Bras. Farmacogn. 2019, 29, 445–448. [Google Scholar] [CrossRef]
- Guo, W.; Wang, L.; Gao, Y.; Zhao, B.N.; Wang, D.J.; Duan, W.J.; Yu, Z.Y. Isolation of isochlorogenic acid isomers in flower buds of Lonicera japonica by high-speed counter-current chromatography and preparative high performance liquid chromatography. J. Chromatogr. B 2015, 981, 27–32. [Google Scholar] [CrossRef]
- Ma, T.Y.; Dong, H.J.; Lu, H.; Zhao, H.Q.; Guo, L.P.; Wang, X. Preparative separation of caffeoylquinic acid isomers from Lonicerae japonicae Flos by pH-zone-refining counter-current chromatography and a strategy for selection of solvent systems with high sample loading capacities. J. Chromatogr. A 2018, 1578, 61–66. [Google Scholar] [CrossRef]
- Yu, X.X.; Wu, J.W.; Liang, Z.K.; Zhao, M.Q.; Xu, X.J. Isolation and purification of isochlorogenic acid A from Lonicera japonica Thunb using high-speed counter-current chromatography. ACTA Chromatogr. 2015, 27, 541–549. [Google Scholar] [CrossRef]
- Liu, X.X.; Sun, S.W.; Yuan, W.J.; Gao, H.; Si, Y.Y.; Liu, K.; Zhang, S.; Liu, Y.; Wang, W. Isolation of tricin as a xanthine oxidase inhibitor from sweet white clover (Melilotus albus) and its distribution in selected Gramineae species. Molecules 2018, 23, 2719. [Google Scholar] [CrossRef]
- Li, J.; Yuan, C.H.; Pan, L.; Benatrehina, P.A.; Chai, H.; Keller, W.J.; Naman, C.B.; Kinghorn, A.D. Bioassay-guide isolation of antioxidant and cytoprotective constituents from a maqui berry (Aristotelia chilensis) dietary supplement ingredient as marker for qualitative and quantitative analysis. J. Agric. Food Chem. 2017, 65, 8634–8642. [Google Scholar] [CrossRef]
- Gao, M.; Wang, X.L.; Gu, M.; Su, Z.G.; Wang, Y.; Janson, J.C. Separation of polyphenols using porous polyamide resin and assessment of mechanism of retention. J. Sep. Sci. 2011, 34, 1853–1858. [Google Scholar] [CrossRef]
- Liu, G.Y.; Zhuang, L.W.; Song, D.D.; Lu, C.L.; Xu, X. Isolation, purification, and identification of the main phenolic compounds from leaves of celery (Apium graveolens L. var. dulce Mill./Pers.). J. Sep. Sci. 2017, 40, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.P.; He, Q.X.; Liu, K.C.; Zhang, Y.; Han, J.; Sun, C.; Lei, Z.; Tian, Q.P.; Han, L.W. The anti-inflammatory activity of isochlorogenic acid A in an in vivo model of zebrafish embryo. Lat. Am. J. Pharm. 2017, 36, 1915–1923. [Google Scholar]
- Liu, Y.; Duan, X.Q.; Yan, F.L.; Pan, C.; Wu, J.C.; Cao, F.; Liang, J. Isochlorogenic acid A inhibits activation of NLRP3/NF-κB in rats with collagen-induced arthritis. Chin. Pharmacol. Bull. 2019, 35, 1415–1419. [Google Scholar] [CrossRef]
- Zha, R.P.; Xu, W.; Wang, W.Y.; Dong, L.; Wang, Y.P. Prevention of lipopolysaccharide-induced injury by 3,5-dicaffeoylquinic acid in endothelial cells. Acta Pharmacol. Sin. 2007, 28, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Szöllősi, A.G.; Vasas, N.; Angyal, Á.; Kistamás, K.; Nánási, P.P.; Mihály, J.; Béke, G.; Herczeg-Lisztes, E.; Szegedi, A.; Kawada, N.; et al. Activation of TRPV3 regulates inflammatory actions of human epidermal keratinocytes. J. Investig. Dermatol. 2018, 138, 365–374. [Google Scholar] [CrossRef]
- Zubcevic, L.; Herzik, M.A., Jr.; Wu, M.; Borschel, W.F.; Hirschi, M.; Song, A.S.; Lander, G.C.; Lee, S.Y. Conformational ensemble of the human TRPV3 ion channel. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Hu, H.Z.; Grandl, J.; Bandell, M.; Petrus, M.; Patapoutian, A. Two amino acid residues determine 2-APB sensitivity of the ion channels TRPV3 and TRPV4. Proc. Natl. Acad. Sci. USA 2009, 106, 1626–1631. [Google Scholar] [CrossRef]
- Chung, M.K.; Lee, H.; Mizuno, A.; Suzuki, M.; Caterina, M.J. 2-Aminoethoxydiphenyl borate activates and sensitizes the heat-gated ion channel TRPV3. J. Neurosci. 2004, 24, 5177–5182. [Google Scholar] [CrossRef]
- Nagai, H.; Nishigori, C.; Takaoka, Y.; Sugano, A.; Nakamachi, Y.; Kawano, S. Identification of a heterozygous p.Gly568Val missense mutation in the TRPV3 gene in a Japanese patient with Olmsted syndrome: In silico analysis of TRPV3. J. Dermatol. 2017, 44, 1059–1062. [Google Scholar] [CrossRef]
- Wang, J.; Gu, D.; Wang, M.; Guo, X.; Li, H.; Dong, Y.; Guo, H.; Wang, Y.; Fan, M.; Yang, Y. Rational approach to solvent system selection for liquid-liquid extraction-assisted sample pretreatment in counter-current chromatography. J. Chromatogr. B 2017, 1053, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Gu, D.Y.; Ji, Z.N.; Fang, C.; Xu, F.; Yang, Y. Comprehensive separation of major compositions from Sophora japonica var. violacea by counter-current chromatography using a liquid-liquid extraction strategy. Ind. Crops Prod. 2018, 124, 363–368. [Google Scholar] [CrossRef]
- Chen, T.; Liu, Y.L.; Zou, D.L.; Chen, C.; You, J.M.; Zhou, G.Y.; Sun, J.; Li, Y.L. Application of an efficient strategy based on liquid-liquid extraction, high-speed counter-current chromatography, and preparative HPLC for the rapid enrichment, separation, and purification of four anthraquinones from Rheum tanguticum. J. Sep. Sci. 2014, 37, 165–170. [Google Scholar] [CrossRef]
- Ito, Y. Golden rules and pitfalls in the selecting optimum conditions for high-speed counter-current chromatography. J. Chromatogr. A 2005, 1065, 145–168. [Google Scholar] [CrossRef]
- Shi, S.Y.; Huang, K.L.; Zhang, Y.P.; Zhao, Y.; Du, Q.Z. Purification and identification of antiviral compounds from Laggera pterodonta by high-speed counter-current chromatography. J. Chromatogr. B 2007, 859, 119–124. [Google Scholar] [CrossRef]
- Sun, S.W.; Dai, X.Y.; Sun, J.; Bu, X.G.; Weng, C.H.; Li, H.; Zhu, H. A diketopiperazine factor from Rheinheimera aquimaris QSI02 exhibits anti-quorum sensing activity. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, O.; Khan, S.; Ha, I.J.; Park, Y.; Tosum, A.; Kim, Y.S. Application of stepwise gradients in counter-current chromatography: A rapid and economical strategy for one-step separation of eight coumarins from Seseli resinosum. J. Chromatogr. A 2013, 1310, 66–73. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds isochlorogenic acids A and B are available from the authors. |



| No. | Solvent System (v/v) | Partition Coefficient (K) | Separation Factor (α) | |||
|---|---|---|---|---|---|---|
| Isochlorogenic Acid B | Isochlorogenic Acid A | Impurity 10 | Isochlorogenic Acids A and B | Isochlorogenic Acids B and Impurity 10 | ||
| 1 | 1:3:4 | 0.21 | 0.58 | 0.30 | 2.63 | 1.43 |
| 2 | 1:3:6 | 0.36 | 1.01 | 0.35 | 2.80 | 1.03 |
| 3 | 1:4:6 | 0.63 | 2.39 | 0.58 | 3.79 | 1.08 |
| 4 | 1:4:8 | 0.74 | 3.10 | 0.66 | 4.18 | 1.12 |
| 5 | 1:4:8 * | 1.59 | 4.12 | 0.52 | 2.59 | 3.06 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.-W.; Wang, R.-R.; Sun, X.-Y.; Fan, J.-H.; Qi, H.; Liu, Y.; Qin, G.-Q.; Wang, W. Identification of Transient Receptor Potential Vanilloid 3 Antagonists from Achillea alpina L. and Separation by Liquid-Liquid-Refining Extraction and High-Speed Counter-Current Chromatography. Molecules 2020, 25, 2025. https://doi.org/10.3390/molecules25092025
Sun S-W, Wang R-R, Sun X-Y, Fan J-H, Qi H, Liu Y, Qin G-Q, Wang W. Identification of Transient Receptor Potential Vanilloid 3 Antagonists from Achillea alpina L. and Separation by Liquid-Liquid-Refining Extraction and High-Speed Counter-Current Chromatography. Molecules. 2020; 25(9):2025. https://doi.org/10.3390/molecules25092025
Chicago/Turabian StyleSun, Shi-Wei, Rong-Rong Wang, Xiao-Ying Sun, Jia-He Fan, Hang Qi, Yang Liu, Guo-Qing Qin, and Wei Wang. 2020. "Identification of Transient Receptor Potential Vanilloid 3 Antagonists from Achillea alpina L. and Separation by Liquid-Liquid-Refining Extraction and High-Speed Counter-Current Chromatography" Molecules 25, no. 9: 2025. https://doi.org/10.3390/molecules25092025
APA StyleSun, S.-W., Wang, R.-R., Sun, X.-Y., Fan, J.-H., Qi, H., Liu, Y., Qin, G.-Q., & Wang, W. (2020). Identification of Transient Receptor Potential Vanilloid 3 Antagonists from Achillea alpina L. and Separation by Liquid-Liquid-Refining Extraction and High-Speed Counter-Current Chromatography. Molecules, 25(9), 2025. https://doi.org/10.3390/molecules25092025







