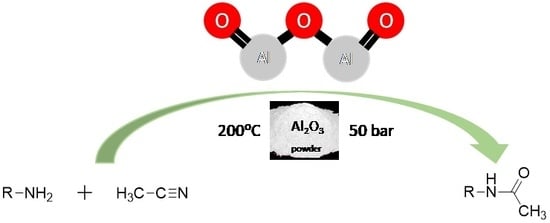
N-Acetylation of Amines in Continuous-Flow with Acetonitrile—No Need for Hazardous and Toxic Carboxylic Acid Derivatives
Abstract
:1. Introduction
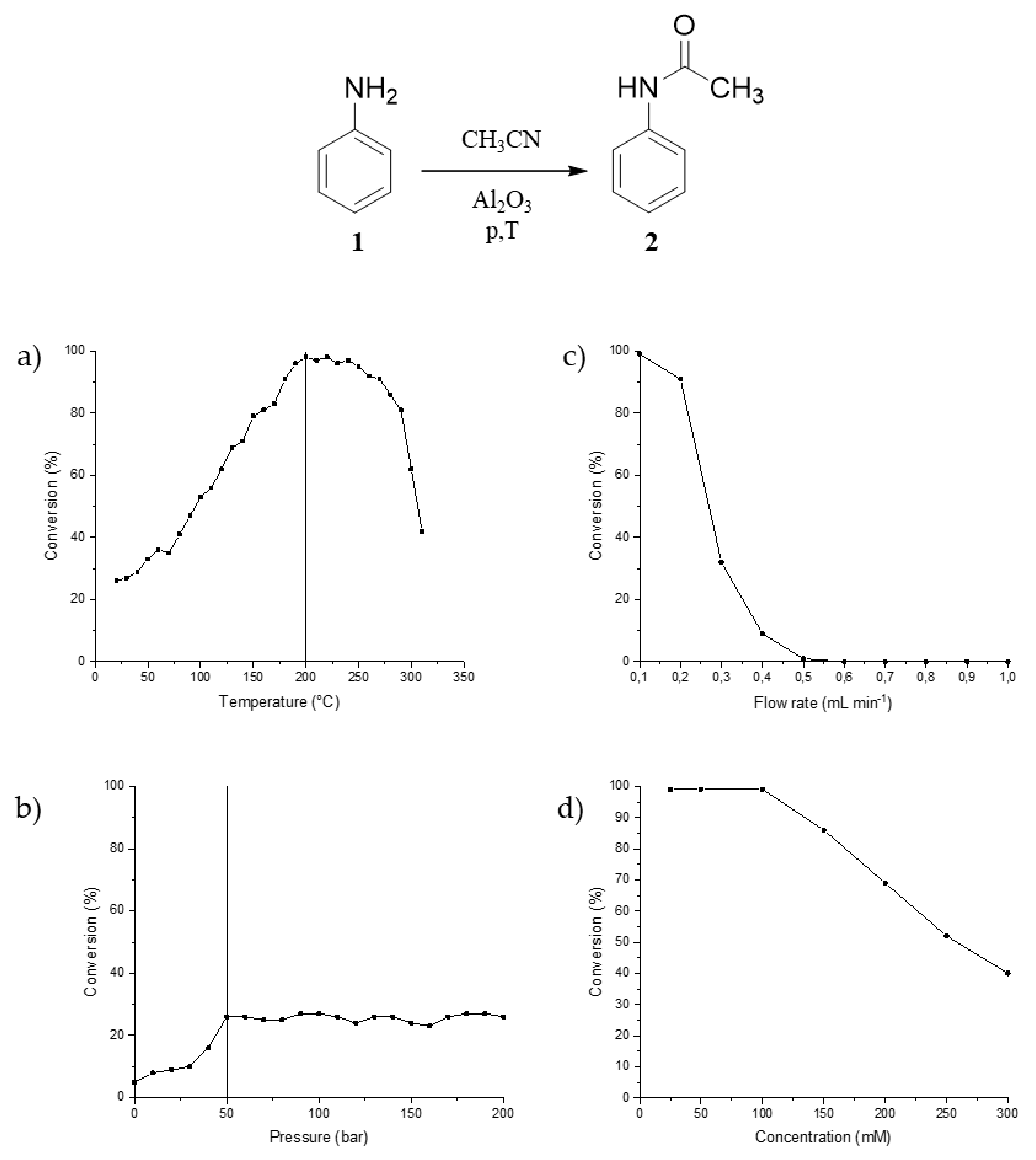
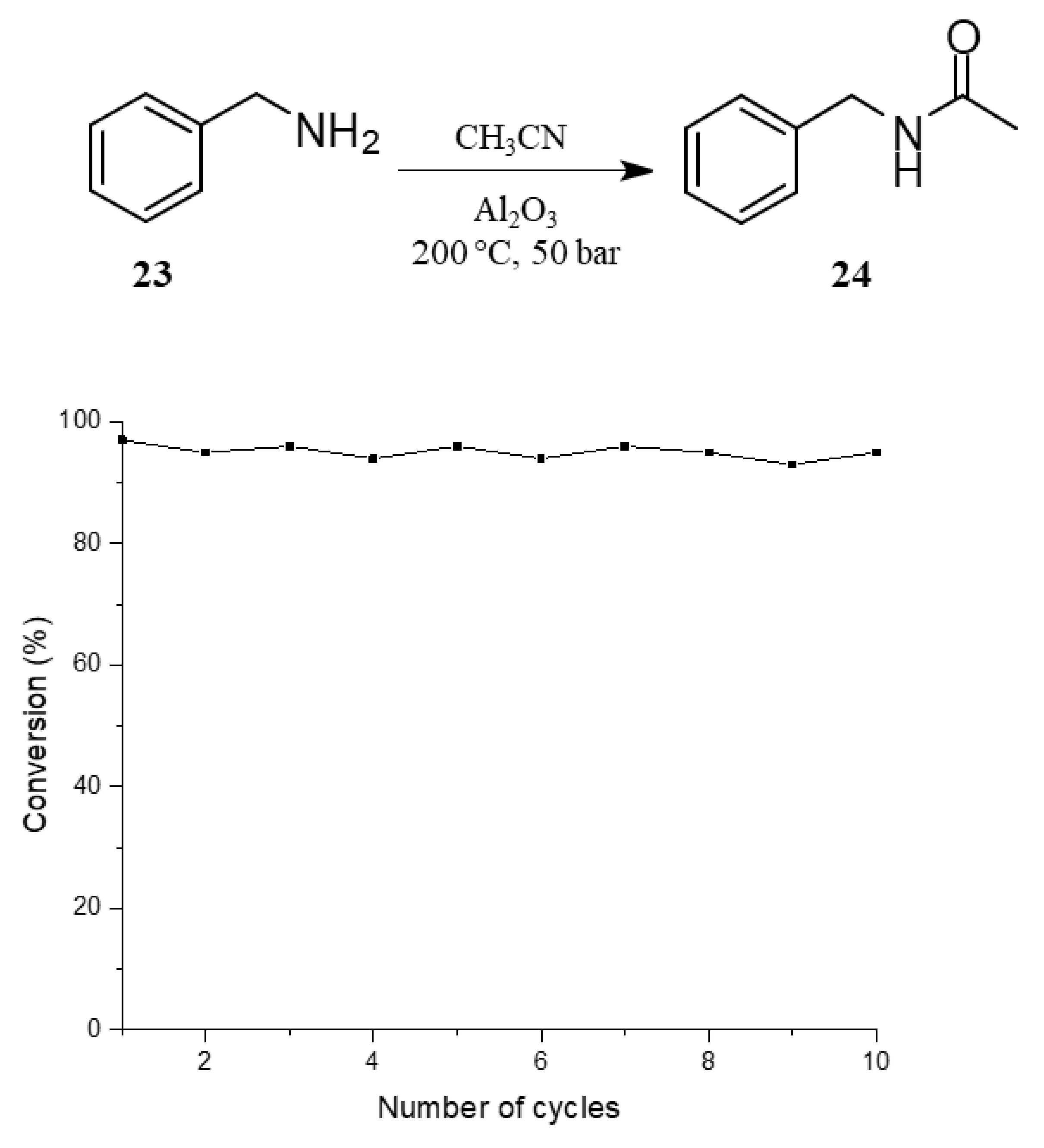
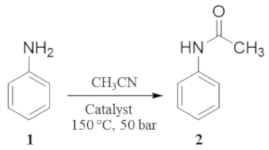
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. General Aspects of the CF Acetylation
3.3. Product Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Amić, A.; Molnar, M. An Improved and Efficient N-acetylation of Amines Using Choline Chloride Based Deep Eutectic Solvents. Org. Prep. Proc. Int. 2017, 49, 249–257. [Google Scholar] [CrossRef]
- Biswas, R.; Mukherjee, A. Introducing the Concept of Green Synthesis in the Undergraduate Laboratory: Two-Step Synthesis of 4-Bromoacetanilide from Aniline. J. Chem. Ed. 2017, 94, 1391–1394. [Google Scholar] [CrossRef]
- Tajbakhsh, M.; Hosseinzadeh, R.; Alinezhad, H.; Rezaee, P.; Tajbakhsh, M. TiO2 NPs as Catalyst for N-Formylation and N-Acetylation of Amines under Solvent-Free Conditions. Lett. Org. Chem. 2013, 10, 657–663. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H. Acetic anhydride as a synthetic reagent. J. Het. Chem. 1976, 13, 179–194. [Google Scholar] [CrossRef]
- Baumann, M.; Baxendale, I.R. The synthesis of active pharmaceutical ingredients (APIs) using continuous flow chemistry. Beilstein J. Org. Chem. 2015, 11, 1194–1219. [Google Scholar] [CrossRef] [Green Version]
- Britton, J.; Raston, C.L. Multi-step continuous-flow synthesis. Chem. Soc. Rev. 2017, 46, 1250–1271. [Google Scholar] [CrossRef] [Green Version]
- Escobar Carlos, A.; Orellana-Vera, J.; Vega, A.; Sicker, D.; Sieler, J. Regioselective N-Acetylation of 4-(2-Hydroxyphenyl)-2-phenyl-2,3-dihydro-1H-1,5-benzodiazepine Using Protection by an Intramolecular Hydrogen Bond. Z. Für Nat. B 2009, 64, 969–972. [Google Scholar] [CrossRef] [Green Version]
- Farina, V.; Reeves, J.T.; Senanayake, C.H.; Song, J.J. Asymmetric Synthesis of Active Pharmaceutical Ingredients. Chem. Rev. 2006, 106, 2734–2793. [Google Scholar] [CrossRef]
- Nakazono, K.; Fukasawa, K.; Sato, T.; Koyama, Y.; Takata, T. Synthesis of acetylene-functionalized [2]rotaxane monomers directed toward side chain-type polyrotaxanes. Polym. J. 2010, 42, 208–215. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.; Tang, D.; Gai, Y.; Wang, G.; Kim, H.; Chen, Z.; Phan, L.T.; Or, Y.S.; Wang, Z. An Efficient Large-Scale Synthesis of EDP-420, a First-in-Class Bridged Bicyclic Macrolide (BBM) Antibiotic Drug Candidate. Org. Proc. Res. Dev. 2010, 14, 504–510. [Google Scholar] [CrossRef]
- Isidro-Llobet, A.; Álvarez, M.; Albericio, F. Amino Acid-Protecting Groups. Chem. Rev. 2009, 109, 2455–2504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, B.K.; Gupta, R.; Baldus, L.; Lyon, D.; Narita, T.; Lammers, M.; Choudhary, C.; Weinert, B.T. Analysis of human acetylation stoichiometry defines mechanistic constraints on protein regulation. Nat. Commun. 2019, 10, 1055. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhou, F.; Bai, M.; Liu, Y.; Zhang, L.; Zhu, Q.; Bi, Y.; Ning, G.; Zhou, L.; Wang, X. The pivotal role of protein acetylation in linking glucose and fatty acid metabolism to β-cell function. Cell Death Dis. 2019, 10, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alleti, R.; Oh, W.S.; Perambuduru, M.; Afrasiabi, Z.; Sinn, E.; Reddy, V.P. Gadolinium triflate immobilized in imidazolium based ionic liquids: A recyclable catalyst and green solvent for acetylation of alcohols and amines. Green Chem. 2005, 7, 203–206. [Google Scholar] [CrossRef]
- El Seoud, O.A.; Koschella, A.; Fidale, L.C.; Dorn, S.; Heinze, T. Applications of Ionic Liquids in Carbohydrate Chemistry: A Window of Opportunities. Biomacromolecules 2007, 8, 2629–2647. [Google Scholar] [CrossRef]
- Bartoli, G.; Dalpozzo, R.; De Nino, A.; Maiuolo, L.; Nardi, M.; Procopio, A.; Tagarelli, A. Per-O-acetylation of sugars catalyzed by Ce(OTf)3. Green Chem. 2004, 6, 191–192. [Google Scholar] [CrossRef]
- Deka, N.; Mariotte, A.-M.; Boumendjel, A. Microwave mediated solvent-free acetylation of deactivated and hindered phenols. Green Chem. 2001, 3, 263–264. [Google Scholar] [CrossRef]
- Snodin, D.J. Genotoxic Impurities: From Structural Alerts to Qualification. Org. Proc. Res. Dev. 2010, 14, 960–976. [Google Scholar] [CrossRef]
- Plutschack, M.B.; Pieber, B.; Gilmore, K.; Seeberger, P.H. The Hitchhiker’s Guide to Flow Chemistry. Chem. Rev. 2017, 117, 11796–11893. [Google Scholar] [CrossRef]
- Vukelić, S.; Koksch, B.; Seeberger, P.H.; Gilmore, K. A Sustainable, Semi-Continuous Flow Synthesis of Hydantoins. Chem. Eur. J. 2016, 22, 13451–13454. [Google Scholar] [CrossRef]
- Ushakov, D.B.; Gilmore, K.; Kopetzki, D.; McQuade, D.T.; Seeberger, P.H. Continuous-Flow Oxidative Cyanation of Primary and Secondary Amines Using Singlet Oxygen. Angew. Chem. Int. Ed. 2014, 45, 557. [Google Scholar] [CrossRef] [PubMed]
- McQuade, D.T.; Seeberger, P.H. Applying Flow Chemistry: Methods, Materials, and Multistep Synthesis. J. Org. Chem. 2013, 78, 6384–6389. [Google Scholar] [CrossRef]
- Watts, K.; Baker, A.; Wirth, T. Electrochemical Synthesis in Microreactors. J. Flow Chem. 2015, 4, 2–11. [Google Scholar] [CrossRef]
- Wiles, C.; Watts, P. Continuous process technology: A tool for sustainable production. Green Chem. 2014, 16, 55–62. [Google Scholar] [CrossRef]
- Wiles, C.; Watts, P. Continuous flow reactors: A perspective. Green Chem. 2012, 14, 38–54. [Google Scholar] [CrossRef]
- Wiles, C.; Watts, P. Solid-Supported Gallium Triflate: An Efficient Catalyst for the Three-Component Ketonic Strecker Reaction. ChemSusChem 2012, 5, 332–338. [Google Scholar] [CrossRef]
- Osorio-Planes, L.; Rodriguez-Escrich, C.; Pericàs, M.A. Removing the Superfluous: A Supported Squaramide Catalyst with a Minimalistic Linker Applied to the Enantioselective Flow Synthesis of Pyranonaphthoquinones. Catal. Sci. Technol. 2016, 6, 4686–4689. [Google Scholar] [CrossRef]
- Llanes, P.; Rodríguez-Escrich, C.; Sayalero, S.; Pericàs, M.A. Organocatalytic Enantioselective Continuous-Flow Cyclopropanation. Org. Lett. 2016, 18, 6292–6295. [Google Scholar] [CrossRef]
- Izquierdo, J.; Pericàs, M.A. A Recyclable, Immobilized Analogue of Benzotetramisole for Catalytic Enantioselective Domino Michael Addition/Cyclization Reactions in Batch and Flow. ACS Catal. 2016, 6, 348–356. [Google Scholar] [CrossRef] [Green Version]
- Pascanu, V.; Hansen, P.R.; Gómez, A.B.; Ayats, C.; Platero-Prats, A.E.; Johansson, M.J.; Pericàs, M.À.; Martín-Matute, B. Highly Functionalized Biaryls via Suzuki–Miyaura Cross-Coupling Catalyzed by Pd@MOF under Batch and Continuous Flow Regimes. ChemSusChem 2015, 8, 123–130. [Google Scholar] [CrossRef]
- Martin-Rapun, R.; Sayalero, S.; Pericàs, M.A. Asymmetric anti-Mannich Reactions in Continuous Flow. Green Chem. 2013, 15, 3295–3301. [Google Scholar] [CrossRef]
- Bottecchia, C.; Rubens, M.; Gunnoo, S.B.; Hessel, V.; Madder, A.; Noel, T. Visible-Light-Mediated Selective Arylation of Cysteine in Batch and Flow. Angew. Chem. Int. Ed. 2017, 56, 12702–12707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Y.; Kuijpers, K.P.L.; König, N.; Shang, M.; Hessel, V.; Noël, T. A Mechanistic Investigation of the Visible-Light Photocatalytic Trifluoromethylation of Heterocycles Using CF3I in Flow. Chem. Eur. J. 2016, 22, 12295–12300. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Kuijpers, K.; Hessel, V.; Noël, T. A Convenient Numbering-Up Strategy for the Scale-Up of Gas-Liquid Photoredox Catalysis in Flow. React. Chem. Eng. 2016, 1, 73–81. [Google Scholar] [CrossRef]
- Straathof, N.J.W.; Cramer, S.E.; Hessel, V.; Noël, T. Practical Photocatalytic Trifluoromethylation and Hydrotrifluoromethylation of Styrenes in Batch and Flow. Angew. Chem. Int. Ed. 2016, 55, 15549–15553. [Google Scholar] [CrossRef] [Green Version]
- Seo, H.; Katcher, M.H.; Jamison, T.F. Photoredox Activation of Carbon Dioxide for Amino Acid Synthesis in Continuous Flow. Nat. Chem. 2016, 9, 453–456. [Google Scholar] [CrossRef] [Green Version]
- McTeague, T.A.; Jamison, T.F. Photoredox Activation of SF6 for Fluorination. Angew. Chem. Int. Ed. 2016, 55, 15072–15075. [Google Scholar] [CrossRef]
- Adamo, A.; Beingessner, R.L.; Behnam, M.; Chen, J.; Jamison, T.F.; Jensen, K.F.; Monbaliu, J.C.M.; Myerson, A.S.; Revalor, E.M.; Snead, D.R. On-Demand Continuous-Flow Production of Pharmaceuticals in a Compact, Reconfigurable System. Science 2016, 352, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Snead, D.R.; Jamison, T.F. A Three-Minute Synthesis and Purification of Ibuprofen: Pushing the Limits of Continuous-Flow Processing. Angew. Chem. Int. Ed. 2015, 54, 983–987. [Google Scholar] [CrossRef]
- Wu, J.; Yang, X.; He, Z.; Mao, X.; Hatton, T.A.; Jamison, T.F. Continuous Flow Synthesis of Ketones from Carbon Dioxide and Organolithium or Grignard Reagents. Angew. Chem. Int. Ed. 2014, 53, 8416–8420. [Google Scholar] [CrossRef]
- Rogers, L.; Jensen, K.F. Continuous manufacturing – the Green Chemistry promise? Green Chem. 2019, 21, 3481–3498. [Google Scholar] [CrossRef] [Green Version]
- Giri, G.; Yang, L.; Mo, Y.; Jensen, K.F. Adding Crystals to Minimize Clogging in Continuous Flow Synthesis. Cryst. Growth Des. 2019, 19, 98–105. [Google Scholar] [CrossRef]
- Coley, C.W.; Thomas, D.A.; Lummiss, J.A.M.; Jaworski, J.N.; Breen, C.P.; Schultz, V.; Hart, T.; Fishman, J.S.; Rogers, L.; Gao, H.; et al. A robotic platform for flow synthesis of organic compounds informed by AI planning. Science 2019, 365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Weeranoppanant, N.; Thomas, D.A.; Tahara, K.; Stelzer, T.; Russell, M.G.; O’Mahony, M.; Myerson, A.S.; Lin, H.; Kelly, L.P.; et al. Advanced Continuous Flow Platform for On-Demand Pharmaceutical Manufacturing. Chem. Eur. J. 2018, 24, 2776–2784. [Google Scholar] [CrossRef]
- Bédard, A.-C.; Adamo, A.; Aroh, K.C.; Russell, M.G.; Bedermann, A.A.; Torosian, J.; Yue, B.; Jensen, K.F.; Jamison, T.F. Reconfigurable system for automated optimization of diverse chemical reactions. Science 2018, 361, 1220–1225. [Google Scholar] [CrossRef] [Green Version]
- Jensen, K.F. Flow chemistry. Microreaction technology comes of age. AIChE J. 2017, 63, 858–869. [Google Scholar] [CrossRef]
- Ollivier, N.; Toupy, T.; Hartkoorn, R.C.; Desmet, R.; Monbaliu, J.-C.M.; Melnyk, O. Accelerated microfluidic native chemical ligation at difficult amino acids toward cyclic peptides. Nat. Commun. 2018, 9, 2847. [Google Scholar] [CrossRef] [Green Version]
- Kreituss, I.; Bode, J.W. Flow chemistry and polymer-supported pseudoenantiomeric acylating agents enable parallel kinetic resolution of chiral saturated N-heterocycles. Nat. Chem. 2016, 9, 446–452. [Google Scholar] [CrossRef] [Green Version]
- Berton, M.; Huck, L.; Alcázar, J. On-demand synthesis of organozinc halides under continuous flow conditions. Nat. Protoc. 2018, 13, 324–334. [Google Scholar] [CrossRef]
- Elvira, K.S.; Solvas, X.C.I.; Wootton, R.C.R.; de Mello, A.J. The past, present and potential for microfluidic reactor technology in chemical synthesis. Nat. Chem. 2013, 5, 905–915. [Google Scholar] [CrossRef]
- Tadele, K.; Verma, S.; Nadagouda, M.N.; Gonzalez, M.A.; Varma, R.S. A rapid flow strategy for the oxidative cyanation of secondary and tertiary amines via C-H activation. Sci. Rep. 2017, 7, 16311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuse, S.; Mifune, Y.; Nakamura, H.; Tanaka, H. Total synthesis of feglymycin based on a linear/convergent hybrid approach using micro-flow amide bond formation. Nat. Commun. 2016, 7, 13491. [Google Scholar] [CrossRef] [PubMed]
- Kirschneck, D. Strategies for Using Microreactors and Flow Chemistry: Drivers and Tools. Chem. Eng. Tech. 2013, 36, 1061–1066. [Google Scholar] [CrossRef]
- Fanelli, F.; Parisi, G.; Degennaro, L.; Luisi, R. Contribution of microreactor technology and flow chemistry to the development of green and sustainable synthesis. Beilstein J. Org. Chem. 2017, 13, 520–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Angelis, S.; Celestini, P.; Purgatorio, R.; Degennaro, L.; Rebuzzini, G.; Luisi, R.; Carlucci, C. Development of a continuous flow synthesis of propranolol: Tackling a competitive side reaction. J. Flow Chem. 2019, 9, 231–236. [Google Scholar] [CrossRef]
- De Angelis, S.; Hone, C.; Degennaro, L.; Celestini, P.; Luisi, R.; Kappe, C.O. Sequential α-lithiation and aerobic oxidation of an arylacetic acid-continuous-flow synthesis of cyclopentyl mandelic acid. J. Flow Chem. 2018, 8, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Movsisyan, M.; Delbeke, E.I.P.; Berton, J.K.E.T.; Battilocchio, C.; Ley, S.V.; Stevens, C.V. Taming hazardous chemistry by continuous flow technology. Chem. Soc. Rev. 2016, 45, 4892–4928. [Google Scholar] [CrossRef]
- Mellmer, M.A.; Sanpitakseree, C.; Demir, B.; Bai, P.; Ma, K.; Neurock, M.; Dumesic, J.A. Solvent-enabled control of reactivity for liquid-phase reactions of biomass-derived compounds. Nat. Catal. 2018, 1, 199–207. [Google Scholar] [CrossRef]
- Xiong, B.; Wang, G.; Xiong, T.; Wan, L.; Zhou, C.; Liu, Y.; Zhang, P.; Yang, C.; Tang, K. Direct synthesis of N-arylamides via the coupling of aryl diazonium tetrafluoroborates and nitriles under transition-metal-free conditions. Tetrahedron Lett. 2018, 59, 3139–3142. [Google Scholar] [CrossRef]
- Lee, Y.M.; Moon, M.E.; Vajpayee, V.; Filimonov, V.D.; Chi, K.-W. Efficient and economic halogenation of aryl amines via arenediazonium tosylate salts. Tetrahedron 2010, 66, 7418–7422. [Google Scholar] [CrossRef]
- Beller, M. First Amidocarbonylation with Nitriles for the Synthesis of N-Acyl Amino Acids. Synlett 1999, 1999, 108–110. [Google Scholar] [CrossRef]
- Enders, D.; Shilvock, J. Some recent applications of α -amino nitrile chemistry. Chem. Soc. Rev. 2000, 29, 359–373. [Google Scholar] [CrossRef]
- Jiang, T.-S.; Wang, G.-W. Synthesis of 3-Acylindoles by Palladium-Catalyzed Acylation of Free (N–H) Indoles with Nitriles. Org. Lett. 2013, 15, 788–791. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.B.; Shin, W.S.; Hong, J.E.; Lee, S.Y.; Oh, D.Y. Synthesis of Ketones with Alkyl Phosphonates and Nitriles as Acyl Cation Equivalent: Application of Dephosphonylation Reaction of β-Functionalized Phosphonate with Hydride. Synth. Commun. 1997, 27, 3333–3339. [Google Scholar] [CrossRef]
- Saikia, U.P.; Hussain, F.L.; Suri, M.; Pahari, P. Selective N-acetylation of aromatic amines using acetonitrile as acylating agent. Tetrahedron Lett. 2016, 57, 1158–1160. [Google Scholar] [CrossRef]
- Brahmayya, M.; Suen, S.-Y.; Dai, S.A. Sulfonated graphene oxide-catalyzed N-acetylation of amines with acetonitrile under sonication. J. Taiwan Inst. Chem. Eng. 2018, 83, 174–183. [Google Scholar] [CrossRef]
- Molnár, Á.; Papp, A. Catalyst recycling—A survey of recent progress and current status. Coord. Chem. Rev. 2017, 349, 1–65. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–28 are available from the authors. |
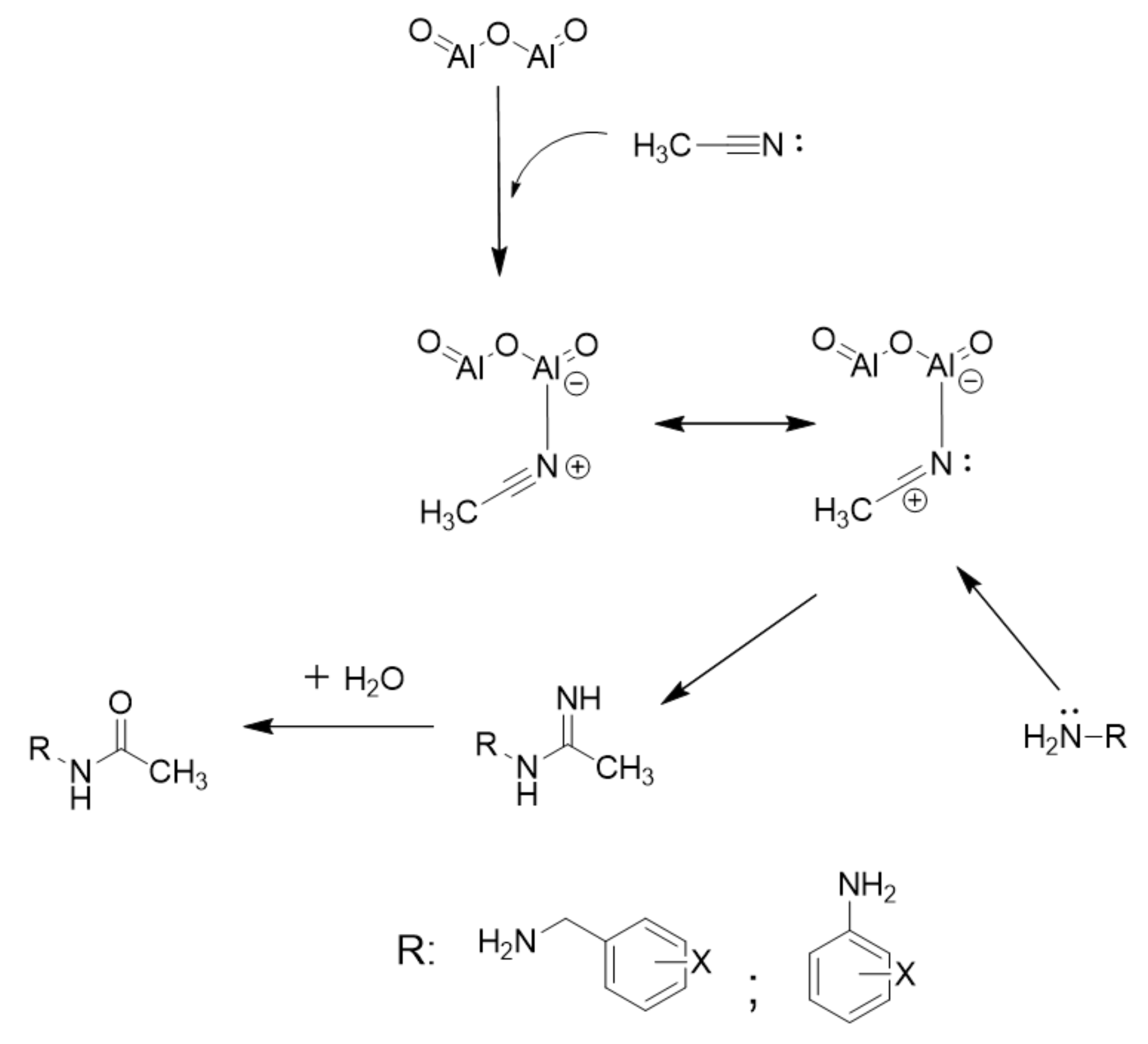
| Entry | Lewis Acids | Solvent | Conversion (%) |
|---|---|---|---|
| 1 | Fe2O3 | Acetonitrile | 0 |
| 2 | Boric acid | Acetonitrile | 3 |
| 3 | AlCl3 | Acetonitrile | 19 |
| 4 | Al2O3 | Acetonitrile | 64 |
| Entry | Substrate | Product | Yield (%) | Space Time Yield (mol kg−1 h−1) |
|---|---|---|---|---|
| 1 | 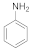 1 | 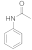 2 | > 99 | 1.5 |
| 2 | 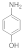 3 | 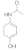 4 | 93 | 1.395 |
| 3 |  5 | 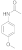 6 | 51 | 0.765 |
| 4 | 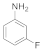 7 |  8 | > 99 | 1.5 |
| 5 |  9 |  10 | > 99 | 1.5 |
| 6 | 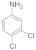 11 | 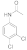 12 | 95 | 1.425 |
| 7 | 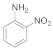 13 | 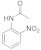 14 | 0 | 0 |
| 8 | 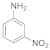 15 |  16 | 0 | 0 |
| 9 |  17 |  18 | 0 | 0 |
| 10 | 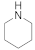 19 | 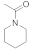 20 | > 99 | 1.5 |
| 11 | 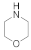 21 | 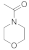 22 | > 99 | 1.5 |
| 12 | 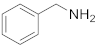 23 | 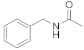 24 | > 99 | 1.5 |
| 13 |  25 | 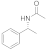 26 | > 99 | 1.5 |
| 14 | 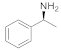 27 |  28 | > 99 | 1.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orsy, G.; Fülöp, F.; Mándity, I.M. N-Acetylation of Amines in Continuous-Flow with Acetonitrile—No Need for Hazardous and Toxic Carboxylic Acid Derivatives. Molecules 2020, 25, 1985. https://doi.org/10.3390/molecules25081985
Orsy G, Fülöp F, Mándity IM. N-Acetylation of Amines in Continuous-Flow with Acetonitrile—No Need for Hazardous and Toxic Carboxylic Acid Derivatives. Molecules. 2020; 25(8):1985. https://doi.org/10.3390/molecules25081985
Chicago/Turabian StyleOrsy, György, Ferenc Fülöp, and István M. Mándity. 2020. "N-Acetylation of Amines in Continuous-Flow with Acetonitrile—No Need for Hazardous and Toxic Carboxylic Acid Derivatives" Molecules 25, no. 8: 1985. https://doi.org/10.3390/molecules25081985