In Vitro and in Vivo Activity of mTOR Kinase and PI3K Inhibitors Against Leishmania donovani and Trypanosoma brucei
Abstract
1. Introduction
2. Results and Discussion
2.1. Intracellular Leishmania Pilot Screening
2.2. Hit Characteristics and Selection of mTOR/PI3K Inhibitor
2.3. In Vitro Activity Against Kinetoplastid Parasite
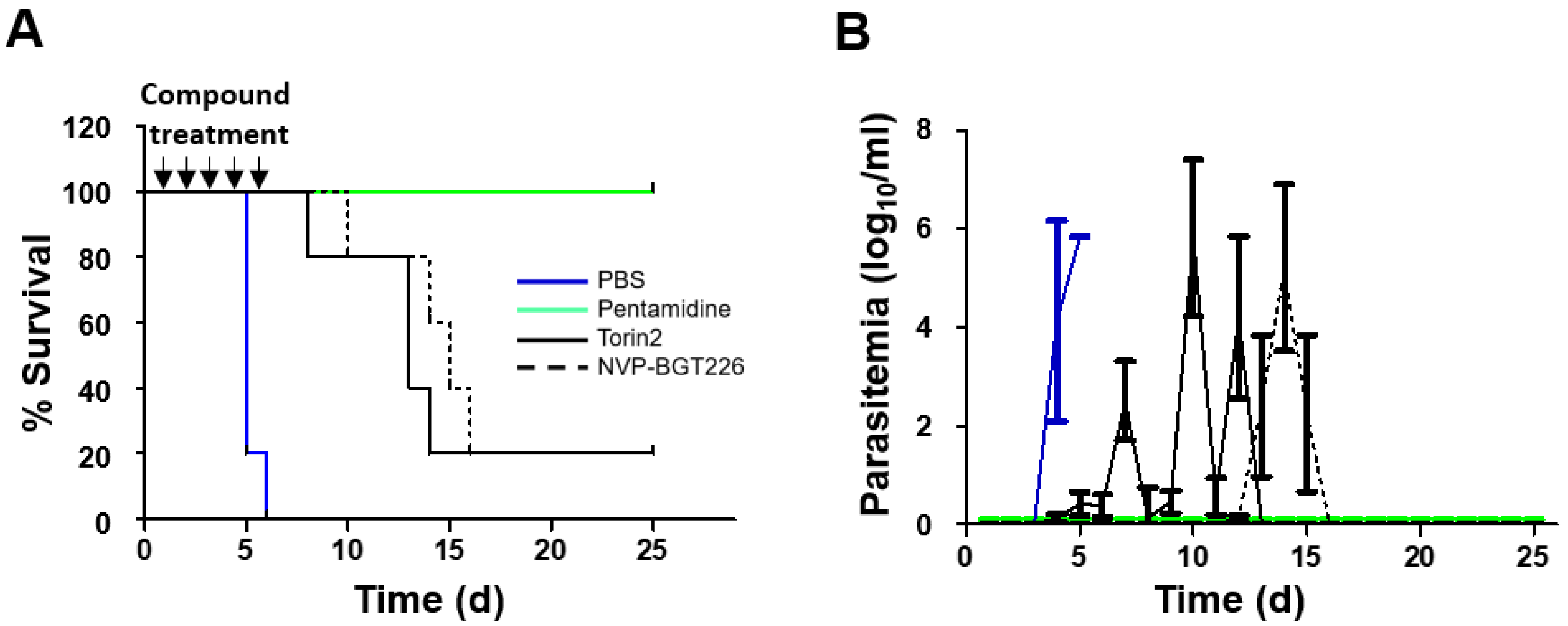
2.4. In Vivo Efficacy of mTOR/IP3K Inhibitors in VL Mouse Model
2.5. In Vivo Efficacy of NVP-BGT226 in T. brucei Mouse Model
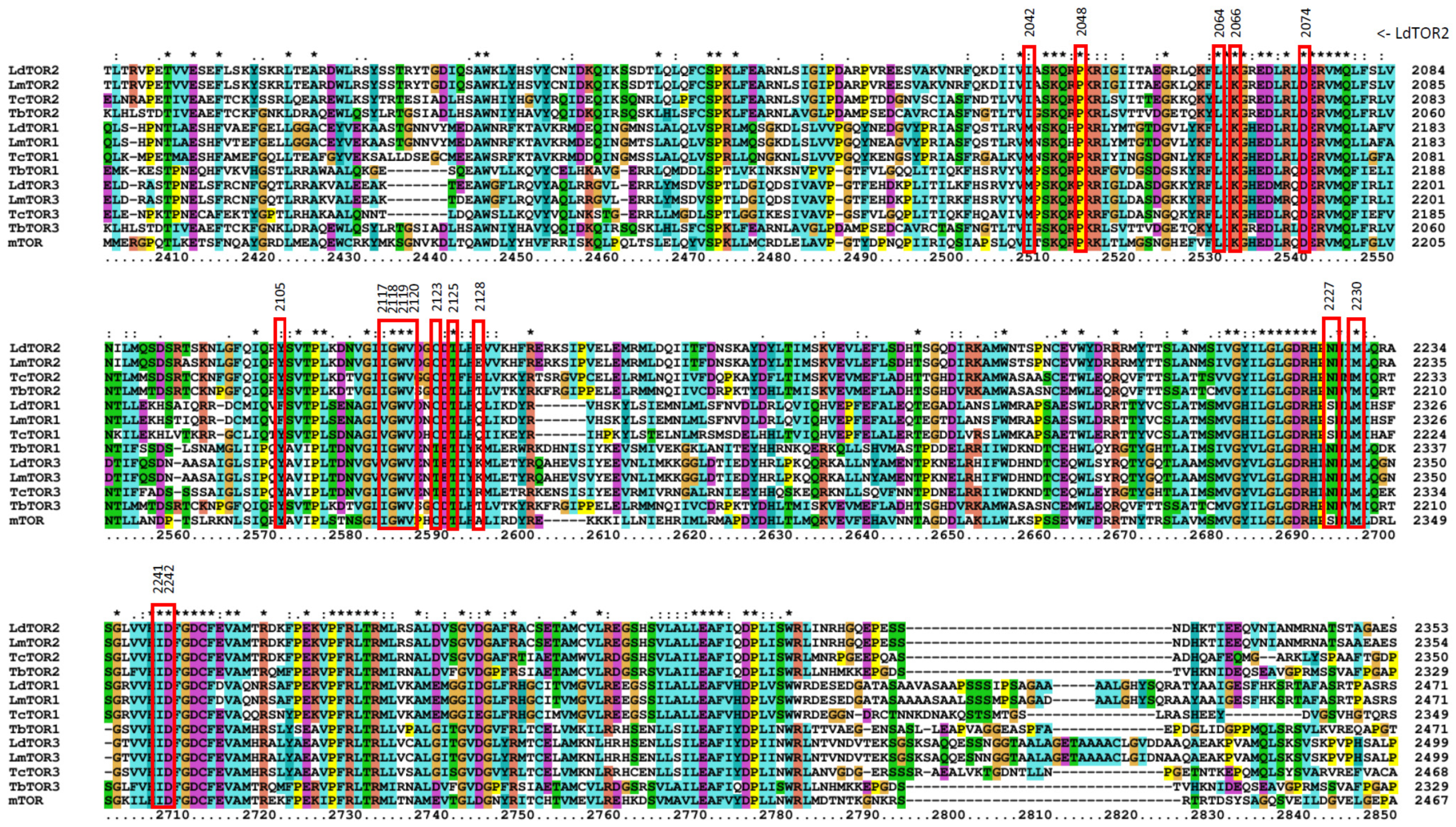
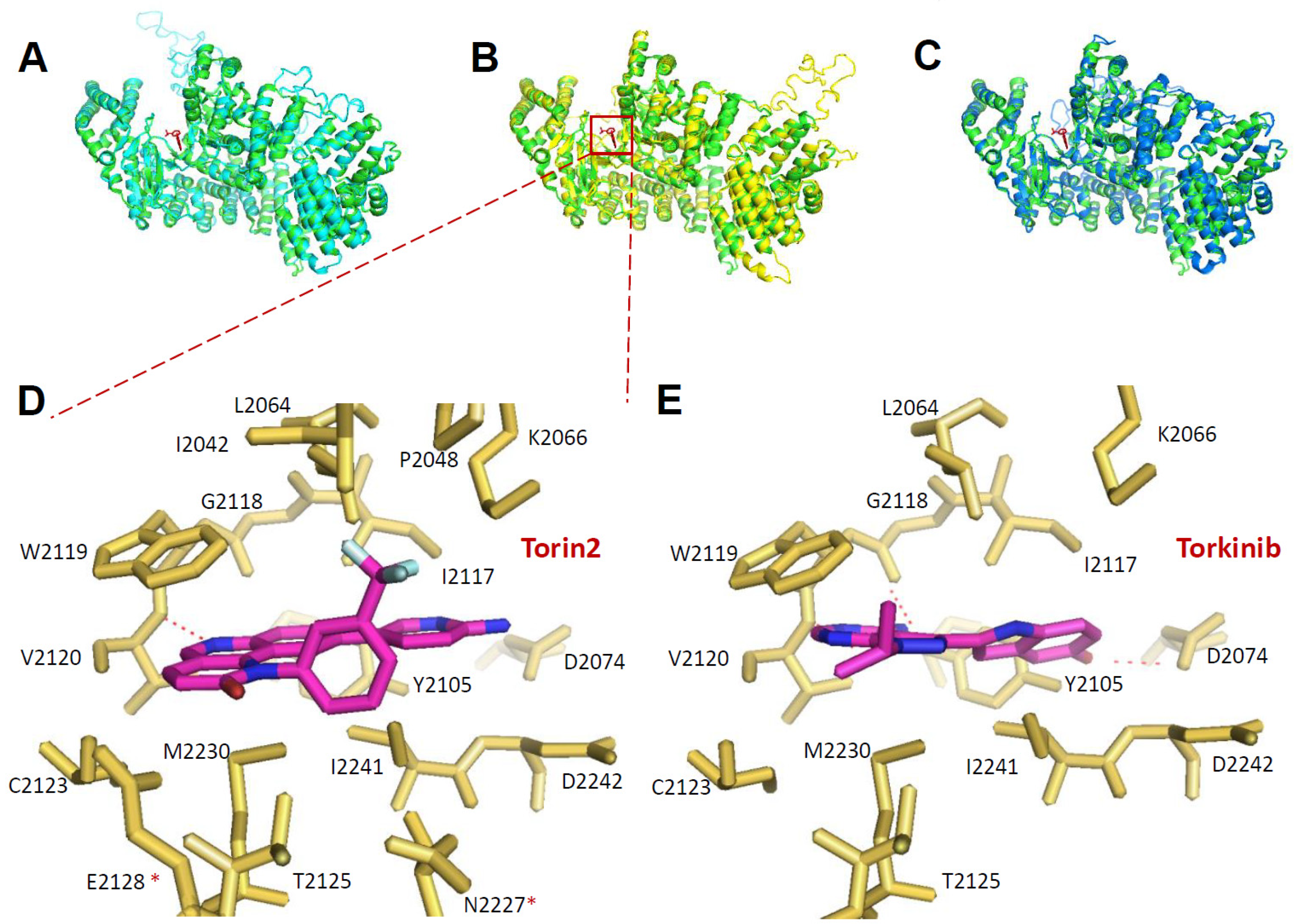
2.6. Prediction of Inhibitor Binding to Kinetoplastid TORs
3. Materials and Methods
3.1. Ethics Statement
3.2. Inhibitors
3.3. Parasite and Cell Cultures
3.4. Screening of Bioactive Compounds Against Intracellular Leishmania
3.5. Parasite Growth Inhibition
3.6. In Vivo Experiments
3.6.1. VL Mouse Model
3.6.2. HAT Mouse Model
3.7. Prediction of Compound Binding Modes
3.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Stuart, K.; Brun, R.; Croft, S.; Fairlamb, A.; Gürtler, R.E.; McKerrow, J.; Reed, S.; Tarleton, R.L. Kinetoplastids: Related protozoan pathogens, different diseases. J. Clin. Investig. 2008, 118, 1301–1310. [Google Scholar] [CrossRef]
- World Health Organization. Leishmaniasis. Fact sheet No. 375, 2017. World Health Organization. Available online: http://www.who.int/mediacentre/factsheets/fs375/en/ (accessed on 23 April 2020).
- Boelaert, M.; Criel, B.; Leeuwenburg, J.; Van Damme, W.; Le Ray, D.; Van Der Stuyft, P. Visceral leishmaniasis control: A public health perspective. Trans. R. Soc. Trop. Med. Hyg. 2000, 94, 465–471. [Google Scholar] [CrossRef]
- World Health Organization. Control of the Leishmaniases; WHO Technical Report Series 949; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Alvar, J.; Velez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; Boer, M.D. Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLOS ONE 2012, 7, e35671. [Google Scholar] [CrossRef]
- Pigott, D.M.; Bhatt, S.; Golding, N.; A Duda, K.; Battle, K.E.; Brady, O.J.; Messina, J.P.; Balard, Y.; Bastien, P.; Pratlong, F.; et al. Global distribution maps of the leishmaniases. eLife 2014, 3, 3. [Google Scholar] [CrossRef]
- No, J.H. Visceral leishmaniasis: Revisiting current treatments and approaches for future discoveries. Acta Trop. 2016, 155, 113–123. [Google Scholar] [CrossRef]
- Doua, F.; Miezan, T.W.; Singaro, J.R.S.; Yapo, F.B.; Baltz, T. The Efficacy of Pentamidine in the Treatment of Early-Late Stage Trypanosoma brucei gambiense Trypanosomiasis *. Am. J. Trop. Med. Hyg. 1996, 55, 586–588. [Google Scholar] [CrossRef]
- Kennedy, P.G. Clinical features, diagnosis, and treatment of human African trypanosomiasis (sleeping sickness). Lancet Neurol. 2013, 12, 186–194. [Google Scholar] [CrossRef]
- Alirol, E.; Schrumpf, D.; Heradi, J.A.; Riedel, A.; De Patoul, C.; Quere, M.; Chappuis, F. Nifurtimox-Eflornithine Combination Therapy for Second-Stage Gambiense Human African Trypanosomiasis: Médecins Sans Frontières Experience in the Democratic Republic of the Congo. Clin. Infect. Dis. 2012, 56, 195–203. [Google Scholar] [CrossRef]
- Lindner, A.K.; Lejon, V.; Chappuis, F.; Seixas, J.; Kazumba, L.; Barrett, M.P.; Mwamba, E.; Erphas, O.; A Akl, E.; Villanueva, G.; et al. New WHO guidelines for treatment of gambiense human African trypanosomiasis including fexinidazole: Substantial changes for clinical practice. Lancet Infect. Dis. 2020, 20, e38–e46. [Google Scholar] [CrossRef]
- Antinori, S.; Galimberti, L.; Bianco, R.; Grande, R.; Galli, M.; Corbellino, M. Chagas disease in Europe: A review for the internist in the globalized world. Eur. J. Intern. Med. 2017, 43, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Zulantay, I.; Apt, W.; Ramos, D.; Godoy, L.; Valencia, C.; Molina, M.; Sepulveda, E.; Thieme, P.; Martinez, G.; Corral, G. The Epidemiological Relevance of Family Study in Chagas Disease. PLOS Neglected Trop. Dis. 2013, 7, e1959. [Google Scholar] [CrossRef] [PubMed]
- De Koning, H.P. Ever-increasing complexities of diamidine and arsenical crossresistance in African trypanosomes. Trends Parasitol. 2008, 24, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Hamill, R.J. Amphotericin B Formulations: A Comparative Review of Efficacy and Toxicity. Drugs 2013, 73, 919–934. [Google Scholar] [CrossRef]
- Ponte-Sucre, A.; Gamarro, F.; Dujardin, J.-C.; Barrett, M.P.; Lopez-Vélez, R.; García-Hernández, R.; Pountain, A.W.; Mwenechanya, R.; Papadopoulou, B. Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLOS Neglected Trop. Dis. 2017, 11, e0006052. [Google Scholar] [CrossRef]
- Wilkinson, S.R.; Taylor, M.C.; Horn, D.; Kelly, J.M.; Cheeseman, I.H. A mechanism for cross-resistance to nifurtimox and benznidazole in trypanosomes. Proc. Natl. Acad. Sci. USA 2008, 105, 5022–5027. [Google Scholar]
- Castro, J.; De Mecca, M.M.; Bartel, L. Toxic side effects of drugs used to treat Chagas’ disease (American trypanosomiasis). Hum. Exp. Toxicol. 2006, 25, 471–479. [Google Scholar] [CrossRef]
- Chappuis, F.; Sundar, S.; Hailu, A.; Ghalib, H.; Rijal, S.; Peeling, R.W.; Alvar, J.; Boelaert, M. Visceral leishmaniasis: What are the needs for diagnosis, treatment and control? Nat. Rev. Microbiol. 2007, 5, 873–882. [Google Scholar] [CrossRef]
- World Health Organization. Human African Trypanosomiasis: A Guide for Drug Supply, 2002. World Health Organization. Available online: https://apps.who.int/iris/handle/10665/67252 (accessed on 23 April 2020).
- Andrews, K.; Walduck, A.; Kelso, M.J.; Fairlie, D.P.; Saul, A.; Parsons, P.G.; Kelso, M.J. Anti-malarial effect of histone deacetylation inhibitors and mammalian tumour cytodifferentiating agents. Int. J. Parasitol. 2000, 30, 761–768. [Google Scholar] [CrossRef]
- Eastman, R.T.; White, J.; Hucke, O.; Bauer, K.; Yokoyama, K.; Nallan, L.; Chakrabarti, D.; Verlinde, C.; Gelb, M.H.; Rathod, P.K.; et al. Resistance to a Protein Farnesyltransferase Inhibitor inPlasmodium falciparum. J. Boil. Chem. 2005, 280, 13554–13559. [Google Scholar] [CrossRef]
- Frearson, J.A.; Brand, S.; McElroy, S.P.; Cleghorn, L.A.T.; Smid, O.; Stojanovski, L.; Price, H.P.; Güther, M.L.S.; Torrie, L.S.; Robinson, D.; et al. N-myristoyltransferase inhibitors as new leads to treat sleeping sickness. Nat. 2010, 464, 728–732. [Google Scholar] [CrossRef]
- Pollastri, M.P.; Campbell, R.K. Target repurposing for neglected diseases. Futur. Med. Chem. 2011, 3, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Sabatini, D.M. mTOR signaling at a glance. J. Cell Sci. 2009, 122, 3589–3594. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Ali, S.M.; Sabatini, D.M. Growing roles for the mTOR pathway. Curr. Opin. Cell Boil. 2005, 17, 596–603. [Google Scholar] [CrossRef]
- Wullschleger, S.; Loewith, R.; Hall, M.N. TOR Signaling in Growth and Metabolism. Cell 2006, 124, 471–484. [Google Scholar] [CrossRef]
- Abraham, R.T. PI 3-kinase related kinases: ‘Big’ players in stress-induced signaling pathways. DNA Repair 2004, 3, 883–887. [Google Scholar] [CrossRef]
- Bakkenist, C.J.; Kastan, M.B. Initiating Cellular Stress Responses. Cell 2004, 118, 9–17. [Google Scholar] [CrossRef]
- Keith, C.T.; Schreiber, S.L. PIK-Related Kinases: DNA Repair, Recombination, and Cell Cycle Checkpoints. Sci. 1995, 270, 50. [Google Scholar] [CrossRef]
- Alzahrani, A.S. PI3K/Akt/mTOR inhibitors in cancer: At the bench and bedside. Semin. Cancer Boil. 2019, 59, 125–132. [Google Scholar] [CrossRef]
- Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T. The PI3K Pathway in Human Disease. Cell 2017, 170, 605–635. [Google Scholar] [CrossRef]
- Hillmann, P.; Fabbro, D. PI3K/mTOR Pathway Inhibition: Opportunities in Oncology and Rare Genetic Diseases. Int. J. Mol. Sci. 2019, 20, 5792. [Google Scholar] [CrossRef]
- Mayer, I.A.; Arteaga, C.L. The PI3K/AKT Pathway as a Target for Cancer Treatment. Annu. Rev. Med. 2016, 67, 11–28. [Google Scholar] [CrossRef]
- Yang, J.; Nie, J.; Ma, X.; Wei, Y.; Peng, Y.; Wei, X. Targeting PI3K in cancer: Mechanisms and advances in clinical trials. Mol. Cancer 2019, 18, 26. [Google Scholar] [CrossRef]
- Janku, F.; Yap, T.A.; Meric-Bernstam, F. Targeting the PI3K pathway in cancer: Are we making headway? Nat. Rev. Clin. Oncol. 2018, 15, 273–291. [Google Scholar] [CrossRef]
- Diaz-Gonzalez, R.; Kuhlmann, F.M.; Galan-Rodriguez, C.; da Silva, L.M.; Saldivia, M.; Karver, C.E.; Rodriguez, A.; Beverley, S.M.; Navarro, M.; Pollastri, M.P. The susceptibility of trypanosomatid pathogens to PI3/mTOR kinase inhibitors affords a new opportunity for drug repurposing. PLoS Negl Trop Dis. 2011, 5, e1297. [Google Scholar] [CrossRef]
- Khadem, F.; Jia, P.; Mou, Z.; Feiz Barazandeh, A.; Liu, D.; Keynan, Y.; Uzonna, J.E. Pharmacological inhibition of p110delta subunit of PI3K confers protection against experimental leishmaniasis. J. Antimicrob Chemother. 2017, 72, 467–477. [Google Scholar] [CrossRef]
- Khadir, F.; Shaler, C.R.; Oryan, A.; Rudak, P.T.; Mazzuca, D.M.; Taheri, T.; Dikeakos, J.D.; Haeryfar, S.M.M.; Rafati, S. Therapeutic control of leishmaniasis by inhibitors of the mammalian target of rapamycin. PLOS Neglected Trop. Dis. 2018, 12, e0006701. [Google Scholar] [CrossRef]
- Barquilla, A.; Crespo, J.L.; Navarro, M. Rapamycin inhibits trypanosome cell growth by preventing TOR complex 2 formation. Proc. Natl. Acad. Sci. USA 2008, 105, 14579–14584. [Google Scholar]
- Barquilla, A.; Navarro, M. Trypanosome TOR complex 2 functions in cytokinesis. Cell Cycle 2009, 8, 697–699. [Google Scholar] [CrossRef]
- De Jesus, T.C.L.; Tonelli, R.R.; Nardelli, S.C.; Augusto, L.D.S.; Motta, M.C.M.; Girard-Dias, W.; Miranda, K.; Ulrich, P.; Jimenez, V.; Barquilla, A.; et al. Target of Rapamycin (TOR)-like 1 Kinase Is Involved in the Control of Polyphosphate Levels and Acidocalcisome Maintenance in Trypanosoma brucei*. J. Boil. Chem. 2010, 285, 24131–24140. [Google Scholar] [CrossRef]
- Da Silva, L.M.; Beverley, S.M. Expansion of the target of rapamycin (TOR) kinase family and function in Leishmania shows that TOR3 is required for acidocalcisome biogenesis and animal infectivity. Proc. Natl. Acad. Sci. USA 2010, 107, 11965–11970. [Google Scholar]
- Liu, Q.; Wang, J.; Kang, S.A.; Thoreen, C.C.; Hur, W.; Ahmed, T.; Sabatini, D.M.; Gray, N.S. Discovery of 9-(6-Aminopyridin-3-yl)-1-(3-(trifluoromethyl)phenyl)benzo[h][1,6]naphthyridin-2(1H)-one (Torin2) as a Potent, Selective, and Orally Available Mammalian Target of Rapamycin (mTOR) Inhibitor for Treatment of Cancer. J. Med. Chem. 2011, 54, 1473–1480. [Google Scholar] [CrossRef]
- Maira, S.-M.; Stauffer, F.; Brueggen, J.; Furet, P.; Schnell, C.; Fritsch, C.; Brachmann, S.; Chène, P.; De Pover, A.; Schoemaker, K.; et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol. Cancer Ther. 2008, 7, 1851–1863. [Google Scholar] [CrossRef]
- Apsel, B.; Blair, J.A.; Gonzalez, B.Z.; Nazif, T.; Feldman, M.E.; Aizenstein, B.; Hoffman, R.; Williams, R.L.; Shokat, K.M.; Knight, Z.A. Targeted polypharmacology: Discovery of dual inhibitors of tyrosine and phosphoinositide kinases. Nat. Methods 2008, 4, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, A.C.; Liu, Y.; Edlind, M.P.; Ingolia, N.T.; Janes, M.R.; Sher, A.; Shi, E.Y.; Stumpf, C.R.; Christensen, C.; Bonham, M.J.; et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature 2012, 485, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Mallon, R.; Hollander, I.; Feldberg, L.; Lucas, J.; Soloveva, V.; Venkatesan, A.; Dehnhardt, C.; Santos, E.D.; Chen, Z.; Dos Santos, O.; et al. Antitumor Efficacy Profile of PKI-402, a Dual Phosphatidylinositol 3-Kinase/Mammalian Target of Rapamycin Inhibitor. Mol. Cancer Ther. 2010, 9, 976–984. [Google Scholar] [CrossRef]
- Markman, B.; Tabernero, J.; Krop, I.; Shapiro, G.I.; Siu, L.; Chen, L.C.; Mita, M.; Cuero, M.M.; Stutvoet, S.; Birle, D.; et al. Phase I safety, pharmacokinetic, and pharmacodynamic study of the oral phosphatidylinositol-3-kinase and mTOR inhibitor BGT226 in patients with advanced solid tumors. Ann. Oncol. 2012, 23, 2399–2408. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Shi, C.; Toral-Barza, L.; Lucas, J.; Shor, B.; Kim, J.-E.; Zhang, W.-G.; Mahoney, R.; Gaydos, C.; Tardio, L.; et al. Beyond Rapalog Therapy: Preclinical Pharmacology and Antitumor Activity of WYE-125132, an ATP-Competitive and Specific Inhibitor of mTORC1 and mTORC2. Cancer Res. 2010, 70, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Keiser, J.; Stich, A.; Burri, C. New drugs for the treatment of human African trypanosomiasis: Research and development. Trends Parasitol. 2001, 17, 42–49. [Google Scholar] [CrossRef]
- Seixas, J.D.; Luengo-Arratta, S.A.; Díaz, R.; Saldivia, M.; Rojas-Barros, D.I.; Manzano, P.; Gonzalez, R.D.; Berlanga, M.; Smith, T.K.; Navarro, M.; et al. Establishment of a Structure–Activity Relationship of 1H-Imidazo[4,5-c]quinoline-Based Kinase Inhibitor NVP-BEZ235 as a Lead for African Sleeping Sickness. J. Med. Chem. 2014, 57, 4834–4848. [Google Scholar] [CrossRef]
- Fang, B.; Kannan, A.; Guo, T.; Gao, L. Simultaneously targeting DNA damage repair pathway and mTORC1/2 results in small cell lung cancer growth arrest via ER stress-induced apoptosis. Int J. Biol Sci. 2018, 14, 1221–1231. [Google Scholar] [CrossRef]
- Kang, M.H.; Reynolds, C.P.; Maris, J.M.; Gorlick, R.; Kolb, E.A.; Lock, R.; Carol, H.; Keir, S.T.; Wu, J.; Lyalin, D.; et al. Initial testing (stage 1) of the investigational mTOR kinase inhibitor MLN0128 by the pediatric preclinical testing program. Pediatr. Blood Cancer 2014, 61, 1486–1489. [Google Scholar] [CrossRef]
- Okada, T.; Lee, A.; Qin, L.-X.; Agaram, N.; Mimae, T.; Shen, Y.; O’Connor, R.; López-Lago, M.A.; Craig, A.; Miller, M.L.; et al. Integrin-α10 Dependency Identifies RAC and RICTOR as Therapeutic Targets in High-Grade Myxofibrosarcoma. Cancer Discov. 2016, 6, 1148–1165. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kanaya, N.; Somlo, G.; Chen, S. Synergistic anti-cancer activity of CDK4/6 inhibitor palbociclib and dual mTOR kinase inhibitor MLN0128 in pRb-expressing ER-negative breast cancer. Breast Cancer Res. Treat. 2019, 174, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Yardley, V.; Vishwakarma, P.; Shivahare, R.; Sharma, B.; Launay, D.; Martin, D.; Puri, S.K. Nitroimidazo-oxazole compound DNDI-VL-2098: An orally effective preclinical drug candidate for the treatment of visceral leishmaniasis. J. Antimicrob. Chemother. 2014, 70, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Khare, S.; Nagle, A.S.; Biggart, A.; Lai, Y.H.; Liang, F.; Davis, L.C.; Barnes, S.W.; Mathison, C.J.N.; Myburgh, E.; Gao, M.-Y.; et al. Proteasome inhibition for treatment of leishmaniasis, Chagas disease and sleeping sickness. Nat. 2016, 537, 229–233. [Google Scholar] [CrossRef]
- Manhas, R.; Tandon, S.; Sen, S.S.; Tiwari, N.; Munde, M.; Madhubala, R. Leishmania donovani Parasites Are Inhibited by the Benzoxaborole AN2690 Targeting Leucyl-tRNA Synthetase. Antimicrob. Agents Chemother. 2018, 62, AAC.00079–18. [Google Scholar] [CrossRef]
- Mowbray, C.; Braillard, S.; Speed, W.; Glossop, P.A.; Whitlock, G.A.; Gibson, K.R.; Mills, J.E.J.; Brown, A.D.; Gardner, J.M.F.; Cao, Y.; et al. Novel Amino-pyrazole Ureas with Potent In Vitro and In Vivo Antileishmanial Activity. J. Med. Chem. 2015, 58, 9615–9624. [Google Scholar] [CrossRef]
- Patterson, S.; Wyllie, S.; Norval, S.; Stojanovski, L.; Simeons, F.R.; Auer, J.L.; Osuna-Cabello, M.; Read, K.D.; Fairlamb, A. The anti-tubercular drug delamanid as a potential oral treatment for visceral leishmaniasis. eLife 2016, 5, 334. [Google Scholar] [CrossRef]
- Saha, S.; Acharya, C.; Pal, U.; Chowdhury, S.R.; Sarkar, K.; Maiti, N.C.; Jaisankar, P.; Majumder, H.K. A Novel Spirooxindole Derivative Inhibits the Growth of Leishmania donovani Parasites both In Vitro and In Vivo by Targeting Type IB Topoisomerase. Antimicrob. Agents Chemother. 2016, 60, 6281–6293. [Google Scholar] [CrossRef]
- Berry, T.; Luther, W.; Bhatnagar, N.; Jamin, Y.; Poon, E.; Sanda, T.; Pei, D.; Sharma, B.; Vetharoy, W.R.; Hallsworth, A.; et al. The ALK(F1174L) mutation potentiates the oncogenic activity of MYCN in neuroblastoma. Cancer Cell 2012, 22, 117–130. [Google Scholar] [CrossRef]
- Chang, K.-Y.; Tsai, S.-Y.; Wu, C.-M.; Yen, C.-J.; Chuang, B.-F.; Chang, J.-Y. Novel Phosphoinositide 3-Kinase/mTOR Dual Inhibitor, NVP-BGT226, Displays Potent Growth-Inhibitory Activity against Human Head and Neck Cancer Cells In Vitro and In Vivo. Clin. Cancer Res. 2011, 17, 7116–7126. [Google Scholar] [CrossRef]
- Myburgh, E.; Coles, J.A.; Ritchie, R.; Kennedy, P.G.E.; McLatchie, A.P.; Rodgers, J.; Taylor, M.C.; Barrett, M.P.; Brewer, J.M.; Mottram, J.C. In Vivo Imaging of Trypanosome-Brain Interactions and Development of a Rapid Screening Test for Drugs against CNS Stage Trypanosomiasis. PLOS Neglected Trop. Dis. 2013, 7, e2384. [Google Scholar] [CrossRef]
- Siqueira-Neto, J.; Moon, S.; Jang, J.; Yang, G.; Lee, C.; Moon, H.K.; Chatelain, E.; Genovesio, A.; Cechetto, J.; Freitas-Junior, L.H. An Image-Based High-Content Screening Assay for Compounds Targeting Intracellular Leishmania donovani Amastigotes in Human Macrophages. PLOS Neglected Trop. Dis. 2012, 6, e1671. [Google Scholar] [CrossRef] [PubMed]
- Vermeersch, M.; Da Luz, R.I.; Toté, K.; Timmermans, J.; Cos, P.; Maes, L. In Vitro Susceptibilities of Leishmania donovani Promastigote and Amastigote Stages to Antileishmanial Reference Drugs: Practical Relevance of Stage-Specific Differences. Antimicrob. Agents Chemother. 2009, 53, 3855–3859. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhu, W.; Wang, Y.; Huang, G.; Byun, S.Y.; Choi, G.; Li, K.; Huang, Z.; Docampo, R.; Oldfield, E.; et al. In Vitro and in Vivo Activity of Multitarget Inhibitors against Trypanosoma brucei. ACS Infect. Dis. 2015, 1, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Bowling, T.; Mercer, L.; Don, R.; Jacobs, R.; Nare, B. Application of a resazurin-based high-throughput screening assay for the identification and progression of new treatments for human African trypanosomiasis. Int. J. Parasitol. Drugs Drug Resist. 2012, 2, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Stauber, L.A. Leishmaniasis in the hamster. In Some Physiological Aspects and Consequences of Parasitism; Rutgers University Press: New Brunswisck, NJ, USA, 1955. [Google Scholar]
- Choi, J.; Chen, J.; Schreiber, S.L.; Clardy, J. Structure of the FKBP12-Rapamycin Complex Interacting with Binding Domain of Human FRAP. Science 1996, 273, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Veverka, V.; Crabbe, T.; Bird, I.; Lennie, G.; Muskett, F.W.; Taylor, R.J.; Carr, M. Structural characterization of the interaction of mTOR with phosphatidic acid and a novel class of inhibitor: Compelling evidence for a central role of the FRB domain in small molecule-mediated regulation of mTOR. Oncogene 2007, 27, 585–595. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available |


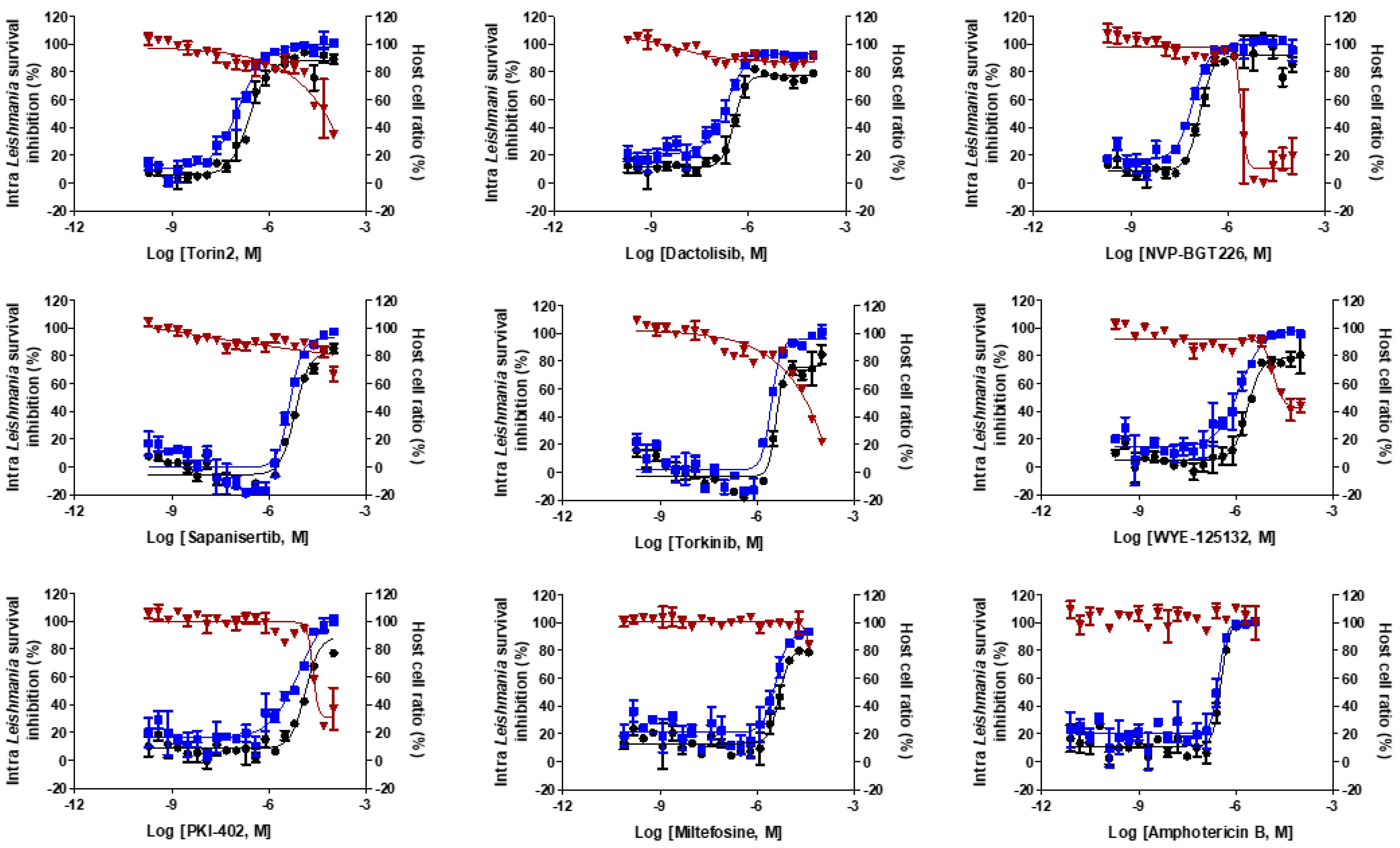
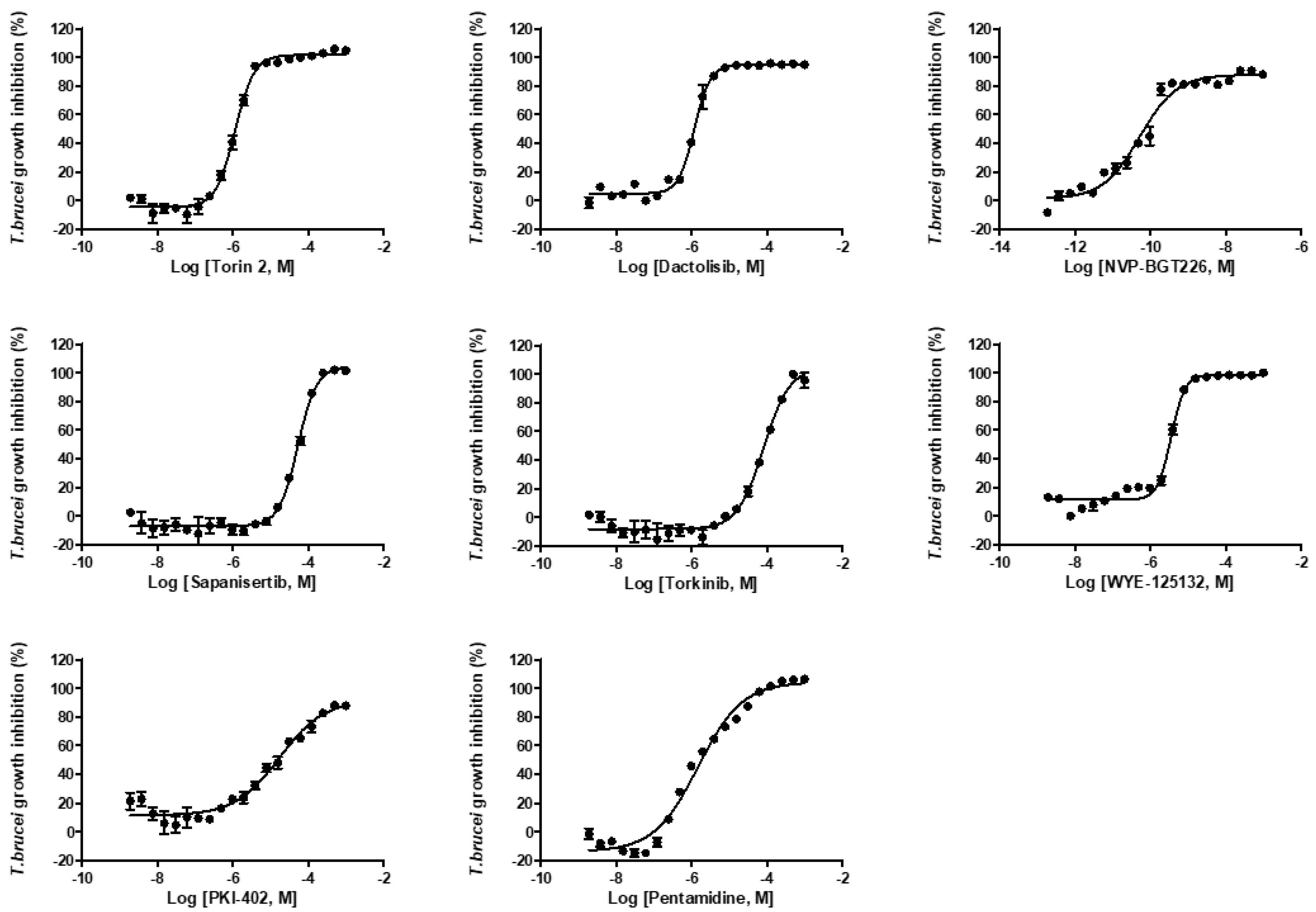
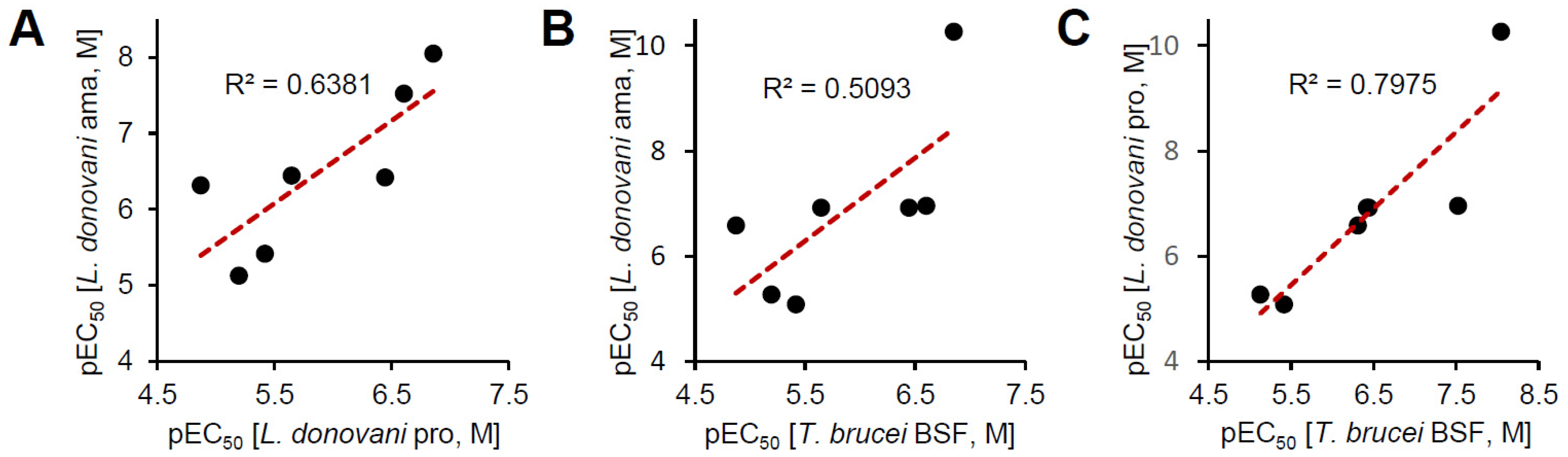
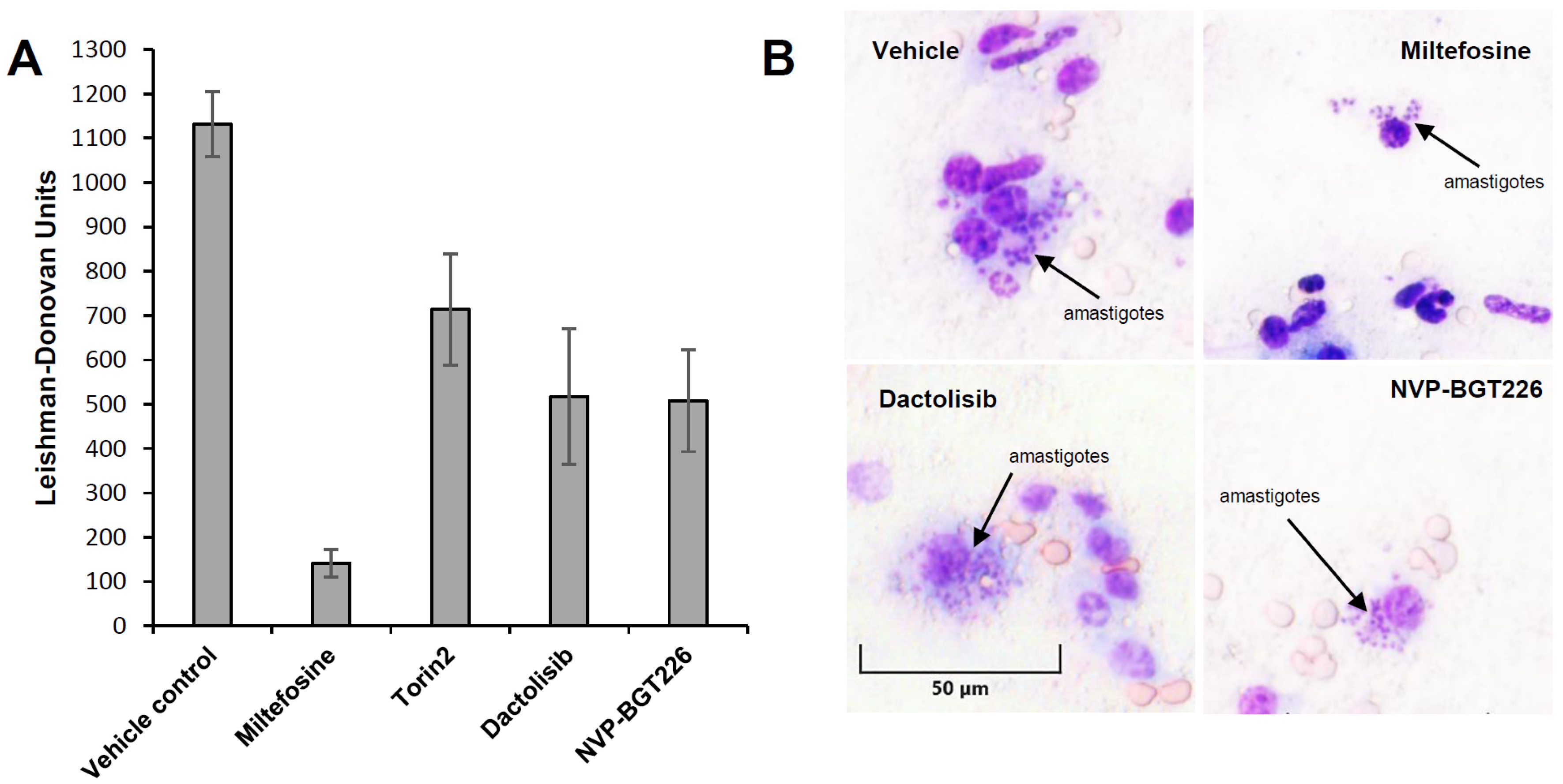
| Compounds | Intracellular L. donovani EC50 ± SD a (μM) | THP-1 Cell CC50 ± SD a (μM) | Selectivity Index (SI) | L. donovani Promastigote EC50 ± SD a (μM) | T. brucei Lister 427 BSF EC50 ± SD a (μM) |
|---|---|---|---|---|---|
| Miltefosine | 4.81 ± 0.81 | >40 | >8.3 | 1.03 ± 0.63 | NA |
| Amphotericin B | 0.34 ± 0.04 | >4 | >11.8 | 0.015 ± 0.003 | NA |
| Pentamidine | NA | NA | NA | NA | 0.15 ± 0.01 |
| Torin2 | 0.25 ± 0.03 | 30.50 ± 1.3 | 122 | 0.03 ± 0.004 | 0.11 ± 0.01 |
| Dactolisib (NVP-BEZ235) | 0.36 ± 0.07 | >100 | ≥277.8 | 0.38 ± 0.06 | 0.12 ± 0.01 |
| NVP-BGT226 | 0.14 ± 0.02 | 2.66 ± 0.46 | 19 | 0.009 ± 0.001 | 0.000054 ± 0.000003 |
| Sapanisertib | 6.39 ± 1.12 | >100 | ≥39 | 7.46 ± 1.04 | 5.34 ± 0.98 |
| Torkinib | 3.83 ± 0.98 | 33.43 ± 1.4 | 8.7 | 3.86 ± 1.11 | 8.16 ± 1.52 |
| WYE-125132 | 2.26 ± 0.71 | 43.27 ± 2.7 | 19 | 0.36 ± 0.04 | 0.12 ± 0.01 |
| PKI-402 | 13.44 ± 2.35 | 28.48 ± 1.2 | 2.1 | 0.49 ± 0.22 | 0.26 ± 0.02 |
| Compounds | Dose (mg/kg) | LDU in the Liver (Mean ± SD) | Inhibition of L. donovani In Vivo (%) (Mean ± SD) |
|---|---|---|---|
| Vehicle | − | 1132 ± 73 | 0 |
| Miltefosine | 30 | 142 ± 24 | 87 ± 2.13 |
| Torin2 | 15 | 735 ± 66 | 35 ± 5.81 |
| Dactolisib (NVP-BEZ235) | 50 | 527 ± 108 | 53 ± 9.50 |
| NVP-BGT226 | 5 | 516 ± 36 | 54 ± 3.18 |
| mTOR | L. donovani | L. major | T. cruzi | T. brucei | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | ||
| Sequence identity (%) | 100 | 39.77 | 36.77 | 32.08 | 39.83 | 36.96 | 32.18 | 38.19 | 36.23 | 33.63 | 33.47 | 35.15 | 34.78 |
| RMSD | 0 | 0.374 | 0.428 | 0.291 | 0.157 | 0.296 | 0.280 | 0.360 | 0.393 | 0.392 | 0.284 | 0.173 | 0.422 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phan, T.-N.; Baek, K.-H.; Lee, N.; Byun, S.Y.; Shum, D.; No, J.H. In Vitro and in Vivo Activity of mTOR Kinase and PI3K Inhibitors Against Leishmania donovani and Trypanosoma brucei. Molecules 2020, 25, 1980. https://doi.org/10.3390/molecules25081980
Phan T-N, Baek K-H, Lee N, Byun SY, Shum D, No JH. In Vitro and in Vivo Activity of mTOR Kinase and PI3K Inhibitors Against Leishmania donovani and Trypanosoma brucei. Molecules. 2020; 25(8):1980. https://doi.org/10.3390/molecules25081980
Chicago/Turabian StylePhan, Trong-Nhat, Kyung-Hwa Baek, Nakyung Lee, Soo Young Byun, David Shum, and Joo Hwan No. 2020. "In Vitro and in Vivo Activity of mTOR Kinase and PI3K Inhibitors Against Leishmania donovani and Trypanosoma brucei" Molecules 25, no. 8: 1980. https://doi.org/10.3390/molecules25081980
APA StylePhan, T.-N., Baek, K.-H., Lee, N., Byun, S. Y., Shum, D., & No, J. H. (2020). In Vitro and in Vivo Activity of mTOR Kinase and PI3K Inhibitors Against Leishmania donovani and Trypanosoma brucei. Molecules, 25(8), 1980. https://doi.org/10.3390/molecules25081980




