Computational and Spectroscopic Studies of Carbon Disulfide
Abstract
1. Introduction
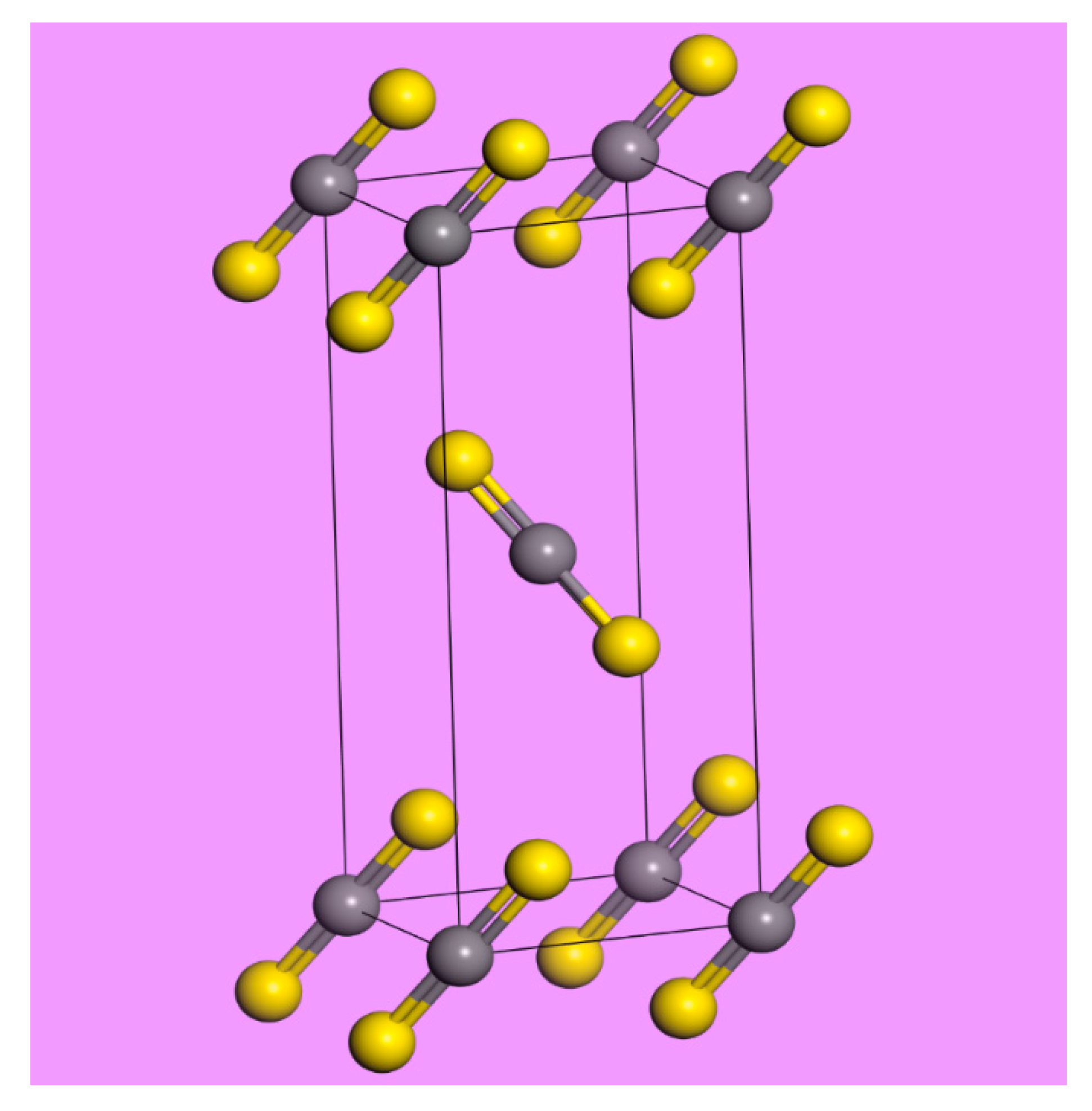
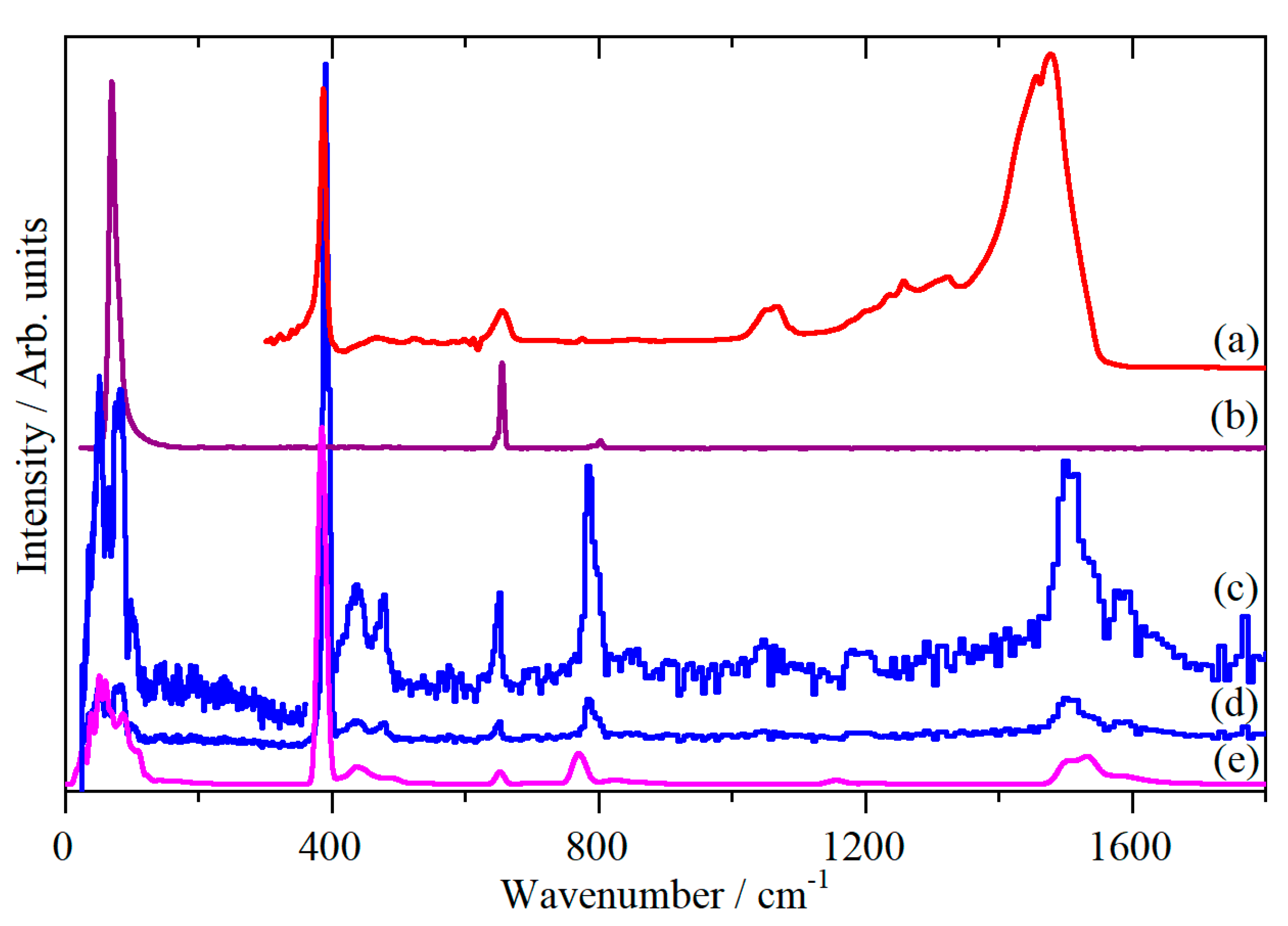
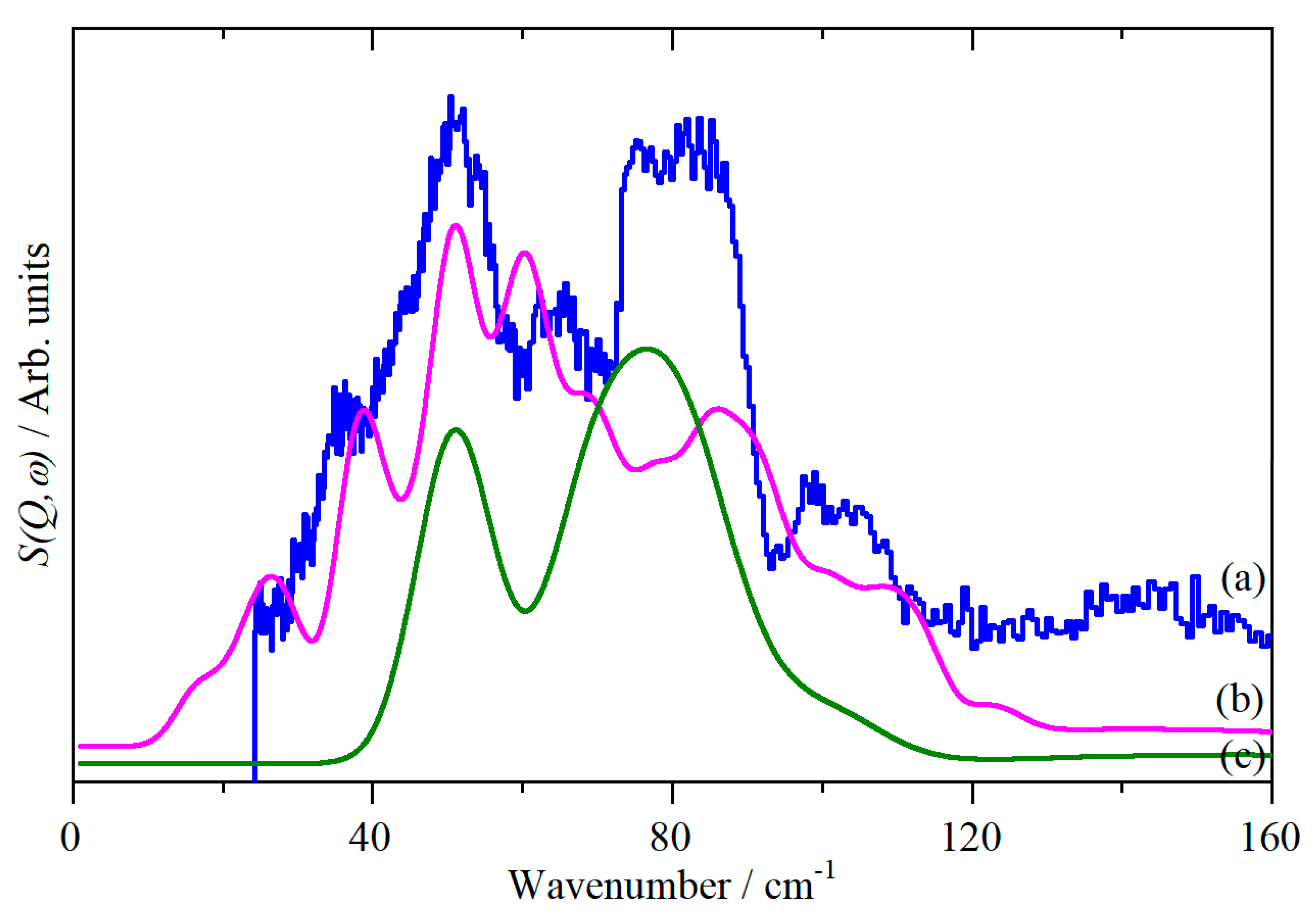
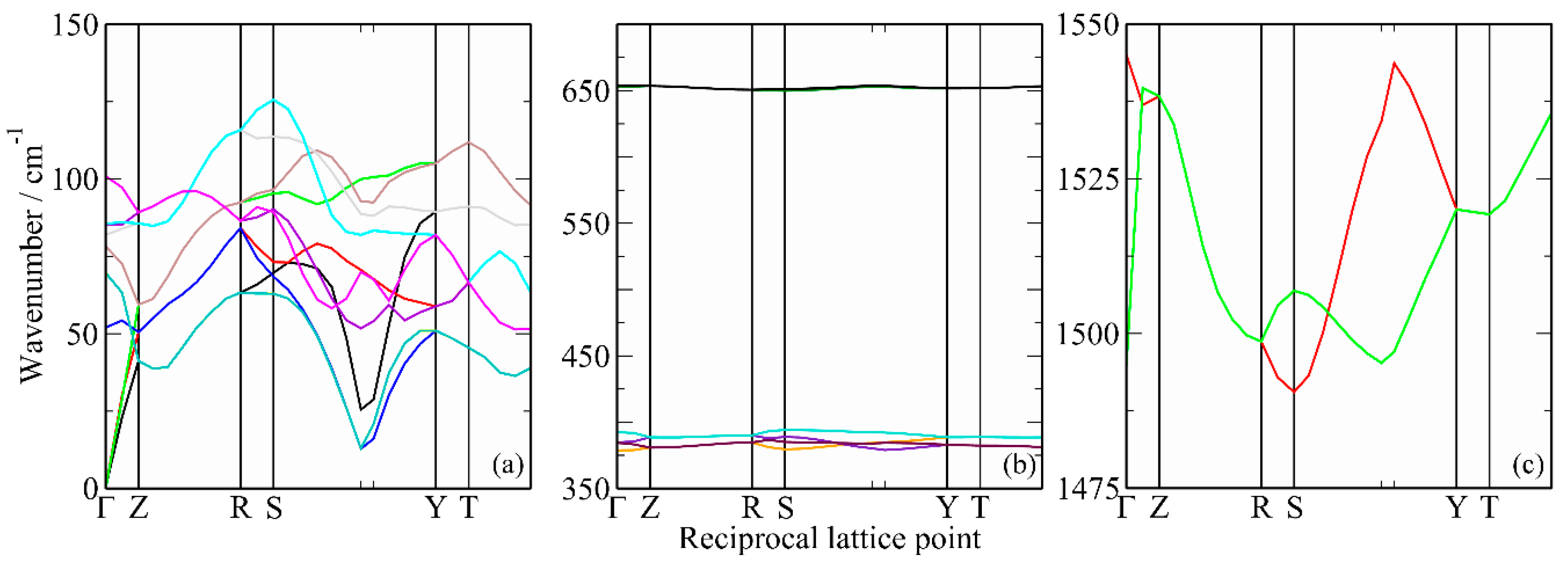
2. Results
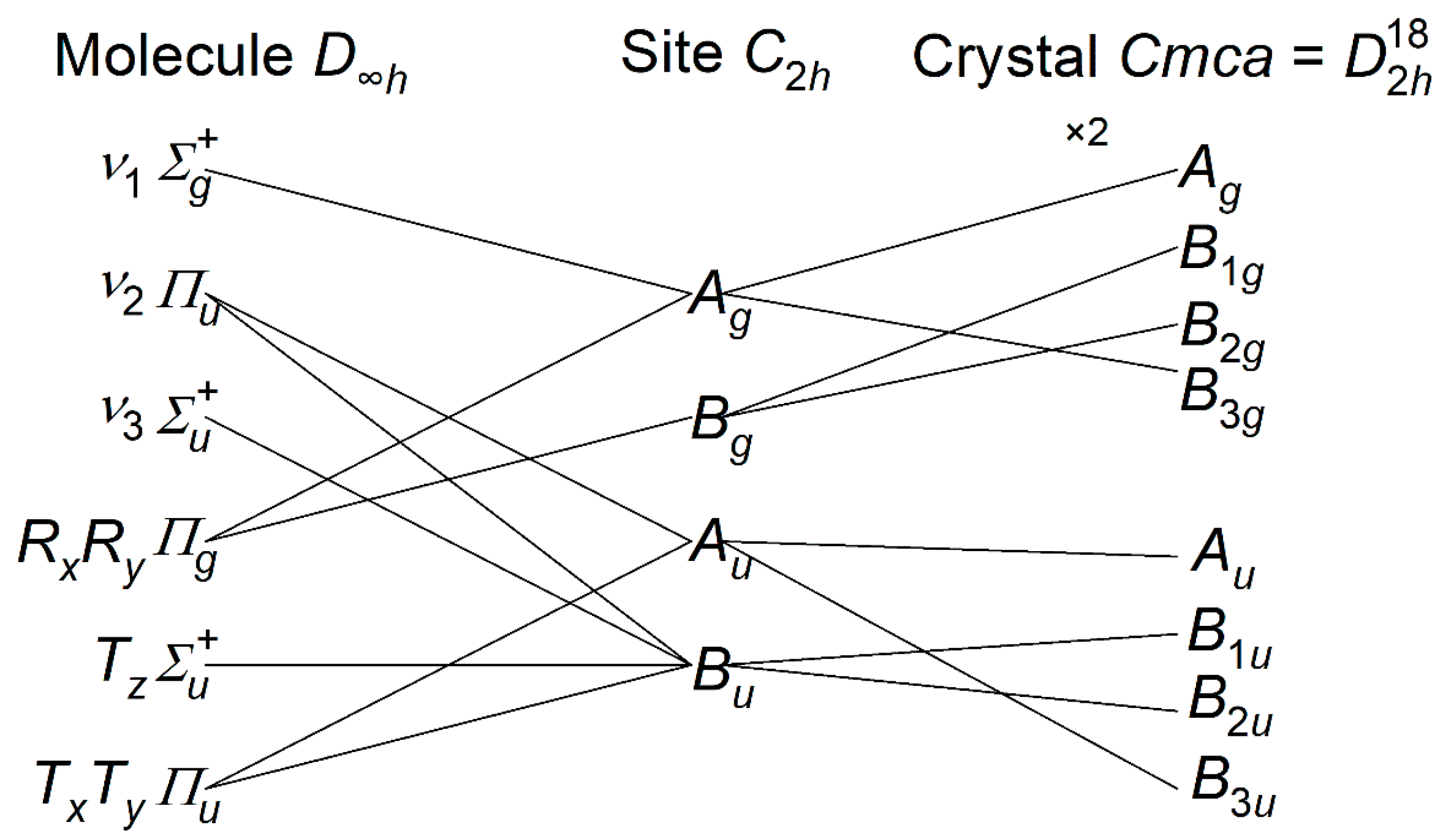
- {Ag + B3g} ν1 symmetric stretch, {Au + B1u + B2u + B3u} ν2 bend,
- {B1u + B2u} ν3 asymmetric stretch, {Ag + B1g + B2g + B3g} libration,
- {B1g + B2g} translation along z, {Au + B1u + B2u + B3u} translation along x,y
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, D.E.; Timmermann, R.W. Carbon Disulfide. In Kirk-Othmer Encyclopedia of Chemical Technology, 4th ed.; John Wiley: New York, NY, USA, 1991; Volume 5, pp. 26–36. [Google Scholar]
- Stoicheff, B.P. High resolution Raman spectroscopy of gases: XI. Spectra of CS2 and CO2. Can. J. Phys. 1958, 36, 218–230. [Google Scholar] [CrossRef]
- Person, W.B.; Hall, L.C. Absolute infrared intensities of CS2 fundamentals in gas and liquid phases. An interpretation of the bond moments of CO2 and CS2. Spectrochim. Acta 1964, 20, 771–779. [Google Scholar] [CrossRef]
- Smith, D.F.; Overend, J. General quartic force field of CS2. J. Chem. Phys. 1971, 54, 3632–3639. [Google Scholar] [CrossRef]
- Lindenmayer, J.; Jones, H. Diode laser spectroscopy of the ν3 band region of four isotopic forms of CS2. J. Mol. Spec. 1985, 110, 65–73. [Google Scholar] [CrossRef]
- Kroto, H.W.; Teixeira-Dias, J.J.C. The effects of intermolecular interactions in the Raman spectrum of liquid CS2. Spectrochim. Acta 1972, 28, 1497–1502. [Google Scholar] [CrossRef]
- Anderson, A.; Grout, P.J.; Leech, J.W.; Sun, T.S. Raman spectra of molecular crystals: Carbon disulphide. Chem. Phys. Lett. 1973, 21, 9–14. [Google Scholar] [CrossRef]
- Yamada, H.; Person, W.B. Absolute infrared intensities of the fundamental absorption bands in solid CS2. J. Chem. Phys. 1964, 40, 309–321. [Google Scholar] [CrossRef]
- Ishi, K.; Takahashi, S.I. Far infrared spectrum of crystalline carbon disulphide. Chem. Phys. Lett. 1977, 45, 460–461. [Google Scholar] [CrossRef]
- Burgos, E.; Righini, R. The effects of anisotropic atom-atom interactions on the crystal structure and lattice dynamics of solid CS2. Chem. Phys. Lett. 1983, 96, 584–590. [Google Scholar] [CrossRef]
- Powell, B.M.; Dolling, G.; Torrie, B.H.; Pawley, G.S. Intermolecular modes of solid carbon disulphide. J. Phys. C: Solid State Phys. 1982, 15, 4265–4274. [Google Scholar] [CrossRef]
- Bier, K.D.; Jodl, H.J.; Loewenschuss, A. The Raman spectrum of amorphous and crystalline solid carbon disulphide. Chem. Phys. Lett. 1985, 115, 34–39. [Google Scholar] [CrossRef]
- Mitchell, P.C.H.; Parker, S.F.; Ramirez-Cuesta, A.J.; Tomkinson, J. Vibrational Spectroscopy with Neutrons, with Applications in Chemistry, Biology, Materials Science and Catalysis; World Scientific: Singapore, Singapore, 2005. [Google Scholar]
- Baenziger, N.C.; Duax, W.L. Crystal structure and molecular motion of solid carbon disulfide. J. Chem. Phys. 1968, 48, 2974–2981. [Google Scholar] [CrossRef]
- Powell, B.M.; Dolling, G.; Torrie, B.H. Structure of solid carbon disulphide between 5 and 150 K. Acta Cryst. 1982, B38, 28–32. [Google Scholar] [CrossRef]
- Grout, P.J.; Leech, J.W. Intermolecular modes of solid carbon disulphide. J. Phys. C: Solid State Phys. 1982, 15, L1083–Ll087. [Google Scholar] [CrossRef]
- Higgs, J.F.; Anderson, A. Dynamical model for the lattice frequencies and crystal field splittings of carbon disulphide. Phys. Stat. Sol. (b) 1986, 137, 39–46. [Google Scholar] [CrossRef]
- Ramirez-Cuesta, A.J. aCLIMAX 4.0.1, The new version of the software for analyzing and interpreting INS spectra. Comput. Phys. Commun. 2004, 157, 226–238. [Google Scholar] [CrossRef]
- Parker, S.F.; Bennington, S.M.; Taylor, J.W.; Herman, H.; Silverwood, I.; Albers, P.; Refson, K. Complete assignment of the vibrational modes of C60 by inelastic neutron scattering spectroscopy and periodic-DFT. Phys. Chem. Chem. Phys. 2011, 13, 7789–7804. [Google Scholar] [CrossRef]
- Parker, S.F.; Jayasooriya, U.A. Assignment of the solid state spectra of the Group VI hexacarbonyls by inelastic neutron scattering spectroscopy. Phys. Chem. Chem. Phys. 2019, 21, 24950–24955. [Google Scholar] [CrossRef]
- Pinna, R.S.; Rudić, S.; Parker, S.F.; Armstrong, J.; Zanetti, M.; Škoro, G.; Waller, S.P.; Zacek, D.; Smith, C.A.; Capstick, M.J.; et al. The neutron guide upgrade of the TOSCA spectrometer. Nucl. Instrum. Methods Phys. Res. A 2018, 896, 68–74. [Google Scholar] [CrossRef]
- ISIS Neutron and Muon Source. Available online: http://www.isis.stfc.ac.uk/ (accessed on 24 March 2020).
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.J.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Krist. 2005, 220, 567–570. [Google Scholar] [CrossRef]
- Refson, K.; Tulip, P.R.; Clark, S.J. Variational density-functional perturbation theory for dielectrics and lattice dynamics. Phys. Rev. B 2006, 73, 155114. [Google Scholar] [CrossRef]
- Perdew, J.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Tkatchenko, A.; Scheffler, M. Accurate molecular van der Waals interactions from ground-state electron density and free-atom reference data. Phys. Rev. Lett. 2009, 102, 073005. [Google Scholar] [CrossRef] [PubMed]
- Milman, V.; Perlov, A.; Refson, K.; Clark, S.J.; Gavartin, J.; Winkler, B. Structural, electronic and vibrational properties of tetragonal zirconia under pressure: A density functional theory study. J. Phys.: Condens. Matter 2009, 21, 485404. [Google Scholar] [CrossRef] [PubMed]
- Gonze, X.; Charlier, J.-C.; Teter, M.P. Interatomic force constants from first principles: The case of α–quartz. Phys. Rev. B 1994, 50, 13035–13038. [Google Scholar] [CrossRef]
Sample Availability: Carbon disulfide is widely available from commercial sources. |
| DFT | INS 1 | Infrared | Raman | Description | |||
|---|---|---|---|---|---|---|---|
| / cm−1 | Symmetry | Infrared / Debye2 Å−2 amu−1 | Raman / Å4 amu−1 | / cm−1 | / cm−1 | / cm−1 | |
| 0.0 | B2u | 0.000 | 0.0 | Acoustic | |||
| 0.0 | B1u | 0.000 | 0.0 | Acoustic | |||
| 0.0 | B3u | 0.000 | 0.0 | Acoustic | |||
| 52.0 | Au | 0.000 | 0.0 | 51 | Translation | ||
| 69.7 | B1u | 0.007 | 0.0 | 66.5 [9] | Translation | ||
| 78.1 | B2u | 0.007 | 0.0 | 68.2 [9] | Translation | ||
| 81.9 | Ag | 0.000 | 231.0 | 75 [7] | Libration | ||
| 85.3 | B1g | 0.000 | 21.9 | 79 [7] | Libration | ||
| 85.6 | B3g | 0.000 | 23.2 | 79 [7] | Libration | ||
| 100.9 | B2g | 0.000 | 109.8 | 85 [7] | Libration | ||
| 378.3 | B3u | 0.202 | 0.0 | 388.7 [8] | ν2 bend | ||
| 384.0 | B2u | 0.197 | 0.0 | 393.4 [8] | ν2 bend | ||
| 384.5 | Au | 0.000 | 0.0 | ν2 bend | |||
| 392.3 | B1u | 0.225 | 0.0 | 390 vs | 400.1 [8] | ν2 bend | |
| 653.1 | Ag | 0.000 | 913.1 | 651 w | 655 s | ν1 symmetric stretch | |
| 653.9 | B3g | 0.000 | 68.2 | 646 m | ν1 symmetric stretch | ||
| 1494.6 | B1u | 42.570 | 0.0 | 1507 w | 1479 vs | ν3 asymmetric stretch | |
| 1536.3 | B2u | 10.132 | 0.0 | 1540 w | 1530 sh | ν3 asymmetric stretch |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adilina, I.B.; Aulia, F.; Fitriady, M.A.; Oemry, F.; Widjaya, R.R.; Parker, S.F. Computational and Spectroscopic Studies of Carbon Disulfide. Molecules 2020, 25, 1901. https://doi.org/10.3390/molecules25081901
Adilina IB, Aulia F, Fitriady MA, Oemry F, Widjaya RR, Parker SF. Computational and Spectroscopic Studies of Carbon Disulfide. Molecules. 2020; 25(8):1901. https://doi.org/10.3390/molecules25081901
Chicago/Turabian StyleAdilina, Indri B., Fauzan Aulia, Muhammad A. Fitriady, Ferensa Oemry, Robert R. Widjaya, and Stewart F. Parker. 2020. "Computational and Spectroscopic Studies of Carbon Disulfide" Molecules 25, no. 8: 1901. https://doi.org/10.3390/molecules25081901
APA StyleAdilina, I. B., Aulia, F., Fitriady, M. A., Oemry, F., Widjaya, R. R., & Parker, S. F. (2020). Computational and Spectroscopic Studies of Carbon Disulfide. Molecules, 25(8), 1901. https://doi.org/10.3390/molecules25081901






