Tuning of the Amount of Se in Rice (Oryza sativa) Grain by Varying the Nature of the Irrigation Method: Development of an ICP-MS Analytical Protocol, Validation and Application to 26 Different Rice Genotypes
Abstract
1. Introduction
2. Results
2.1. Methods Assessment
2.2. Validation
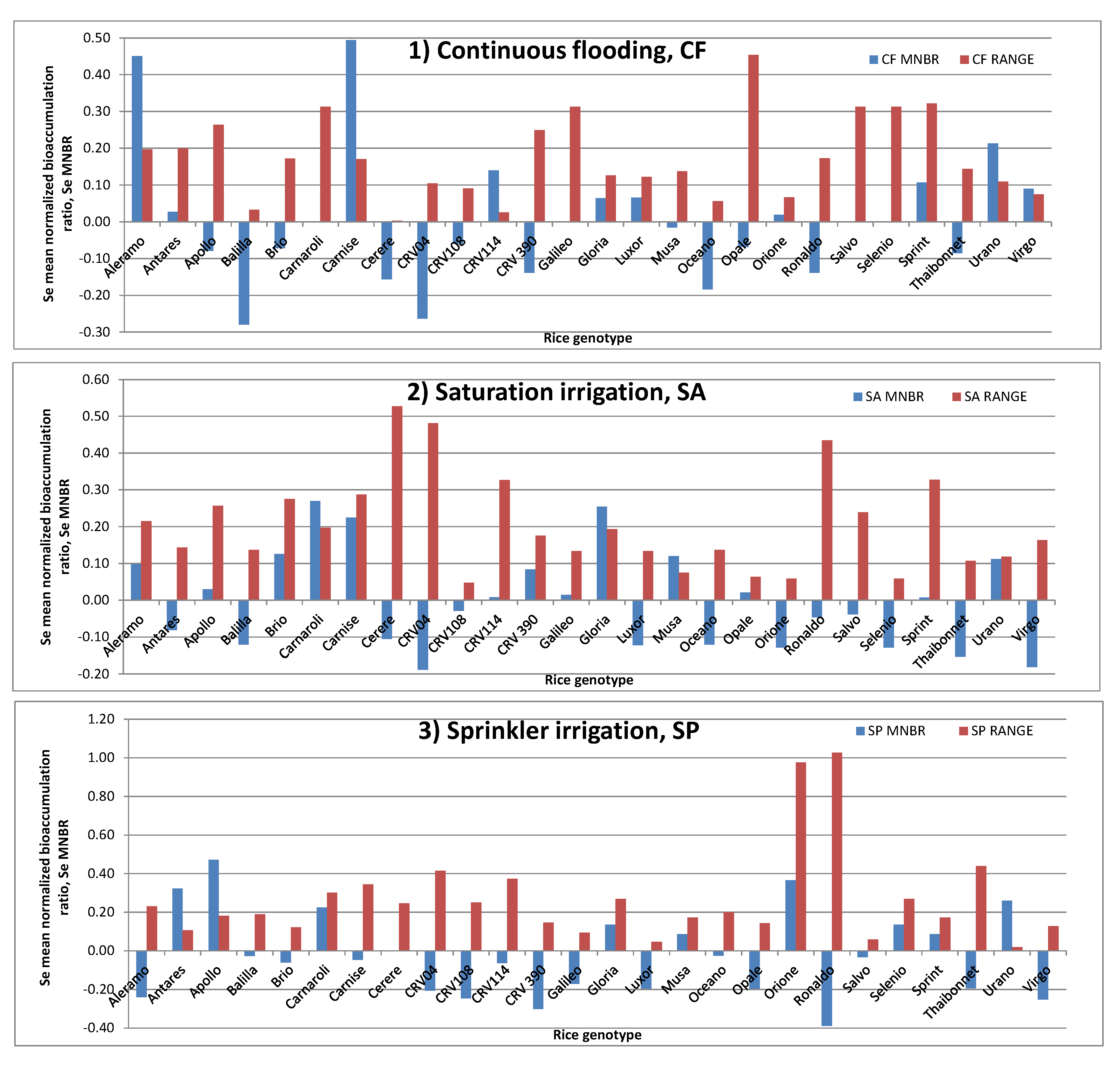
2.3. Se in Rice Grain
2.4. Se in Soils and Irrigation Waters
3. Discussion
4. Materials and Methods
4.1. Site, Soils, Irrigation Water and Rice Genotypes
4.2. Description of Irrigation Methods
4.2.1. Continuous Flooding Irrigation, CF
4.2.2. Sprinkler Irrigation, SP
4.2.3. Saturation Irrigation, SA
4.3. Experimental Design
4.4. Crop Management
4.5. Harvesting and Sampling of Rice
4.6. Instrumentation
4.7. Reagents
4.8. Analytical Methods
4.8.1. Disgregation of Rice Samples
4.8.2. Disgregation of Soils
4.8.3. Pre-Treatment of Irrigation Water
4.8.4. ICP-MS Determination of Se in Disgregated Matrices and Irrigation Water
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sunde, R.A. Selenium. In Handbook of Nutritionally Essential Mineral Elements, 1st ed.; O’Dell, B.D., Sunde, R.A., Eds.; CRC Press: Boca Raton, FL, USA, 1997; pp. 493–556. ISBN 9780824793128. [Google Scholar]
- U.S. Department of Health & Human Services. National Institutes of Health, Office of Dietary Supplements. Health Information. Selenium. Available online: https://ods.od.nih.gov/factsheets/selenium-HealthProfessional (accessed on 1 March 2020).
- Ullah, H.; Liu, G.; Yousaf, B.; Ali, M.U.; Abbas, Q.; Munir, M.A.M.; Mian, M.M. Developmental Selenium Exposure and Health Risk in Daily Foodstuffs: A Systematic Review and Meta-Analysis. Ecotoxicol. Environ. Saf. 2018, 149, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Mazokopakis, E.E.; Papadakis, J.A.; Papadomanolaki, M.G.; Batistakis, A.G.; Giannakopoulos, T.G.; Protopapadakis, E.E.; Ganotakis, E.S. Effects of 12 Months Treatment with L-Selenomethionine on Serum Anti-TPO Levels in Patients with Hashimoto’s Thyroiditis. Thyroid 2007, 17, 609–612. [Google Scholar] [CrossRef] [PubMed]
- Penglase, S.; Hamre, K.; Ellingsen, S. Selenium Prevents Downregulation of Antioxidant Selenoprotein Genes by Methylmercury. Free Radic. Biol. Med. 2014, 75, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Reilly, C. Selenium in Food and Health, 2nd ed.; Springer: New York, NY, USA, 2006; ISBN 978-0-387-33244-4. [Google Scholar]
- Foster, L.H.; Sumar, S. Selenium in Health and Disease: A Review. Crit. Rev. Food Sci. Nutr. 1997, 37, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Ellis, D.R.; Salt, D.E. Plants, Selenium and Human Health. Curr. Opin. Plant Biol. 2003, 6, 273–279. [Google Scholar] [CrossRef]
- Sunde, R.A. Selenium. In Present Knowledge in Nutrition. Volume 1, 9th ed.; Bowman, B., Russell, R., Eds.; International Life Sciences Institute: Washington, DC, USA, 2006; pp. 480–497. ISBN 9781578811984. [Google Scholar]
- National Research Council. Selenium in Nutrition, Revised Ed.; National Academy Press: Washington, DC, USA, 1983; ISBN 0309567793. [Google Scholar]
- World Health Organization; Food and Agricultural Organization of the United Nations. Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; Report of a Joint FAO—WHO Expert Consultation, Bangkok, Thailand, 21–30 September 1998; WHO: Geneva, Switzerland, 2004; pp. 194–216. ISBN 9241546123. Available online: http://apps.who.int/iris/bitstream/handle/10665/42716/9241546123.pdf?ua=1 (accessed on 1 March 2020).
- McLaughlin, M.; Parker, D.; Clarke, J. Metals and Micronutrients—Food Safety Issues. Field Crops Res. 1999, 60, 143–163. [Google Scholar] [CrossRef]
- Barclay, M.N.I.; MacPherson, A.; Dixon, J. Selenium Content of a Range of UK Foods. J. Food Compos. Anal. 1995, 8, 307–318. [Google Scholar] [CrossRef]
- Fordyce, F. Selenium Geochemistry and Health. AMBIO J. Hum. Environ. 2007, 36, 94–97. [Google Scholar] [CrossRef]
- Mayland, H.F.; James, L.F.; Panter, K.E.; Sonderegger, J.L. Selenium in Agriculture and the Environment; Jacobs, L.W., Ed.; SSSA: Madison, WI, USA, 1989; pp. 15–50. [Google Scholar] [CrossRef]
- Läuchli, A. Selenium in Plants: Uptake, Functions, and Environmental Toxicity. Bot. Acta 1993, 106, 455–468. [Google Scholar] [CrossRef]
- Klapec, T.; Mandić, M.L.; Grgić, J.; Primorac, L.; Perl, A.; Krstanović, V. Selenium in Selected Foods Grown or Purchased in Eastern Croatia. Food Chem. 2004, 85, 445–452. [Google Scholar] [CrossRef]
- Lemire, M.; Fillion, M.; Barbosa, F., Jr.; Guimarães, J.R.D.; Mergler, D. Elevated levels of selenium in the typical diet of Amazonian riverside populations. Sci. Total Environ. 2010, 408, 4076–4084. [Google Scholar] [CrossRef] [PubMed]
- Fageria, N.K. Yield Physiology of Rice. J. Plant Nutr. 2007, 30, 843–879. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C. Methodology for Evaluation of Lowland Rice Genotypes for Nitrogen Use Efficiency. J. Plant Nutr. 2003, 26, 1315–1333. [Google Scholar] [CrossRef]
- UN Food and Agriculture Organization, Corporate Statistical Database (FAOSTAT). Crops/Regions/World List/Production Quantity (Pick Lists), Rice (Paddy). 2017. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 1 March 2020).
- Marin, A.R.; Masscheleyn, P.H.; Patrick, W.H., Jr. Soil redox-pH stability of arsenic species and its influence on arsenic uptake by rice. Plant Soil 1993, 152, 245–253. [Google Scholar] [CrossRef]
- Meharg, A.A.; Rahman, M. Arsenic contamination of Bangladesh paddy field soils: Implications for rice contribution to arsenic consumption. Environ. Sci. Technol. 2003, 37, 229–234. [Google Scholar] [CrossRef]
- Meharg, A.A.; Williams, P.N.; Adomako, E.; Lawgali, Y.Y.; Deacon, J.C.; Villada, A.; Robert, C.J.; Cambell, R.C.J.; Sun, G.; Zhu, Y.-G.; et al. Geographical variation in total and inorganic arsenic content of polished (white) rice. Environ. Sci. Technol. 2009, 43, 1612–1617. [Google Scholar] [CrossRef]
- Kobayashi, J. Pollution by cadmium and the itai-itai disease in Japan. In Toxicity of Heavy Metals in the Environment; Oeheme, F.W., Ed.; Marcel Dekker: New York, NY, USA, 1978; pp. 199–260. ISBN 0824767187. [Google Scholar]
- Kawada, T.; Suzuki, S. A review on the cadmium content of rice, daily cadmium intake, and accumulation in the kidneys. J. Occup. Health 1998, 40, 264–269. [Google Scholar] [CrossRef]
- Yu, H.; Wang, J.; Fang, W.; Yuan, J.; Yang, Z. Cadmium Accumulation in Different Rice Cultivars and Screening for Pollution-Safe Cultivars of Rice. Sci. Total Environ. 2006, 370, 302–309. [Google Scholar] [CrossRef]
- Horvat, M.; Nolde, N.; Fajon, V.; Jereb, V.; Logar, M.; Lojen, S.; Jacimovic, R.; Falnoga, I.; Liya, Q.; Faganeli, J.; et al. Total Mercury, Methylmercury and Selenium in Mercury Polluted Areas in the Province Guizhou, China. Sci. Total Environ. 2003, 304, 231–256. [Google Scholar] [CrossRef]
- Feng, X.; Li, P.; Qiu, G.; Wang, S.; Li, G.; Shang, L.; Meng, B.; Jiang, H.; Bai, W.; Li, Z.; et al. Human Exposure To Methylmercury through Rice Intake in Mercury Mining Areas, Guizhou Province, China. Environ. Sci. Technol. 2008, 42, 326–332. [Google Scholar] [CrossRef]
- Meng, B.; Feng, X.; Qiu, G.; Liang, P.; Li, P.; Chen, C.; Shang, L. The Process of Methylmercury Accumulation in Rice (Oryza sativa L.). Environ. Sci. Technol. 2011, 45, 2711–2717. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.N.; Lombi, E.; Sun, G.-X.; Scheckel, K.; Zhu, Y.-G.; Feng, X.; Zhu, J.; Carey, A.-M.; Adomako, E.; Lawgali, Y.; et al. Selenium Characterization in the Global Rice Supply Chain. Environ. Sci. Technol. 2009, 43, 6024–6030. [Google Scholar] [CrossRef] [PubMed]
- Gbadebo, A.M.; Babalola, O.O.; Ajigbotesho, F.L. Selenium Concentration in Food and Blood of Residents of Abeokuta Metropolis, Southwestern Nigeria. J. Geochem. Explor. 2010, 107, 175–179. [Google Scholar] [CrossRef]
- Waegeneers, N.; Thiry, C.; De Temmerman, L.; Ruttens, A. Predicted Dietary Intake of Selenium by the General Adult Population in Belgium. Food Addit. Contam. Part A 2013, 30, 278–285. [Google Scholar] [CrossRef]
- Giacosa, A.; Faliva, M.; Perna, S.; Minoia, C.; Ronchi, A.; Rondanelli, M. Selenium Fortification of an Italian Rice Cultivar via Foliar Fertilization with Sodium Selenate and Its Effects on Human Serum Selenium Levels and on Erythrocyte Glutathione Peroxidase Activity. Nutrients 2014, 6, 1251–1261. [Google Scholar] [CrossRef]
- Huang, G.; Ding, C.; Yu, X.; Yang, Z.; Zhang, T.; Wang, X. Characteristics of Time-Dependent Selenium Biofortification of Rice (Oryza Sativa L.). J. Agric. Food Chem. 2018, 66, 12490–12497. [Google Scholar] [CrossRef]
- Lockyer, S.; White, A.; Buttriss, J.L. Biofortified Crops for Tackling Micronutrient Deficiencies—What Impact Are These Having in Developing Countries and Could They Be of Relevance within Europe? Nutr. Bull. 2018, 43, 319–357. [Google Scholar] [CrossRef]
- Zhang, M.; Xing, G.; Tang, S.; Pang, Y.; Yi, Q.; Huang, Q.; Huang, X.; Huang, J.; Li, P.; Fu, H. Improving Soil Selenium Availability as a Strategy to Promote Selenium Uptake by High-Se Rice Cultivar. Environ. Exp. Bot. 2019, 163, 45–54. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, W.; Wang, X.; Zhou, X. Genotypic Differences in Selenium Accumulation in Rice Seedlings at Early Growth Stage and Analysis of Dominant Factors Influencing Selenium Content in Rice Seeds. J. Plant Nutr. 2006, 29, 1601–1618. [Google Scholar] [CrossRef]
- Dhillon, S.K.; Dhillon, K.S. Phytoremediation of Selenium-Contaminated Soils: The Efficiency of Different Cropping Systems. Soil Use Manag. 2009, 25, 441–453. [Google Scholar] [CrossRef]
- Duxbury, J.M.; Panaullah, G. Remediation of Arsenic for Agriculture Sustainability, Food Security and Health in Bangladesh; FAO Water Working Paper; FAO: Rome, Italy, 2007; Available online: www.fao.org/nr/water/docs/FAOWATER_ARSENIC.pdf (accessed on 1 March 2020).
- Xu, X.Y.; McGrath, S.P.; Meharg, A.A.; Zhao, F.J. Growing Rice Aerobically Markedly Decreases Arsenic Accumulation. Environ. Sci. Technol. 2008, 42, 5574–5579. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Basu, B.; Kundu, C.K.; Patra, P.K. Deficit Irrigation: An Option to Mitigate Arsenic Load of Rice Grain in West Bengal, India. Agric. Ecosyst. Environ. 2012, 146, 147–152. [Google Scholar] [CrossRef]
- Spanu, A.; Daga, L.; Orlandoni, A.M.; Sanna, G. The Role of Irrigation Techniques in Arsenic Bioaccumulation in Rice (Oryza sativa L.). Environ. Sci. Technol. 2012, 46, 8333–8340. [Google Scholar] [CrossRef] [PubMed]
- Arao, T.; Kawasaki, A.; Baba, K.; Mori, S.; Matsumoto, S. Effects of Water Management on Cadmium and Arsenic Accumulation and Dimethylarsinic Acid Concentrations in Japanese Rice. Environ. Sci. Technol. 2009, 43, 9361–9367. [Google Scholar] [CrossRef] [PubMed]
- Rodda, M.S.; Reid, R.J. Examination of the Role of Iron Deficiency Response in the Accumulation of Cd by Rice Grown in Paddy Soil with Variable Irrigation Regimes. Plant Soil 2013, 371, 219–236. [Google Scholar] [CrossRef]
- Yang, J.; Huang, D.; Duan, H.; Tan, G.; Zhang, J. Alternate Wetting and Moderate Soil Drying Increases Grain Yield and Reduces Cadmium Accumulation in Rice Grains. J. Sci. Food Agric. 2009, 89, 1728–1736. [Google Scholar] [CrossRef]
- Spanu, A.; Valente, M.; Langasco, I.; Barracu, F.; Orlandoni, A.M.; Sanna, G. Sprinkler Irrigation Is Effective in Reducing Cadmium Concentration in Rice (Oryza Sativa L.) Grain: A New Twist on an Old Tale? Sci. Total Environ. 2018, 628–629, 1567–1581. [Google Scholar] [CrossRef]
- Li, H.-F.; Lombi, E.; Stroud, J.L.; McGrath, S.P.; Zhao, F.-J. Selenium Speciation in Soil and Rice: Influence of Water Management and Se Fertilization. J. Agric. Food Chem. 2010, 58, 11837–11843. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Y.; Lai, F. Effects of Different Water Management on Absorption and Accumulation of Selenium in Rice. Saudi J. Biol. Sci. 2018, 25, 1178–1182. [Google Scholar] [CrossRef]
- Locatelli, C. Heavy Metals in Matrices of Food Interest: Sequential Voltammetric Determination at Trace and Ultratrace Level of Copper, Lead, Cadmium, Zinc, Arsenic, Selenium, Manganese and Iron in Meals. Electroanalysis 2004, 16, 1478–1486. [Google Scholar] [CrossRef]
- Panigati, M.; Falciola, L.; Mussini, P.; Beretta, G.; Facino, R. Determination of Selenium in Italian Rices by Differential Pulse Cathodic Stripping Voltammetry. Food Chem. 2007, 105, 1091–1098. [Google Scholar] [CrossRef]
- Krawczyk-Coda, M. Determination of Selenium in Food Samples by High-Resolution Continuum Source Atomic Absorption Spectrometry After Preconcentration on Halloysite Nanotubes Using Ultrasound-Assisted Dispersive Micro Solid-Phase Extraction. Food Anal. Methods 2018, 12, 128–135. [Google Scholar] [CrossRef]
- Sadiq, N.; Beauchemin, D. Optimization of the Operating Conditions of Solid Sampling Electrothermal Vaporization Coupled to Inductively Coupled Plasma Optical Emission Spectrometry for the Sensitive Direct Analysis of Powdered Rice. Anal. Chim. Acta 2014, 851, 23–29. [Google Scholar] [CrossRef]
- Moon, J.H.; Kim, S.H.; Kim, K.S.; Lee, O. INAA for the Evaluation of Selenium Contents in Grain Foods Consumed by Korean. J. Radioanal. Nucl. Chem. 2016, 309, 337–341. [Google Scholar] [CrossRef]
- Ahmed, M.K.; Shaheen, N.; Islam, M.S.; Habibullah-Al-Mamun, M.; Islam, S.; Banu, C.P. Trace Elements in Two Staple Cereals (Rice and Wheat) and Associated Health Risk Implications in Bangladesh. Environ. Monit. Assess. 2015, 187, 326. [Google Scholar] [CrossRef]
- Jorhem, L.; Åstrand, C.; Sundström, B.; Baxter, M.; Stokes, P.; Lewis, J.; Grawé, K.P. Elements in Rice on the Swedish Market: Part 2. Chromium, Copper, Iron, Manganese, Platinum, Rubidium, Selenium and Zinc. Food Addit. Contam. Part A 2008, 25, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Joy, E.J.M.; Louise Ander, E.; Broadley, M.R.; Young, S.D.; Chilimba, A.D.C.; Hamilton, E.M.; Watts, M.J. Elemental Composition of Malawian Rice. Environ. Geochem. Health 2016, 39, 835–845. [Google Scholar] [CrossRef]
- Londonio, A.; Morzán, E.; Smichowski, P. Determination of Toxic and Potentially Toxic Elements in Rice and Rice-Based Products by Inductively Coupled Plasma-Mass Spectrometry. Food Chem. 2019, 284, 149–154. [Google Scholar] [CrossRef]
- Song, T.; Su, X.; He, J.; Liang, Y.; Zhou, T.; Liu, C. Selenium (Se) Uptake and Dynamic Changes of Se Content in Soil–plant Systems. Environ. Sci. Pollut. Res. 2018, 25, 34343–34350. [Google Scholar] [CrossRef]
- Sun, G.-X.; Liu, X.; Williams, P.N.; Zhu, Y.-G. Distribution and Translocation of Selenium from Soil to Grain and Its Speciation in Paddy Rice (Oryza sativa L.). Environ. Sci. Technol. 2010, 44, 6706–6711. [Google Scholar] [CrossRef]
- Al-Rmalli, S.W.; Jenkins, R.O.; Haris, P.V. Intake of Arsenic and Selenium in a Bangladeshi Population Investigated Using Inductively Coupled Plasma Mass Spectrometry. Biomed. Spectrosc. Imaging 2017, 5, 373–391. [Google Scholar] [CrossRef]
- Rodrigo, S.; Santamaria, O.; Perez-Izquierdo, L.; Poblaciones, M.J. Arsenic and Selenium Levels in Rice Fields from South-West of Spain: Influence of the Years of Monoculture. Plant Soil Environ. 2017, 63, 184–188. [Google Scholar] [CrossRef]
- Huang, Q.; Xu, Y.; Liu, Y.; Qin, X.; Huang, R.; Liang, X. Selenium Application Alters Soil Cadmium Bioavailability and Reduces Its Accumulation in Rice Grown in Cd-Contaminated Soil. Environ. Sci. Pollut. Res. 2018, 25, 31175–31182. [Google Scholar] [CrossRef] [PubMed]
- Currie, L.A. Nomenclature in Evaluation of Analytical Methods Including Detection and Quantification capabilities. Anal. Chim. Acta 1999, 391, 105–126. [Google Scholar] [CrossRef]
- Horwitz, W. Evaluation of Analytical Methods Used for Regulation of Foods and Drugs. Anal. Chem. 1982, 54, 67–76. [Google Scholar] [CrossRef]
- David, E.; Eleazu, C.; Igweibor, N.; Ugwu, C.; Enwefa, G.; Nwigboji, N. Comparative Study on the Nutrients, Heavy Metals and Pesticide Composition of Some Locally Produced and Marketed Rice Varieties in Nigeria. Food Chem. 2019, 278, 617–624. [Google Scholar] [CrossRef]
- Runge, J.; Heringer, O.A.; Ribeiro, J.S.; Biazati, L.B. Multi-Element Rice Grains Analysis by ICP OES and Classification by Processing Types. Food Chem. 2019, 271, 419–424. [Google Scholar] [CrossRef]
- Rybicka, I.; Krawczyk, M.; Stanisz, E.; Gliszczyńska-Świgło, A. Selenium in Gluten-Free Products. Plant Foods Hum. Nutr. 2015, 70, 128–134. [Google Scholar] [CrossRef]
- Jo, G.; Todorov, T.I. Distribution of Nutrient and Toxic Elements in Brown and Polished Rice. Food Chem. 2019, 289, 299–307. [Google Scholar] [CrossRef]
- Gazzetta Ufficiale Serie Generale n. 248 del 21-10-1999. Suppl. Ordinario n. 185. Metodi Ufficiali di Analisi Chimica del Suolo. Available online: http://www.gazzettaufficiale.it/eli/id/1992/05/25/092A2322/sg (accessed on 1 March 2020). (In Italian).
- Pansu, M.; Gautheyrou, J. Handbook of Soil Analysis. Mineralogical, Organic and Inorganic Methods; Springer: Berlin, Germany, 2006; pp. 581–591. ISBN 9783540312116. [Google Scholar]
- Spanu, A.; Pruneddu, G.; Deidda, M. Primi risultati sulla coltivazione del riso (Oryza sativa L.) irrigato per aspersione. Riv. Agron. 1989, 23, 378–384. Available online: http://eprints.uniss.it/4634 (accessed on 1 March 2020).
- Spanu, A.; Murtas, A.; Ballone, F. Water use and crop coefficients in sprinkler irrigated rice. Ital. J. Agron. 2009, 2, 47–58. [Google Scholar] [CrossRef]
- Spanu, A.; Murtas, A.; Ledda, L.; Ballone, F. Innovative Agronomic Techniques for Rice Cultivation. In Proceedings of the Challenger and Opportunities for Sustainable Rice-Based Production Systems, Torino, Italy, 13–15 September 2004; pp. 207–216. Available online: https://www.researchgate.net/profile/Francesco_Vidotto/publication/264874823_CHALLENGES_AND_OPPORTUNITIES_FOR_SUSTAINABLE_RICE-BASED_PRODUCTION_SYSTEMS/links/540d88a70cf2d8daaacb2ca6/CHALLENGES-AND-OPPORTUNITIES-FOR-SUSTAINABLE-RICE-BASED-PRODUCTION-SYSTEMS.pdf (accessed on 1 March 2020).
- Spanu, A.; Murtas, A.; Ledda, L.; Ballone, F. Root System Development in Flooded and Sprinkler Irrigated Rice Cultivars. In Proceedings of the Conference Proceedings of Challenges and Opportunities for Sustainable Rice-Based Production Systems, Torino, Italy, 13–15 September 2004; pp. 217–225. Available online: https://www.researchgate.net/profile/Francesco_Vidotto/publication/264874823_CHALLENGES_AND_OPPORTUNITIES_FOR_SUSTAINABLE_RICE-BASED_PRODUCTION_SYSTEMS/links/540d88a70cf2d8daaacb2ca6/CHALLENGES-AND-OPPORTUNITIES-FOR-SUSTAINABLE-RICE-BASED-PRODUCTION-SYSTEMS.pdf (accessed on 1 March 2020).
- Weller, S.; Janz, B.; Jörg, L.; Kraus, D.; Racela, H.S.U.; Wassmann, R.; Butterbach-Bahl, K.; Kiese, R. Greenhouse Gas Emissions and Global Warming Potential of Traditional and Diversified Tropical Rice Rotation Systems. Glob. Chang. Biol. 2015, 22, 432–448. [Google Scholar] [CrossRef] [PubMed]
- Birke, M.; Reimann, C.; Demetriades, A.; Rauch, U.; Lorenz, H.; Harazim, B.; Glatte, W. Determination of Major and Trace Elements in European Bottled Mineral Water—Analytical Methods. J. Geochem. Explor. 2010, 107, 217–226. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| LoD a (μg kg−1) | LoQ (μg kg−1) | Linearity | Repeatability b | Intermediate Precisionc | Trueness | |||
|---|---|---|---|---|---|---|---|---|
| 0.73 | 2.4 | Concentration range (μg kg−1): 2.4–240 | CRM rice flour | CV | CRM rice flour | CV | CRM (Concentration ± s e, μg kg−1) | Recovery d (% ± s e) |
| Y = (a ± sa)X + (b ± sb) a = 0.0016; sa = 0.0001; b = 0.00001; sb = 0.00001; R2 = 0.9999 | NIST 1568 a | 2.4 | NIST 1568a | 8.2 | NIST 1568a (380 ± 40) | 99 ± 2 | ||
| NCSZC 73008 | 3.5 | NCSZC 73008 | 7.0 | NCSZC 73,008 (61 ± 15) | 100 ± 2 | |||
| NCSZC 11007 | 12 | NCSZC 11007 | 14 | NCSZC 11,007 (140 ± 20) f | -- | |||
| IRMM 804 | 11 | IRMM 804 | 20 | IRMM 804 (60 ± 10) f | -- | |||
| CF | SA | SP | ||||
|---|---|---|---|---|---|---|
| Rice Genotype | Year 1 | Year 2 | Year 1 | Year 2 | Year 1 | Year 2 |
| Aleramo | 110 ± 60 | 96 ± 7 | 115 ± 10 | 110 ± 20 | 7 ± 1 | 6.6 ± 0.9 |
| Antares | 88 ± 3 | 80 ± 10 | 80 ± 20 | 110 ± 30 | 11 ± 2 | 13 ± 1 |
| Apollo | 100 ± 70 | 56 ± 4 | 110 ± 20 | 100 ± 10 | 11 ± 2 | 16.0 ± 0.7 |
| Balilla | 70 ± 30 | 50 ± 20 | 90 ± 15 | 90 ± 20 | 9 ± 3 | 9 ± 2 |
| Brio | 80 ± 25 | 72 ± 9 | 120 ± 20 | 110 ± 30 | 8 ± 2 | 9 ± 1 |
| Carnaroli | 80 ± 10 | 82 ± 4 | 130 ± 20 | 130 ± 25 | 11 ± 2 | 11 ± 1 |
| Carnise | 150 ± 60 | 100 ± 20 | 130 ± 10 | 120 ± 30 | 9 ± 3 | 8 ± 1 |
| Cerere | 80 ± 20 | 60 ± 5 | 110 ± 30 | 70 ± 30 | 9 ± 4 | 9 ± 2 |
| CRV04 | 65 ± 30 | 56 ± 8 | 100 ± 30 | 63 ± 6 | 8 ± 3 | 6 ± 1.5 |
| CRV108 | 85 ± 50 | 70 ± 20 | 90 ± 10 | 110 ± 30 | 5 ± 1 | 9.0 ± 0.7 |
| CRV114 | 110 ± 40 | 80 ± 20 | 80 ± 20 | 130 ± 30 | 6 ± 4 | 11.5 ± 0.9 |
| CRV 390 | 70 ± 35 | 70 ± 20 | 95 ± 25 | 130 ± 20 | 5 ± 2 | 7.9 ± 0.8 |
| Galileo | 110 ± 20 | 60 ± 10 | 90 ± 20 | 120 ± 40 | 7 ± 3 | 8 ± 2 |
| Gloria | 95 ± 40 | 80 ± 25 | 110 ± 20 | 150 ± 50 | 8 ± 3 | 13 ± 1 |
| Luxor | 95 ± 50 | 80 ± 10 | 90 ± 20 | 90 ± 10 | 6.6 ± 0.8 | 8 ± 1 |
| Musa | 100 ± 30 | 65 ± 20 | 110 ± 20 | 120 ± 30 | 8 ± 2 | 12 ± 1.5 |
| Oceano | 75 ± 20 | 60 ± 15 | 90 ± 30 | 90 ± 20 | 7 ± 1 | 9.6 ± 0.9 |
| Opale | 110 ± 40 | 50 ± 10 | 100 ± 30 | 110 ± 3 | 7 ± 3 | 11 ± 1 |
| Orione | 100 ± 50 | 70 ± 20 | 80 ± 20 | 100 ± 30 | 7.0 ± 0.7 | 7.5 ± 0.8 |
| Ronaldo | 90 ± 40 | 55 ± 20 | 70 ± 20 | 130 ± 30 | 9 ± 2 | 19 ± 2 |
| Salvo | 110 ± 50 | 60 ± 10 | 80 ± 30 | 120 ± 60 | 8 ± 2 | 10.3 ± 0.9 |
| Selenio | 110 ± 60 | 60 ± 20 | 80 ± 20 | 100 ± 30 | 8 ± 2 | 13.0 ± 0.9 |
| Sprint | 90 ± 15 | 90 ± 20 | 80 ± 10 | 130 ± 20 | 8 ± 4 | 12 ± 1.5 |
| Thaibonnet | 80 ± 20 | 70 ± 10 | 75 ± 20 | 100 ± 20 | 8.2 ± 0.5 | 6 ± 1 |
| Urano | 110 ± 40 | 90 ± 30 | 100 ± 20 | 130 ± 40 | 10 ± 4 | 13 ± 2 |
| Virgo | 100 ± 50 | 80 ± 9 | 70 ± 20 | 100 ± 10 | 6.5 ± 2 | 7 ± 1.5 |
| Average Indica genotypes | 94 | 71 | 89 | 112 | 9 | 11 |
| Average Japonica genotypes | 95 | 71 | 97 | 110 | 8 | 10 |
| Average all genotypes | 95 | 71 | 95 | 111 | 8 | 10 |
| Parameter | Irrigation Methods | ||
|---|---|---|---|
| CF | SA | SP | |
| pH | 7.75 | 7.56 | 7.65 |
| Eh a (mV vs. SCE) | −225 | −100 b; 390 c | 130 d |
| Carbonates (% as CaCO3) | <0.01 | <0.01 | <0.01 |
| Total nitrogen (%) | 0.04 | 0.03 | 0.14 |
| Organic carbon (%) | 1.3 | 1.3 | 1.1 |
| Assimilable phosphorous (mg kg−1 as P2O5) | 111 | 112 | 64 |
| Exchangeable potassium (mg kg−1 as K2O) | 220 | 230 | 210 |
| Field capacity (%, v/v) | e | e | 34.6 |
| Permanent wilting point (%, v/v) | e | e | 20.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spanu, A.; Langasco, I.; Valente, M.; Deroma, M.A.; Spano, N.; Barracu, F.; Pilo, M.I.; Sanna, G. Tuning of the Amount of Se in Rice (Oryza sativa) Grain by Varying the Nature of the Irrigation Method: Development of an ICP-MS Analytical Protocol, Validation and Application to 26 Different Rice Genotypes. Molecules 2020, 25, 1861. https://doi.org/10.3390/molecules25081861
Spanu A, Langasco I, Valente M, Deroma MA, Spano N, Barracu F, Pilo MI, Sanna G. Tuning of the Amount of Se in Rice (Oryza sativa) Grain by Varying the Nature of the Irrigation Method: Development of an ICP-MS Analytical Protocol, Validation and Application to 26 Different Rice Genotypes. Molecules. 2020; 25(8):1861. https://doi.org/10.3390/molecules25081861
Chicago/Turabian StyleSpanu, Antonino, Ilaria Langasco, Massimiliano Valente, Mario Antonello Deroma, Nadia Spano, Francesco Barracu, Maria Itria Pilo, and Gavino Sanna. 2020. "Tuning of the Amount of Se in Rice (Oryza sativa) Grain by Varying the Nature of the Irrigation Method: Development of an ICP-MS Analytical Protocol, Validation and Application to 26 Different Rice Genotypes" Molecules 25, no. 8: 1861. https://doi.org/10.3390/molecules25081861
APA StyleSpanu, A., Langasco, I., Valente, M., Deroma, M. A., Spano, N., Barracu, F., Pilo, M. I., & Sanna, G. (2020). Tuning of the Amount of Se in Rice (Oryza sativa) Grain by Varying the Nature of the Irrigation Method: Development of an ICP-MS Analytical Protocol, Validation and Application to 26 Different Rice Genotypes. Molecules, 25(8), 1861. https://doi.org/10.3390/molecules25081861









