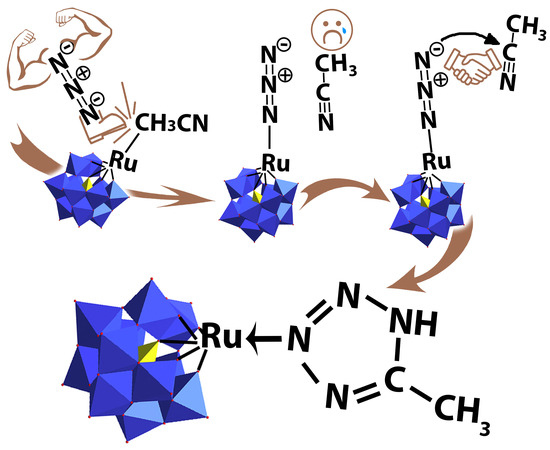
Easy Ligand Activation in the Coordination Sphere of Ru inside the [PW11O39]7– Backbone
Abstract
1. Introduction
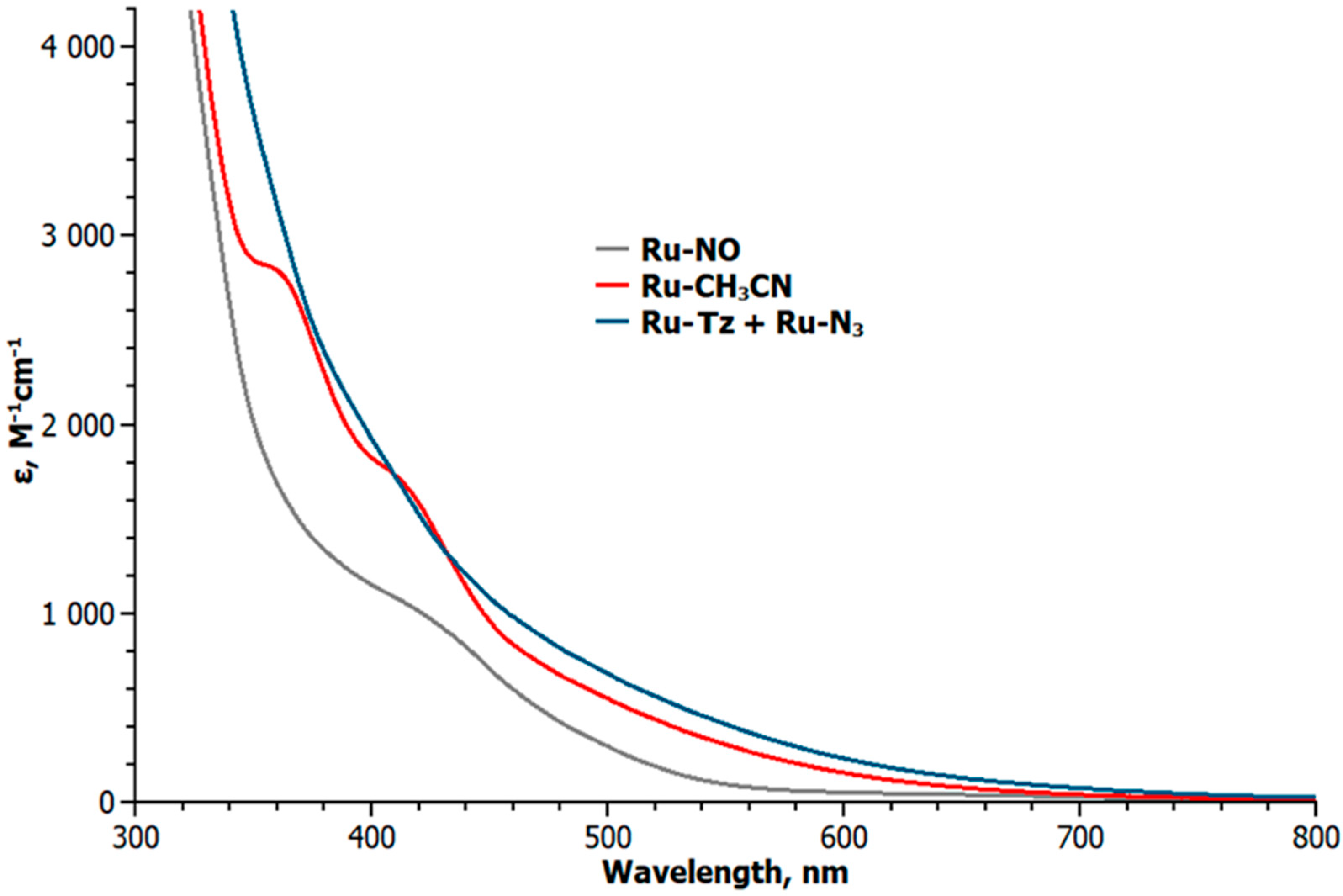
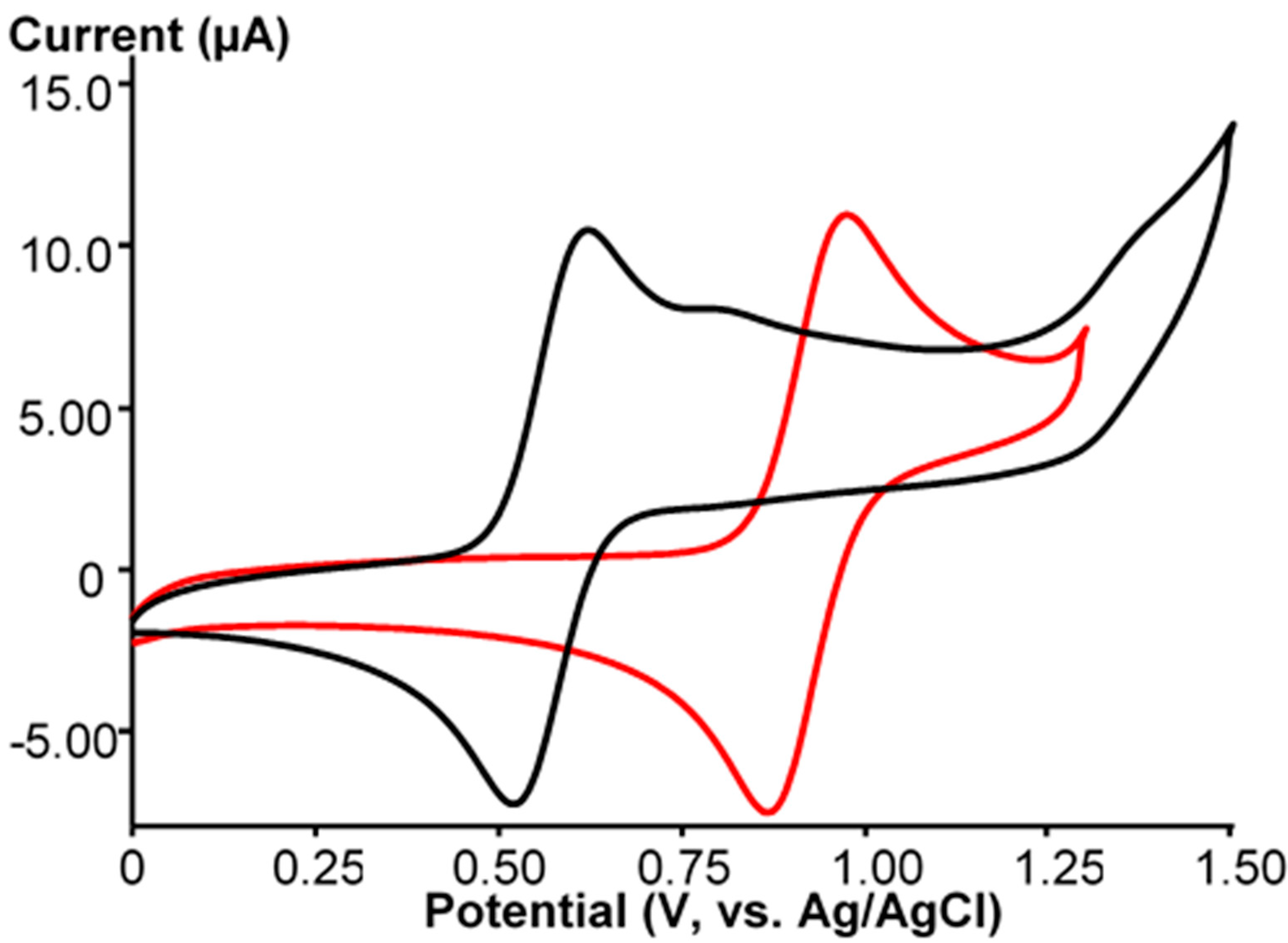
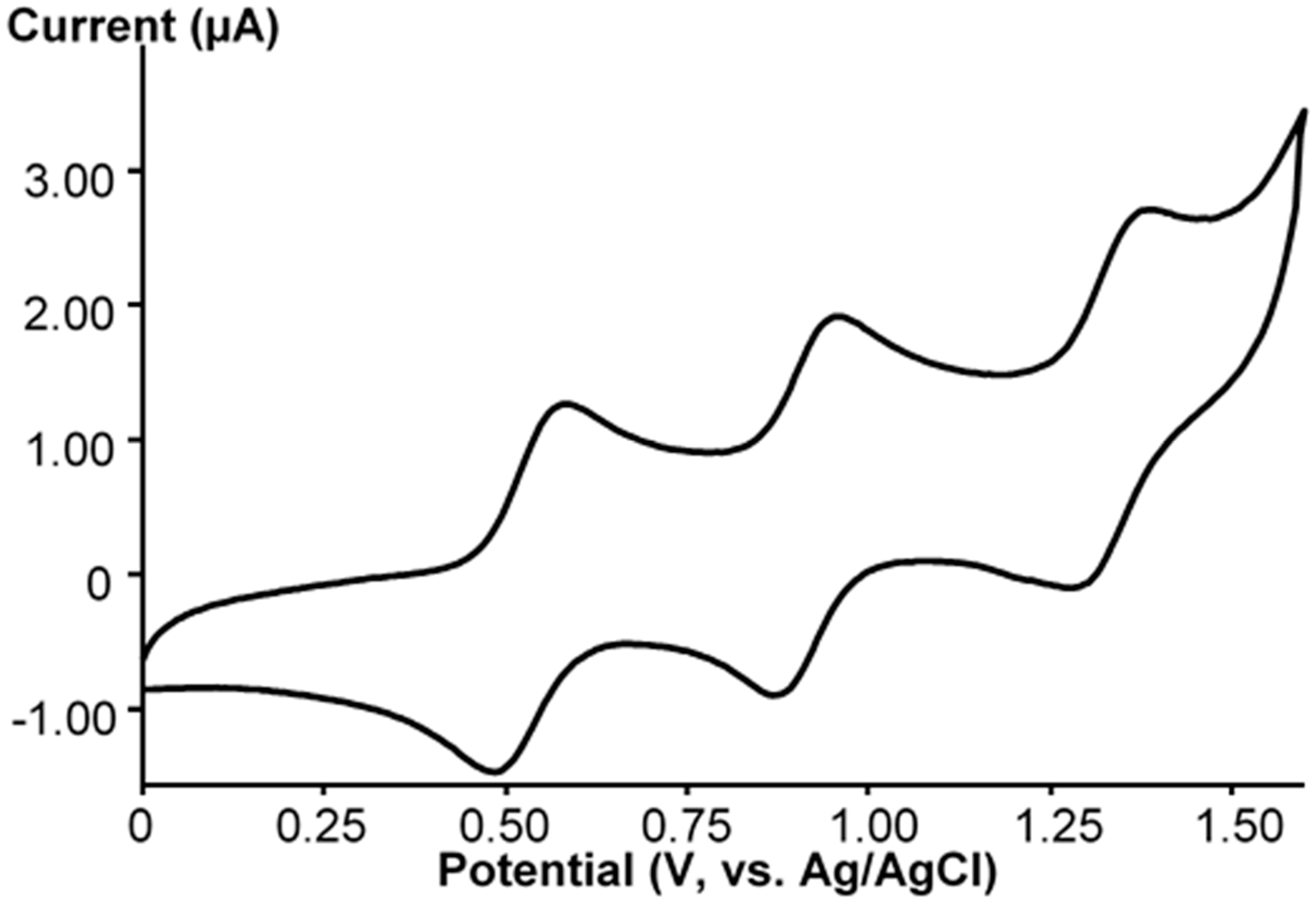
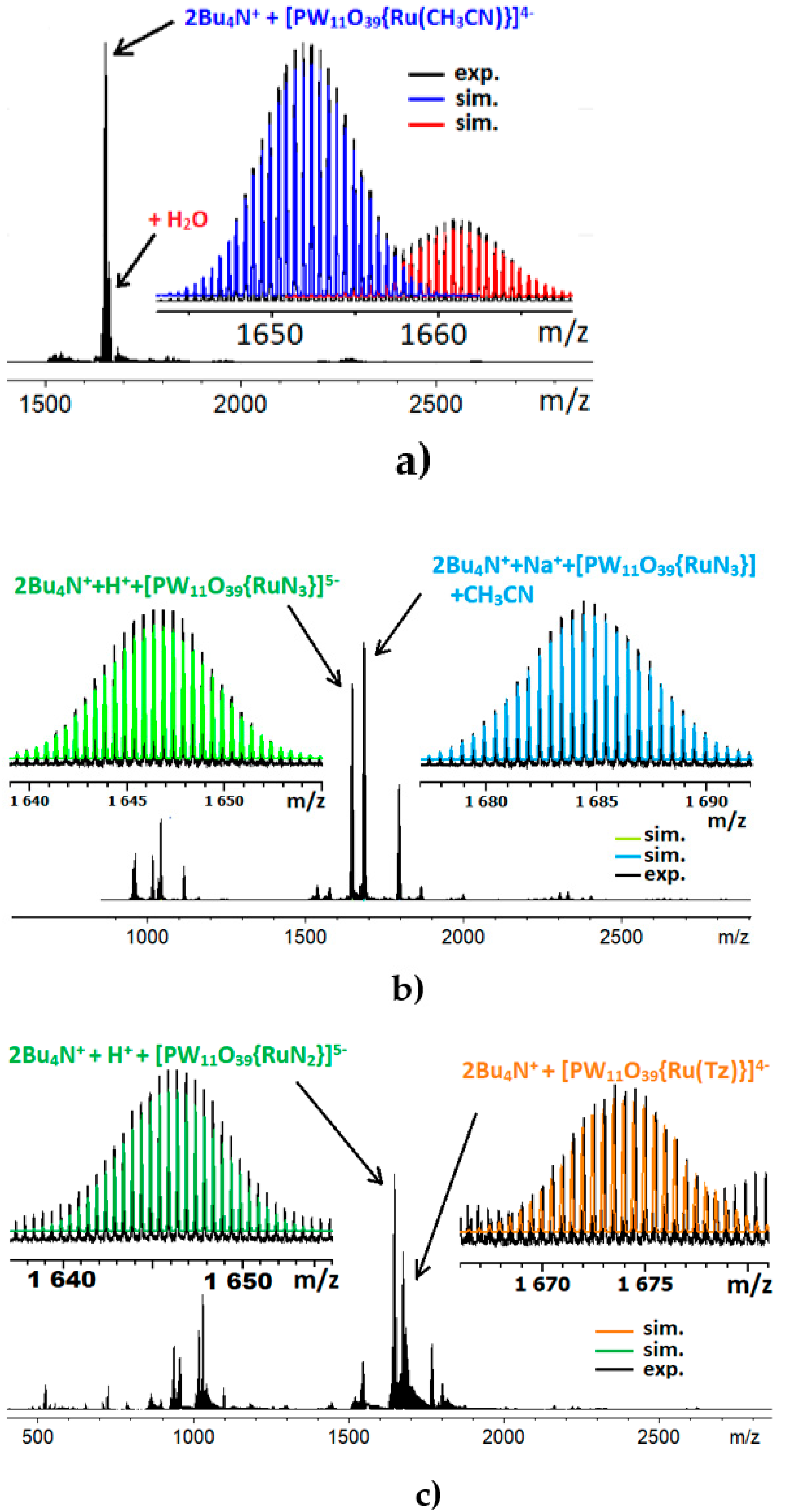
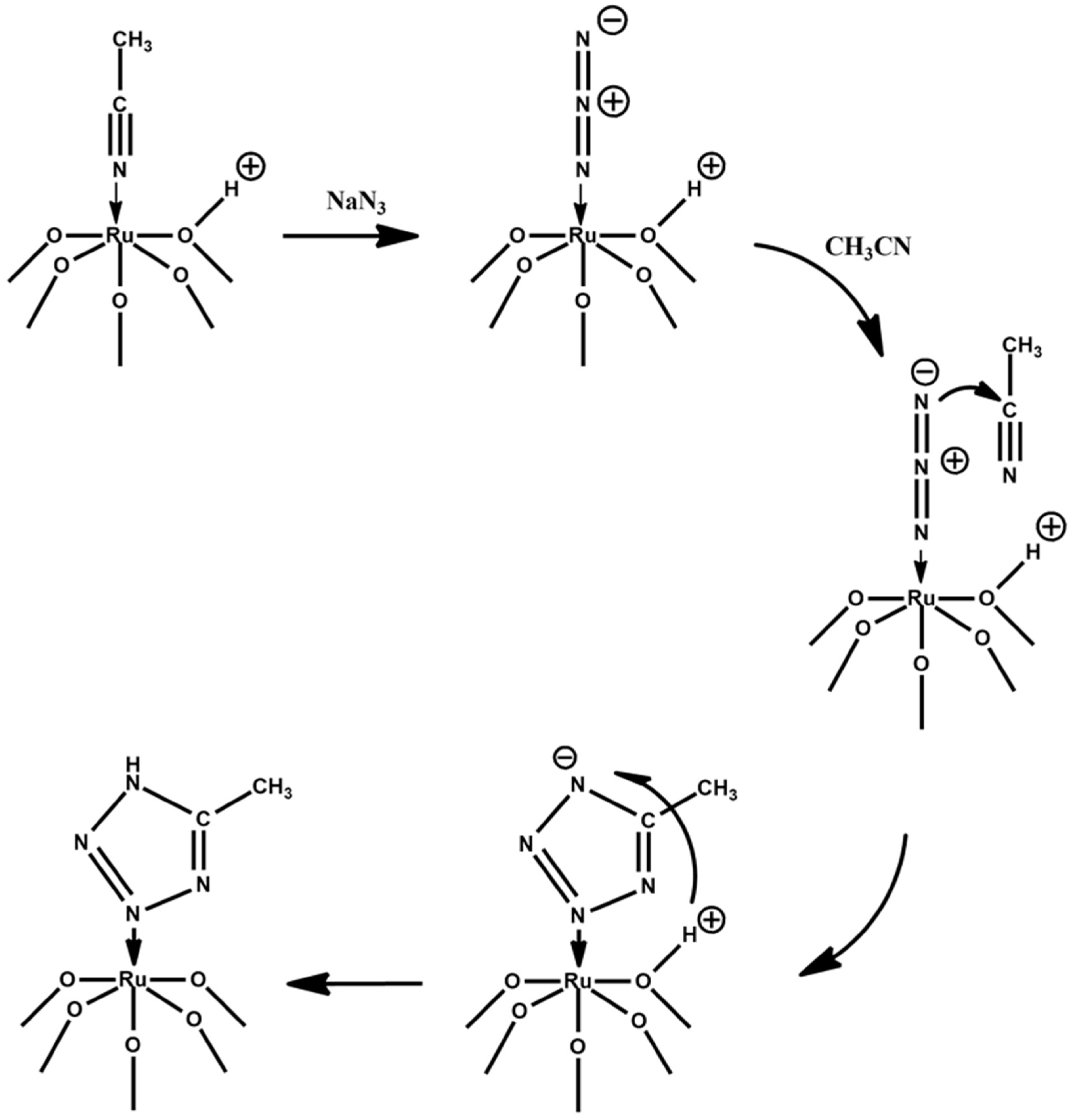
2. Results
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Izarova, N.V.; Pope, M.T.; Kortz, U. Noble metals in polyoxometalates. Angew. Chem. Int. Ed. 2012, 51, 9492–9510. [Google Scholar] [CrossRef]
- Putaj, P.; Lefebvre, F. Polyoxometalates containing late transition and noble metal atoms. Coord. Chem. Rev. 2011, 255, 1642–1685. [Google Scholar] [CrossRef]
- Bagno, A.; Bonchio, M.; Sartorel, A.; Scorrano, G. Microwave-Assisted Rapid Incorporation of Ruthenium into Lacunary Keggin-Type Polyoxotungstates: One-Step Synthesis, 99Ru, 183W NMR Characterization and Catalytic Activity of [PW11O39RuII(DMSO)]5−. Eur. J. Inorg. Chem. 2000, 2000, 17–20. [Google Scholar] [CrossRef]
- Neumann, R.; Abu-Gnim, C. Alkene oxidation catalyzed by a ruthenium-substituted heteropolyanion, SiRu(L)W11O39: The mechanism of the periodate-mediated oxidative cleavage. J. Am. Chem. Soc. 1990, 112, 6025–6031. [Google Scholar] [CrossRef]
- Steckhan, E.; Kandzia, C. Ruthenium-Catalysed, Electrochemical Cleavage of Aryl Olefins for the Synthesis of Benzaldehydes. Synlett 1992, 1992, 139–140. [Google Scholar] [CrossRef]
- Murakami, M.; Hong, D.; Suenobu, T.; Yamaguchi, S.; Ogura, T.; Fukuzumi, S. Catalytic Mechanism of Water Oxidation with Single-Site Ruthenium–Heteropolytungstate Complexes. J. Am. Chem. Soc. 2011, 133, 11605–11613. [Google Scholar] [CrossRef]
- Ogo, S.; Miyamoto, M.; Ide, Y.; Sano, T.; Sadakane, M. Hydrothermal and solid-state transformation of ruthenium-supported Keggin-type heteropolytungstates [XW11O39{Ru(ii)(benzene)(H2O)}]n− (X = P (n = 5), Si (n = 6), Ge (n = 6)) to ruthenium-substituted Keggin-type heteropolytungstates. Dalton Trans. 2012, 41, 9901–9907. [Google Scholar] [CrossRef]
- Sadakane, M.; Rinn, N.; Moroi, S.; Kitatomi, H.; Ozeki, T.; Kurasawa, M.; Itakura, M.; Hayakawa, S.; Kato, K.; Miyamoto, M.; et al. Preparation and structural characterization of RuII-DMSO and RuIII-DMSO− substituted α-Keggin-type phosphotungstates, [PW11O39RuIIDMSO]5− and [PW 11O39RuIIIDMSO]4−, and catalytic activity for water oxidation. Z. Anorg. Allg. Chem. 2011, 637, 1467–1474. [Google Scholar] [CrossRef]
- Sartorel, A.; Miró, P.; Carraro, M.; Berardi, S.; Bortolini, O.; Bagno, A.; Bo, C.; Bonchio, M. Oxygenation by Ruthenium Monosubstituted Polyoxotungstates in Aqueous Solution: Experimental and Computational Dissection of a Ru(III)-Ru(V) Catalytic Cycle. Chem. Eur. J. 2014, 20, 10932–10943. [Google Scholar] [CrossRef]
- Rong, C.; Pope, M.T. Lacunary polyoxometalate anions are. pi.-acceptor ligands. Characterization of some tungstoruthenate(II,III,IV,V) heteropolyanions and their atom-transfer reactivity. J. Am. Chem. Soc. 1992, 114, 2932–2938. [Google Scholar] [CrossRef]
- Yokoyama, A.; Ohkubo, K.; Ishizuka, T.; Kojima, T.; Fukuzumi, S. Remarkable enhancement of catalytic activity of a 2/: 1 complex between a non-planar Mo(v)–porphyrin and a ruthenium-substituted Keggin-type heteropolyoxometalate in catalytic oxidation of benzyl alcohols. Dalton Trans. 2012, 41, 10006–10013. [Google Scholar] [CrossRef]
- Bart, J.C.; Anson, F.C. Coordination, electron transfer and catalytic chemistry of a ruthenium-substituted heteropolytungstate anion as revealed in its electrochemical behavior. J. Electroanal. Chem. 1995, 390, 11–19. [Google Scholar] [CrossRef]
- Khenkin, A.M.; Efremenko, I.; Weiner, L.; Martin, J.M.L.; Neumann, R. Photochemical Reduction of Carbon Dioxide Catalyzed by a Ruthenium-Substituted Polyoxometalate. Chem. Eur. J. 2010, 16, 1356–1364. [Google Scholar] [CrossRef]
- Shi, D.; He, C.; Qi, B.; Chen, C.; Niu, J.; Duan, C. Merging of the photocatalysis and copper catalysis in metal–organic frameworks for oxidative C–C bond formation. Chem. Sci. 2015, 6, 1035–1042. [Google Scholar] [CrossRef]
- Sadakane, M.; Moroi, S.; Iimuro, Y.; Izarova, N.; Kortz, U.; Hayakawa, S.; Kato, K.; Ogo, S.; Ide, Y.; Ueda, W.; et al. Stabilization of high-valence ruthenium with silicotungstate ligands: Preparation, structural characterization, and redox studies of ruthenium(III)-substituted a-keggin-type silicotungstates with pyridine ligands, [SiW 11O 39Ru III(Py)] 5−. Chem. Asian J. 2012, 7, 1331–1339. [Google Scholar] [CrossRef]
- Ogo, S.; Moroi, S.; Ueda, T.; Komaguchi, K.; Hayakawa, S.; Ide, Y.; Sano, T.; Sadakane, M. Preparation of tetrabutylammonium salt of a mono-Ru(iii)-substituted α-Keggin-type silicotungstate with a 4,4′-bipyridine ligand and its electrochemical behaviour in organic solvents. Dalton Trans. 2013, 42, 7190. [Google Scholar] [CrossRef]
- Sadakane, M.; Tsukuma, D.; Dickman, M.H.; Bassil, B.; Kortz, U.; Higashijima, M.; Ueda, W. Structural characterization of mono-ruthenium substituted Keggin-type silicotungstates. Dalton Trans. 2006, 4271–4276. [Google Scholar] [CrossRef]
- Ogo, S.; Shimizu, N.; Ozeki, T.; Kobayashi, Y.; Ide, Y.; Sano, T.; Sadakane, M. Determination of α-Keggin structure of [GeW 11 O 39 Ru III (H 2 O)] 5−. Reaction of [GeW 11 O 39 Ru III (H 2 O)]5− with dimethyl sulfoxide to form [GeW 11 O 39 Ru III (dmso)]5− and their structural characterization. Dalton Trans. 2013, 42, 2540–2545. [Google Scholar] [CrossRef]
- Filipek, K. Synthesis, characterization and reactivity of ruthenium complexes of the lacunary Keggin polyoxoanion: [SiW11O39RuL]n−, L = H2O, NO, N2. Inorg. Chim. Acta 1995, 231, 237–239. [Google Scholar] [CrossRef]
- Sokolov, M.N.; Adonin, S.A.; Mainichev, D.A.; Sinkevich, P.L.; Vicent, C.; Kompankov, N.B.; Gushchin, A.L.; Nadolinny, V.A.; Fedin, V.P. New {RuNO} Polyoxometalate [PW 11 O 39 Ru II (NO)]4−/: Synthesis and Reactivity. Inorg. Chem. 2013, 52, 9675–9682. [Google Scholar] [CrossRef]
- Shmakova, A.A.; Volchek, V.V.; Abramov, P.A.; Sokolov, M.N. Reaction of K2[Ru(NO)Cl5] with K8[γ-SiW10O36] Under Hydrothermal Conditions: Synthesis of [SiW11O39{Ru(NO)}]5–. J. Struct. Chem. 2018, 59, 1427–1432. [Google Scholar] [CrossRef]
- Liu, B.; Yan, J.; Wang, Y.-F.; Yi, X.-Y. Redox chemistry of ruthenium ions in mono-substituted Keggin tungstophosphate: A new synthetic extension for ruthenium derivatives based on [PW 11 O 39 Ru VI N]4−. Dalton Trans. 2015, 44, 16882–16887. [Google Scholar] [CrossRef] [PubMed]
- Sadakane, M.; Iimuro, Y.; Tsukuma, D.; Bassil, B.S.; Dickman, M.H.; Kortz, U.; Zhang, Y.; Ye, S.; Ueda, W. Carbonyl-ruthenium substituted alpha-Keggin-tungstosilicate, [alpha-SiW(11)O(39)Ru(II)(CO)](6-): Synthesis, structure, redox studies and reactivity. Dalton Trans. 2008, 6692–6698. [Google Scholar] [CrossRef] [PubMed]
- Nishiki, K.; Ota, H.; Ogo, S.; Sano, T.; Sadakane, M. Preparation and Structural Characterization of Mono-Ru-Substituted α 2 -Dawson-Type Phosphotungstate with a Carbonyl Ligand and Other Ru(CO)-Substituted Heteropolytungstates. Eur. J. Inorg. Chem. 2015, 2015, 2714–2723. [Google Scholar] [CrossRef]
- Vicent, C.; Adonin, S.A.; Anyushin, A.V.; Mainichev, D.A.; Sokolov, M.N. Gas-Phase Fragmentation Reactions of Keggin-Type {PW 11 O 39 M} (M = Rh, Ir, and Ru) Polyoxometalates as Fingerprints of the Ligands Attached at the Noble Metal Site. Eur. J. Inorg. Chem. 2014, 2014, 5618–5624. [Google Scholar] [CrossRef]
- Sadakane, M.; Tsukuma, D.; Dickman, M.H.; Bassil, B.S.; Kortz, U.; Capron, M.; Ueda, W. Dimerization of mono-ruthenium substituted α-Keggin-type tungstosilicate [α-SiW 11 O 39 Ru III (H 2 O)]5− to µ-oxo-bridged dimer in aqueous solution: Synthesis, structure, and redox studies. Dalton Trans. 2007, 2833–2838. [Google Scholar] [CrossRef]
- Sadakane, M.; Higashijima, M. Synthesis and electrochemical behavior of [SiW11O39RuIII(H2O)]5– and its oxo-bridged dimeric complex [SiW11O39RuIVORuIIISiW11O39]11–. Dalton Trans. 2003, 659–664. [Google Scholar] [CrossRef]
- Sokolov, M.N.; Adonin, S.A.; Sinkevich, P.L.; Vicent, C.; Mainichev, D.A.; Fedin, V.P. Organometallic derivatives of Rh- and Ir-substituted polyoxotungstates with Keggin structure: Reactivity screening by electrospray ionization mass-spectrometry. Dalton Trans. 2012, 41, 9889–9892. [Google Scholar] [CrossRef]
- Sokolov, M.N.; Adonin, S.A.; Mainichev, D.A.; Vicent, C.; Zakharchuk, N.F.; Danilenko, A.M.; Fedin, V.P. Synthesis and characterization of [PW11O39Ir(H2O)]4−: Successful incorporation of Ir into polyoxometalate framework and study of the substitutional lability at the Ir(iii) site. Chem. Commun. 2011, 47, 7833–7835. [Google Scholar] [CrossRef]
- Sokolov, M.N.; Adonin, S.A.; Sinkevich, P.L.; Vicent, C.; Mainichev, D.A.; Fedin, V.P. Keggin-type Polyoxometalates [PW 11 O 39 M Cl]5− with Noble Metals (M = Rh and Ir): Novel Synthetic Entries and ESI-MS Directed Reactivity Screening. Z. Anorg. Allg. Chem. 2014, 640, 122–127. [Google Scholar] [CrossRef]
- Mukhacheva, A.A.; Shmakova, A.A.; Volchek, V.V.; Romanova, T.E.; Benassi, E.; Gushchin, A.L.; Yanshole, V.; Sheven, D.G.; Kompankov, N.B.; Abramov, P.A.; et al. Reactions of [Ru(NO)Cl5 ]2− with pseudotrilacunary {XW 9 O 33 }9− (X = As III, Sb III) anions. Dalton Trans. 2019, 48, 15989–15999. [Google Scholar] [CrossRef]
- Blackman, A. Reactions of Coordinated Ligands. In Advances in Heterocyclic Chemistry, V. 58; Academic Press: San Diego, CA, USA, 1993; pp. 123–170. [Google Scholar]
- Kukushkin, V.Y.; Tudela, D.; Pombeiro, A.J.L. Metal-ion assisted reactions of oximes and reactivity of oxime-containing metal complexes. Coord. Chem. Rev. 1996, 156, 333–362. [Google Scholar] [CrossRef]
- Bolotin, D.S.; Bokach, N.A.; Kukushkin, V.Y. Coordination chemistry and metal-involving reactions of amidoximes: Relevance to the chemistry of oximes and oxime ligands. Coord. Chem. Rev. 2016, 313, 62–93. [Google Scholar] [CrossRef]
- Bolotin, D.S. Reactions of Amidoximes with Metal-Activated Nitriles. Russ. J. Coord. Chem. 2018, 44, 243–251. [Google Scholar] [CrossRef]
- Bokach, N.A.; Kukushkin, V.Y. Addition of HO-nucleophiles to free and coordinated nitriles. Russ. Chem. Rev. 2005, 74, 153–170. [Google Scholar] [CrossRef]
- Kukushkin, V.Y.; Pombeiro, A.J.L. Additions to Metal-Activated Organonitriles†. Chem. Rev. 2002, 102, 1771–1802. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.D.; Dawoodi, Z.; Foust, D.F.; Segmueller, B.E. Polynuclear coordination and ligand activation. The structure, bonding, and desulfurization of thioformamido ligands about a trinuclear site. Organometallics 1983, 2, 315–323. [Google Scholar] [CrossRef]
- Adams, R.D.; Kiprotich, E.J.; Smith, M.D. Multiple cluster CH activations and transformations of furan by triosmium carbonyl complexes. Chem. Commun. 2018, 54, 3464–3467. [Google Scholar] [CrossRef]
- Afonin, M.Y.; Savkov, B.Y.; Virovets, A.V.; Korenev, V.S.; Golovin, A.V.; Maksakov, V.A. Transformation of Chlorohydrocarbons and Amines in the Coordination Sphere of [(µ-H)2Os3(CO)10]. Eur. J. Inorg. Chem. 2017, 2017, 3105–3114. [Google Scholar] [CrossRef]
- Afonin, M.J.; Maksakov, V.A.; Kirin, V.P.; Scheludyakova, L.A.; Golovin, A.V.; Vasil’ev, V.G. Transformations of allylamine and N-allylacetamide on triosmium cluster complexes with hemilabile ligands. Polyhedron 2009, 28, 2754–2758. [Google Scholar] [CrossRef]
- Whitmire, K.H. Iron Compounds without Hydrocarbon Ligands. In Comprehensive Organometallic Chemistry II.; Pergamon Press: London, UK, 1995; pp. 1–99. [Google Scholar]
- Adams, R.D.; Selegue, J.P. Osmium. In Comprehensive Organometallic Chemistry; Pergamon Press: London, UK, 1982; pp. 967–1064. [Google Scholar]
- Yersin, H.; Vogler, A. Photochemistry and Photophysics of Coordination Compounds; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 1987; ISBN 978-3-540-17808-8. [Google Scholar]
- Havrylyuk, D.; Deshpande, M.; Parkin, S.; Glazer, E.C. Ru (ii) complexes with diazine ligands: Electronic modulation of the coordinating group is key to the design of “dual action” photoactivated agents. Chem. Commun. 2018, 54, 12487–12490. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.P.; Ren, Y.; Berry, J.; Knott, S.A.; McLauchlan, C.C.; Szczepura, L.F. Small molecule activation of nitriles coordinated to the [Re 6 Se 8 ]2+ core: Formation of oxazine, oxazoline and carboxamide complexes. Dalton Trans. 2018, 47, 4653–4660. [Google Scholar] [CrossRef] [PubMed]
- Walther, D.; Ruben, M.; Rau, S. Carbon dioxide and metal centres: From reactions inspired by nature to reactions in compressed carbon dioxide as solvent. Coord. Chem. Rev. 1999, 182, 67–100. [Google Scholar] [CrossRef]
- Knowles, C.; Knowles, A. Standards in Absorption Spectrometry; Springer: Amsterdam, The Netherlands, 1981; ISBN 978-0-412-22470-6. [Google Scholar]
- Huisgen, R. Proceedings of the Chemical Society. October 1961. Proc. Chem. Soc. 1961, 357–396. [Google Scholar] [CrossRef]
- Meldal, M.; Tornøe, C.W. Cu-Catalyzed Azide−Alkyne Cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.; Xue, P.; Sun, H.H.Y.; Williams, I.D.; Sharpless, K.B.; Fokin, V.V.; Jia, G. Ruthenium-Catalyzed Cycloaddition of Alkynes and Organic Azides. J. Am. Chem. Soc. 2005, 127, 15998–15999. [Google Scholar] [CrossRef]
- Boren, B.C.; Narayan, S.; Rasmussen, L.K.; Zhang, L.; Zhao, H.; Lin, Z.; Jia, G.; Fokin, V.V. Ruthenium-Catalyzed Azide−Alkyne Cycloaddition: Scope and Mechanism. J. Am. Chem. Soc. 2008, 130, 8923–8930. [Google Scholar] [CrossRef]
- McNulty, J.; Keskar, K.; Vemula, R. The First Well-Defined Silver(I)-Complex-Catalyzed Cycloaddition of Azides onto Terminal Alkynes at Room Temperature. Chem. Eur. J. 2011, 17, 14727–14730. [Google Scholar] [CrossRef]
- McNulty, J.; Keskar, K. Discovery of a Robust and Efficient Homogeneous Silver(I) Catalyst for the Cycloaddition of Azides onto Terminal Alkynes. Eur. J. Org. Chem. 2012, 2012, 5462–5470. [Google Scholar] [CrossRef]
- Allen, A.D.; Senoff, C.V. Nitrogenopentammineruthenium(II) complexes. Chem. Commun. 1965, 621. [Google Scholar] [CrossRef]
- Ibers, J.A.; Davids, B.R. Bonding of molecular nitrogen. II. Crystal and molecular structure of azidodinitrogenbis(ethylenediamine)ruthenium(II) hexafluorophosphate. Inorg. Chem. 1970, 9, 2768–2774. [Google Scholar] [CrossRef]
- Chaudret, B.; Devillers, J.; Poilblanc, R. Preparation, characterization and X-ray crystal structure of Ru 2 H 6 N 2 (PPH 3) 4, a compound containing four bridging hydrides and a ruthenium–ruthenium double bond. J. Chem. Soc., Chem. Commun. 1983, 641–643. [Google Scholar] [CrossRef]
- Emelyanov, V.A.; Khranenko, S.P.; Belyaev, A.V. Nitrosation of ruthenium chloro complexes. Russ. J. Inorg. Chem. 2001, 46, 346–351. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukhacheva, A.A.; Gushchin, A.L.; Yanshole, V.V.; Abramov, P.A.; Sokolov, M.N. Easy Ligand Activation in the Coordination Sphere of Ru inside the [PW11O39]7– Backbone. Molecules 2020, 25, 1859. https://doi.org/10.3390/molecules25081859
Mukhacheva AA, Gushchin AL, Yanshole VV, Abramov PA, Sokolov MN. Easy Ligand Activation in the Coordination Sphere of Ru inside the [PW11O39]7– Backbone. Molecules. 2020; 25(8):1859. https://doi.org/10.3390/molecules25081859
Chicago/Turabian StyleMukhacheva, Anna A., Artem L. Gushchin, Vadim V. Yanshole, Pavel A. Abramov, and Maksim N. Sokolov. 2020. "Easy Ligand Activation in the Coordination Sphere of Ru inside the [PW11O39]7– Backbone" Molecules 25, no. 8: 1859. https://doi.org/10.3390/molecules25081859
APA StyleMukhacheva, A. A., Gushchin, A. L., Yanshole, V. V., Abramov, P. A., & Sokolov, M. N. (2020). Easy Ligand Activation in the Coordination Sphere of Ru inside the [PW11O39]7– Backbone. Molecules, 25(8), 1859. https://doi.org/10.3390/molecules25081859