Antiproliferative Properties of Papaver rhoeas Ovule Extracts and Derived Fractions Tested on HL60 Leukemia Human Cells
Abstract
1. Introduction
2. Results
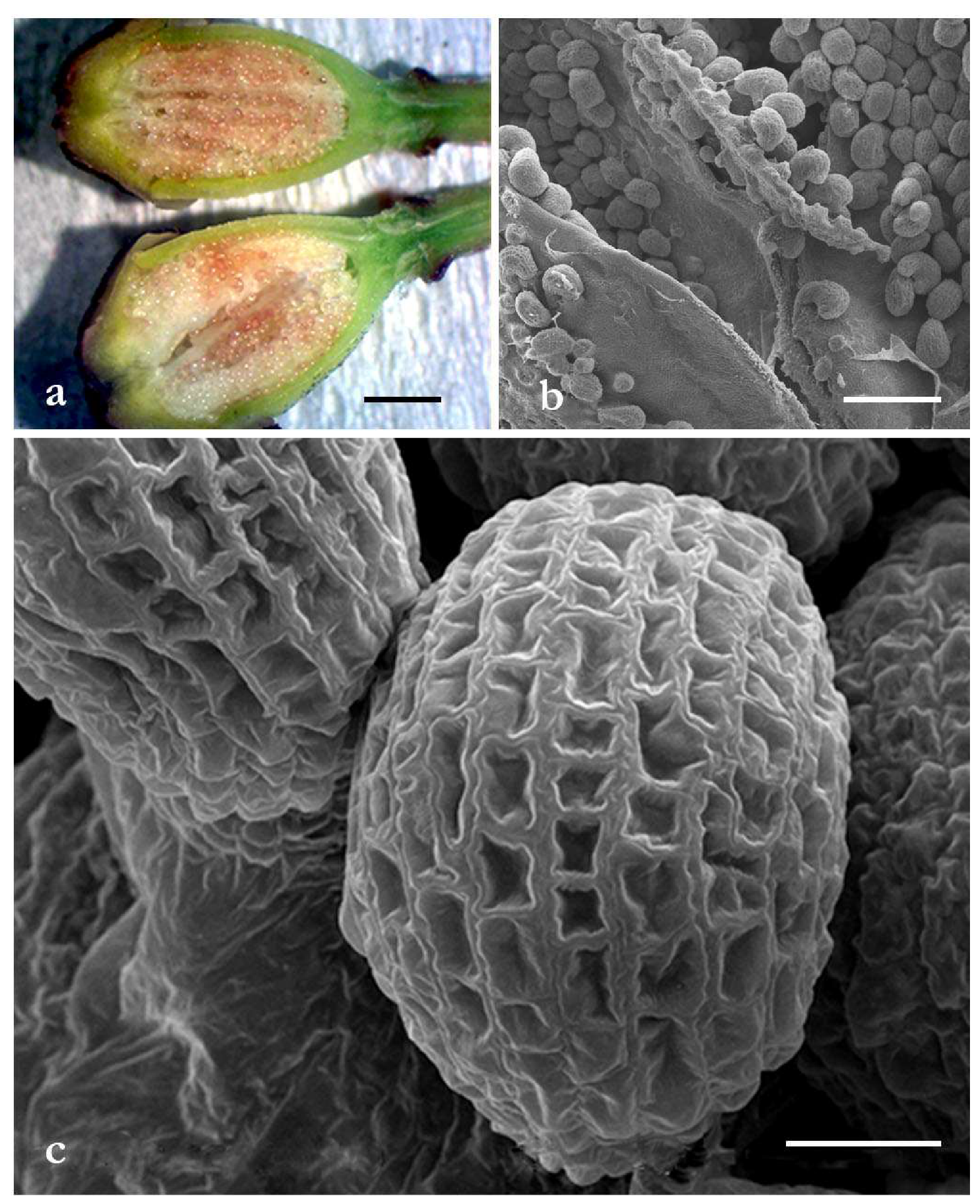
2.1. Optical Microscopy and Scanning Electron Microscopy Investigations of P. rhoeas Ovary and Ovules
2.2. Thin-Layer Chromatography Investigation and MTT Tests
2.3. GC-MS Investigation of Spots Showing Cytotoxic Activity
3. Discussion
4. Materials and Methods
4.1. Sample Preparation and Optical Microscopy and Scanning Electron Microscopy Investigations of P. rhoeas Ovules
4.2. P. rhoeas Ovule Extract Preparation and Thin-Layer Chromatography (TLC) Investigations
4.3. HL60 Cell Culture and MTT Assay
4.4. GC-MS Chemical Investigations
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Bernardini, S.; Tiezzi, A.; Laghezza Masci, V.; Ovidi, E. Natural products for human health: An historical overview of the drug discovery approaches. Nat. Prod. Res. 2017, 6419, 1–25. [Google Scholar] [CrossRef]
- Bernardini, S.; Osorio, M.S.; Tiezzi, A. Plants: An Infinite Source of Molecules Useful for Pharmaceuticals. Curr. Tradit. Med. 2018, 4, 157–165. [Google Scholar] [CrossRef]
- Günaydin, Y.K.; Dündar, Z.D.; Çekmen, B.; Akilli, N.B.; Köylü, R.; Cander, B. Intoxication due to Papaver rhoeas (Corn Poppy): Five case reports. Case Rep. Med. 2015, 2015. [Google Scholar] [CrossRef]
- Çoban, İ.; Toplan, G.G.; Özbek, B.; Gürer, Ç.U.; Sarıyar, G. Variation of alkaloid contents and antimicrobial activities of Papaver rhoeas L. growing in Turkey and northern Cyprus. Pharm. Biol. 2017, 55, 1894–1898. [Google Scholar] [CrossRef]
- Oh, J.H.; Ha, I.J.; Lee, M.Y.; Kim, E.O.; Park, D.; Lee, J.H.; Lee, S.G.; Kim, D.W.; Lee, T.H.; Lee, E.J.; et al. Identification and metabolite profiling of alkaloids in aerial parts of Papaver rhoeas by liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry. J. Sep. Sci. 2018, 41, 2517–2527. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Eaves, D.J.; Sanchez-Moran, E.; Franklin, F.C.H.; Franklin-Tong, V.E. The Papaver rhoeas S determinants confer self-incompatibility to Arabidopsis thaliana in planta. Science 2015, 350, 684–687. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, V.R.M.; Carrera, I.; Cacabelos, R. In vitro screening for cytotoxic activity of herbal extracts. Evid. Based Complement. Altern. Med. 2017, 2017. [Google Scholar] [CrossRef]
- Conforti, F.; Ioele, G.; Statti, G.A.; Marrelli, M.; Ragno, G.; Menichini, F. Antiproliferative activity against human tumor cell lines and toxicity test on Mediterranean dietary plants. Food Chem. Toxicol. 2008, 46, 3325–3332. [Google Scholar] [CrossRef] [PubMed]
- Hasplova, K.; Hudecova, A.; Miadokova, E.; Magdolenova, Z.; Galova, E.; Vaculcikova, L.; Gregan, F.; Dusinska, M. Biological activity of plant extract isolated from Papaver rhoeas on human lymfoblastoid cell line. Neoplasma 2011, 58, 386–391. [Google Scholar] [CrossRef]
- Manayi, A.; Kurepaz-Mahmoodabadi, M.; Gohari, A.R.; Ajani, Y.; Saeidnia, S. Presence of phthalate derivatives in the essential oils of a medicinal plant Achillea tenuifolia. DARU J. Pharm. Sci. 2014, 22, 78. [Google Scholar] [CrossRef]
- Bai, S.; Bharti, P.; Seasotiya, L.; Malik, A.; Dalal, S. GC-MS analysis of chloroform extract of Acacia nilotica L. leaves. J. Pharmacogn. Phytochem. 2014, 2, 79–82. [Google Scholar]
- Mikautadze, E.; Avaliani, N.; Kuchiashvili, N.; Nozadze, M.; Kiguradze, T.; Pkhakadze, V.; Mamulaishvili, I.; Mikeladze, E.; Solomonia, R. Anti-epileptic properties of oleamide. BMC Proc. 2008, 2, P42. [Google Scholar] [CrossRef]
- Mattern, K.L.; Evans, C.A.; Lara, J.C. Selective antibacterial action of 2-mercaptoethanol on propionibacteria in skin cultures. Appl. Environ. Microbiol. 1979, 37, 177–179. [Google Scholar] [CrossRef]
- Malek, S.N.A.; Shin, S.K.; Wahab, N.A.; Yaacob, H. Cytotoxic components of Pereskia bleo (Kunth) DC. (Cactaceae) leaves. Molecules 2009, 14, 1713. [Google Scholar] [CrossRef]
- Varsha, K.K.; Devendra, L.; Shilpa, G.; Priya, S.; Pandey, A.; Nampoothiri, K.M. 2,4-Di-tert-butyl phenol as the antifungal, antioxidant bioactive purified from a newly isolated Lactococcus sp. Int. J. Food Microbiol. 2015, 211, 44–50. [Google Scholar] [CrossRef]
- Belghit, S.; Driche, E.H.; Bijani, C.; Zitouni, A.; Sabaou, N.; Badji, B.; Mathieu, F. Activity of 2,4-Di-tert-butylphenol produced by a strain of Streptomyces mutabilis isolated from a Saharan soil against Candida albicans and other pathogenic fungi. J. Mycol. Med. 2016, 26, 160–169. [Google Scholar] [CrossRef]
- Kadoma, Y.; Ito, S.; Atsumi, T.; Fujisawa, S. Mechanisms of cytotoxicity of 2- or 2,6-di-tert-butylphenols and 2-methoxyphenols in terms of inhibition rate constant and a theoretical parameter. Chemosphere 2009, 74, 626–632. [Google Scholar] [CrossRef]
- Konoz, E.; Abbasi, A.; Parastar, H.; Moazeni, R.S.; Jalali-Heravi, M. Analysis of olive fruit essential oil: Application of gas chromatography-mass spectrometry combined with chemometrics. Int. J. Food Prop. 2015, 18, 316–331. [Google Scholar] [CrossRef][Green Version]
- Abbey, M.; Nestel, P.J. Plasma cholesteryl ester transfer protein activity is increased when trans-elaidic acid is substituted for cis-oleic acid in the diet. Atherosclerosis 1994, 106, 99–107. [Google Scholar] [CrossRef]
- Agoramoorthy, G.; Chandrasekaran, M.; Venkatesalu, V.; Hsu, M.J. Antibacterial and antifungal activities of fatty acid methyl esters of the blind-your-eye mangrove from India. Brazilian J. Microbiol. 2007, 38, 739–742. [Google Scholar] [CrossRef]
- Ponnamma, S.U.; Manjunath, K. GC-MS analysis of phytocomponents in the methanolic extract of Justicia wynaadensis (NEES) T. Anders. Int. J. Pharma Bio Sci. 2012, 3, 570–576. [Google Scholar]
- Aparna, V.; Dileep, K.V.; Mandal, P.K.; Karthe, P.; Sadasivan, C.; Haridas, M. Anti-Inflammatory Property of n-Hexadecanoic Acid: Structural Evidence and Kinetic Assessment. Chem. Biol. Drug Des. 2012, 80, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Rahuman, A.A.; Gopalakrishnan, G.; Ghouse, B.S.; Arumugam, S.; Himalayan, B. Effect of Feronia limonia on mosquito larvae. Fitoterapia 2000, 71, 553–555. [Google Scholar] [CrossRef]
- Ravi, L.; Krishnan, K. Cytotoxic Potential of N-hexadecanoic Acid Extracted from Kigelia pinnata Leaves. Asian J. Cell Biol. 2016, 12, 20–27. [Google Scholar]
- Jananie, R.; Priya, V.; Vijayalakshmi, K. Determination of bioactive components of Cynodon dactylon by GC-MS analysis. New York Sci. J. 2011, 4, 16–20. [Google Scholar]
- Donoso-Fierro, C.; Tiezzi, A.; Ovidi, E.; Ceccarelli, D.; Triggiani, D.; Mastrogiovanni, F.; Taddei, A.R.; Pérez, C.; Becerra, J.; Silva, M.; et al. Antiproliferative activity of yatein isolated from Austrocedrus chilensis against murine myeloma cells: Cytological studies and chemical investigations. Pharm. Biol. 2015, 53, 378–385. [Google Scholar] [CrossRef]
- Garzoli, S.; Masci, V.L.; Ovidi, E.; Turchetti, G.; Zago, D.; Tiezzi, A. Chemical Investigation of a Biologically Active Schinus molle L. Leaf Extract. J. Anal. Methods Chem. 2019, 2019. [Google Scholar] [CrossRef]
Sample Availability: Samples are available from the authors. |
| Samples | EC50 µg/mL (Mean ± SD) |
|---|---|
| Extract | 119.233 ± 42.755 |
| S6 | 127.363 ± 41.932 |
| S7 | 5.235 ± 1.501 |
| S8 | 12.100 ± 0.823 |
| VBL | 0.019 ± 0.002 |
| Component | LRI 1 | LRIlit 2 | S6 (%) | S7 (%) | S8 (%) |
|---|---|---|---|---|---|
| hexanal | 1075 | 1081 | - | - | 0.80 ± 0.14 |
| 2-heptenal, (Z)- | 1280 | 1287 | - | - | 1.78 ± 0.03 |
| 3,5-octadien-2-ol | 1480 | * | - | - | 10.40 ± 0.14 |
| 1-hexanol,2-ethyl- | 1491 | 1494 | 3.93 ± 0.03 | 0.68 ± 0.03 | - |
| 2-mercaptoethanol | 1495 | 1498 ° | 15.00 ± 0.28 | 1.38 ± 0.03 | - |
| hexacosane | 1505 | * | - | 7.90 ± 0.14 | - |
| 2-decenal, (E)- | 1645 | 1650 | - | - | 3.40 ± 0.28 |
| hexadecanoic acid, methyl ester | 2246 | 2251 | - | - | 3.15 ± 0.21 |
| hexadecanoic acid, ethyl ester | 2280 | 2288 | - | 2.65 ± 0.21 | 15.90 ± 0.14 |
| 2,4-di-tert-butylphenol | 2317 | 2321 | - | 2.50 ± 0.14 | - |
| 2,6-di-tert-butylphenol | 2327 + | - | - | 29.95 ± 0.21 | |
| diethylene glycol hexyl ether | 2400 | * | 21.95 ± 0.21 | 12.40 ± 0.14 | - |
| elaidic acid, methyl ester | 2445 + | - | - | 22.15 ± 0.35 | |
| 9,12-octadienoic acid, ethyl ester | 2555 | 2560 | - | 22.50 ± 1.41 | - |
| dibutyl phthalate | 2610 | 2618 | 4.00 ± 0.14 | - | - |
| n-hexadecanoic acid | 2920 | 2928 | - | - | 11.75 ± 0.21 |
| dodecanoic acid, 3-hydroxy | 3000 | * | - | 8.90 ± 0.28 | - |
| 9-octadecenamide, (Z)- | 3260 | 3265 + | - | 17.15 ± 0.49 | - |
| 2-ethylhexyl fumarate | 3300 | * | 53.90 ± 1.13 | - | - |
| dipalmitin | 3500 | * | - | 22.30 ± 0.56 | - |
| Sum | 98.78 | 98.36 | 99.28 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ovidi, E.; Laghezza Masci, V.; Garzoli, S.; Gambellini, G.; Keita, S.V.; Zago, D.; Turchetti, G.; Modesti, L.; Tiezzi, A. Antiproliferative Properties of Papaver rhoeas Ovule Extracts and Derived Fractions Tested on HL60 Leukemia Human Cells. Molecules 2020, 25, 1850. https://doi.org/10.3390/molecules25081850
Ovidi E, Laghezza Masci V, Garzoli S, Gambellini G, Keita SV, Zago D, Turchetti G, Modesti L, Tiezzi A. Antiproliferative Properties of Papaver rhoeas Ovule Extracts and Derived Fractions Tested on HL60 Leukemia Human Cells. Molecules. 2020; 25(8):1850. https://doi.org/10.3390/molecules25081850
Chicago/Turabian StyleOvidi, Elisa, Valentina Laghezza Masci, Stefania Garzoli, Gabriella Gambellini, Saran Vittoria Keita, Daniele Zago, Giovanni Turchetti, Lorenzo Modesti, and Antonio Tiezzi. 2020. "Antiproliferative Properties of Papaver rhoeas Ovule Extracts and Derived Fractions Tested on HL60 Leukemia Human Cells" Molecules 25, no. 8: 1850. https://doi.org/10.3390/molecules25081850
APA StyleOvidi, E., Laghezza Masci, V., Garzoli, S., Gambellini, G., Keita, S. V., Zago, D., Turchetti, G., Modesti, L., & Tiezzi, A. (2020). Antiproliferative Properties of Papaver rhoeas Ovule Extracts and Derived Fractions Tested on HL60 Leukemia Human Cells. Molecules, 25(8), 1850. https://doi.org/10.3390/molecules25081850









